Zingiber officinale Root Capsule Extract Synergistically Enhance the Anti-Inflammatory Effects of Diclofenac Sodium in Experimental Acute Inflammation
Abstract
:1. Introduction
2. Results
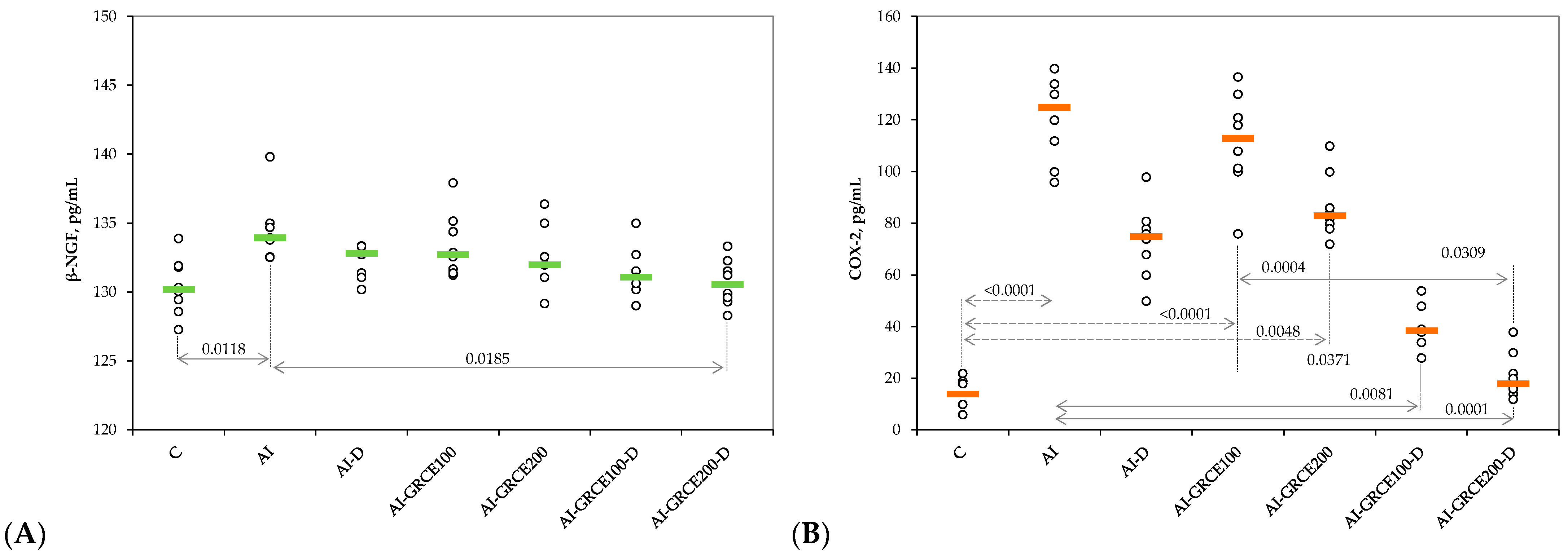
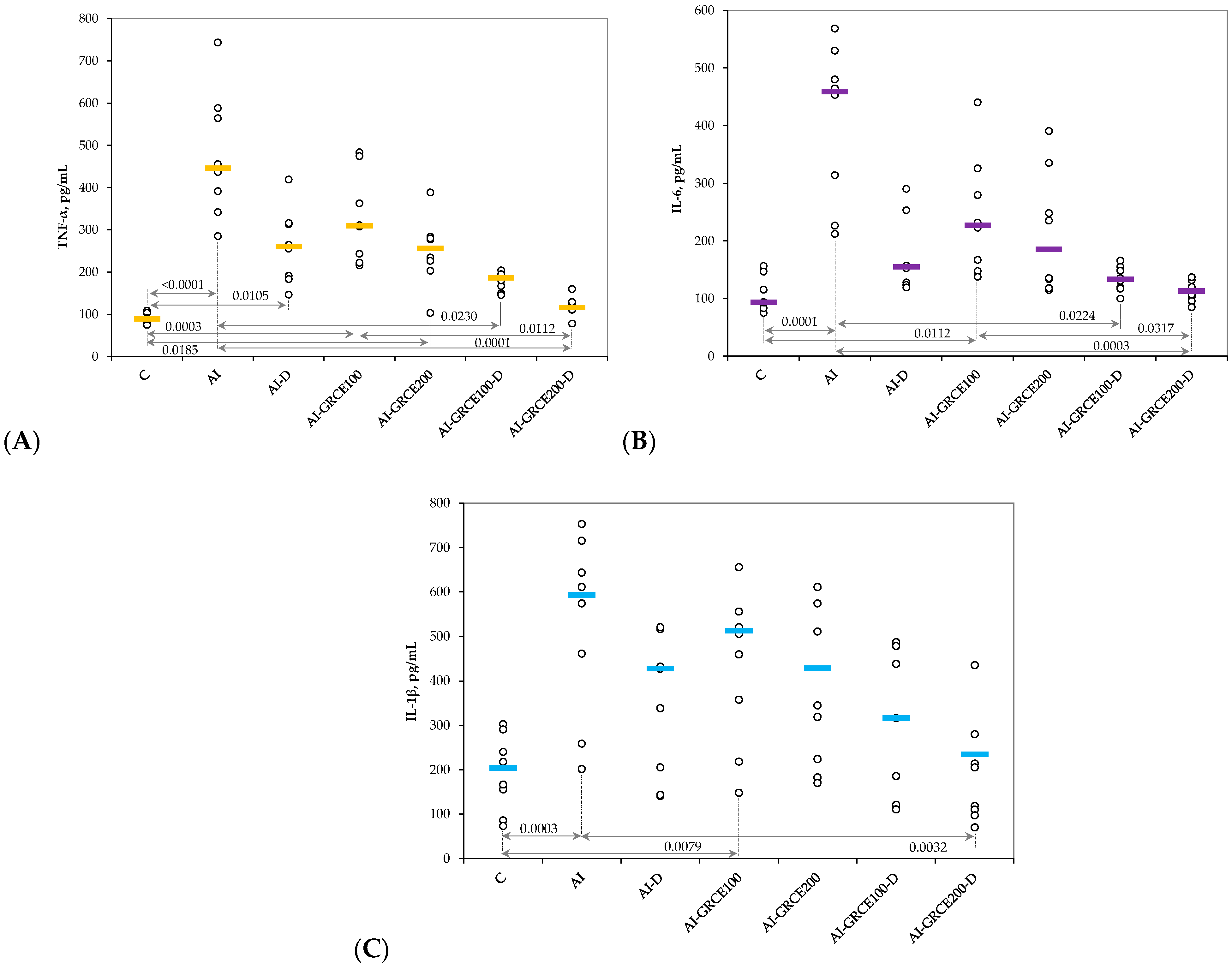
2.1. Results on Serum Biomarkers
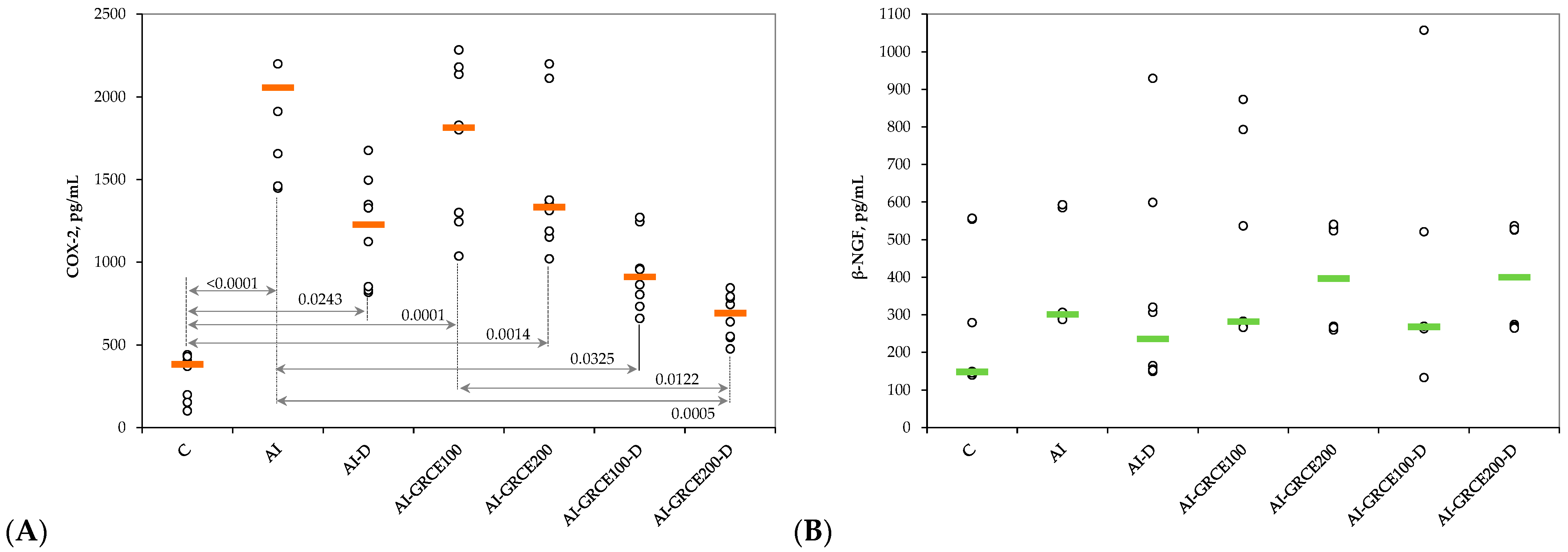
2.2. Results on Tissue Biomarkers
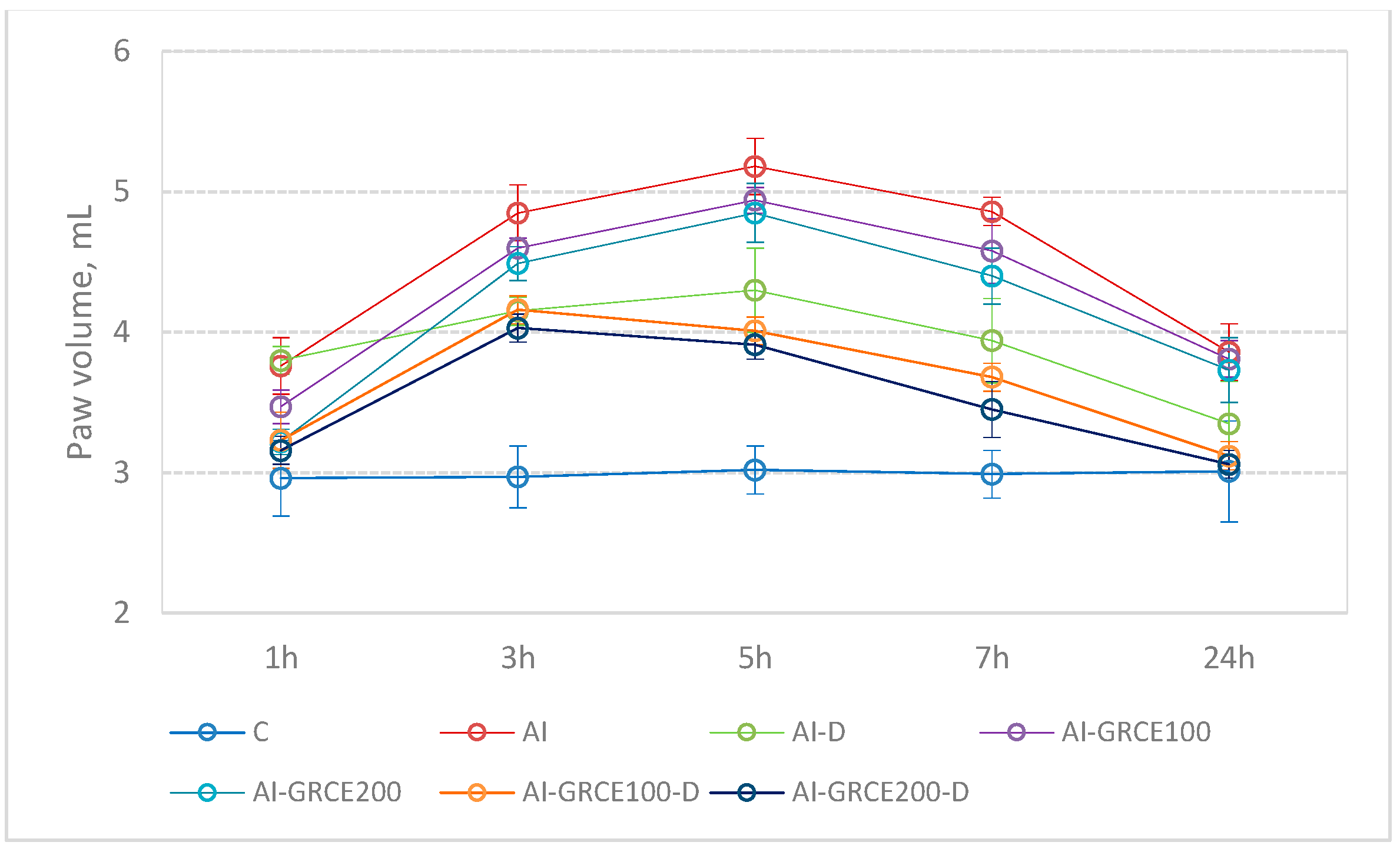
2.3. Edema Reduction
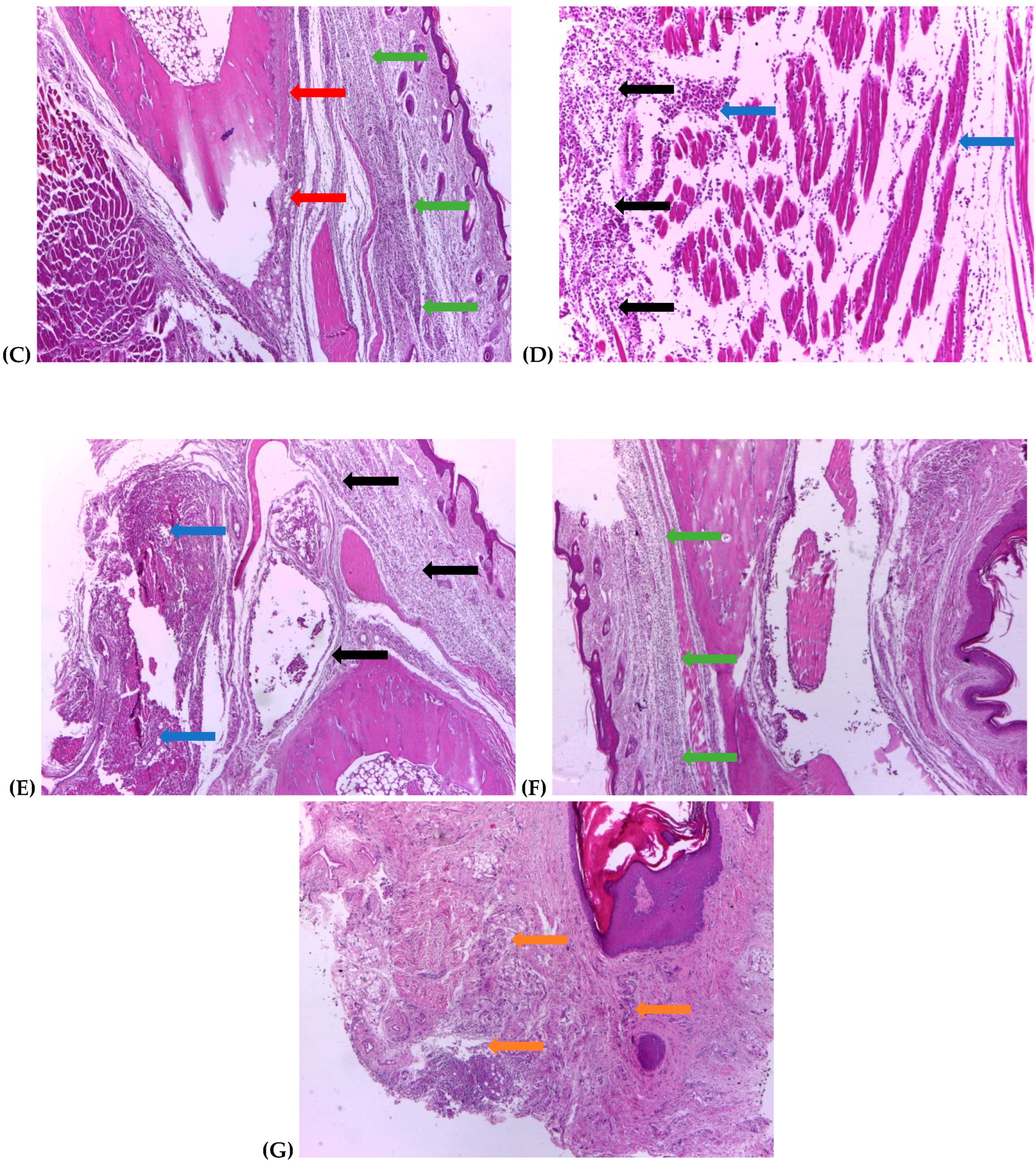
2.4. Histological Assessment
3. Discussion
3.1. Serum and Tissue Biomarkers
3.2. Edema Reduction
3.3. Histological Changes
4. Materials and Methods
4.1. Chemicals and Drugs
4.2. Animal Grouping and Experimental Design
4.3. Inflammatory Edema Assessment
4.4. Blood Samples Collection
4.5. Tissue Homogenate
4.6. Biochemical Assays
4.7. Histopathological Examination
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Punchard, N.A.; Whelan, C.J.; Adcock, I. The journal of inflammation. J. Inflamm. 2004, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Di Paola, R.; Viviani, B.; Genovese, T.; Mazzon, E.; Lucchi, L.; Cuzzocrea, S. Increased carrageenan-induced acute lung inflammation in old rats. Immunology 2005, 115, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.A.; Risely, E.A.; Nuss, G.W. Carrageenan induced edema in hind paw of the rat as an assay for anti-inflammatory. Proc. Soc. Exp. Biol. Med. 1962, 111, 544–547. [Google Scholar] [CrossRef]
- Boarescu, I.; Pop, R.M.; Boarescu, P.-M.; Bocșan, I.C.; Gheban, D.; Râjnoveanu, R.-M.; Râjnoveanu, A.; Bulboacă, A.E.; Buzoianu, A.D.; Bolboacă, S.D. Anti-Inflammatory and Analgesic Effects of Curcumin Nanoparticles Associated with Diclofenac Sodium in Experimental Acute Inflammation. Int. J. Mol. Sci. 2022, 23, 11737. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A.; Ghorbanzadeh, B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian. J. Pharmacol. 2015, 47, 292. [Google Scholar] [CrossRef] [PubMed]
