Association of Alpha-Crystallin with Human Cortical and Nuclear Lens Lipid Membrane Increases with the Grade of Cortical and Nuclear Cataract
Abstract
:1. Introduction
2. Results
2.1. MSO by α-Crystallin in Individual Eye Lens Cortical and Nuclear Membranes with Varying Cataract Grade
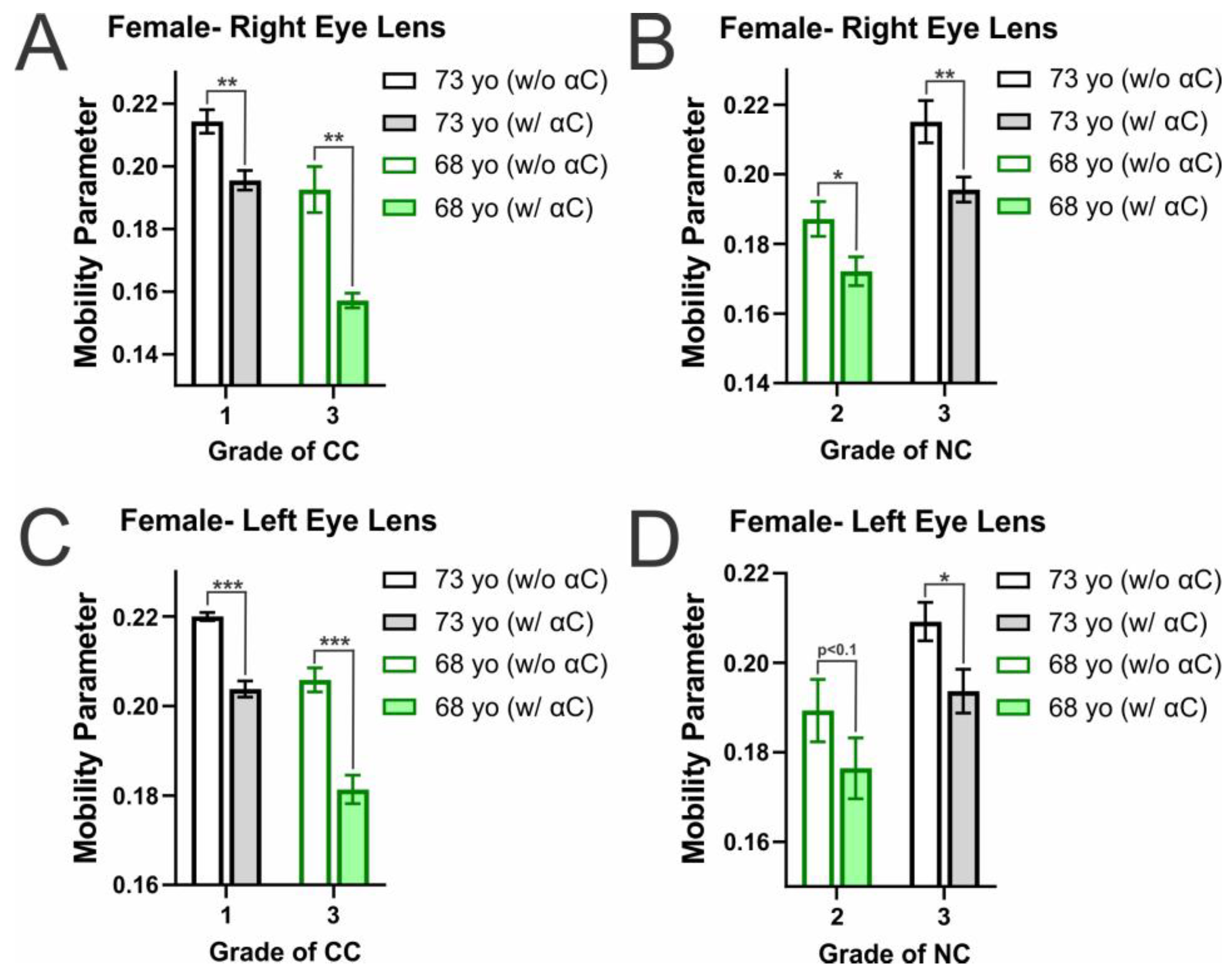
2.2. Membrane Mobility on the Surface of Individual Eye Lens Cortical and Nuclear Membranes with α-Crystallin Binding
2.3. Membrane Order near the Surface of Individual Eye Lens Cortical and Nuclear Membranes with α-Crystallin Binding
2.4. Hydrophobicity on the Surface of Individual Eye Lens Cortical and Nuclear Membranes with α-Crystallin Binding
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Isolation of Cortical and Nuclear Lipids from Single Human Lens
4.3. Preparation of Small Unilamellar Vesicles (SUVs) from Cortical and Nuclear Lipids Isolated from a Single Human Lens
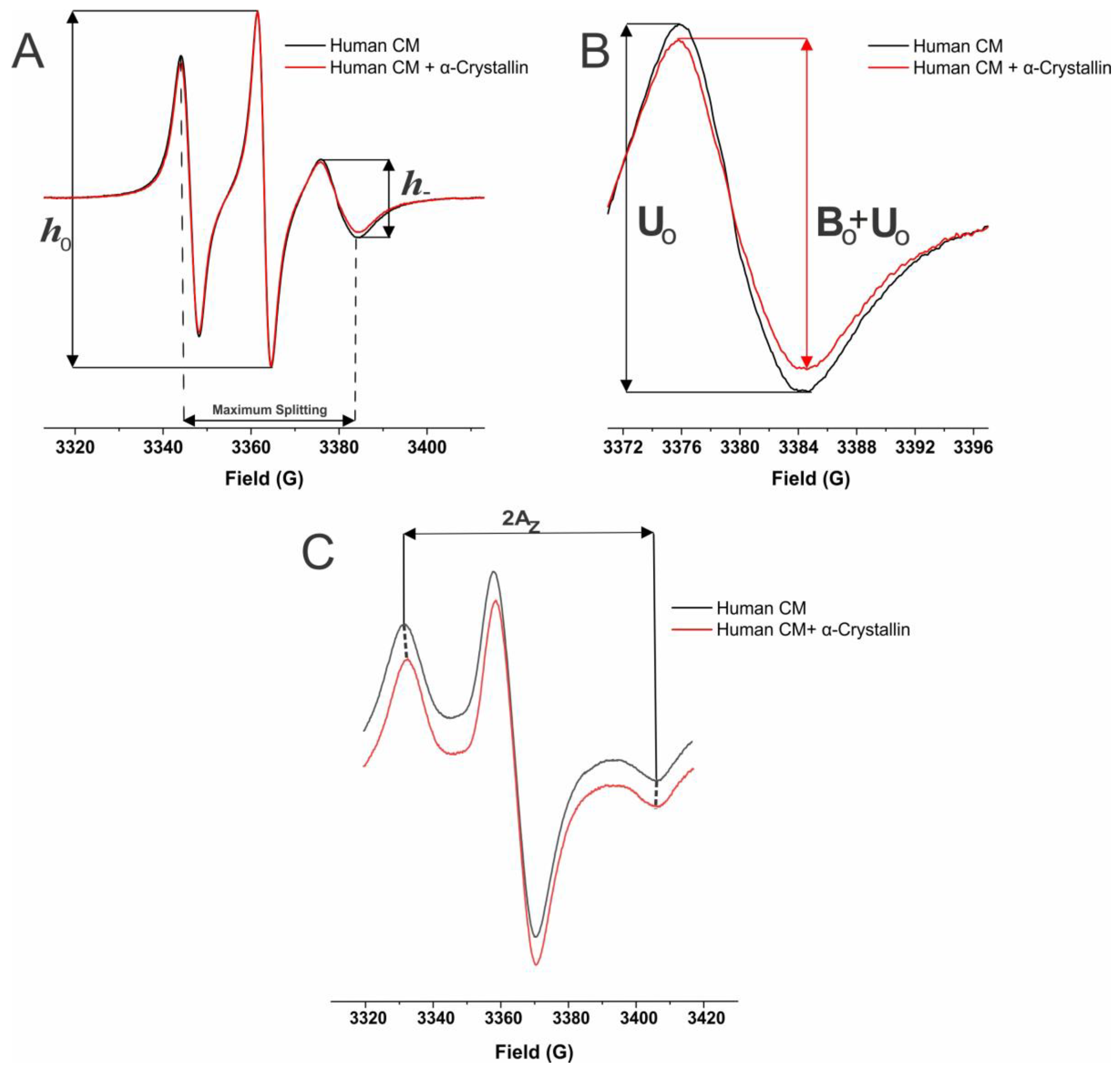
4.4. The Electron Paramagnetic Resonance (EPR) Spin-Labeling Method to Investigate the α-Crystallin Binding to Human Cortical and Nuclear Membranes Isolated from a Single Lens
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPR | electron paramagnetic resonance |
| CM | cortical membrane |
| NM | nuclear membrane |
| CC | cortical cataract |
| NC | nuclear cataract |
| MSO | percentage of membrane surface occupied |
| 4PT | 4-palmitamido-TEMPO |
| PL | phospholipid |
| SL | sphingolipid |
| Chol | cholesterol |
| CBD | cholesterol bilayer domain |
References
- Derham, B.K.; Harding, J.J. α-Crystallin as a Molecular Chaperone. Prog. Retin. Eye Res. 1999, 18, 463–509. [Google Scholar] [CrossRef] [PubMed]
- Derham, B.K.; Harding, J.J. Effects of Modifications of Alpha-Crystallin on Its Chaperone and Other Properties. Biochem. J. 2002, 364, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J. Alpha-Crystallin. Exp. Eye Res. 2003, 76, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.; Bova, M.P.; Ding, L.-L.; Haley, D.A.; Stewart, P.L. Lens α-Crystallin: Function and Structure. Eye 1999, 13, 403–408. [Google Scholar] [CrossRef]
- Bloemendal, H.; De Jong, W.; Jaenicke, R.; Lubsen, N.H.; Slingsby, C.; Tardieu, A. Ageing and Vision: Structure, Stability and Function of Lens Crystallins. Prog. Biophys. Mol. Biol. 2004, 86, 407–485. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.A.; Sprague-Piercy, M.A.; Kwok, A.O.; Roskamp, K.W.; Martin, R.W. Chemical Properties Determine Solubility and Stability in Βγ-Crystallins of the Eye Lens. ChemBioChem Eur. J. Chem. Biol. 2021, 22, 1329–1346. [Google Scholar] [CrossRef]
- Andley, U.P. AlphaA-Crystallin R49Cneo Mutation Influences the Architecture of Lens Fiber Cell Membranes and Causes Posterior and Nuclear Cataracts in Mice. BMC Ophthalmol. 2009, 9, 4. [Google Scholar] [CrossRef]
- Cobb, B.A.; Petrash, J.M. Structural and Functional Changes in the αA-Crystallin R116C Mutant in Hereditary Cataracts. Biochemistry 2000, 39, 15791–15798. [Google Scholar] [CrossRef]
- De Bruyne, S.; van Schie, L.; Himpe, J.; De Somer, F.; Everaert, I.; Derave, W.; Van den Broecke, C.; Huizing, M.; Bostan, N.; Speeckaert, M.; et al. A Potential Role for Fructosamine-3-Kinase in Cataract Treatment. Int. J. Mol. Sci. 2021, 22, 3841. [Google Scholar] [CrossRef]
- Brown, Z.; Ponce, A.; Lampi, K.; Hancock, L.; Takemoto, L. Differential Binding of Mutant (R116C) and Wildtype AlphaA Crystallin to Actin. Curr. Eye Res. 2007, 32, 1051–1054. [Google Scholar] [CrossRef]
- Grosas, A.B.; Carver, J.A. Eye Lens Crystallins: Remarkable Long-Lived Proteins. In Long-Lived Proteins in Human Aging and Disease; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2021; pp. 59–96. ISBN 978-3-527-82675-9. [Google Scholar]
- Kamei, A.; Hamaguchi, T.; Matsuura, N.; Masuda, K. Does Post-Translational Modification Influence Chaperone-like Activity of Alpha-Crystallin? I. Study on Phosphorylation. Biol. Pharm. Bull. 2001, 24, 96–99. [Google Scholar] [CrossRef]
- Kamei, A.; Iwase, H.; Masuda, K. Cleavage of Amino Acid Residue(s) from the N-Terminal Region of Alpha A- and Alpha B-Crystallins in Human Crystalline Lens during Aging. Biochem. Biophys. Res. Commun. 1997, 231, 373–378. [Google Scholar] [CrossRef]
- Blakytny, R.; Carver, J.A.; Harding, J.J.; Kilby, G.W.; Sheil, M.M. A Spectroscopic Study of Glycated Bovine Alpha-Crystallin: Investigation of Flexibility of the C-Terminal Extension, Chaperone Activity and Evidence for Diglycation. Biochim. Biophys. Acta 1997, 1343, 299–315. [Google Scholar] [CrossRef]
- van Boekel, M.A.; Hoogakker, S.E.; Harding, J.J.; de Jong, W.W. The Influence of Some Post-Translational Modifications on the Chaperone-like Activity of Alpha-Crystallin. Ophthalmic Res. 1996, 28 (Suppl. S1), 32–38. [Google Scholar] [CrossRef]
- Harrington, V.; McCall, S.; Huynh, S.; Srivastava, K.; Srivastava, O.P. Crystallins in Water Soluble-High Molecular Weight Protein Fractions and Water Insoluble Protein Fractions in Aging and Cataractous Human Lenses. Mol. Vis. 2004, 10, 476–489. [Google Scholar]
- Hanson, S.R.; Hasan, A.; Smith, D.L.; Smith, J.B. The Major in Vivo Modifications of the Human Water-Insoluble Lens Crystallins Are Disulfide Bonds, Deamidation, Methionine Oxidation and Backbone Cleavage. Exp. Eye Res. 2000, 71, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Wilmarth, P.A.; Tanner, S.; Dasari, S.; Nagalla, S.R.; Riviere, M.A.; Bafna, V.; Pevzner, P.A.; David, L.L. Age-Related Changes in Human Crystallins Determined from Comparative Analysis of Post-Translational Modifications in Young and Aged Lens: Does Deamidation Contribute to Crystallin Insolubility? J. Proteome Res. 2006, 5, 2554–2566. [Google Scholar] [CrossRef] [PubMed]
