Photodynamic Therapy for Atherosclerosis
Abstract
:1. Introduction
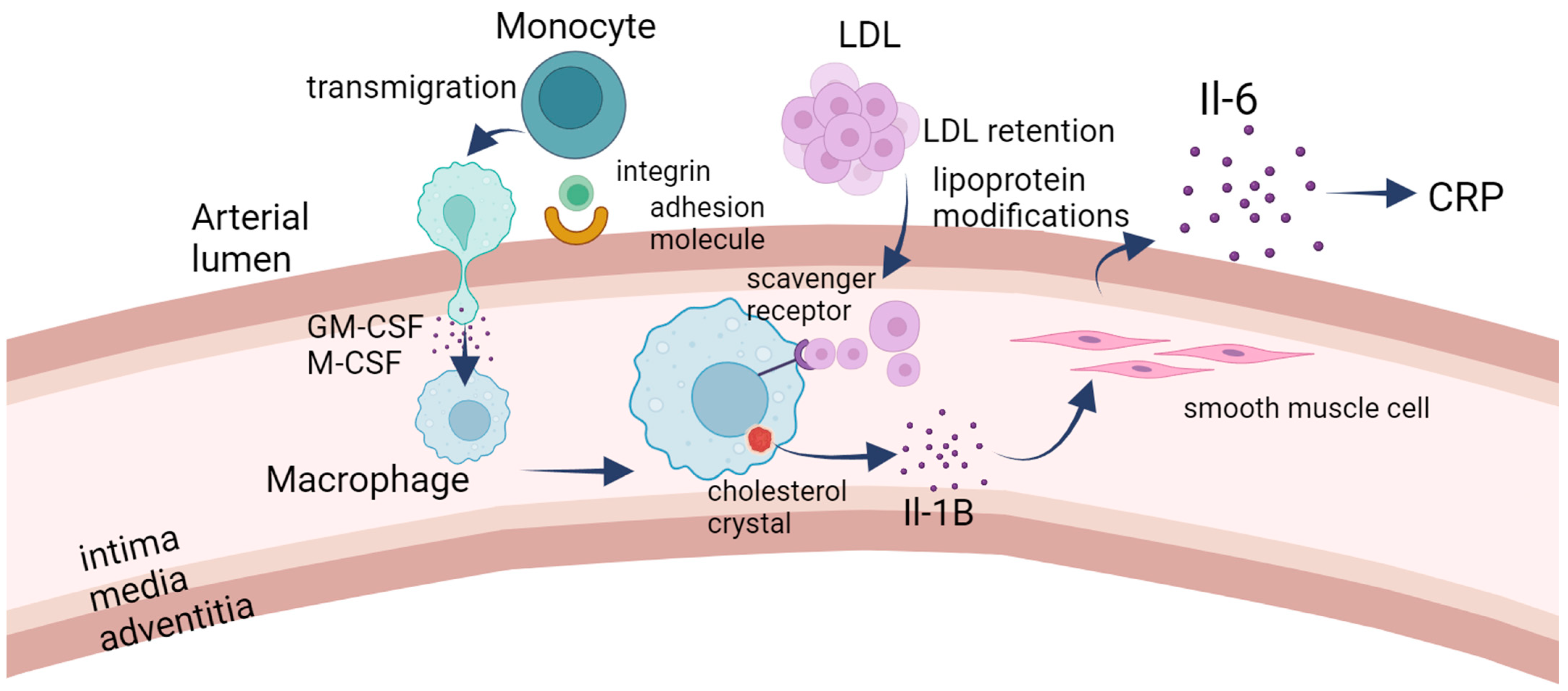
1.1. Pathogenesis of Atherosclerosis
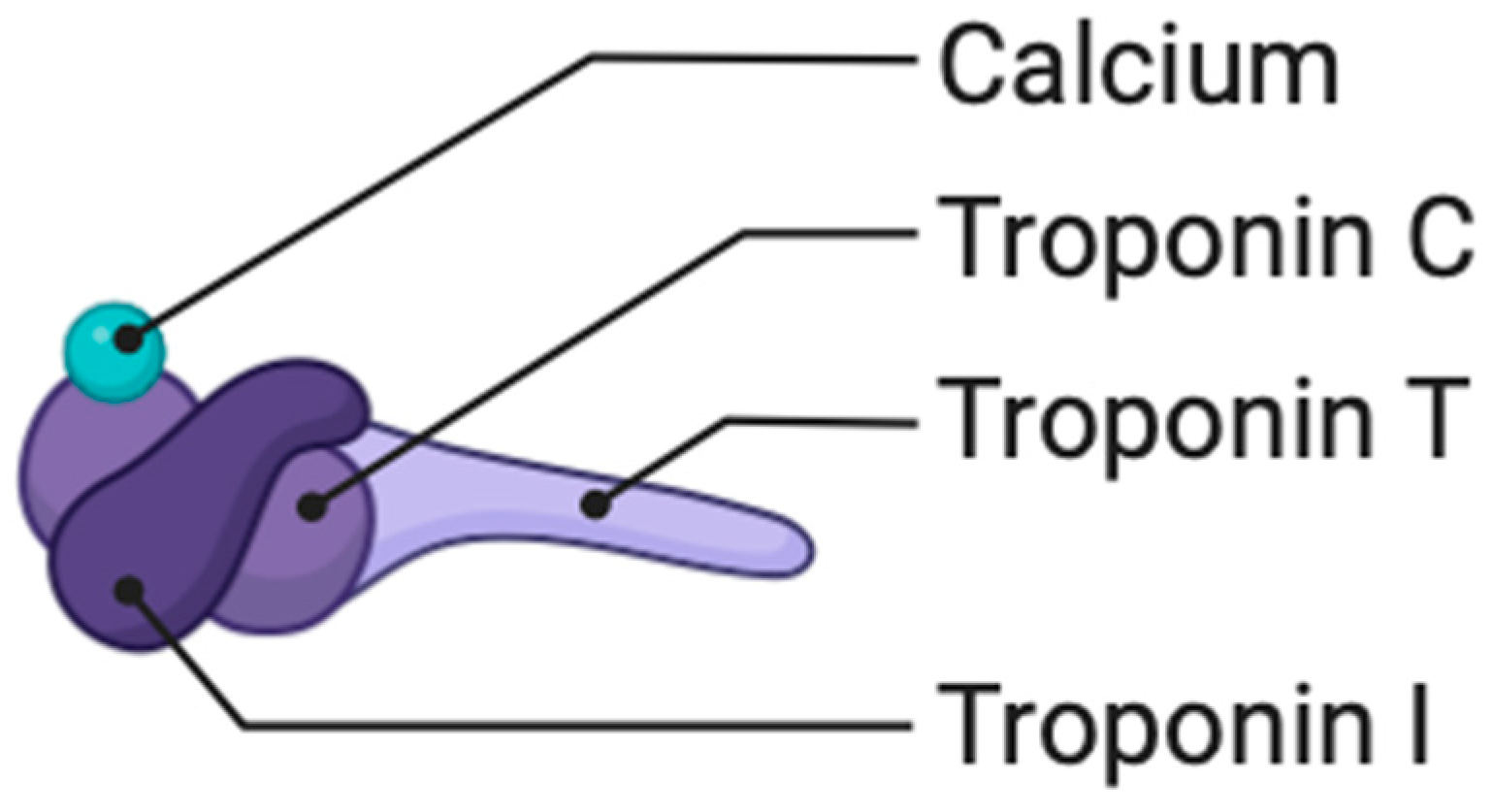
1.2. Biomarkers in Atherosclerosis
2. Atherosclerosis Treatment and Photodynamic Therapy
Nanoparticles
3. A Review of the Literature
3.1. In Vitro
3.2. In Vivo in Mice Models
3.3. In Vivo on Rabbits
3.4. In Vivo on Pigs
3.5. In Vivo on Humans
4. Comparative Analysis of Costs and Side Effects: Photodynamic Therapy vs. Statin Therapy in Atherosclerosis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharif, H.; Akash, M.S.H.; Rehman, K.; Irshad, K.; Imran, I. Pathophysiology of atherosclerosis: Association of risk factors and treatment strategies using plant-based bioactive compounds. J. Food Biochem. 2020, 44, e13449. [Google Scholar] [CrossRef]
- Fan, J.; Watanabe, T. Atherosclerosis: Known and unknown. Pathol. Int. 2022, 72, 151–160. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Emini Veseli, B.; Perrotta, P.; De Meyer, G.R.A.; Roth, L.; Van der Donckt, C.; Martinet, W.; De Meyer, G.R.Y. Animal models of atherosclerosis. Eur. J. Pharmacol. 2017, 816, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Mushenkova, N.V.; Summerhill, V.I.; Zhang, D.; Romanenko, E.B.; Grechko, A.V.; Orekhov, A.N. Current Advances in the Diagnostic Imaging of Atherosclerosis: Insights into the Pathophysiology of Vulnerable Plaque. Int. J. Mol. Sci. 2020, 21, 2992. [Google Scholar] [CrossRef] [PubMed]
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406. [Google Scholar] [CrossRef] [PubMed]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Mechanisms of acute coronary syndromes. N. Engl. J. Med. 2013, 369, 883–884. [Google Scholar] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc. Pathol. 2013, 22, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Stary, H.C. Natural history and histological classification of atherosclerotic lesions: An update. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1177–1178. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Weber, C.; Noels, H. Atherosclerosis: Current pathogenesis and therapeutic options. Nat. Med. 2011, 17, 1410–1422. [Google Scholar] [CrossRef]
