Post-Transplant Cyclophosphamide Combined with Brilliant Blue G Reduces Graft-versus-Host Disease without Compromising Graft-versus-Leukaemia Immunity in Humanised Mice
Abstract
1. Introduction
2. Results
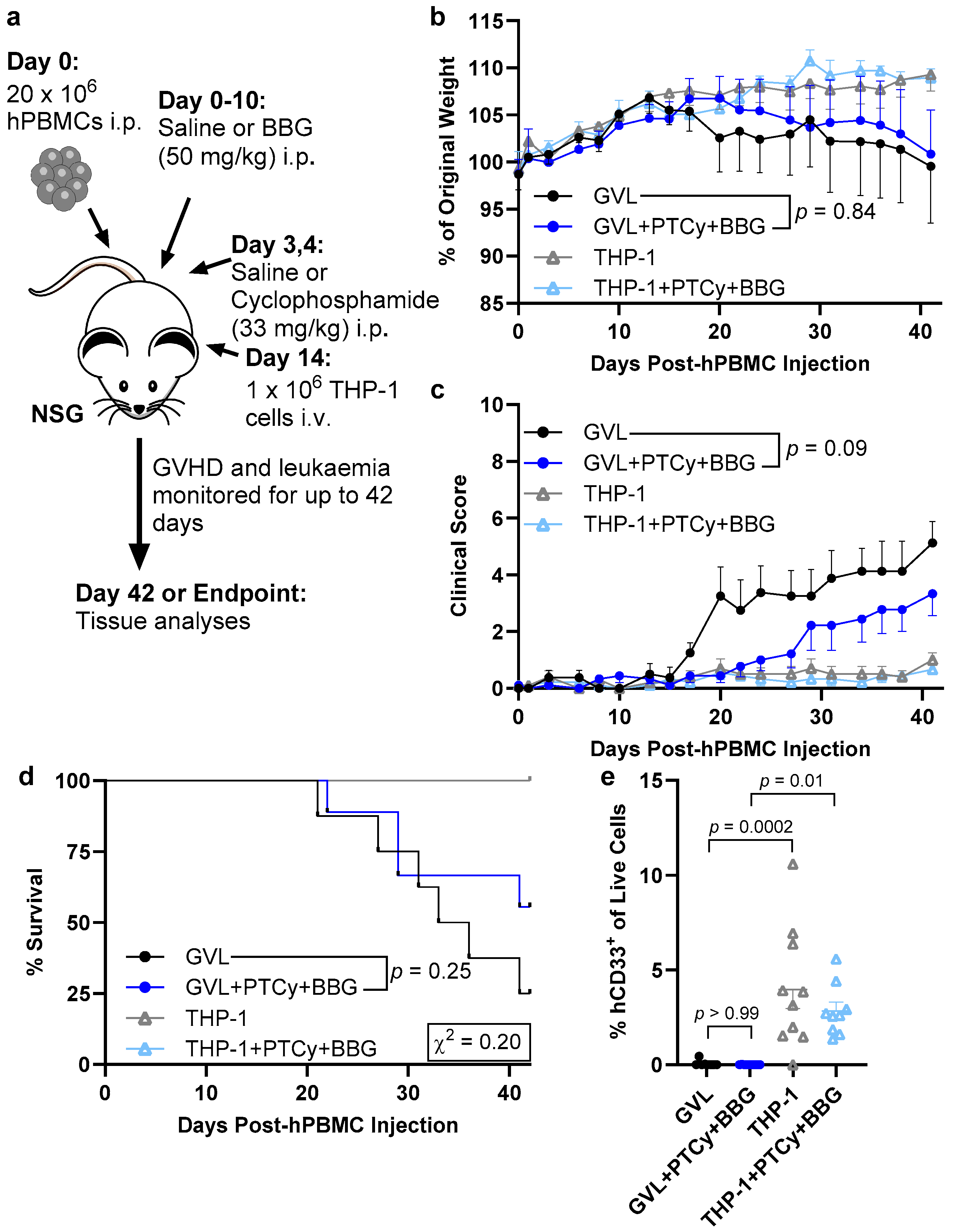
2.1. PTCy with BBG Does Not Reduce Clinical GVHD Compared to PTCy Alone
2.2. PTCy with BBG Reduces Liver GVHD Compared to PTCy Alone at Endpoint
2.3. PTCy with BBG Reduces Serum Human Interferon-γ Concentrations Compared to PTCy Alone at Endpoint
2.4. PTCy with BBG Increases Human CD39+ Tregs Compared to PTCy Alone on Day 21
2.5. PTCy with BBG Does Not Compromise GVL Immunity
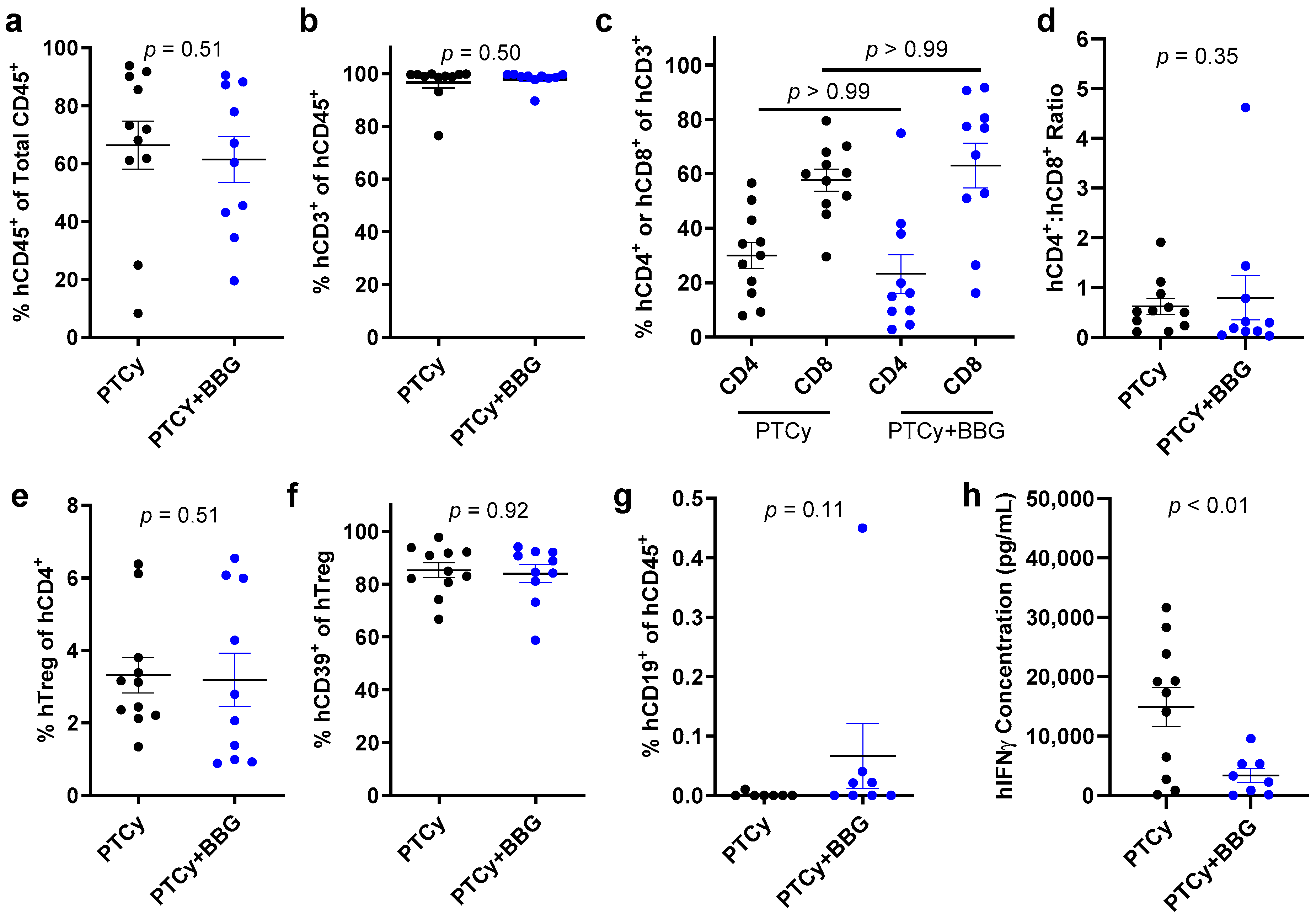
2.6. PTCy with BBG Does Not Alter Proportions of Human Immune Cell Subsets Compared to PTCy in GVL Immunity at Endpoint
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Human Cells
4.3. NSG Mice
4.4. Humanised Mouse Model of GVHD
4.5. Humanised Mouse Model of GVL Immunity
4.6. Histological Analysis of Tissues
4.7. Flow Cytometric Analysis of Cells
4.8. Flow Cytometric Analysis of Human Cytokines
4.9. Data Presentation and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niederwieser, D.; Baldomero, H.; Bazuaye, N.; Bupp, C.; Chaudhri, N.; Corbacioglu, S.; Elhaddad, A.; Frutos, C.; Galeano, S.; Hamad, N.; et al. One and a half million hematopoietic stem cell transplants: Continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica 2022, 107, 1045–1053. [Google Scholar] [CrossRef]
- Blazar, B.R.; Hill, G.R.; Murphy, W.J. Dissecting the biology of allogeneic HSCT to enhance the GvT effect whilst minimizing GvHD. Nat. Rev. Clin. Oncol. 2020, 17, 475–492. [Google Scholar] [CrossRef]
- Shouval, R.; Fein, J.A.; Labopin, M.; Kröger, N.; Duarte, R.F.; Bader, P.; Chabannon, C.; Kuball, J.; Basak, G.W.; Dufour, C.; et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: A European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019, 6, e573–e584. [Google Scholar] [CrossRef]
- Jiang, H.; Fu, D.; Bidgoli, A.; Paczesny, S. T Cell Subsets in Graft Versus Host Disease and Graft Versus Tumor. Front. Immunol. 2021, 12, 761448. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, N.; Zhang, X.; Cao, Y.; Zhang, L.; Liu, A.; Zhang, Y. Post-transplantation cyclophosphamide as GVHD prophylaxis in allogenic hematopoietic stem cell transplantation: Recent advances and modification. Blood Rev. 2023, 62, 101078. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, P.; Geraghty, N.J.; Adhikary, S.R.; Bird, K.M.; Fuller, S.J.; Watson, D.; Sluyter, R. Purinergic Signalling in Allogeneic Haematopoietic Stem Cell Transplantation and Graft-versus-Host Disease. Int. J. Mol. Sci. 2021, 22, 8343. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R.; Cuthbertson, P.; Elhage, A.; Sligar, C.; Watson, D. Purinergic signalling in graft-versus-host disease. Curr. Opin. Pharmacol. 2023, 68, 102346. [Google Scholar] [CrossRef] [PubMed]