- Halici, Z.; Dengiz, G.O.; Odabasoglu, F.; Suleyman, H.; Cadirci, E.; Halici, M. Amiodarone has anti-inflammatory and anti-oxidative properties: An experimental study in rats with carrageenan-induced paw edema. J. Pharmacol. 2007, 566, 215–221. [Google Scholar] [CrossRef]
- Ronchetti, D.; Borghi, V.; Gaitan, G.; Herrero, J.F.; Impagnatiello, F. NCX 2057, a novel NO-releasing derivative of ferulic acid, suppresses inflammatory and nociceptive responses in in vitro and in vivo models. Br. J. Pharmacol. 2009, 158, 569–579. [Google Scholar] [CrossRef]
- Atkinson, T.J.; Fudin, J. Nonsteroidal antiinflammatory drugs for acute and chronic pain. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 219–231. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef]
- Vonkeman, H.E.; van de Laar, M.A. Nonsteroidal anti-inflammatory drugs: Adverse effects and their prevention. Semin. Arthritis Rheum. 2010, 39, 294–312. [Google Scholar] [CrossRef]
- Kołodziejska, J.; Kołodziejczyk, M. Diclofenac in the treatment of pain in patients with rheumatic diseases. Reumatologia 2018, 56, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Yarishkin, O.V.; Hwang, E.M.; Kim, D.; Yoo, J.C.; Kang, S.S.; Kim, D.R.; Chung, H.J.; Jeong, H.S.; Kang, D.; Han, J.; et al. Diclofenac, a non-steroidal anti-inflammatory drug, inhibits L-type Ca2+ channels in neonatal rat ventricular cardiomyocytes. Korean J. Physiol. Pharmacol. 2009, 13, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Stoner, G.D. Ginger: Is it ready for prime time? Cancer Prev. Res. 2013, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Kumar, N.V.; Murthy, P.S.; Manjunatha, J.R.; Bettadaiah, B.K. Synthesis and quorum sensing inhibitory activity of key phenolic compounds of ginger and their derivatives. Food Chem. 2014, 159, 451–457. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Ind. Crop. Prod. 2015, 70, 238–244. [Google Scholar] [CrossRef]
- Citronberg, J.; Bostick, R.; Ahearn, T.; Turgeon, D.K.; Ruffin, M.T.; Djuric, Z.; Sen, A.; Brenner, D.E.; Zick, S.M. Effects of ginger supplementation on cell-cycle biomarkers in the normal-appearing colonic mucosa of patients at increased risk for colorectal cancer: Results from a pilot, randomized, and controlled trial. Cancer Prev. Res. 2013, 6, 271–281. [Google Scholar] [CrossRef]
- Grzanna, R.; Lindmark, L.; Frondoza, C.G. Ginger—An herbal medicinal product with broad anti-inflammatory actions. J. Med. Food 2005, 8, 125–132. [Google Scholar] [CrossRef]
- Yücel, Ç.; Karatoprak, G.Ş.; Açıkara, Ö.B.; Akkol, E.K.; Barak, T.H.; Sobarzo-Sánchez, E.; Aschner, M.; Shirooie, S. Immunomodulatory and anti-inflammatory therapeutic potential of gingerols and their nanoformulations. Front. Pharmacol. 2022, 13, 902551. [Google Scholar] [CrossRef]
- Boarescu, I.; Pop, R.M.; Boarescu, P.-M.; Bocșan, I.C.; Gheban, D.; Bulboacă, A.E.; Buzoianu, A.D.; Bolboacă, S.D. Ginger (Zingiber officinale) Root Capsules Enhance Analgesic and Antioxidant Efficacy of Diclofenac Sodium in Experimental Acute Inflammation. Antioxidants 2023, 12, 745. [Google Scholar] [CrossRef]
- Calimag, K.P.D.; Arbis, C.C.H.; Collantes, T.M.A.; Bariuan, J.V.; Ang, M.J.C.; Cervancia, C.A.; Desamero, M.J.M.; Estacio, M.A.C. Attenuation of carrageenan-induced hind paw edema and plasma TNF-α level by Philippine stingless bee (Tetragonula biroi Friese) propolis. Exp. Anim. 2021, 70, 185–193. [Google Scholar] [CrossRef]