- Heys, K.R.; Friedrich, M.G.; Truscott, R.J.W. Presbyopia and Heat: Changes Associated with Aging of the Human Lens Suggest a Functional Role for the Small Heat Shock Protein, Alpha-Crystallin, in Maintaining Lens Flexibility. Aging Cell 2007, 6, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Aarts, H.J.; Lubsen, N.H.; Schoenmakers, J.G. Crystallin Gene Expression during Rat Lens Development. Eur. J. Biochem. 1989, 183, 31–36. [Google Scholar] [CrossRef]
- Su, S.-P.; McArthur, J.D.; Truscott, R.J.W.; Aquilina, J.A. Truncation, Cross-Linking and Interaction of Crystallins and Intermediate Filament Proteins in the Aging Human Lens. Biochim. Biophys. Acta 2011, 1814, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Hoehenwarter, W.; Klose, J.; Jungblut, P.R. Eye Lens Proteomics. Amino Acids 2006, 30, 369–389. [Google Scholar] [CrossRef]
- McFall-Ngai, M.J.; Ding, L.L.; Takemoto, L.J.; Horwitz, J. Spatial and Temporal Mapping of the Age-Related Changes in Human Lens Crystallins. Exp. Eye Res. 1985, 41, 745–758. [Google Scholar] [CrossRef]
- Roy, D.; Spector, A. Absence of Low-Molecular-Weight Alpha Crystallin in Nuclear Region of Old Human Lenses. Proc. Natl. Acad. Sci. USA 1976, 73, 3484–3487. [Google Scholar] [CrossRef]
- Datiles, M.B., III; Ansari, R.R.; Yoshida, J.; Brown, H.; Zambrano, A.I.; Tian, J.; Vitale, S.; Zigler, J.S., Jr.; Ferris, F.L., III; West, S.K.; et al. Longitudinal Study of Age-Related Cataract Using Dynamic Light Scattering: Loss of α-Crystallin Leads to Nuclear Cataract Development. Ophthalmology 2016, 123, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Mainali, L. Association of Alpha-Crystallin with Fiber Cell Plasma Membrane of the Eye Lens Accompanied by Light Scattering and Cataract Formation. Membranes 2021, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Boyle, D.L.; Takemoto, L. EM Immunolocalization of Alpha-Crystallins: Association with the Plasma Membrane from Normal and Cataractous Human Lenses. Curr. Eye Res. 1996, 15, 577–582. [Google Scholar] [CrossRef]
- Cenedella, R.J.; Fleschner, C.R. Selective Association of Crystallins with Lens “native” Membrane during Dynamic Cataractogenesis. Curr. Eye Res. 1992, 11, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Truscott, R.J.W. Large-Scale Binding of α-Crystallin to Cell Membranes of Aged Normal Human Lenses: A Phenomenon That Can Be Induced by Mild Thermal Stress. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5145–5152. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekher, G.; Cenedella, R.J. Protein Associated with Human Lens “native” Membrane during Aging and Cataract Formation. Exp. Eye Res. 1995, 60, 707–717. [Google Scholar] [CrossRef]
- Cobb, B.A.; Petrash, J.M. Alpha-Crystallin Chaperone-like Activity and Membrane Binding in Age-Related Cataracts. Biochemistry 2002, 41, 483–490. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Truscott, R.J.W. Membrane Association of Proteins in the Aging Human Lens: Profound Changes Take Place in the Fifth Decade of Life. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4786–4793. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J. Age-Related Nuclear Cataract: A Lens Transport Problem. Ophthalmic Res. 2000, 32, 185–194. [Google Scholar] [CrossRef]
- Borchman, D. Lipid Conformational Order and the Etiology of Cataract and Dry Eye. J. Lipid Res. 2021, 62, 100039. [Google Scholar] [CrossRef]
- Borchman, D.; Stimmelmayr, R.; George, J.C. Whales, Lifespan, Phospholipids, and Cataracts. J. Lipid Res. 2017, 58, 2289–2298. [Google Scholar] [CrossRef]
- Deeley, J.M.; Mitchell, T.W.; Wei, X.; Korth, J.; Nealon, J.R.; Blanksby, S.J.; Truscott, R.J.W. Human Lens Lipids Differ Markedly from Those of Commonly Used Experimental Animals. Biochim. Biophys. Acta 2008, 1781, 288–298. [Google Scholar] [CrossRef]
- Stimmelmayr, R.; Borchman, D. Lens Lipidomes among Phocidae and Odobenidae. Aquat. Mamm. 2018, 43, 506–518. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of Membranes Derived from the Total Lipids Extracted from the Human Lens Cortex and Nucleus. Biochim. Biophys. Acta 2013, 1828, 1432–1440. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Changes in the Properties and Organization of Human Lens Lipid Membranes Occurring with Age. Curr. Eye Res. 2017, 42, 721–731. [Google Scholar] [CrossRef]
- Widomska, J.; Subczynski, W.K. Why Is Very High Cholesterol Content Beneficial for the Eye Lens but Negative for Other Organs? Nutrients 2019, 11, 1083. [Google Scholar] [CrossRef]
- Yappert, M.C.; Rujoi, M.; Borchman, D.; Vorobyov, I.; Estrada, R. Glycero- versus Sphingo-Phospholipids: Correlations with Human and Non-Human Mammalian Lens Growth. Exp. Eye Res. 2003, 76, 725–734. [Google Scholar] [CrossRef]