- Elhage, A.; Cuthbertson, P.; Sligar, C.; Watson, D.; Sluyter, R. A Species-Specific Anti-Human P2X7 Monoclonal Antibody Reduces Graft-versus-Host Disease in Humanised Mice. Pharmaceutics 2023, 15, 2263. [Google Scholar] [CrossRef]
- Fowler, B.J.; Gelfand, B.D.; Kim, Y.; Kerur, N.; Tarallo, V.; Hirano, Y.; Amarnath, S.; Fowler, D.H.; Radwan, M.; Young, M.T.; et al. Nucleoside reverse transcriptase inhibitors possess intrinsic anti-inflammatory activity. Science 2014, 346, 1000–1003. [Google Scholar] [CrossRef]
- Geraghty, N.J.; Belfiore, L.; Ly, D.; Adhikary, S.R.; Fuller, S.J.; Varikatt, W.; Sanderson-Smith, M.L.; Sluyter, V.; Alexander, S.I.; Sluyter, R.; et al. The P2X7 receptor antagonist Brilliant Blue G reduces serum human interferon-γ in a humanized mouse model of graft-versus-host disease. Clin. Exp. Immunol. 2017, 190, 79–95. [Google Scholar] [CrossRef]
- Koehn, B.H.; Saha, A.; McDonald-Hyman, C.; Loschi, M.; Thangavelu, G.; Ma, L.; Zaiken, M.; Dysthe, J.; Krepps, W.; Panthera, J.; et al. Danger-associated extracellular ATP counters MDSC therapeutic efficacy in acute GVHD. Blood 2019, 134, 1670–1682. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.; Ganesan, J.; Müller, T.; Dürr, C.; Grimm, M.; Beilhack, A.; Krempl, C.D.; Sorichter, S.; Gerlach, U.V.; Jüttner, E.; et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010, 16, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Barrera-Avalos, C.; Briceño, P.; Valdés, D.; Imarai, M.; Leiva-Salcedo, E.; Rojo, L.E.; Milla, L.A.; Huidobro-Toro, J.P.; Robles-Planells, C.; Escobar, A.; et al. P2X7 receptor is essential for cross-dressing of bone marrow-derived dendritic cells. iScience 2021, 24, 103520. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.V.; Li, Y.; Liu, X.; Xia, S.; Shi, P.; Li, L.; Chen, Z.; Yin, C.; Eriguchi, M.; Chen, Y.; et al. ATP release drives heightened immune responses associated with hypertension. Sci. Immunol. 2019, 4, eaau6426. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; La Sala, A.; Chiozzi, P.; Morelli, A.; Falzoni, S.; Girolomoni, G.; Idzko, M.; Dichmann, S.; Norgauer, J.; Di Virgilio, F. The P2 purinergic receptors of human dendritic cells: Identification and coupling to cytokine release. FASEB J. 2000, 14, 2466–2476. [Google Scholar] [CrossRef]
