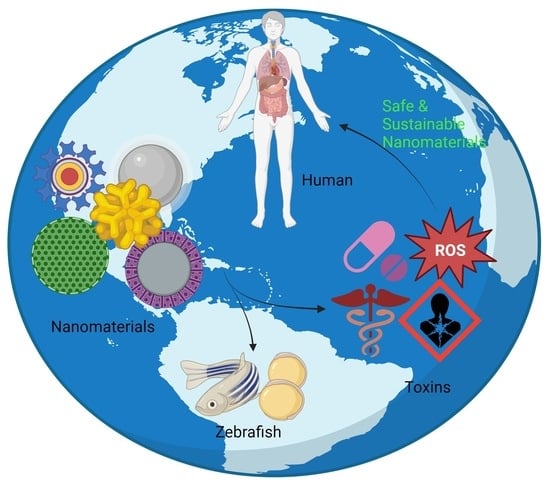
Zebrafish Insights into Nanomaterial Toxicity: A Focused Exploration on Metallic, Metal Oxide, Semiconductor, and Mixed-Metal Nanoparticles
Abstract
:1. Introduction
2. Metal Nanoparticles
2.1. Gold Nanoparticles
2.2. Silver Nanoparticles
2.3. Copper Nanoparticles
2.4. Platinum Nanoparticles
2.5. Bimetallic Nanoparticles
2.5.1. Gold–Silver Nanoparticles
2.5.2. Ruthenium–Ferrocene
2.6. Metal–Semiconductor Nanoparticles
2.6.1. Iron–Zinc Oxide Nanoparticles
2.6.2. Cobalt and Cobalt Oxide Nanoparticles
3. Semiconductor- and Carbon-Based Nanomaterials
3.1. Titanium Dioxide Nanoparticles
3.2. Zinc Oxide Nanoparticles
3.3. Copper Oxide Nanoparticles
3.4. Iron Oxide Nanoparticles
3.5. Graphene Oxide
3.6. Copper Sulfide Nanoparticles
3.7. Zirconium Dioxide Nanoparticles
3.8. Molybdenum Disulfide Nanosheets
4. Challenges and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mutalik, C.; Okoro, G.; Chou, H.-L.; Lin, I.H.; Yougbaré, S.; Chang, C.-C.; Kuo, T.-R. Phase-Dependent 1T/2H-MoS2 Nanosheets for Effective Photothermal Killing of Bacteria. ACS Sustain. Chem. Eng. 2022, 10, 8949–8957. [Google Scholar] [CrossRef]
- Hsu, I.-L.; Yeh, F.H.; Chin, Y.-C.; Cheung, C.I.; Chia, Z.C.; Yang, L.-X.; Chen, Y.-J.; Cheng, T.-Y.; Wu, S.-P.; Tsai, P.-J. Multiplex antibacterial processes and risk in resistant phenotype by high oxidation-state nanoparticles: New killing process and mechanism investigations. Chem. Eng. J. 2021, 409, 128266. [Google Scholar] [CrossRef]
- Siboro, P.Y.; Nguyen, V.K.T.; Miao, Y.B.; Sharma, A.K.; Mi, F.L.; Chen, H.L.; Chen, K.H.; Yu, Y.T.; Chang, Y.; Sung, H.W. Ultrasound-Activated, Tumor-Specific in Situ Synthesis of a Chemotherapeutic Agent Using ZIF-8 Nanoreactors for Precision Cancer Therapy. ACS Nano 2022, 16, 12403–12414. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-C.; Yang, L.-X.; Hsu, F.-T.; Hsu, C.-W.; Chang, T.-W.; Chen, H.-Y.; Chen, L.Y.-C.; Chia, Z.C.; Hung, C.-H.; Su, W.-C.; et al. Iron oxide@chlorophyll clustered nanoparticles eliminate bladder cancer by photodynamic immunotherapy-initiated ferroptosis and immunostimulation. J. Nanobiotechnol. 2022, 20, 373. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-W.; Ko, H.; Huang, W.-S.; Chiu, Y.-C.; Yang, L.-X.; Chia, Z.-C.; Chin, Y.-C.; Chen, Y.-J.; Tsai, Y.-T.; Hsu, C.-W. Tannic acid-induced interfacial ligand-to-metal charge transfer and the phase transformation of Fe3O4 nanoparticles for the photothermal bacteria destruction. Chem. Eng. J. 2022, 428, 131237. [Google Scholar] [CrossRef]
- Mutalik, C.; Krisnawati, D.I.; Patil, S.B.; Khafid, M.; Atmojo, D.S.; Santoso, P.; Lu, S.-C.; Wang, D.-Y.; Kuo, T.-R. Phase-Dependent MoS2 Nanoflowers for Light-Driven Antibacterial Application. ACS Sustain. Chem. Eng. 2021, 9, 7904–7912. [Google Scholar] [CrossRef]
- Mutalik, C.; Okoro, G.; Krisnawati, D.I.; Jazidie, A.; Rahmawati, E.Q.; Rahayu, D.; Hsu, W.-T.; Kuo, T.-R. Copper sulfide with morphology-dependent photodynamic and photothermal antibacterial activities. J. Colloid Interface Sci. 2022, 607, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-C.; Tan, S.-H.; Hsiao, Y.-C.; Mutalik, C.; Chen, H.-M.; Yougbaré, S.; Kuo, T.-R. Unveiling the Antibacterial Mechanism of Gold Nanoclusters via In Situ Transmission Electron Microscopy. ACS Sustain. Chem. Eng. 2022, 10, 464–471. [Google Scholar] [CrossRef]
- Zhang, D.; Atochina-Vasserman, E.N.; Lu, J.; Maurya, D.S.; Xiao, Q.; Liu, M.; Adamson, J.; Ona, N.; Reagan, E.K.; Ni, H.; et al. The Unexpected Importance of the Primary Structure of the Hydrophobic Part of One-Component Ionizable Amphiphilic Janus Dendrimers in Targeted mRNA Delivery Activity. J. Am. Chem. Soc. 2022, 144, 4746–4753. [Google Scholar] [CrossRef]
- Qin, J.X.; Yang, X.G.; Lv, C.F.; Li, Y.Z.; Liu, K.K.; Zang, J.H.; Yang, X.; Dong, L.; Shan, C.X. Nanodiamonds: Synthesis, properties, and applications in nanomedicine. Mater. Des. 2021, 210, 110091. [Google Scholar] [CrossRef]
- Machado, S.; González-Ballesteros, N.; Gonçalves, A.; Magalhães, L.; Sárria Pereira de Passos, M.; Rodríguez-Argüelles, M.C.; Castro Gomes, A. Toxicity in vitro and in Zebrafish Embryonic Development of Gold Nanoparticles Biosynthesized Using Cystoseira Macroalgae Extracts. Int. J. Nanomed. 2021, 16, 5017–5036. [Google Scholar] [CrossRef]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Ward, A.C. Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials 2018, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Mross, K.; Niemann, B.; Massing, U.; Drevs, J.; Unger, C.; Bhamra, R.; Swenson, C.E. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: An open-label, single-dose study. Cancer Chemother. Pharmacol. 2004, 54, 514–524. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar]