- Boarescu, P.-M.; Boarescu, I.; Bulboacă, A.E.; Bocșan, I.C.; Pop, R.M.; Gheban, D.; Râjnoveanu, R.-M.; Râjnoveanu, A.; Roşian, Ş.H.; Buzoianu, A.D.; et al. Multi-Organ Protective Effects of Curcumin Nanoparticles on Drug-Induced Acute Myocardial Infarction in Rats with Type 1 Diabetes Mellitus. Appl. Sci. 2021, 11, 5497. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNFα and the TNF receptor superfamily: Structure-function relationship (s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Bester, J.; Pretorius, E. The inflammatory effects of TNF-α and complement component 3 on coagulation. Sci. Rep. 2018, 8, 1812. [Google Scholar] [CrossRef]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Annamalai, P.; Thangam, E.B. Local and systemic profiles of inflammatory cytokines in carrageenan-induced paw inflammation in rats. Immunol. Investig. 2017, 46, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Torres, R. Role of interleukin-1β during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef]
- Boarescu, P.-M.; Boarescu, I.; Pop, R.M.; Roşian, Ş.H.; Bocșan, I.C.; Rus, V.; Mada, R.O.; Popa, I.D.; Neagu, N.; Bulboacă, A.E.; et al. Evaluation of Oxidative Stress Biomarkers, Pro-Inflammatory Cytokines, and Histological Changes in Experimental Hypertension, Dyslipidemia, and Type 1 Diabetes Mellitus. Int. J. Mol. Sci. 2022, 23, 1438. [Google Scholar] [CrossRef]
- Ramos, A.G.B.; de Menezes, I.R.A.; da Silva, M.S.A.; Torres Pessoa, R.; de Lacerda Neto, L.J.; Rocha Santos Passos, F.; Melo Coutinho, H.D.; Iriti, M.; Quintans-Júnior, L.J. Antiedematogenic and Anti-Inflammatory Activity of the Monoterpene Isopulegol and Its β-Cyclodextrin (β-CD) Inclusion Complex in Animal Inflammation Models. Foods 2020, 9, 630. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Yang, S.