- Deeley, J.M.; Hankin, J.A.; Friedrich, M.G.; Murphy, R.C.; Truscott, R.J.W.; Mitchell, T.W.; Blanksby, S.J. Sphingolipid Distribution Changes with Age in the Human Lens. J. Lipid Res. 2010, 51, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of Membranes Derived from the Total Lipids Extracted from Clear and Cataractous Lenses of 61–70-Year-Old Human Donors. Eur. Biophys. J. EBJ 2015, 44, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Estrada, R.; Yappert, M.C.; Borchman, D. Oxidation-Induced Changes in Human Lens Epithelial Cells: 1. Phospholipids. Free Radic. Biol. Med. 2006, 41, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tang, D.; Yappert, M.C.; Borchman, D. Oxidation-Induced Changes in Human Lens Epithelial Cells 2. Mitochondria and the Generation of Reactive Oxygen Species. Free Radic. Biol. Med. 2006, 41, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Yappert, M.C.; Jumblatt, M.M.; Borchman, D. Hyperoxia and Thyroxine Treatment and the Relationships between Reactive Oxygen Species Generation, Mitochondrial Membrane Potential, and Cardiolipin in Human Lens Epithelial Cell Cultures. Curr. Eye Res. 2008, 33, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Witting, L.A. Lipid Peroxidationin Vivo. J. Am. Oil Chem. Soc. 1965, 42, 908–913. [Google Scholar] [CrossRef]
- Oborina, E.M.; Yappert, M.C. Effect of Sphingomyelin versus Dipalmitoylphosphatidylcholine on the Extent of Lipid Oxidation. Chem. Phys. Lipids 2003, 123, 223–232. [Google Scholar] [CrossRef]
- Mainali, L.; O’Brien, W.J.; Subczynski, W.K. Detection of Cholesterol Bilayer Domains in Intact Biological Membranes: Methodology Development and Its Application to Studies of Eye Lens Fiber Cell Plasma Membranes. Exp. Eye Res. 2019, 178, 72–81. [Google Scholar] [CrossRef]
- Shin, S.; Zhou, H.; He, C.; Wei, Y.; Wang, Y.; Shingu, T.; Zeng, A.; Wang, S.; Zhou, X.; Li, H.; et al. Qki Activates Srebp2-Mediated Cholesterol Biosynthesis for Maintenance of Eye Lens Transparency. Nat. Commun. 2021, 12, 3005. [Google Scholar] [CrossRef]
- Cobb, B.A.; Petrash, J.M. Factors Influencing Alpha-Crystallin Association with Phospholipid Vesicles. Mol. Vis. 2002, 8, 85–93. [Google Scholar]
- Borchman, D.; Tang, D. Binding Capacity of Alpha-Crystallin to Bovine Lens Lipids. Exp. Eye Res. 1996, 63, 407–410. [Google Scholar] [CrossRef]
- Grami, V.; Marrero, Y.; Huang, L.; Tang, D.; Yappert, M.C.; Borchman, D. α-Crystallin Binding in Vitro to Lipids from Clear Human Lenses. Exp. Eye Res. 2005, 81, 138–146. [Google Scholar] [CrossRef]
- Cobb, B.A.; Petrash, J.M. Characterization of Alpha-Crystallin-Plasma Membrane Binding. J. Biol. Chem. 2000, 275, 6664–6672. [Google Scholar] [CrossRef]
- Su, S.-P.; McArthur, J.D.; Friedrich, M.G.; Truscott, R.J.W.; Aquilina, J.A. Understanding the α-Crystallin Cell Membrane Conjunction. Mol. Vis. 2011, 17, 2798–2807. [Google Scholar] [PubMed]
- Chandrasekher, G.; Cenedella, R.J. Properties of α-Crystallin Bound to Lens Membrane: Probing Organization at the Membrane Surface. Exp. Eye Res. 1997, 64, 423–430. [Google Scholar] [CrossRef]
- Ifeanyi, F.; Takemoto, L. Differential Binding of α-Crystallins to Bovine Lens Membrane. Exp. Eye Res. 1989, 49, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ifeanyi, F.; Takemoto, L. Specificity of Alpha Crystallin Binding to the Lens Membrane. Curr. Eye Res. 1990, 9, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ifeanyi, F.; Takemoto, L. Alpha Crystallin from Human Cataractous vs. Normal Lenses: Change in Binding to Lens Membrane. Exp. Eye Res. 1990, 50, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Mulders, J.W.M.; Stokkermans, J.; Leunissen, J.A.M.; Benedetti, E.L.; Bloemendal, H.; De Jong, W.W. Interaction of α-Crystallin with Lens Plasma Membranes. Eur. J. Biochem. 1985, 152, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Z.; Augusteyn, R.C. On the Interaction of α-Crystallin with Membranes. Curr. Eye Res. 1994, 13, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Borchman, D.; Ozaki, Y.; Lamba, O.P.; Byrdwell, C.W.; Yappert, M.C.; Paterson, C.A. Lipid –Protein Interactions in Human and Bovine Lens Membranes by Fourier Transform Raman and Infrared Spectroscopies. Exp. Eye Res. 1996, 62, 47–54. [Google Scholar] [CrossRef]
- Liang, J.J.N.; Li, X.-Y. Spectroscopic Studies on the Interaction of Calf Lens Membranes with Crystallins. Exp. Eye Res. 1992, 54, 719–724. [Google Scholar] [CrossRef]
- Mulders, J.W.M.; Wojcik, E.; Bloemendal, H.; de Jong, W.W. Loss of High-Affinity Membrane Binding of Bovine Nuclear α-Crystallin. Exp. Eye Res. 1989, 49, 149–152. [Google Scholar] [CrossRef]
- Tang, D.; Borchman, D.; Yappert, M.C.; Cenedella, R.J. Influence of Cholesterol on the Interaction of Alpha-Crystallin with Phospholipids. Exp. Eye Res. 1998, 66, 559–567. [Google Scholar] [CrossRef]
- Mainali, L.; O’Brien, W.J.; Timsina, R. Interaction of Alpha-Crystallin with Phospholipid Membranes. Curr. Eye Res. 2021, 46, 185–194. [Google Scholar] [CrossRef]
- Timsina, R.; Trossi-Torres, G.; O’Dell, M.; Khadka, N.K.; Mainali, L. Cholesterol and Cholesterol Bilayer Domains Inhibit Binding of Alpha-Crystallin to the Membranes Made of the Major Phospholipids of Eye Lens Fiber Cell Plasma Membranes. Exp. Eye Res. 2021, 206, 108544. [Google Scholar] [CrossRef]
- Timsina, R.; Khadka, N.K.; Maldonado, D.; Mainali, L. Interaction of Alpha-Crystallin with Four Major Phospholipids of Eye Lens Membranes. Exp. Eye Res. 2021, 202, 108337. [Google Scholar] [CrossRef]
- Cenedella, R.J.; Chandrasekher, G. High Capacity Binding of Alpha Crystallins to Various Bovine Lens Membrane Preparations. Curr. Eye Res. 1993, 12, 1025–1038. [Google Scholar] [CrossRef] [PubMed]