- Passero, F.C., Jr.; Grapsa, D.; Syrigos, K.N.; Saif, M.W. The safety and efficacy of Onivyde (irinotecan liposome injection) for the treatment of metastatic pancreatic cancer following gemcitabine-based therapy. Expert Rev. Anticancer Ther. 2016, 16, 697–703. [Google Scholar] [CrossRef]
- Sabaawy, H.E.; Azuma, M.; Embree, L.J.; Tsai, H.J.; Starost, M.F.; Hickstein, D.D. TEL-AML1 transgenic zebrafish model of precursor B cell acute lymphoblastic leukemia. Proc. Natl. Acad. Sci. USA 2006, 103, 15166–15171. [Google Scholar] [CrossRef]
- Mercatali, L.; Vanni, S.; Miserocchi, G.; Liverani, C.; Spadazzi, C.; Cocchi, C.; Calabrese, C.; Gurrieri, L.; Fausti, V.; Riva, N.; et al. The emerging role of cancer nanotechnology in the panorama of sarcoma. Front. Bioeng. Biotechnol. 2022, 10, 953555. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Q.; Liu, D.; Liu, T.; Xing, L. Neurotoxicity of nanoparticles: Insight from studies in zebrafish. Ecotoxicol. Environ. Saf. 2022, 242, 113896. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.-R.; Zhu, Y.-X.; Duan, Q.-Y.; Chen, Z.; Wu, F.-G. Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery applications. J. Control. Release 2019, 311–312, 301–318. [Google Scholar] [CrossRef]
- Saleem, S.; Kannan, R.R. Zebrafish: A Promising Real-Time Model System for Nanotechnology-Mediated Neurospecific Drug Delivery. Nanoscale Res. Lett. 2021, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Fernandes, A.R.; Baptista, P.V. Gold Nanoparticle Approach to the Selective Delivery of Gene Silencing in Cancer—The Case for Combined Delivery? Genes 2017, 8, 94. [Google Scholar] [CrossRef]
- Megarajan, S.; Ameen, F.; Singaravelu, D.; Islam, M.A.; Veerappan, A. Synthesis of N-myristoyltaurine stabilized gold and silver nanoparticles: Assessment of their catalytic activity, antimicrobial effectiveness and toxicity in zebrafish. Environ. Res. 2022, 212, 113159. [Google Scholar] [CrossRef]
- Ren, B.; Jia, B.; Zhang, X.; Wang, J.; Li, Y.; Liang, H.; Liang, H. Influence of multi-walled carbon nanotubes on enantioselective bioaccumulation and oxidative stress toxicity of indoxacarb in zebrafish (Danio rerio). Chemosphere 2021, 267, 128872. [Google Scholar] [CrossRef] [PubMed]
- Bek, J.W.; De Clercq, A.; De Saffel, H.; Soenens, M.; Huysseune, A.; Witten, P.E.; Coucke, P.J.; Willaert, A. Photoconvertible fluorescent proteins: A versatile tool in zebrafish skeletal imaging. J. Fish Biol. 2021, 98, 1007–1017. [Google Scholar] [CrossRef]
- Chousidis, I.; Stalikas, C.D.; Leonardos, I.D. Induced toxicity in early-life stage zebrafish (Danio rerio) and its behavioral analysis after exposure to non-doped, nitrogen-doped and nitrogen, sulfur-co doped carbon quantum dots. Environ. Toxicol. Pharmacol. 2020, 79, 232. [Google Scholar] [CrossRef]
- He, X.; Deng, Z.; Xu, W.; Li, Y.; Xu, C.; Chen, H.; Shen, J. A novel dual-response chemosensor for bioimaging of Exogenous/Endogenous hypochlorite and hydrazine in living cells, Pseudomonas aeruginosa and zebrafish. Sens. Actuators B Chem. 2020, 321, 128450. [Google Scholar] [CrossRef]
- d’Amora, M.; Raffa, V.; De Angelis, F.; Tantussi, F. Toxicological profile of plasmonic nanoparticles in zebrafish model. Int. J. Mol. Sci. 2021, 22, 6372. [Google Scholar] [CrossRef]
- Mutalik, C.; Wang, D.Y.; Krisnawati, D.I.; Jazidie, A.; Yougbare, S.; Kuo, T.R. Light-Activated Heterostructured Nanomaterials for Antibacterial Applications. Nanomaterials 2020, 10, 643. [Google Scholar] [CrossRef] [PubMed]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on metal nanoparticles as nanocarriers: Current challenges and perspectives in drug delivery systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.; Tang, M. Toxicological study of metal and metal oxide nanoparticles in zebrafish. J. Appl. Toxicol. 2020, 40, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Shi, L.; Xu, J.; Duan, G.; Yang, H. A focus on the genotoxicity of gold nanoparticles. Nanomedicine 2020, 15, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-Y.; Kuo, T.-R.; Yougbaré, S.; Lin, L.-Y.; Xiao, C.-Y. Novel direct growth of ZIF-67 derived Co3O4 and N-doped carbon composites on carbon cloth as supercapacitor electrodes. J. Colloid Interface Sci. 2022, 608, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhao, Y.; Xia, T.; Meng, H.; Ji, Z.; Liu, R.; George, S.; Xiong, S.; Wang, X.; Zhang, H.; et al. High Content Screening in Zebrafish Speeds up Hazard Ranking of Transition Metal Oxide Nanoparticles. ACS Nano 2011, 5, 7284–7295. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; He, Y.; Wang, X.; Cheng, Y.; Zhang, H.; Savolainen, K.; Mädler, L.; Pokhrel, S.; Lin, S. Redox Activity and Nano–Bio Interactions Determine the Skin Injury Potential of Co3O4-Based Metal Oxide Nanoparticles toward Zebrafish. ACS Nano 2020, 14, 4166–4177. [Google Scholar] [CrossRef]