-F.; Huang, F.-M.; Liu, C.-M.; Tai, K.-W.; Hsieh, Y.-S. Proinflammatory cytokines induce cyclooxygenase-2 mRNA and protein expression in human pulp cell cultures. J. Endod. 2003, 29, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Masala, I.F.; Sarzi-Puttini, P. A review of chronic musculoskeletal pain: Central and peripheral effects of diclofenac. Pain. Ther. 2018, 7, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, F.A.; Bradley, M.J.; Hueman, M.T.; Schobel, S.A.; Gaucher, B.J.; Styrmisdottir, E.L.; Potter, B.K.; Forsberg, J.A.; Elster, E.A. Nonsteroidal anti-inflammatory drugs may affect cytokine response and benefit healing of combat-related extremity wounds. Surgery 2017, 161, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Kim, M.H.; Hong, J.; Kim, S.H.; Yang, W.M. Dried ginger (Zingiber officinalis) inhibits inflammation in a lipopolysaccharide-induced mouse model. Evid. Based Complement. Alternat Med. 2013, 2013, 914563. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, S.M.; Ezzat, M.I.; Okba, M.M.; Menze, E.T.; Abdel-Naim, A.B. The hidden mechanism beyond ginger (zingiber officinale rosc.) potent in vivo and in vitro anti-inflammatory activity. J. Ethnopharmacol. 2018, 214, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Gad, S.S. Effect of ginger as anti-inflammatory agent on serum nitric oxide, tumor necrotic factor α (TNF-α) and interleukin 4 (IL-4) in albino rats with carrageenan induced paw edema. Virol. Immunol. J. 2018, 2, 1–15. [Google Scholar] [CrossRef]
- Ebendal, T. Function and evolution in the NGF family and its receptors. J. Neurosci. Res. 1992, 32, 461–470. [Google Scholar] [CrossRef]
- Yuan, H.; Du, S.; Chen, L.; Xu, X.; Wang, Y.; Ji, F. Hypomethylation of nerve growth factor (NGF) promotes binding of C/EBPα and contributes to inflammatory hyperalgesia in rats. J. Neuroinflammation 2020, 17, 34. [Google Scholar] [CrossRef]