- Timsina, R.; Trossi-Torres, G.; Thieme, J.; O’Dell, M.; Khadka, N.K.; Mainali, L. Alpha-Crystallin Association with the Model of Human and Animal Eye Lens-Lipid Membranes Is Modulated by Surface Hydrophobicity of Membranes. Curr. Eye Res. 2022, 47, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Trossi-Torres, G.; Timsina, R.; Mainali, L. Alpha-Crystallin-Membrane Association Modulated by Phospholipid Acyl Chain Length and Degree of Unsaturation. Membranes 2022, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Borchman, D.; Yappert, M.C. Alpha-Crystallin/Lens Lipid Interactions Using Resonance Energy Transfer. Ophthalmic Res. 1999, 31, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkova, N.M.; Horváth, I.; Török, Z.; Wolkers, W.F.; Balogi, Z.; Shigapova, N.; Crowe, L.M.; Tablin, F.; Vierling, E.; Crowe, J.H.; et al. Small Heat-Shock Proteins Regulate Membrane Lipid Polymorphism. Proc. Natl. Acad. Sci. USA 2002, 99, 13504–13509. [Google Scholar] [CrossRef] [PubMed]
- Bloemendal, H.; Berbers, G.A.; De Jong, W.W.; Ramaekers, F.C.; Vermorken, A.J.; Dunia, I.; Benedetti, E.L. Interaction of Crystallins with the Cytoskeletal-Plasma Membrane Complex of the Bovine Lens. Ciba Found. Symp. 1984, 106, 177–190. [Google Scholar] [CrossRef]
- Timsina, R.; Wellisch, S.; Haemmerle, D.; Mainali, L. Binding of Alpha-Crystallin to Cortical and Nuclear Lens Lipid Membranes Derived from a Single Lens. Int. J. Mol. Sci. 2022, 23, 11295. [Google Scholar] [CrossRef]
- The Risk of Cataract among Users of Inhaled Steroids—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/11246585/ (accessed on 9 November 2023).
- Beebe, D.C.; Holekamp, N.M.; Shui, Y.-B. Oxidative Damage and the Prevention of Age-Related Cataracts. Ophthalmic Res. 2010, 44, 155–165. [Google Scholar] [CrossRef]
- Raju, P.; George, R.; Ramesh, S.V.; Arvind, H.; Baskaran, M.; Vijaya, L. Influence of Tobacco Use on Cataract Development. Br. J. Ophthalmol. 2006, 90, 1374–1377. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; He, J.; Wang, C.; Wu, H.; Shi, X.; Zhang, H.; Xie, J.; Lee, S.Y. Smoking and Risk of Age-Related Cataract: A Meta-Analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3885–3895. [Google Scholar] [CrossRef]
- Foster, P.J.; Wong, T.Y.; Machin, D.; Johnson, G.J.; Seah, S.K.L. Risk Factors for Nuclear, Cortical and Posterior Subcapsular Cataracts in the Chinese Population of Singapore: The Tanjong Pagar Survey. Br. J. Ophthalmol. 2003, 87, 1112–1120. [Google Scholar] [CrossRef]
- West, S.K.; Valmadrid, C.T. Epidemiology of Risk Factors for Age-Related Cataract. Surv. Ophthalmol. 1995, 39, 323–334. [Google Scholar] [CrossRef]
- West, S.; Munoz, B.; Emmett, E.A.; Taylor, H.R. Cigarette Smoking and Risk of Nuclear Cataracts. Arch. Ophthalmol. 1989, 107, 1166–1169. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Hsu, W.-M.; Cheng, C.-Y.; Liu, J.-H.; Chou, P. Epidemiologic Study of Age-Related Cataracts among an Elderly Chinese Population in Shih-Pai, Taiwan. Ophthalmology 2003, 110, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Rouhiainen, P.; Rouhiainen, H.; Salonen, J.T. Association between Low Plasma Vitamin E Concentration and Progression of Early Cortical Lens Opacities. Am. J. Epidemiol. 1996, 144, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, S.; Vilas, K.; Shamanna, B.R.; Rao, G.N.; Thomas, R.; Balasubramanian, D. Smoking and Its Association with Cataract: Results of the Andhra Pradesh Eye Disease Study from India. Investig. Ophthalmol. Vis. Sci. 2005, 46, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Nirmalan, P.K.; Robin, A.L.; Katz, J.; Tielsch, J.M.; Thulasiraj, R.D.; Krishnadas, R.; Ramakrishnan, R. Risk Factors for Age Related Cataract in a Rural Population of Southern India: The Aravind Comprehensive Eye Study. Br. J. Ophthalmol. 2004, 88, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, H.; Theodoratou, E.; Chan, K.Y.; Rudan, I. The National and Subnational Prevalence of Cataract and Cataract Blindness in China: A Systematic Review and Meta-Analysis. J. Glob. Health 2018, 8, 010804. [Google Scholar] [CrossRef]
- Vashist, P.; Talwar, B.; Gogoi, M.; Maraini, G.; Camparini, M.; Ravindran, R.D.; Murthy, G.V.; Fitzpatrick, K.E.; John, N.; Chakravarthy, U.; et al. Prevalence of Cataract in an Older Population in India. Ophthalmology 2011, 118, 272–278.e2. [Google Scholar] [CrossRef]
- McCarty, C.A.; Nanjan, M.B.; Taylor, H.R. Attributable Risk Estimates for Cataract to Prioritize Medical and Public Health Action. Investig. Ophthalmol. Vis. Sci. 2000, 41, 3720–3725. [Google Scholar]
- Lawal, Y.; Bello, F.; Kaoje, Y.S. Prediabetes Deserves More Attention: A Review. Clin. Diabetes Publ. Am. Diabetes Assoc. 2020, 38, 328–338. [Google Scholar] [CrossRef]
- Kiziltoprak, H.; Tekin, K.; Inanc, M.; Goker, Y.S. Cataract in Diabetes Mellitus. World J. Diabetes 2019, 10, 140–153. [Google Scholar] [CrossRef]
- Nielsen, N.V.; Vinding, T. The Prevalence of Cataract in Insulin-Dependent and Non-Insulin-Dependent-Diabetes Mellitus. Acta Ophthalmol. 1984, 62, 595–602. [Google Scholar] [CrossRef]
- Klein, B.E.K.; Klein, R.; Moss, S.E. Prevalence of Cataracts in a Population-Based Study of Persons with Diabetes Mellitus. Ophthalmology 1985, 92, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Correlation between Adult Diabetic Cataracts and Red Blood Cell Aldose Reductase Levels—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16639016/ (accessed on 9 November 2023).