- Gakis, G.P.; Aviziotis, I.G.; Charitidis, C.A. Metal and metal oxide nanoparticle toxicity: Moving towards a more holistic structure–activity approach. Environ. Sci. Nano 2023, 10, 761–780. [Google Scholar] [CrossRef]
- Notter, D.A.; Mitrano, D.M.; Nowack, B. Are nanosized or dissolved metals more toxic in the environment? A meta-analysis. Environ. Toxicol. Chem. 2014, 33, 2733–2739. [Google Scholar] [CrossRef]
- Cassano, D.; Mapanao, A.-K.; Summa, M.; Vlamidis, Y.; Giannone, G.; Santi, M.; Guzzolino, E.; Pitto, L.; Poliseno, L.; Bertorelli, R.; et al. Biosafety and Biokinetics of Noble Metals: The Impact of Their Chemical Nature. ACS Appl. Bio Mater. 2019, 2, 4464–4470. [Google Scholar] [CrossRef]
- Yougbare, S.; Chou, H.L.; Yang, C.H.; Krisnawati, D.I.; Jazidie, A.; Nuh, M.; Kuo, T.R. Facet-dependent gold nanocrystals for effective photothermal killing of bacteria. J. Hazard. Mater. 2021, 407, 124617. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-K.; Cheng, T.-M.; Chu, H.-L.; Tan, S.-H.; Kuo, J.-C.; Hsu, P.-H.; Su, C.-Y.; Chen, H.-M.; Lee, C.-M.; Kuo, T.-R. Metabolic mechanism investigation of antibacterial active cysteine-conjugated gold nanoclusters in Escherichia coli. ACS Sustain. Chem. Eng. 2019, 7, 15479–15486. [Google Scholar] [CrossRef]
- Draviana, H.T.; Fitriannisa, I.; Khafid, M.; Krisnawati, D.I.; Widodo; Lai, C.-H.; Fan, Y.-J.; Kuo, T.-R. Size and charge effects of metal nanoclusters on antibacterial mechanisms. J. Nanobiotechnol. 2023, 21, 428. [Google Scholar] [CrossRef] [PubMed]
- Okoro, G.; Husain, S.; Saukani, M.; Mutalik, C.; Yougbaré, S.; Hsiao, Y.-C.; Kuo, T.-R. Emerging Trends in Nanomaterials for Photosynthetic Biohybrid Systems. ACS Mater. Lett. 2023, 5, 95–115. [Google Scholar] [CrossRef]
- Li, C.-H.; Kuo, T.-R.; Su, H.-J.; Lai, W.-Y.; Yang, P.-C.; Chen, J.-S.; Wang, D.-Y.; Wu, Y.-C.; Chen, C.-C. Fluorescence-guided probes of aptamer-targeted gold nanoparticles with computed tomography imaging accesses for in vivo tumor resection. Sci. Rep. 2015, 5, 15675. [Google Scholar] [CrossRef]
- Jia, Y.-P.; Ma, B.-Y.; Wei, X.-W.; Qian, Z.-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin. Chem. Lett. 2017, 28, 691–702. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Kaur, N.; Aditya, R.N.; Singh, A.; Kuo, T.R. Biomedical Applications for Gold Nanoclusters: Recent Developments and Future Perspectives. Nanoscale Res. Lett. 2018, 13, 302. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 990. [Google Scholar] [CrossRef]
- Patibandla, S.; Zhang, Y.; Tohari, A.M.; Gu, P.; Reilly, J.; Chen, Y.; Shu, X. Comparative analysis of the toxicity of gold nanoparticles in zebrafish. J. Appl. Toxicol. 2018, 38, 1153–1161. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X. Gold nanoparticles for skin drug delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef]
- Horng, J.-l.; Lee, Y.-S.; Lin, L.-Y. Exposure to silver impairs the osmoregulatory capability of euryhaline medaka (Oryzias latipes) subjected to salinity changes. Aquat. Toxicol. 2023, 260, 106592. [Google Scholar] [CrossRef]
- Fu, C.-W.; Horng, J.-L.; Tong, S.-K.; Cherng, B.-W.; Liao, B.-K.; Lin, L.-Y.; Chou, M.-Y. Exposure to silver impairs learning and social behaviors in adult zebrafish. J. Hazard. Mater. 2021, 403, 124031. [Google Scholar] [CrossRef]
- Horng, J.-L.; Lee, C.-Y.; Liu, S.-T.; Hung, G.-Y.; Lin, L.-Y. Differential effects of silver nanoparticles on two types of mitochondrion-rich ionocytes in zebrafish embryos. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 252, 109244. [Google Scholar] [CrossRef] [PubMed]
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Orbea, A.; González-Soto, N.; Lacave, J.M.; Barrio, I.; Cajaraville, M.P. Developmental and reproductive toxicity of PVP/PEI-coated silver nanoparticles to zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2017, 199, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dumitrescu, E.; Kumar, A.; Austin, D.; Goia, D.; Wallace, K.N.; Andreescu, S. Differential lethal and sublethal effects in embryonic zebrafish exposed to different sizes of silver nanoparticles. Environ. Pollut. 2019, 248, 627–634. [Google Scholar] [CrossRef] [PubMed]