- Amores-Iniesta, J.; Barberà-Cremades, M.; Martínez, C.M.; Pons, J.A.; Revilla-Nuin, B.; Martínez-Alarcón, L.; Di Virgilio, F.; Parrilla, P.; Baroja-Mazo, A.; Pelegrín, P. Extracellular ATP Activates the NLRP3 Inflammasome and Is an Early Danger Signal of Skin Allograft Rejection. Cell Rep. 2017, 21, 3414–3426. [Google Scholar] [CrossRef]
- Yang, Y.; Story, M.E.; Hao, X.; Sumpter, T.L.; Mathers, A.R. P2X7 Receptor Expression and Signaling on Dendritic Cells and CD4(+) T Cells is Not Required but Can Enhance Th17 Differentiation. Front. Cell Dev. Biol. 2022, 10, 687659. [Google Scholar] [CrossRef]
- Schenk, U.; Frascoli, M.; Proietti, M.; Geffers, R.; Traggiai, E.; Buer, J.; Ricordi, C.; Westendorf, A.M.; Grassi, F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 2011, 4, ra12. [Google Scholar] [CrossRef]
- Elhage, A.; Sligar, C.; Cuthbertson, P.; Watson, D.; Sluyter, R. Insights into mechanisms of graft-versus-host disease through humanised mouse models. Biosci. Rep. 2022, 42, BSR20211986. [Google Scholar] [CrossRef]
- Adhikary, S.R.; Cuthbertson, P.; Nicholson, L.; Bird, K.M.; Sligar, C.; Hu, M.; O’Connell, P.J.; Sluyter, R.; Alexander, S.I.; Watson, D. Post-transplant cyclophosphamide limits reactive donor T cells and delays the development of graft-versus-host disease in a humanized mouse model. Immunology 2021, 164, 332–347. [Google Scholar] [CrossRef]
- Sligar, C.; Cuthbertson, P.; Miles, N.A.; Adhikary, S.R.; Elhage, A.; Zhang, G.; Alexander, S.I.; Sluyter, R.; Watson, D. Tocilizumab increases regulatory T cells, reduces natural killer cells and delays graft-versus-host disease development in humanized mice treated with post-transplant cyclophosphamide. Immunol. Cell Biol. 2023, 101, 639–656. [Google Scholar] [CrossRef]
- Cuthbertson, P.; Geraghty, N.J.; Adhikary, S.R.; Casolin, S.; Watson, D.; Sluyter, R. P2X7 receptor antagonism increases regulatory T cells and reduces clinical and histological graft-versus-host disease in a humanised mouse model. Clin. Sci. 2021, 135, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Cuthbertson, P.; Adhikary, S.R.; Geraghty, N.J.; Guy, T.V.; Hadjiashrafi, A.; Fuller, S.J.; Ly, D.; Watson, D.; Sluyter, R. Increased P2X7 expression in the gastrointestinal tract and skin in a humanised mouse model of graft-versus-host disease. Clin. Sci. 2020, 134, 207–223. [Google Scholar] [CrossRef]
- Borsellino, G.; Kleinewietfeld, M.; Di Mitri, D.; Sternjak, A.; Diamantini, A.; Giometto, R.; Höpner, S.; Centonze, D.; Bernardi, G.; Dell’Acqua, M.L.; et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 2007, 110, 1225–1232. [Google Scholar] [CrossRef]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Ritacco, C.; Köse, M.C.; Courtois, J.; Canti, L.; Beguin, C.; Dubois, S.; Vandenhove, B.; Servais, S.; Caers, J.; Beguin, Y.; et al. Post-transplant cyclophosphamide prevents xenogeneic graft-versus-host disease while depleting proliferating regulatory T cells. iScience 2023, 26, 106085. [Google Scholar] [CrossRef]