- Mrugacz, M.; Pony-Uram, M.; Bryl, A.; Zorena, K. Current Approach to the Pathogenesis of Diabetic Cataracts. Int. J. Mol. Sci. 2023, 24, 6317. [Google Scholar] [CrossRef]
- Taurine Supplementation Protects Lens against Glutathione Depletion—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/34286494/ (accessed on 9 November 2023).
- Snow, A.; Shieh, B.; Chang, K.-C.; Pal, A.; Lenhart, P.; Ammar, D.; Ruzycki, P.; Palla, S.; Reddy, G.B.; Petrash, J.M. Aldose Reductase Expression as a Risk Factor for Cataract. Chem. Biol. Interact. 2015, 234, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Haroon, H.B.; Perumalsamy, V.; Nair, G.; Anand, D.K.; Kolli, R.; Monichen, J.; Prabha, K. Repression of Polyol Pathway Activity by Hemidesmus indicus Var. Pubescens R.Br. Linn Root Extract, an Aldose Reductase Inhibitor: An In Silico and Ex Vivo Study. Nat. Prod. Bioprospect. 2021, 11, 315–324. [Google Scholar] [CrossRef]
- Kumar, C.U.; Suryavanshi, U.; Sontake, V.; Reddy, P.Y.; Sankhala, R.S.; Swamy, M.J.; Reddy, G.B. Effect of Sorbitol on Alpha-Crystallin Structure and Function. Biochem. Mosc. 2022, 87, 131–140. [Google Scholar] [CrossRef]
- Lim, S.A.; Joo, C.-K.; Kim, M.S.; Chung, S.K. Expression of P53 and Caspase-8 in Lens Epithelial Cells of Diabetic Cataract. J. Cataract Refract. Surg. 2014, 40, 1102–1108. [Google Scholar] [CrossRef]
- Funatsu, H.; Yamashita, H.; Noma, H.; Shimizu, E.; Mimura, T.; Hori, S. Prediction of Macular Edema Exacerbation after Phacoemulsification in Patients with Nonproliferative Diabetic Retinopathy. J. Cataract Refract. Surg. 2002, 28, 1355. [Google Scholar] [CrossRef]
- Schaumberg, D.A.; Ridker, P.M.; Glynn, R.J.; Christen, W.G.; Dana, M.R.; Hennekens, C.H. High Levels of Plasma C-Reactive Protein and Future Risk of Age-Related Cataract. Ann. Epidemiol. 1999, 9, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.K.; Klein, R.; Lee, K.E.; Knudtson, M.D.; Tsai, M.Y. Markers of Inflammation, Vascular Endothelial Dysfunction, and Age-Related Cataract. Am. J. Ophthalmol. 2006, 141, 116–122. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R.; Jensen, S.C.; Linton, K.L. Hypertension and Lens Opacities from the Beaver Dam Eye Study. Am. J. Ophthalmol. 1995, 119, 640–646. [Google Scholar] [CrossRef]
- Sreenivas, V.; Prabhakar, A.K.; Badrinath, S.S.; Fernandez, T.; Roy, I.S.; Sharma, T.; Sheh, B. A Rural Population Based Case-Control Study of Senile Cataract in India. J. Epidemiol. 1999, 9, 327–336. [Google Scholar] [CrossRef]
- Angra, S.K.; Pushker, N.; Sachdev, M.S.; Jaffery, N.F. Risk Factors for Age-Related Posterior Subcapsular Cataract. Ann. Ophthalmol. 2000, 32, 101–106. [Google Scholar] [CrossRef]
- Sabanayagam, C.; Wang, J.J.; Mitchell, P.; Tan, A.G.; Tai, E.S.; Aung, T.; Saw, S.-M.; Wong, T.Y. Metabolic Syndrome Components and Age-Related Cataract: The Singapore Malay Eye Study. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Mukesh, B.N.; Le, A.; Dimitrov, P.N.; Ahmed, S.; Taylor, H.R.; McCarty, C.A. Development of Cataract and Associated Risk Factors: The Visual Impairment Project. Arch. Ophthalmol. 2006, 124, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Cumming, R.G.; Mitchell, P. Medications and Cataract: The Blue Mountains Eye Study. Ophthalmology 1998, 105, 1751–1758. [Google Scholar] [CrossRef]
- Kanthan, G.L.; Wang, J.J.; Rochtchina, E.; Mitchell, P. Use of Antihypertensive Medications and Topical Beta-Blockers and the Long-Term Incidence of Cataract and Cataract Surgery. Br. J. Ophthalmol. 2009, 93, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Spector, A.; Chiesa, R.; Sredy, J.; Garner, W. cAMP-Dependent Phosphorylation of Bovine Lens Alpha-Crystallin. Proc. Natl. Acad. Sci. USA 1985, 82, 4712–4716. [Google Scholar] [CrossRef] [PubMed]
- Ireland, M.E.; Shanbom, S. Lens Beta-Adrenergic Receptors. Functional Coupling to Adenylate Cyclase and Photoaffinity Labeling. Investig. Ophthalmol. Vis. Sci. 1991, 32, 541–548. [Google Scholar]
- Hazen, P.; Trossi-Torres, G.; Khadka, N.K.; Timsina, R.; Mainali, L. Binding of βL-Crystallin with Models of Animal and Human Eye Lens-Lipid Membrane. Int. J. Mol. Sci. 2023, 24, 13600. [Google Scholar] [CrossRef]
- Huang, L.; Grami, V.; Marrero, Y.; Tang, D.; Yappert, M.C.; Rasi, V.; Borchman, D. Human Lens Phospholipid Changes with Age and Cataract. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1682–1689. [Google Scholar] [CrossRef]
- Jacob, R.F.; Cenedella, R.J.; Mason, R.P. Evidence for Distinct Cholesterol Domains in Fiber Cell Membranes from Cataractous Human Lenses. J. Biol. Chem. 2001, 276, 13573–13578. [Google Scholar] [CrossRef]
- Hales, A.M.; Chamberlain, C.G.; Murphy, C.R.; McAvoy, J.W. Estrogen Protects Lenses against Cataract Induced by Transforming Growth Factor-β (TGFβ). J. Exp. Med. 1997, 185, 273–280. [Google Scholar] [CrossRef]
- Lee, S.-M.; Tseng, L.-M.; Li, A.-F.; Liu, H.-C.; Liu, T.-Y.; Chi, C.-W. Polymorphism of Estrogen Metabolism Genes and Cataract. Med. Hypotheses 2004, 63, 494–497. [Google Scholar] [CrossRef]