- van Aerle, R.; Lange, A.; Moorhouse, A.; Paszkiewicz, K.; Ball, K.; Johnston, B.D.; de-Bastos, E.; Booth, T.; Tyler, C.R.; Santos, E.M. Molecular Mechanisms of Toxicity of Silver Nanoparticles in Zebrafish Embryos. Environ. Sci. Technol. 2013, 47, 8005–8014. [Google Scholar] [CrossRef]
- Lee, K.J.; Browning, L.M.; Nallathamby, P.D.; Desai, T.; Cherukuri, P.K.; Xu, X.-H.N. In Vivo Quantitative Study of Sized-Dependent Transport and Toxicity of Single Silver Nanoparticles Using Zebrafish Embryos. Chem. Res. Toxicol. 2012, 25, 1029–1046. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Arabeyyat, Z.H.; Xin, Q.; Paunov, V.N.; Dale, I.J.F.; Lloyd Mills, R.I.; Rotchell, J.M.; Cheng, J. Silver Nanoparticles in Zebrafish (Danio rerio) Embryos: Uptake, Growth and Molecular Responses. Int. J. Mol. Sci. 2020, 21, 1876. [Google Scholar] [CrossRef]
- Lu, C.; Lv, Y.; Kou, G.; Liu, Y.; Liu, Y.; Chen, Y.; Wu, X.; Yang, F.; Luo, J.; Yang, X. Silver nanoparticles induce developmental toxicity via oxidative stress and mitochondrial dysfunction in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 243, 113993. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Krishnan, M.; Shukla, S.; Lee, H.; Min, S.H.; Choi, D.K.; Cho, Y.; Bajpai, V.K.; Huh, Y.S.; et al. The effect of biogenic manufactured silver nanoparticles on human endothelial cells and zebrafish model. Sci. Total Environ. 2019, 679, 365–377. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Horng, J.-L.; Liu, S.-T.; Lin, L.-Y. Exposure to copper nanoparticles impairs ion uptake, and acid and ammonia excretion by ionocytes in zebrafish embryos. Chemosphere 2020, 261, 128051. [Google Scholar] [CrossRef]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Wu, F.; Harper, B.J.; Crandon, L.E.; Harper, S.L. Assessment of Cu and CuO nanoparticle ecological responses using laboratory small-scale microcosms. Environ. Sci. Nano 2020, 7, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Denluck, L.; Wu, F.; Crandon, L.E.; Harper, B.J.; Harper, S.L. Reactive oxygen species generation is likely a driver of copper based nanomaterial toxicity. Environ. Sci. Nano 2018, 5, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, T.; Sun, H.; Liu, J.-X. Copper nanoparticles induce zebrafish intestinal defects via endoplasmic reticulum and oxidative stress. Metallomics 2019, 12, 12–22. [Google Scholar] [CrossRef]
- Li, L.; Tai, Z.; Liu, W.; Luo, Y.; Wu, Y.; Lin, S.; Liu, M.; Gao, B.; Liu, J.X. Copper overload impairs hematopoietic stem and progenitor cell proliferation via prompting HSF1/SP1 aggregation and the subsequently downregulating FOXM1-Cytoskeleton axis. iScience 2023, 26, 106406. [Google Scholar] [CrossRef] [PubMed]
- Skeeters, S.S.; Rosu, A.C.; Divyanshi; Yang, J.; Zhang, K. Comparative Determination of Cytotoxicity of Sub-10 nm Copper Nanoparticles to Prokaryotic and Eukaryotic Systems. ACS Appl. Mater. Interfaces 2020, 12, 50203–50211. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Vijayaraghavan, P.; Chiang, W.-H.; Chen, H.-H.; Liu, T.-I.; Shen, M.-Y.; Omoto, A.; Kamimura, M.; Soga, K.; Chiu, H.-C. Targeted Delivery of Functionalized Upconversion Nanoparticles for Externally Triggered Photothermal/Photodynamic Therapies of Brain Glioblastoma. Theranostics 2018, 8, 1435–1448. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Ameer, F.S.; Raval, Y.S.; Huang, G.; Tzeng, T.J.; Anker, J.N.; Brumaghim, J.L. Polyphenol effects on CuO-nanoparticle-mediated DNA damage, reactive oxygen species generation, and fibroblast cell death. Toxicol. Vitr. 2022, 78, 105252. [Google Scholar] [CrossRef] [PubMed]
- Matos, T.P.; Gutiérrez, M.F.; Hanzen, T.A.; Malaquias, P.; de Paula, A.M.; de Souza, J.J.; Hass, V.; Fernández, E.; Reis, A.; Loguercio, A.D. 18-month clinical evaluation of a copper-containing universal adhesive in non-carious cervical lesions: A double-blind, randomized controlled trial. J. Dent. 2019, 90, 103219. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sun, H.; Zhang, T.; Liu, J.X. Copper induce zebrafish retinal developmental defects via triggering stresses and apoptosis. Cell Commun. Signal. 2020, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- Razmara, P.; Pyle, G.G. Impact of Copper Nanoparticles and Copper Ions on Transcripts Involved in Neural Repair Mechanisms in Rainbow Trout Olfactory Mucosa. Arch. Environ. Contam. Toxicol. 2023, 84, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, M.; Gurunathan, S.; Qasim, M.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials 2019, 9, 1719. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, S.N.; Engelbrekt, C.; Lützhøft, H.-C.H.; Jiménez-Lamana, J.; Noori, J.S.; Alatraktchi, F.A.; Delgado, C.G.; Slaveykova, V.I.; Baun, A. A Multimethod Approach for Investigating Algal Toxicity of Platinum Nanoparticles. Environ. Sci. Technol. 2016, 50, 10635–10643. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Baijal, G.; Somashekar, R.; Iyer, S.; Nayak, V. One Pot Synthesis of PEGylated Bimetallic Gold-Silver Nanoparticles for Imaging and Radiosensitization of Oral Cancers. Int. J. Nanomed. 2021, 16, 7103–7121. [Google Scholar] [CrossRef]