- Hackam, D.G.; Anand, S.S. Emerging Risk Factors for Atherosclerotic Vascular Disease: A Critical Review of the Evdence. JAMA 2003, 290, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Hagström, E.; Steg, P.G.; Szarek, M.; Bhatt, D.L.; Bittner, V.A.; Danchin, N.; Diaz, R.; Goodman, S.G.; Harrington, R.A.; Jukema, J.W.; et al. Apolipoprotein B, Residual Cardiovascular Risk After Acute Coronary Syndrome, and Effects of Alirocumab. Circulation 2022, 146, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Nomura, S.O.; Steffen, B.T.; Guan, W.; Remaley, A.T.; Karger, A.B.; Ouyang, P.; Michos, E.D.; Tsai, M.Y. Apolipoprotein B discordance with low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol in relation to coronary artery calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). J. Clin. Lipidol. 2020, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Ambale-Venkatesh, B.; Yang, X.; Wu, C.O.; Liu, K.; Hundley, W.G.; McClelland, R.; Gomes, A.S.; Folsom, A.R.; Shea, S.; Guallar, E.; et al. Cardiovascular Event Prediction by Machine Learning: The Multi-Ethnic Study of Atherosclerosis. Circ. Res. 2017, 121, 1092–1101. [Google Scholar] [CrossRef]
- Tabas, I.; Bornfeldt, K.E. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ. Res. 2016, 118, 653–667. [Google Scholar] [CrossRef]
- Jenkins, S.J.; Ruckerl, D.; Cook, P.C.; Jones, L.H.; Finkelman, F.D.; van Rooijen, N.; MacDonald, A.S.; Allen, J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 2011, 332, 1284–1288. [Google Scholar] [CrossRef]
- Gabriela, A.T.; Lorena, C.; Vasile, N.; Olimpia, P.I.; Claudia, L.C.; Dan, T.R.; Para, I.; Popovici, I.; Cheregi, C. Risk factors of subclinical atherosclerosis in obesity and overweight. J. Pak. Med. Assoc. 2020, 70, 840–844. [Google Scholar]