- Amann, R.; Schuligoi, R. Inhibition of carrageenan-induced edema by indomethacin or sodium salicylate does not prevent the increase of nerve growth factor in the rat hind paw. Neurosci. Lett. 2000, 278, 173–176. [Google Scholar] [CrossRef]
- Lee, Y.; Rodriguez, C.; Dionne, R.A. The role of COX-2 in acute pain and the use of selective COX-2 inhibitors for acute pain relief. Curr. Pharm. Des. 2005, 11, 1737–1755. [Google Scholar] [CrossRef]
- Hunter, T.S.; Robison, C.; Gerbino, P.P. Emerging evidence in NSAID pharmacology: Important considerations for product selection. Am. J. Manag. Care 2015, 21, S139–S147. [Google Scholar]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef]
- Pop, R.M.; Sabin, O.; Suciu, Ș.; Vesa, S.C.; Socaci, S.A.; Chedea, V.S.; Bocsan, I.C.; Buzoianu, A.D. Nigella Sativa’s Anti-Inflammatory and Antioxidative Effects in Experimental Inflammation. Anti Oxid. 2020, 9, 921. [Google Scholar] [CrossRef]
- Begum, R.; Sharma, M.; Pillai, K.K.; Aeri, V.; Sheliya, M.A. Inhibitory effect of Careya arborea on inflammatory biomarkers in carrageenan-induced inflammation. Pharm. Biol. 2015, 53, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Penna, S.C.; Medeiros, M.V.; Aimbire, F.S.C.; Faria-Neto, H.C.C.; Sertié, J.A.A.; Lopes-Martins, R.A.B. Anti-inflammatory effect of the hydralcoholic extract of Zingiber officinale rhizomes on rat paw and skin edema. Phytomedicine 2003, 10, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Dzoyem, J.P.; McGaw, L.J.; Kuete, V.; Bakowsky, U. Anti-inflammatory and anti-nociceptive activities of African medicinal spices and vegetables. Med. Spices Veg. Afr. 2017, 2017, 239–270. [Google Scholar] [CrossRef]