- Yang, P.; Freeman, Z.T.; Dysko, R.C.; Hoenerhoff, M.J. Degenerative Myelopathy and Neuropathy in NOD.Cg-Prkdc(scid) Il2rg(tm1Wjl)/SzJ (NSG) Mice Caused by Lactate Dehydrogenase-Elevating Virus (LDV). Toxicol. Pathol. 2022, 50, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Pallarès, V.; Núñez, Y.; Sánchez-García, L.; Falgàs, A.; Serna, N.; Unzueta, U.; Gallardo, A.; Alba-Castellón, L.; Álamo, P.; Sierra, J.; et al. Antineoplastic effect of a diphtheria toxin-based nanoparticle targeting acute myeloid leukemia cells overexpressing CXCR4. J. Control. Release 2021, 335, 117–129. [Google Scholar] [CrossRef]
- Lewis, D.I. Animal experimentation: Implementation and application of the 3Rs. Emerg. Top. Life Sci. 2019, 3, 675–679. [Google Scholar]
- Gilman, K.E.; Cracchiolo, M.J.; Matiatos, A.P.; Davini, D.W.; Simpson, R.J.; Katsanis, E. Partially replacing cyclophosphamide with bendamustine in combination with cyclosporine A improves survival and reduces xenogeneic graft-versus-host-disease. Front. Immunol. 2023, 13, 1045710. [Google Scholar] [CrossRef]
- Wachsmuth, L.P.; Patterson, M.T.; Eckhaus, M.A.; Venzon, D.J.; Kanakry, C.G. Optimized Timing of Post-Transplantation Cyclophosphamide in MHC-Haploidentical Murine Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2020, 26, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Markey, K.A.; MacDonald, K.P.; Hill, G.R. The biology of graft-versus-host disease: Experimental systems instructing clinical practice. Blood 2014, 124, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.E.; Nunes, N.S.; Patterson, M.T.; Vinod, N.; Khan, S.M.; Mendu, S.K.; Li, X.; de Paula Pohl, A.; Wachsmuth, L.P.; Choo-Wosoba, H.; et al. Posttransplantation cyclophosphamide expands functional myeloid-derived suppressor cells and indirectly influences Tregs. Blood Adv. 2023, 7, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhu, F.; Qiao, J.; Zhao, K.; Zhu, S.; Zeng, L.; Chen, X.; Xu, K. The impact of P2X7 receptor antagonist, brilliant blue G on graft-versus-host disease in mice after allogeneic hematopoietic stem cell transplantation. Cell. Immunol. 2016, 310, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, N.J.; Watson, D.; Sluyter, R. Long-term treatment with the P2X7 receptor antagonist Brilliant Blue G reduces liver inflammation in a humanized mouse model of graft-versus-host disease. Cell. Immunol. 