- Davis, V.L.; Chan, C.-C.; Schoen, T.J.; Couse, J.F.; Chader, G.J.; Korach, K.S. An Estrogen Receptor Repressor Induces Cataract Formation in Transgenic Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 9427–9432. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.R.; Koo, E.; Agrón, E.; Hallak, J.; Clemons, T.; Azar, D.; Sperduto, R.D.; Ferris, F.L.; Chew, E.Y. Risk Factors Associated with Incident Cataracts and Cataract Surgery in the Age Related Eye Disease Study (AREDS). AREDS Report Number 32. Ophthalmology 2011, 118, 2113–2119. [Google Scholar] [CrossRef]
- Bigsby, R.M.; Cardenas, H.; Caperell–Grant, A.; Grubbs, C.J. Protective Effects of Estrogen in a Rat Model of Age-Related Cataracts. Proc. Natl. Acad. Sci. USA 1999, 96, 9328–9332. [Google Scholar] [CrossRef] [PubMed]
- Nuzzi, R.; Caselgrandi, P. Sex Hormones and Their Effects on Ocular Disorders and Pathophysiology: Current Aspects and Our Experience. Int. J. Mol. Sci. 2022, 23, 3269. [Google Scholar] [CrossRef] [PubMed]
- Truscott, R.J.W. Age-Related Nuclear Cataract-Oxidation Is the Key. Exp. Eye Res. 2005, 80, 709–725. [Google Scholar] [CrossRef]
- Stryker, W.S.; Kaplan, L.A.; Stein, E.A.; Stampfer, M.J.; Sober, A.; Willett, W.C. The Relation of Diet, Cigarette Smoking, and Alcohol Consumption to Plasma Beta-Carotene and Alpha-Tocopherol Levels. Am. J. Epidemiol. 1988, 127, 283–296. [Google Scholar] [CrossRef]
- Ross, M.A.; Crosley, L.K.; Brown, K.M.; Duthie, S.J.; Collins, A.C.; Arthur, J.R.; Duthie, G.G. Plasma Concentrations of Carotenoids and Antioxidant Vitamins in Scottish Males: Influences of Smoking. Eur. J. Clin. Nutr. 1995, 49, 861–865. [Google Scholar] [PubMed]
- Shalini, V.K.; Luthra, M.; Srinivas, L.; Rao, S.H.; Basti, S.; Reddy, M.; Balasubramanian, D. Oxidative Damage to the Eye Lens Caused by Cigarette Smoke and Fuel Smoke Condensates. Indian J. Biochem. Biophys. 1994, 31, 261–266. [Google Scholar]
- Rácz, P.; Erdöhelyi, A. Cadmium, Lead and Copper Concentrations in Normal and Senile Cataractous Human Lenses. Ophthalmic Res. 1988, 20, 10–13. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Sulochana, K.N.; Selvaraj, T.; Abdul Rahim, A.; Lakshmi, M.; Arunagiri, K. Smoking of Beedies and Cataract: Cadmium and Vitamin C in the Lens and Blood. Br. J. Ophthalmol. 1995, 79, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Beswick, H.T.; Harding, J.J. Conformational Changes Induced in Bovine Lens Alpha-Crystallin by Carbamylation. Relevance to Cataract. Biochem. J. 1984, 223, 221–227. [Google Scholar] [CrossRef]
- Ganea, E.; Rixon, K.C.; Harding, J.J. Binding of Glucose, Galactose and Pyridoxal Phosphate to Lens Crystallins. Biochim. Biophys. Acta 1994, 1226, 286–290. [Google Scholar] [CrossRef]
- Riley, M.L.; Harding, J.J. The Reaction of Malondialdehyde with Lens Proteins and the Protective Effect of Aspirin. Biochim. Biophys. Acta 1993, 1158, 107–112. [Google Scholar] [CrossRef]
- Irie, H.; Chubachi, S.; Sato, M.; Sasaki, M.; Kameyama, N.; Inoue, T.; Oyamada, Y.; Nakamura, H.; Asano, K.; Betsuyaku, T. Impact of Cataract on Health-Related Quality of Life in a Longitudinal Japanese Chronic Obstructive Pulmonary Cohort. Chron. Respir. Dis. 2018, 15, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Auladell-Rispau, A.; Monteagudo, M.; Vázquez-Niebla, J.C.; Mohammed, J.; Nuñez, A.; Urrútia, G. Systematic Review on Long-Term Adverse Effects of Inhaled Corticosteroids in the Treatment of COPD. Eur. Respir. Rev. 2021, 30, 210075. [Google Scholar] [CrossRef] [PubMed]
- Kendzerska, T.; Aaron, S.D.; To, T.; Licskai, C.; Stanbrook, M.; Vozoris, N.T.; Hogan, M.-E.; Tan, W.C.; Bourbeau, J.; Gershon, A.S. Effectiveness and Safety of Inhaled Corticosteroids in Older Individuals with Chronic Obstructive Pulmonary Disease and/or Asthma. A Population Study. Ann. Am. Thorac. Soc. 2019, 16, 1252–1262. [Google Scholar] [CrossRef]
- Miller, D.P.; Watkins, S.E.; Sampson, T.; Davis, K.J. Long-Term Use of Fluticasone Propionate/Salmeterol Fixed-Dose Combination and Incidence of Cataracts and Glaucoma among Chronic Obstructive Pulmonary Disease Patients in the UK General Practice Research Database. Int. J. Chron. Obstruct. Pulmon. Dis. 2011, 6, 467–476. [Google Scholar] [CrossRef]
- Mylona, I.; Dermenoudi, M.; Ziakas, N.; Tsinopoulos, I. Hypertension Is the Prominent Risk Factor in Cataract Patients. Medicina 2019, 55, 430. [Google Scholar] [CrossRef]
- Yu, X.; Lyu, D.; Dong, X.; He, J.; Yao, K. Hypertension and Risk of Cataract: A Meta-Analysis. PLoS ONE 2014, 9, e114012. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Xue, L.; Cao, X.; Huang, L.; Song, Y. Apoptosis of Lens Epithelial Cells and Expression of NLRP3-Related Proteins in Patients with Diabetes and Cataract. Ocul. Immunol. Inflamm. 2023, 31, 1103–1110. [Google Scholar] [CrossRef]