- Zammel, N.; Saeed, M.; Bouali, N.; Elkahoui, S.; Alam, J.M.; Rebai, T.; Kausar, M.A.; Adnan, M.; Siddiqui, A.J.; Badraoui, R. Antioxidant and Anti-Inflammatory Effects of Zingiber officinale roscoe and Allium subhirsutum: In Silico, Biochemical and Histological Study. Foods 2021, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, M.F.; Asaad, G.F.; Ragab, T.I.M.; Ahmed, R.F.; El Gendy, A.E.-N.G.; Abd El-Rahman, S.S.; Elgamal, A.M.; Elshamy, A.I. Oral and Topical Anti-Inflammatory and Antipyretic Potentialities of Araucaria bidiwillii Shoot Essential Oil and Its Nanoemulsion in Relation to Chemical Composition. Molecules 2021, 26, 5833. [Google Scholar] [CrossRef] [PubMed]
- Boarescu, I.; Boarescu, P.-M.; Pop, R.M.; Bocșan, I.C.; Gheban, D.; Râjnoveanu, R.-M.; Râjnoveanu, A.; Bulboacă, A.E.; Buzoianu, A.D.; Bolboacă, S.D. Curcumin Nanoparticles Enhance Antioxidant Efficacy of Diclofenac Sodium in Experimental Acute Inflammation. Biomedicines 2022, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Weissgerber, T.L.; Milic, N.M.; Winham, S.J.; Garovic, V.D. Beyond bar and line graphs: Time for a new data presentation paradigm. PLoS Biol. 2015, 13, e1002128. [Google Scholar] [CrossRef] [PubMed]

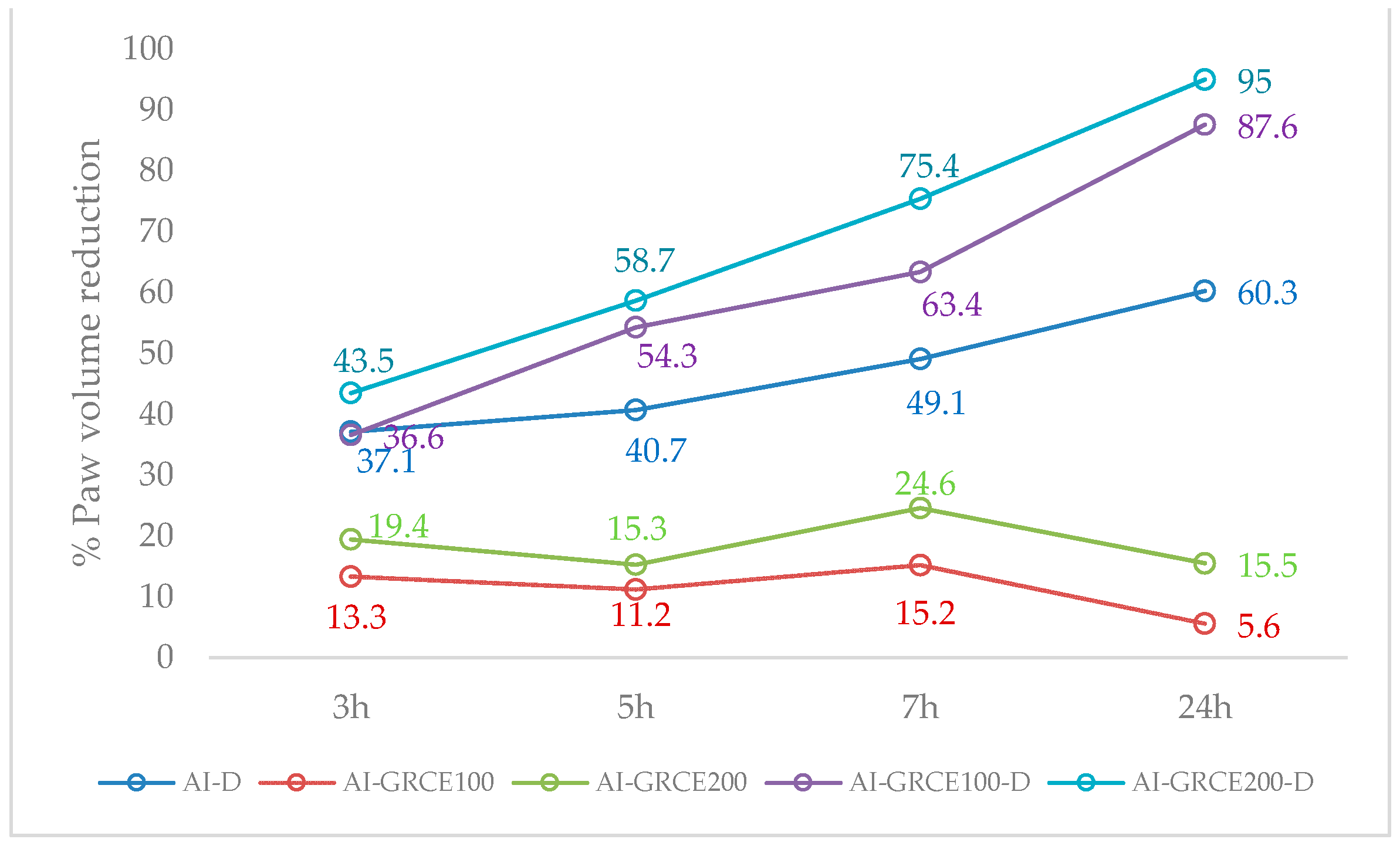

| Group. | Abb. | Intervention|Treatment |
|---|---|---|
| 1. Control group | C | none|saline solution |
| 2. Acute inflammation (AI) model group | AI | acute paw inflammation (API)|saline solution |
| 3. AI treated with Diclofenac sodium (D) | AI-D | API|5 mg/kg b.w. D after API |
| 4. AI treated with Ginger root capsule extract (GRCE) in a dose of 100 mg/kg b.w. | AI-GRCE100 | API|100 mg/kg b.w. GRCE and after API |
| 5. AI treated with GRCE in a dose of 200 mg/kg b.w. | AI-GRCE200 | API|200 mg/kg b.w. GRCE and after API |