2019, 336, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Zhai, N.; Liu, W.; Jin, C.H.; Ding, Y.; Sun, L.; Zhang, D.; Wang, Z.; Tang, Y.; Zhao, W.; LeGuern, C.; et al. Lack of IFN-γ Receptor Signaling Inhibits Graft-versus-Host Disease by Potentiating Regulatory T Cell Expansion and Conversion. J. Immunol. 2023, 211, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Brock, N.; Hohorst, H.J. Metabolism of cyclophosphamide. Cancer 1967, 20, 900–904. [Google Scholar] [CrossRef]
- Gu, J.; Chen, C.S.; Wei, Y.; Fang, C.; Xie, F.; Kannan, K.; Yang, W.; Waxman, D.J.; Ding, X. A mouse model with liver-specific deletion and global suppression of the NADPH-cytochrome P450 reductase gene: Characterization and utility for in vivo studies of cyclophosphamide disposition. J. Pharmacol. Exp. Ther. 2007, 321, 9–17. [Google Scholar] [CrossRef]
- Modi, D.; Ye, J.C.; Surapaneni, M.; Singh, V.; Chen, W.; Jang, H.; Deol, A.; Ayash, L.; Alavi, A.; Ratanatharathorn, V.; et al. Liver Graft-Versus-Host Disease is associated with poor survival among allogeneic hematopoietic stem cell transplant recipients. Am. J. Hematol. 2019, 94, 1072–1080. [Google Scholar] [CrossRef]
- Donnelly-Roberts, D.L.; Namovic, M.T.; Han, P.; Jarvis, M.F. Mammalian P2X7 receptor pharmacology: Comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 2009, 157, 1203–1214. [Google Scholar] [CrossRef]
- Pukhalsky, A.L.; Toptygina, A.P.; Viktorov, V.V. Pharmacokinetics of alkylating metabolites of cyclophosphamide in different strains of mice. Int. J. Immunopharmacol. 1990, 12, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Said, R.; Abdel-Rehim, M.; Sadeghi, B.; Al-Hashemi, S.; Hassan, Z.; Hassan, M. Cyclophosphamide Pharmacokinetics in Mice: A Comparison Between Retro Orbital Sampling Versus Serial Tail Vein Bleeding. Open Pharmacol. J. 2007, 1, 30–35. [Google Scholar]
- Gadeock, S.; Tran, J.N.; Georgiou, J.G.; Jalilian, I.; Taylor, R.M.; Wiley, J.S.; Sluyter, R. TGF-β1 prevents up-regulation of the P2X7 receptor by IFN-γ and LPS in leukemic THP-1 monocytes. Biochim. Biophys. Acta 2010, 1798, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, B.D.; Dubyak, G.R. Induction of the P2z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-gamma in the human THP-1 monocytic cell line. J. Immunol. 1996, 157, 5627–5637. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wan, J.; Yang, X.; Zhang, X.; Huang, D.; Li, X.; Zou, Y.; Chen, C.; Yu, Z.; Xie, L.; et al. Bone marrow niche ATP levels determine leukemia-initiating cell activity via P2X7 in leukemic models. J. Clin. Invest. 2021, 131, e140242. [Google Scholar] [CrossRef]