- Chu, Z.; Chen, L.; Wang, X.; Yang, Q.; Zhao, Q.; Huang, C.; Huang, Y.; Yang, D.-P.; Jia, N. Ultrasmall Au–Ag Alloy Nanoparticles: Protein-Directed Synthesis, Biocompatibility, and X-ray Computed Tomography Imaging. ACS Biomater. Sci. Eng. 2019, 5, 1005–1015. [Google Scholar] [CrossRef]
- Manikandan, M.; Gadre, S.; Chhatar, S.; Chakraborty, G.; Ahmed, N.; Patra, C.; Patra, M. Potent Ruthenium–Ferrocene Bimetallic Antitumor Antiangiogenic Agent That Circumvents Platinum Resistance: From Synthesis and Mechanistic Studies to In Vivo Evaluation in Zebrafish. J. Med. Chem. 2022, 65, 16353–16371. [Google Scholar]
- Xia, T.; Zhao, Y.; Sager, T.; George, S.; Pokhrel, S.; Li, N.; Schoenfeld, D.; Meng, H.; Lin, S.; Wang, X.; et al. Decreased Dissolution of ZnO by Iron Doping Yields Nanoparticles with Reduced Toxicity in the Rodent Lung and Zebrafish Embryos. ACS Nano 2011, 5, 1223–1235. [Google Scholar] [CrossRef]
- Verma, S.K.; Panda, P.K.; Kumari, P.; Patel, P.; Arunima, A.; Jha, E.; Husain, S.; Prakash, R.; Hergenröder, R.; Mishra, Y.K.; et al. Determining factors for the nano-biocompatibility of cobalt oxide nanoparticles: Proximal discrepancy in intrinsic atomic interactions at differential vicinage. Green Chem. 2021, 23, 3439–3458. [Google Scholar] [CrossRef]
- Rethi, L.; Mutalik, C.; Rethi, L.; Chiang, W.H.; Lee, H.L.; Pan, W.Y.; Yang, T.S.; Chiou, J.F.; Chen, Y.J.; Chuang, E.Y.; et al. Molecularly Targeted Photothermal Ablation of Epidermal Growth Factor Receptor-Expressing Cancer Cells with a Polypyrrole–Iron Oxide–Afatinib Nanocomposite. Cancers 2022, 14, 5043. [Google Scholar] [CrossRef] [PubMed]
- Dembélé, J.; Liao, J.H.; Liu, T.P.; Chen, Y.P. Overcoming Cytosolic Delivery Barriers of Proteins Using Denatured Protein-Conjugated Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2023, 15, 432–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chou, C.M.; Chang, T.Y.; Ting, H.; Dembélé, J.; Chu, Y.T.; Liu, T.P.; Changou, C.A.; Liu, C.W.; Chen, C.T. Bridging Size and Charge Effects of Mesoporous Silica Nanoparticles for Crossing the Blood–Brain Barrier. Front. Chem. 2022, 10, 931584. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chen, C.T.; Liu, T.P.; Chien, F.C.; Wu, S.H.; Chen, P.; Mou, C.Y. Catcher in the rel: Nanoparticles-antibody conjugate as NF-κB nuclear translocation blocker. Biomaterials 2020, 246, 119997. [Google Scholar] [CrossRef]
- Huang, G.Y.; Chang, W.J.; Lu, T.W.; Tsai, I.L.; Wu, S.J.; Ho, M.H.; Mi, F.L. Electrospun CuS nanoparticles/chitosan nanofiber composites for visible and near-infrared light-driven catalytic degradation of antibiotic pollutants. Chem. Eng. J. 2022, 431, 134059. [Google Scholar] [CrossRef]
- Lu, H.T.; Huang, G.Y.; Chang, W.J.; Lu, T.W.; Huang, T.W.; Ho, M.H.; Mi, F.L. Modification of chitosan nanofibers with CuS and fucoidan for antibacterial and bone tissue engineering applications. Carbohydr. Polym. 2022, 281, 119035. [Google Scholar] [CrossRef]
- Irshad, M.A.; Rehman, M.Z.u.; Anwar-ul-Haq, M.; Rizwan, M.; Nawaz, R.; Shakoor, M.B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Ali, S. Effect of green and chemically synthesized titanium dioxide nanoparticles on cadmium accumulation in wheat grains and potential dietary health risk: A field investigation. J. Hazard. Mater. 2021, 415, 125585. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Shih, Y.-S.; Chen, Z.-Y.; Cheng, F.-Y.; Lu, J.-Y.; Wu, Y.-H.; Wang, Y.-J. Toxic effects and mechanisms of silver and zinc oxide nanoparticles on zebrafish embryos in aquatic ecosystems. Nanomaterials 2022, 12, 717. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Zhou, Y.; Ma, C.; Feng, Y.; Hao, Y.; Rui, Y.; Wu, W.; Gui, X.; Han, Y.; Wang, Y. Jointed toxicity of TiO2 NPs and Cd to rice seedlings: NPs alleviated Cd toxicity and Cd promoted NPs uptake. Plant Physiol. Biochem. 2017, 110, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Yang, Y.-C.; Wang, B., Jr.; Cheng, F.-Y.; Lee, Y.-L.; Lee, Y.-H.; Wang, Y.-J. Comparing different surface modifications of zinc oxide nanoparticles in the developmental toxicity of zebrafish embryos and larvae. Ecotoxicol. Environ. Saf. 2022, 243, 113967. [Google Scholar] [CrossRef]