| 6. AI with GRCE in a dose of 100 mg/kg b.w. and D | AI-GRCE100-D | API|100 mg/kg b.w. GRCE and5 mg/kg b.w. D after API |
| 7. AI with GRCE in a dose of 200 mg/kg b.w. and D | AI-GRCE200-D | API|200 mg/kg b.w. GRCE and5 mg/kg b.w. D after API |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boarescu, I.; Boarescu, P.-M.; Pop, R.M.; Bocșan, I.C.; Gheban, D.; Bulboacă, A.E.; Buzoianu, A.D.; Bolboacă, S.D. Zingiber officinale Root Capsule Extract Synergistically Enhance the Anti-Inflammatory Effects of Diclofenac Sodium in Experimental Acute Inflammation. Int. J. Mol. Sci. 2024, 25, 1781. https://doi.org/10.3390/ijms25031781
Boarescu I, Boarescu P-M, Pop RM, Bocșan IC, Gheban D, Bulboacă AE, Buzoianu AD, Bolboacă SD. Zingiber officinale Root Capsule Extract Synergistically Enhance the Anti-Inflammatory Effects of Diclofenac Sodium in Experimental Acute Inflammation. International Journal of Molecular Sciences. 2024; 25(3):1781. https://doi.org/10.3390/ijms25031781
Chicago/Turabian StyleBoarescu, Ioana, Paul-Mihai Boarescu, Raluca Maria Pop, Ioana Corina Bocșan, Dan Gheban, Adriana Elena Bulboacă, Anca Dana Buzoianu, and Sorana D. Bolboacă. 2024. "Zingiber officinale Root Capsule Extract Synergistically Enhance the Anti-Inflammatory Effects of Diclofenac Sodium in Experimental Acute Inflammation" International Journal of Molecular Sciences 25, no. 3: 1781. https://doi.org/10.3390/ijms25031781
APA StyleBoarescu, I., Boarescu, P.-M., Pop, R. M., Bocșan, I. C., Gheban, D., Bulboacă, A. E., Buzoianu, A. D., & Bolboacă, S. D. (2024). Zingiber officinale Root Capsule Extract Synergistically Enhance the Anti-Inflammatory Effects of Diclofenac Sodium in Experimental Acute Inflammation. International Journal of Molecular Sciences, 25(3), 1781. https://doi.org/10.3390/ijms25031781