- Pegoraro, A.; Orioli, E.; De Marchi, E.; Salvestrini, V.; Milani, A.; Di Virgilio, F.; Curti, A.; Adinolfi, E. Differential sensitivity of acute myeloid leukemia cells to daunorubicin depends on P2X7A versus P2X7B receptor expression. Cell Death Dis. 2020, 11, 876. [Google Scholar] [CrossRef]
- Watson, D.; Adhikary, S.R.; Cuthbertson, P.; Geraghty, N.J.; Bird, K.M.; Elhage, A.; Sligar, C.; Sluyter, R. Humanized Mouse Model to Study the P2X7 Receptor in Graft-Versus-Host Disease. Methods Mol. Biol. 2022, 2510, 315–340. [Google Scholar]





| Score 1 | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Acute weight loss 2 | <5% | 5–9.9% | 10–14.9% | >15% |
| Chronic weight loss 3 | <5% | 5–6.9% | 7–14.9% | >15% |
| Posture | Normal | Slight hunching | Moderate hunching | Severe hunching |
| Activity | Normal | Slightly reduced activity | Reduced activity and slow movement | Slow to no movement with reduced gait or shuffling |
| Hindlimb function | Normal gait | Unable to stand on hindlimbs | Mild paresis | Severe paresis |
| Fur | Normal | Mild to moderate ruffling | Severe ruffling and hair loss | >50% hair loss |
| Skin integrity | Normal | Scaling of paws and/or tail | Scaling of additional areas | Denuded skin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuthbertson, P.; Button, A.; Sligar, C.; Elhage, A.; Vine, K.L.; Watson, D.; Sluyter, R. Post-Transplant Cyclophosphamide Combined with Brilliant Blue G Reduces Graft-versus-Host Disease without Compromising Graft-versus-Leukaemia Immunity in Humanised Mice. Int. J. Mol. Sci. 2024, 25, 1775. https://doi.org/10.3390/ijms25031775
Cuthbertson P, Button A, Sligar C, Elhage A, Vine KL, Watson D, Sluyter R. Post-Transplant Cyclophosphamide Combined with Brilliant Blue G Reduces Graft-versus-Host Disease without Compromising Graft-versus-Leukaemia Immunity in Humanised Mice. International Journal of Molecular Sciences. 2024; 25(3):1775. https://doi.org/10.3390/ijms25031775
Chicago/Turabian StyleCuthbertson, Peter, Amy Button, Chloe Sligar, Amal Elhage, Kara L. Vine, Debbie Watson, and Ronald Sluyter. 2024. "Post-Transplant Cyclophosphamide Combined with Brilliant Blue G Reduces Graft-versus-Host Disease without Compromising Graft-versus-Leukaemia Immunity in Humanised Mice" International Journal of Molecular Sciences 25, no. 3: 1775. https://doi.org/10.3390/ijms25031775
APA StyleCuthbertson, P., Button, A., Sligar, C., Elhage, A., Vine, K. L., Watson, D., & Sluyter, R. (2024). Post-Transplant Cyclophosphamide Combined with Brilliant Blue G Reduces Graft-versus-Host Disease without Compromising Graft-versus-Leukaemia Immunity in Humanised Mice. International Journal of Molecular Sciences, 25(3), 1775. https://doi.org/10.3390/ijms25031775