- Quevedo, A.C.; Ellis, L.-J.A.; Lynch, I.; Valsami-Jones, E. Mechanisms of silver nanoparticle uptake by embryonic zebrafish cells. Nanomaterials 2021, 11, 2699. [Google Scholar] [CrossRef]
- Robichaud, C.O.; Uyar, A.E.; Darby, M.R.; Zucker, L.G.; Wiesner, M.R. Estimates of Upper Bounds and Trends in Nano-TiO2 Production as a Basis for Exposure Assessment. Environ. Sci. Technol. 2009, 43, 4227–4233. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Hsiao, Y.-C.; Chang, Y.-H.; Krisnawati, D.I.; Alimansur, M.; Jazidie, A.; Nuh, M.; Chang, C.-C.; Wang, D.-Y.; Kuo, T.-R. High UV-VIS-NIR light-induced antibacterial activity by heterostructured TiO2-FeS2 nanocomposites. Int. J. Nanomed. 2020, 15, 8911. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Guo, M.; Huang, C.; Wang, X.; Zhu, Y.; Wang, L.; Wang, Z.; Zhou, L.; Fan, D.; Shi, L.; et al. Titanium dioxide nanoparticle affects motor behavior, neurodevelopment and axonal growth in zebrafish (Danio rerio) larvae. Sci. Total Environ. 2021, 754, 142315. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, C.; Wang, L.; Wang, X.; He, H.; Wu, S.; Zhao, X. Insights into the combined effects of environmental concentration of difenoconazole and tebuconazole on zebrafish early life stage. Sci. Total Environ. 2022, 830, 154687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Liu, C.; Wang, J.; Zou, L.; Yang, F.; Chi, X.; Zhu, J. Nano-TiO2 aggravates bioaccumulation and developmental neurotoxicity of difenoconazole in zebrafish larvae via oxidative stress and apoptosis: Protective role of vitamin C. Ecotoxicol. Environ. Saf. 2023, 251, 114554. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lei, L.; Chen, P.; Guo, W.; Guo, Y.; Yang, L.; Han, J.; Hu, B.; Zhou, B. Effects of nano-TiO2 on the bioavailability and toxicity of bis(2-ethylhexyl)-2,3,4,5-tetrabromophthalate (TBPH) in developing zebrafish. Chemosphere 2022, 295, 133862. [Google Scholar] [CrossRef] [PubMed]
- Bouazizi, N.; Vieillard, J.; Samir, B.; Le Derf, F. Advances in amine-surface functionalization of inorganic adsorbents for water treatment and antimicrobial activities: A review. Polymers 2022, 14, 378. [Google Scholar] [CrossRef]
- Chahoud, I.; Buschmann, J.; Clark, R.; Druga, A.; Falke, H.; Faqi, A.; Hansen, E.; Heinrich–Hirsch, B.; Hellwig, J.; Lingk, W. Classification terms in developmental toxicology: Need for harmonisation. Reprod. Toxicol. 1999, 13, 77–82. [Google Scholar] [CrossRef]
- George, S.; Yin, H.; Liu, Z.; Shen, S.; Cole, I.; Khiong, C.W. Hazard profiling of a combinatorial library of zinc oxide nanoparticles: Ameliorating light and dark toxicity through surface passivation. J. Hazard. Mater. 2022, 434, 128825. [Google Scholar] [CrossRef]
- Cózar, A.; Echevarría, F.; González-Gordillo, J.I.; Irigoien, X.; Úbeda, B.; Hernández-León, S.; Palma, Á.T.; Navarro, S.; García-de-Lomas, J.; Ruiz, A. Plastic debris in the open ocean. Proc. Natl. Acad. Sci. USA 2014, 111, 10239–10244. [Google Scholar] [CrossRef]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Chen, X.; Peng, L.-B.; Wang, D.; Zhu, Q.-L.; Li, J.; Han, T. Particles rather than released Zn2+ from ZnO nanoparticles aggravate microplastics toxicity in early stages of exposed zebrafish and their unexposed offspring. J. Hazard. Mater. 2022, 424, 127589. [Google Scholar] [CrossRef]
- Kansara, K.; Paruthi, A.; Misra, S.K.; Karakoti, A.S.; Kumar, A. Montmorillonite clay and humic acid modulate the behavior of copper oxide nanoparticles in aqueous environment and induces developmental defects in zebrafish embryo. Environ. Pollut. 2019, 255, 113313. [Google Scholar] [CrossRef]
- Pereira, S.P.P.; Boyle, D.; Nogueira, A.; Handy, R.D. Differences in toxicity and accumulation of metal from copper oxide nanomaterials compared to copper sulphate in zebrafish embryos: Delayed hatching, the chorion barrier and physiological effects. Ecotoxicol. Environ. Saf. 2023, 253, 114613. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.R.; Adeleye, A.S.; Gardea-Torresdey, J.; Keller, A.A. Aggregation, Dissolution, and Transformation of Copper Nanoparticles in Natural Waters. Environ. Sci. Technol. 2015, 49, 2749–2756. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.-J.; Huang, C.P.; Lam, C.-C.; Hua, L.-C.; Chang, S.-H.; Huang, C. Transformation of copper oxide nanoparticles as affected by ionic strength and its effects on the toxicity and bioaccumulation of copper in zebrafish embryo. Ecotoxicol. Environ. Saf. 2021, 225, 112759. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, Z.; Zeng, W.; Yang, T.; Cao, Y.; Mei, C.; Kuang, Y. Toxicity assessment of nanoparticles in various systems and organs. Nanotechnol. Rev. 2017, 6, 279–289. [Google Scholar] [CrossRef]