- Lee, S.M.; Lin, S.Y.; Li, M.J.; Liang, R.C. Possible Mechanism of Exacerbating Cataract Formation in Cataractous Human Lens Capsules Induced by Systemic Hypertension or Glaucoma. Ophthalmic Res. 1997, 29, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ainsbury, E.A.; Bouffler, S.D.; Dörr, W.; Graw, J.; Muirhead, C.R.; Edwards, A.A.; Cooper, J. Radiation Cataractogenesis: A Review of Recent Studies. Radiat. Res. 2009, 172, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P. A Review of Non-Cancer Effects, Especially Circulatory and Ocular Diseases. Radiat. Environ. Biophys. 2013, 52, 435–449. [Google Scholar] [CrossRef]
- Nuzzi, R.; Trossarello, M.; Bartoncini, S.; Marolo, P.; Franco, P.; Mantovani, C.; Ricardi, U. Ocular Complications after Radiation Therapy: An Observational Study. Clin. Ophthalmol. 2020, 14, 3153–3166. [Google Scholar] [CrossRef]
- Hamada, N.; Azizova, T.V.; Little, M.P. An Update on Effects of Ionizing Radiation Exposure on the Eye. Br. J. Radiol. 2020, 93, 20190829. [Google Scholar] [CrossRef]
- Ainsbury, E.A.; Barnard, S.; Bright, S.; Dalke, C.; Jarrin, M.; Kunze, S.; Tanner, R.; Dynlacht, J.R.; Quinlan, R.A.; Graw, J.; et al. Ionizing Radiation Induced Cataracts: Recent Biological and Mechanistic Developments and Perspectives for Future Research. Mutat. Res. Mutat. Res. 2016, 770, 238–261. [Google Scholar] [CrossRef]
- Uwineza, A.; Kalligeraki, A.A.; Hamada, N.; Jarrin, M.; Quinlan, R.A. Cataractogenic Load—A Concept to Study the Contribution of Ionizing Radiation to Accelerated Aging in the Eye Lens. Mutat. Res. Mutat. Res. 2019, 779, 68–81. [Google Scholar] [CrossRef]
- Mackay, D.S.; Andley, U.P.; Shiels, A. Cell Death Triggered by a Novel Mutation in the alphaA-Crystallin Gene Underlies Autosomal Dominant Cataract Linked to Chromosome 21q. Eur. J. Hum. Genet. 2003, 11, 784–793. [Google Scholar] [CrossRef]
- Santhiya, S.T.; Soker, T.; Klopp, N.; Illig, T.; Prakash, M.V.S.; Selvaraj, B.; Gopinath, P.M.; Graw, J. Identification of a Novel, Putative Cataract-Causing Allele in CRYAA (G98R) in an Indian Family. Mol. Vis. 2006, 12, 768–773. [Google Scholar] [PubMed]
- Litt, M.; Kramer, P.; LaMorticella, D.M.; Murphey, W.; Lovrien, E.W.; Weleber, R.G. Autosomal Dominant Congenital Cataract Associated with a Missense Mutation in the Human Alpha Crystallin Gene CRYAA. Hum. Mol. Genet. 1998, 7, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Vanita, V.; Singh, J.R.; Hejtmancik, J.F.; Nuernberg, P.; Hennies, H.C.; Singh, D.; Sperling, K. A Novel Fan-Shaped Cataract-Microcornea Syndrome Caused by a Mutation of CRYAA in an Indian Family. Mol. Vis. 2006, 12, 518–522. [Google Scholar]
- Beby, F.; Commeaux, C.; Bozon, M.; Denis, P.; Edery, P.; Morlé, L. New Phenotype Associated with an Arg116Cys Mutation in the CRYAA Gene: Nuclear Cataract, Iris Coloboma, and Microphthalmia. Arch. Ophthalmol. 2007, 125, 213–216. [Google Scholar] [CrossRef]
- Vicart, P.; Caron, A.; Guicheney, P.; Li, Z.; Prévost, M.C.; Faure, A.; Chateau, D.; Chapon, F.; Tomé, F.; Dupret, J.M.; et al. A Missense Mutation in the alphaB-Crystallin Chaperone Gene Causes a Desmin-Related Myopathy. Nat. Genet. 1998, 20, 92–95. [Google Scholar] [CrossRef]
- Andley, U.P.; Hamilton, P.D.; Ravi, N.; Weihl, C.C. A Knock-in Mouse Model for the R120G Mutation of αB-Crystallin Recapitulates Human Hereditary Myopathy and Cataracts. PLoS ONE 2011, 6, e17671. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Luo, L.; Wu, M.; Zeng, R.; Cheng, G.; Hu, B.; Liu, B.; Liang, J.J.; Shang, F. A Novel alphaB-Crystallin Mutation Associated with Autosomal Dominant Congenital Lamellar Cataract. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1069–1075. [Google Scholar] [CrossRef]
- Tjondro, H.C.; Xi, Y.-B.; Chen, X.-J.; Su, J.-T.; Yan, Y.-B. Membrane Insertion of αA-Crystallin Is Oligomer-Size Dependent. Biochem. Biophys. Res. Commun. 2016, 473, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yaldo, L.M.; Dallo, F.J.; Ruterbusch, J.; Schwartz, K.; Jamil, H.J. The Burden of and Factors Associated with Age-Related Eye Diseases in Arab American Adults. J. Immigr. Minor. Health 2022, 24, 1095–1102. [Google Scholar] [CrossRef]
- Kanakamedala, A.; Go, J.A.; Wendt, S.; Ugoh, P.; Khan, M.; Al-Mohtaseb, Z. Systemic and Ocular Comorbidities of Black, Hispanic, and White Women with Cataracts. J. Womens Health 2002 2022, 31, 117–124. [Google Scholar] [CrossRef]
- Kar, R.; Batra, N.; Riquelme, M.A.; Jiang, J.X. Biological Role of Connexin Intercellular Channels and Hemichannels. Arch. Biochem. Biophys. 2012, 524, 2–15. [Google Scholar] [CrossRef]
- Ifeanyi, F.; Takemoto, L. Interaction of Lens Crystallins with Lipid Vesicles. Exp. Eye Res. 1991, 52, 535–538. [Google Scholar] [CrossRef]
- Byrdwell, W.C.; Sato, H.; Schwarz, A.K.; Borchman, D.; Yappert, M.C.; Tang, D. 31P NMR Quantification and Monophasic Solvent Purification of Human and Bovine Lens Phospholipids. Lipids 2002, 37, 1087–1092. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.-J.; Zhu, J.; Xi, Y.-B.; Yang, X.; Hu, L.-D.; Ouyang, H.; Patel, S.H.; Jin, X.; Lin, D.; et al. Lanosterol Reverses Protein Aggregation in Cataracts. Nature 2015, 523, 607–611. [Google Scholar] [CrossRef]