- Pereira, A.C.; Gonçalves, B.B.; Brito, R.d.S.; Vieira, L.G.; Lima, E.C.d.O.; Rocha, T.L. Comparative developmental toxicity of iron oxide nanoparticles and ferric chloride to zebrafish (Danio rerio) after static and semi-static exposure. Chemosphere 2020, 254, 126792. [Google Scholar] [CrossRef]
- Jurewicz, A.; Ilyas, S.; Uppal, J.K.; Ivandic, I.; Korsching, S.; Mathur, S. Evaluation of Magnetite Nanoparticle-Based Toxicity on Embryo–Larvae Stages of Zebrafish (Danio rerio). ACS Appl. Nano Mater. 2020, 3, 1621–1629. [Google Scholar] [CrossRef]
- Qin, W.; Yang, C.; Yi, R.; Gao, G. Hydrothermal synthesis and characterization of single-crystalline α-Fe2O3 nanocubes. J. Nanomater. 2011, 2011, 159259. [Google Scholar] [CrossRef]
- Madhubala, V.; Kalaivani, T.; Kirubha, A.; Prakash, J.S.; Manigandan, V.; Dara, H.K. Study of structural and magnetic properties of hydro/solvothermally synthesized α-Fe2O3 nanoparticles and its toxicity assessment in zebrafish embryos. Appl. Surf. Sci. 2019, 494, 391–400. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, C.; Khan, I.A.; Tang, Y.; Liu, S.; Yang, M. Toxic effects of different-sized graphene oxide particles on zebrafish embryonic development. Ecotoxicol. Environ. Saf. 2020, 197, 110608. [Google Scholar] [CrossRef]
- Ouyang, S.; Hu, X.; Zhou, Q. Envelopment–internalization synergistic effects and metabolic mechanisms of graphene oxide on single-cell Chlorella vulgaris are dependent on the nanomaterial particle size. ACS Appl. Mater. Interfaces 2015, 7, 18104–18112. [Google Scholar] [CrossRef]
- Bangeppagari, M.; Park, S.H.; Kundapur, R.R.; Lee, S.J. Graphene oxide induces cardiovascular defects in developing zebrafish (Danio rerio) embryo model: In-vivo toxicity assessment. Sci. Total Environ. 2019, 673, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Wang, X.; Li, X.; Liu, J.; Huang, X.; Qin, Y.; Che, X.; Zhou, H.; Martyniuk, C.J.; Yan, B. Aggravated toxicity of copper sulfide nanoparticles via hypochlorite-induced nanoparticle dissolution. Environ. Sci. Nano 2022, 9, 1439–1452. [Google Scholar] [CrossRef]
- Schaffie, M.; Hosseini, M. Biological process for synthesis of semiconductor copper sulfide nanoparticle from mine wastewaters. J. Environ. Chem. Eng. 2014, 2, 386–391. [Google Scholar] [CrossRef]
- Rajeshkumar, S.; Santhoshkumar, J.; Vanaja, M.; Sivaperumal, P.; Ponnanikajamideen, M.; Ali, D.; Arunachalam, K. Evaluation of Zebrafish Toxicology and Biomedical Potential of Aeromonas hydrophila Mediated Copper Sulfide Nanoparticles. Oxid. Med. Cell. Longev. 2022, 2022, 7969825. [Google Scholar] [CrossRef] [PubMed]
- Marlow, F.; Gonzalez, E.M.; Yin, C.; Rojo, C.; Solnica-Krezel, L. No tail co-operates with non-canonical Wnt signaling to regulate posterior body morphogenesis in zebrafish. Development 2004, 131, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, L.; Gao, Q.; Zhang, X.; Lin, Y.; Huang, S.; Chen, D. Toxicity of polymer-modified CuS nanoclusters on zebrafish embryo development. J. Appl. Toxicol. 2022, 42, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-X.; Liu, Y.-Y.; Wei, Z.-B.; Yang, L.-Y.; Miao, A.-J. Waterborne and dietborne toxicity of inorganic arsenic to the freshwater zooplankton Daphnia magna. Environ. Sci. Technol. 2018, 52, 8912–8919. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, J.; Gao, Y.; Xiong, H.; Zhang, W.; Yan, B. The ZrO2 NPs enhanced the risk of arsenate by promoting its accumulation and reducing its detoxification during food chain transfer from Daphnia magna to zebrafish. J. Hazard. Mater. 2022, 424, 127338. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Annadurai, G.; Rajeshkumar, S. Characterization and toxicology evaluation of zirconium oxide nanoparticles on the embryonic development of zebrafish, Danio rerio. Drug Chem. Toxicol. 2018, 42, 104–111. [Google Scholar]
- Zou, W.; Zhou, Q.; Zhang, X.; Hu, X. Dissolved Oxygen and Visible Light Irradiation Drive the Structural Alterations and Phytotoxicity Mitigation of Single-Layer Molybdenum Disulfide. Environ. Sci. Technol. 2019, 53, 7759–7769. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-W.; Lai, Y.-H.; Chen, J.-L.; Chen, C. The role of transformation in the risks of chemically exfoliated molybdenum disulfide nanosheets to the aquatic environment. J. Environ. Manag. 2022, 324, 116278. [Google Scholar] [CrossRef]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, J.; Chen, Y.-p. Ecotoxicity assessment of a molybdenum mining effluent using acute lethal, oxidative stress, and osmoregulatory endpoints in zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2021, 28, 5137–5148. [Google Scholar] [CrossRef]
















| Nanomaterial | Models | Toxicology | Reference |
|---|---|---|---|
| Au | Zebrafish | Size directly proportional to toxicity | [30] |
| ZnO, CuO, and NiO | Zebrafish | Delayed hatching of embryos | [36] |
| TMs doped Co3O4 and PdO-Co3O4 | Zebrafish | Severe skin damage | [37] |
| Ag | Zebrafish | Size dependent intestinal toxicity | [57] |
| Ag | Zebrafish | Size dependent toxicity | [58] |
| Ag | Zebrafish | Dosage dependent toxicity | [59] |
| RU-Ag | Zebrafish | Malformation, imbalanced heart rate, and severe morphological changes | [60] |
| Cu | Zebrafish | Decreased pigmentation at 85 ppm | [69] |
| Cu | Zebrafish | Intestinal damage by ROS | [67] |
| CuO | Zebrafish | DNA damage and cytotoxicity | [71] |
| Pt | Pseudokirchneriella subcapitata Chlamydomonas reinhardtii | Growth rate suppression and oxidative stress | [76] |
| Au-Ag | Early-stage zebrafish embryos | Superior biocompatibility and decreased cytotoxicity | [78] |
| Ru-Fc | Zebrafish | Mitochondrial malfunction, ER stress, and necroptotic cell death | [79] |
| Fe-ZnO | Zebrafish and Mouse | Reduced inflammation and lactate dehydrogenase release | [80] |
| NH2-ZnO | Zebrafish | Induced hepatotoxicity by ROS | [91] |
| TiO2 | Zebrafish | 1.0 mg/L nano-TiO2 significantly reduced the body length and weight | [95] |
| TiO2 | Zebrafish | Caused neurotoxicity and abnormal growth | [96] |
| TBPH-TiO2 | Zebrafish | Reduced toxicity and anomalies | [97] |
| ZnO-SCs | Zebrafish | Reduced toxicity and anomalies | [101] |
| MPs and ZnO | Zebrafish | Growth inhibition, oxidative stress, apoptosis, and disruption of the growth hormone/insulin growth factor axis | [102] |
| CuO-clay-HA | Zebrafish | Reduced toxicity and anomalies | [105] |
| CuSO4-CuO ENMs | Zebrafish | Reduced toxicity and anomalies by limiting oxidative stress | [106] |
| CuO | Zebrafish | The highest number of delayed hatching embryos (81.3%) and opaque yolk deformation (36.3%) | [107] |
| IO | Zebrafish | Reduced heartbeat, blood accumulation in the heart, and pericardial edema | [110] |
| Congo red@IO | Zebrafish | Delayed hatching and increased mortality | [111] |
| α-IO | Zebrafish | Concentration dependent toxicity | [112] |
| GO | Zebrafish | Size dependent developmental toxicity | [114] |
| GO | Zebrafish | Delayed hatching, reduced body length, altered heart rate and blood flow, changes in swimming activity and responses to photoperiod stimulation, and increased activity of enzymes and genes related to oxidative stress and apoptosis. | [115] |
| ZrO2 | Daphna magna and Zebrafish | DNA damage and toxic anomalies | [123] |
| MoS2 | Zebrafish | Reduced toxicity with NOM combination | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutalik, C.; Nivedita; Sneka, C.; Krisnawati, D.I.; Yougbaré, S.; Hsu, C.-C.; Kuo, T.-R. Zebrafish Insights into Nanomaterial Toxicity: A Focused Exploration on Metallic, Metal Oxide, Semiconductor, and Mixed-Metal Nanoparticles. Int. J. Mol. Sci. 2024, 25, 1926. https://doi.org/10.3390/ijms25031926
Mutalik C, Nivedita, Sneka C, Krisnawati DI, Yougbaré S, Hsu C-C, Kuo T-R. Zebrafish Insights into Nanomaterial Toxicity: A Focused Exploration on Metallic, Metal Oxide, Semiconductor, and Mixed-Metal Nanoparticles. International Journal of Molecular Sciences. 2024; 25(3):1926. https://doi.org/10.3390/ijms25031926
Chicago/Turabian StyleMutalik, Chinmaya, Nivedita, Chandrasekaran Sneka, Dyah Ika Krisnawati, Sibidou Yougbaré, Chuan-Chih Hsu, and Tsung-Rong Kuo. 2024. "Zebrafish Insights into Nanomaterial Toxicity: A Focused Exploration on Metallic, Metal Oxide, Semiconductor, and Mixed-Metal Nanoparticles" International Journal of Molecular Sciences 25, no. 3: 1926. https://doi.org/10.3390/ijms25031926
APA StyleMutalik, C., Nivedita, Sneka, C., Krisnawati, D. I., Yougbaré, S., Hsu, C.-C., & Kuo, T.-R. (2024). Zebrafish Insights into Nanomaterial Toxicity: A Focused Exploration on Metallic, Metal Oxide, Semiconductor, and Mixed-Metal Nanoparticles. International Journal of Molecular Sciences, 25(3), 1926. https://doi.org/10.3390/ijms25031926