- Estrada, R.; Yappert, M.C. Regional Phospholipid Analysis of Porcine Lens Membranes by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. J. Mass Spectrom. JMS 2004, 39, 1531–1540. [Google Scholar] [CrossRef]
- Rujoi, M.; Jin, J.; Borchman, D.; Tang, D.; Yappert, M.C. Isolation and Lipid Characterization of Cholesterol-Enriched Fractions in Cortical and Nuclear Human Lens Fibers. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1634–1642. [Google Scholar] [CrossRef]
- Buboltz, J.T.; Feigenson, G.W. A Novel Strategy for the Preparation of Liposomes: Rapid Solvent Exchange. Biochim. Biophys. Acta BBA Biomembr. 1999, 1417, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Buboltz, J.T. A More Efficient Device for Preparing Model-Membrane Liposomes by the Rapid Solvent Exchange Method. Rev. Sci. Instrum. 2009, 80, 124301. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Buboltz, J.T.; Feigenson, G.W. Maximum Solubility of Cholesterol in Phosphatidylcholine and Phosphatidylethanolamine Bilayers. Biochim. Biophys. Acta BBA Biomembr. 1999, 1417, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Khadka, N.K.; Mortimer, M.-F.; Marosvari, M.; Timsina, R.; Mainali, L. Membrane Elasticity Modulated by Cholesterol in Model of Porcine Eye Lens-Lipid Membrane. Exp. Eye Res. 2022, 220, 109131. [Google Scholar] [CrossRef]
- Mainali, L.; Pasenkiewicz-Gierula, M.; Subczynski, W.K. Formation of Cholesterol Bilayer Domains Precedes Formation of Cholesterol Crystals in Membranes Made of the Major Phospholipids of Human Eye Lens Fiber Cell Plasma Membranes. Curr. Eye Res. 2020, 45, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Raguz, M.; Mainali, L.; Widomska, J.; Subczynski, W.K. Using Spin-Label Electron Paramagnetic Resonance (EPR) to Discriminate and Characterize the Cholesterol Bilayer Domain. Chem. Phys. Lipids 2011, 164, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Phases and Domains in Sphingomyelin-Cholesterol Membranes: Structure and Properties Using EPR Spin-Labeling Methods. Eur. Biophys. J. 2012, 41, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Phase-Separation and Domain-Formation in Cholesterol-Sphingomyelin Mixture: Pulse-EPR Oxygen Probing. Biophys. J. 2011, 101, 837–846. [Google Scholar] [CrossRef]


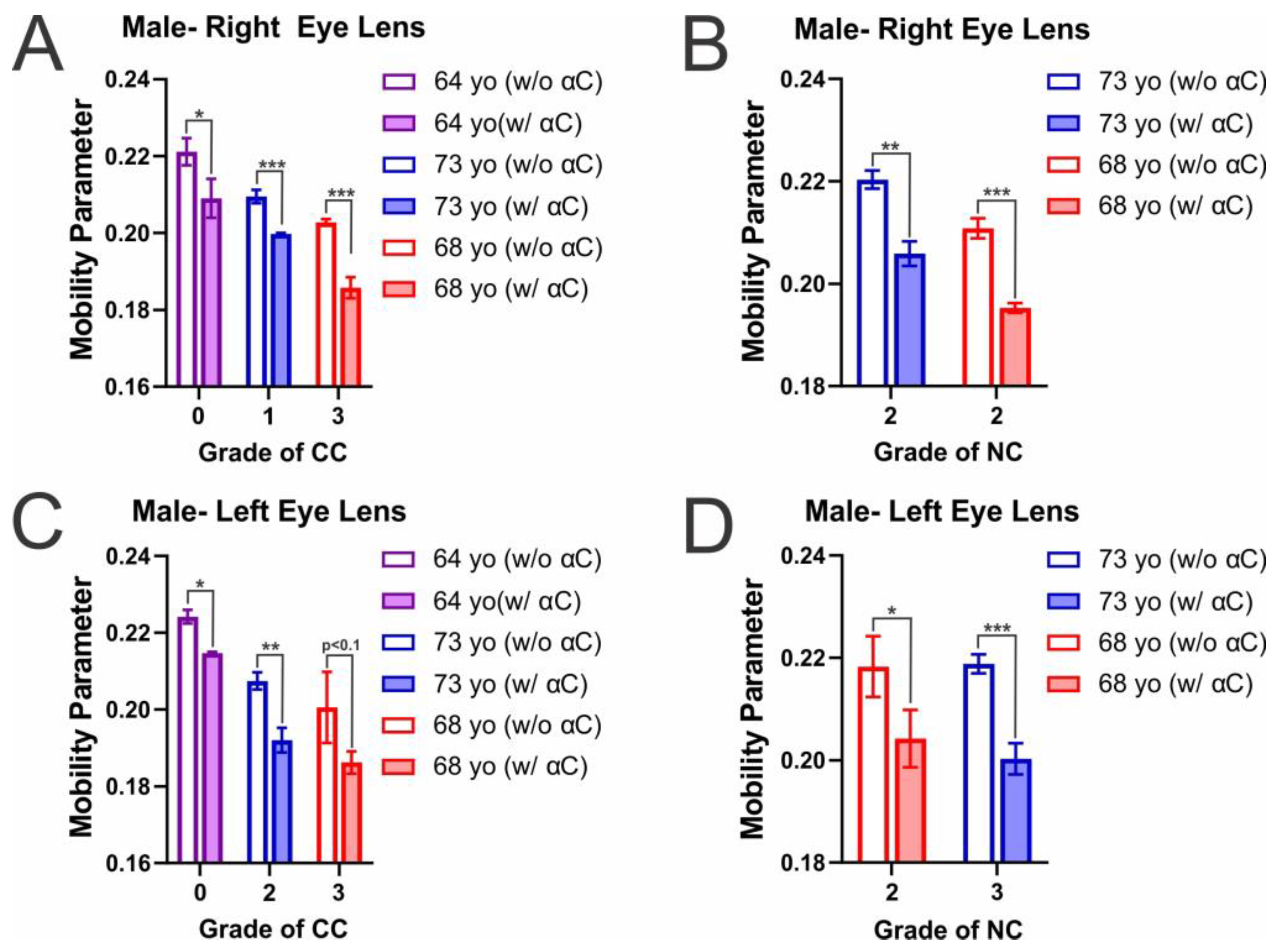


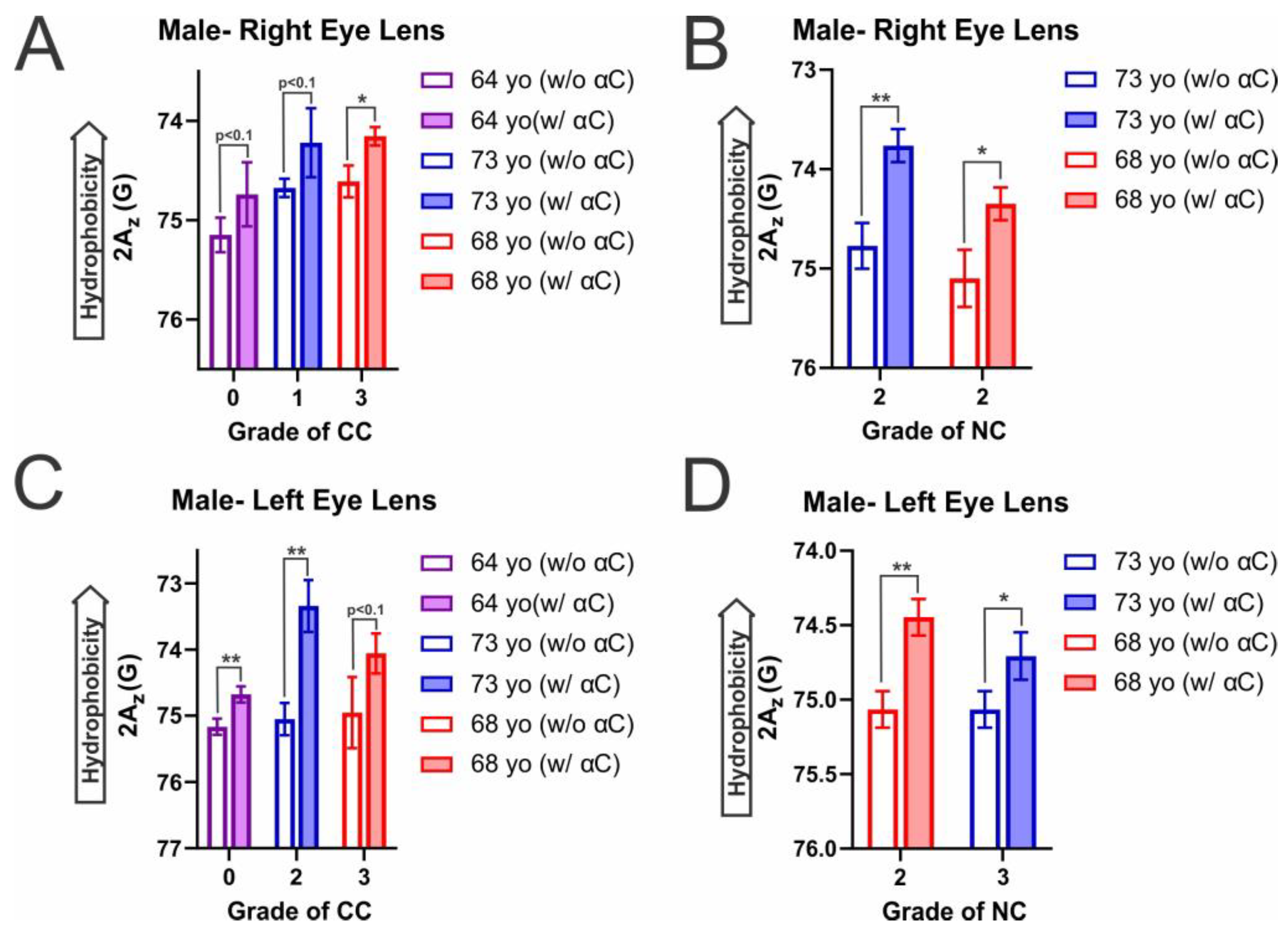

| Male- Right Eye Lens | Male- Left Eye Lens | ||
| Increase in cataract grade | Increase in MSO | Increase in cataract grade | Increase in MSO |
| CC (0) a to CC (1) | 3.62 | CC (0) to CC (2) | 3.73 |
| CC (0) to CC (3) | 6.41 | CC (0) to CC (3) | 10.13 |
| CC (1) to CC (3) | 2.79 | CC (2) to CC (3) | 6.40 |
| NC (2) to NC (2) | 0.02 | NC (2) to NC (3) | 6.35 |
| Female- Right Eye Lens | Female- Left Eye Lens | ||
| Increase in cataract grade | Increase in MSO | Increase in cataract grade | Increase in MSO |
| CC (1) to CC (3) | 15.53 | CC (1) to CC (3) | 9.64 |
| NC (2) to NC (3) | 3.74 | NC (2) to NC (3) | 3.59 |
| Male- Right Eye Lens | Male- Left Eye Lens | ||
| Increase in cataract grade | Change in MP | Increase in cataract grade | Change in MP |
| CC (0) a to CC (1) | 0.011 (decrease) | CC (0) to CC (2) | 0.016 (decrease) |
| CC (0) to CC (3) | 0.018 (decrease) | CC (0) to CC (3) | 0.023 (decrease) |
| CC (1) to CC (2) | 0.007 (decrease) | CC (2) to CC (3) | 0.007 (decrease) |
| NC (2) to NC (2) | 0.009 (decrease) | NC (2) to NC (3) | 0.001 (increase) |
| Female- Right Eye Lens | Female- Left Eye Lens | ||
| Increase in cataract grade | Change in MP | Increase in cataract grade | Change in MP |
| CC (1) to CC (3) | 0.021 (decrease) | CC (1) to CC (3) | 0.014 (decrease) |
| NC (2) to NC (3) | 0.028 (increase) | NC (2) to NC (3) | 0.02 (increase) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hazen, P.; Trossi-Torres, G.; Timsina, R.; Khadka, N.K.; Mainali, L. Association of Alpha-Crystallin with Human Cortical and Nuclear Lens Lipid Membrane Increases with the Grade of Cortical and Nuclear Cataract. Int. J. Mol. Sci. 2024, 25, 1936. https://doi.org/10.3390/ijms25031936
Hazen P, Trossi-Torres G, Timsina R, Khadka NK, Mainali L. Association of Alpha-Crystallin with Human Cortical and Nuclear Lens Lipid Membrane Increases with the Grade of Cortical and Nuclear Cataract. International Journal of Molecular Sciences. 2024; 25(3):1936. https://doi.org/10.3390/ijms25031936
Chicago/Turabian StyleHazen, Preston, Geraline Trossi-Torres, Raju Timsina, Nawal K. Khadka, and Laxman Mainali. 2024. "Association of Alpha-Crystallin with Human Cortical and Nuclear Lens Lipid Membrane Increases with the Grade of Cortical and Nuclear Cataract" International Journal of Molecular Sciences 25, no. 3: 1936. https://doi.org/10.3390/ijms25031936
APA StyleHazen, P., Trossi-Torres, G., Timsina, R., Khadka, N. K., & Mainali, L. (2024). Association of Alpha-Crystallin with Human Cortical and Nuclear Lens Lipid Membrane Increases with the Grade of Cortical and Nuclear Cataract. International Journal of Molecular Sciences, 25(3), 1936. https://doi.org/10.3390/ijms25031936