- Shi, Y.; Guo, L.; Chen, Y.; Xie, Q.; Yan, Z.; Liu, Y.; Kang, J.; Li, S. Risk factors for ischemic stroke: Differences between cerebral small vessel and large artery atherosclerosis aetiologies. Folia Neuropathol. 2021, 59, 378–385. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Pedersen, T.R.; Saver, J.L.; Sever, P.S.; Keech, A.C.; Bohula, E.A.; Murphy, S.A.; Wasserman, S.M.; Honarpour, N.; Wang, H.; et al. Stroke Prevention with the PCSK9 (Proprotein Convertase Subtilisin-Kexin Type 9) Inhibitor Evolocumab Added to Statin in High-Risk Patients With Stable Atherosclerosis. Stroke 2020, 51, 1546–1554. [Google Scholar] [CrossRef]
- Fujii, K.; Abe, I.; Ohya, Y.; Ohta, Y.; Arima, H.; Akasaki, T.; Yoshinari, M.; Iida, M. Risk factors for the progression of early carotid atherosclerosis in a male working population. Hypertens. Res. 2003, 26, 465–471. [Google Scholar] [CrossRef]
- Criqui, M.H.; Kamineni, A.; Allison, M.A.; Ix, J.H.; Carr, J.J.; Cushman, M.; Detrano, R.; Post, W.; Wong, N.D. Risk factor differences for aortic versus coronary calcified atherosclerosis: The multiethnic study of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2289–2296. [Google Scholar] [CrossRef]
- van Capelleveen, J.C.; Bochem, A.E.; Boekholdt, S.M.; Mora, S.; Hoogeveen, R.C.; Ballantyne, C.M.; Ridker, P.M.; Sun, W.; Barter, P.J.; Tall, A.R.; et al. Association of High-Density Lipoprotein-Cholesterol Versus Apolipoprotein A-I With Risk of Coronary Heart Disease: The European Prospective Investigation into Cancer-Norfolk Prospective Population Study, the Atherosclerosis Risk in Communities Study, and the Women’s Health Study. J. Am. Heart Assoc. 2017, 6, e006636. [Google Scholar]
- Haarmann, H.; Koch, J.; Bonsch, N.; Mende, M.; Werhahn, S.M.; Lüers, C.; Stahrenberg, R.; Edelmann, F.; Holzendorf, V.; von Haehling, S.; et al. Morbidity and mortality in patients with cardiovascular risk factors and obstructive sleep apnoea: Results from the DIAST-CHF cohort. Respir. Med. 2019, 154, 127–132. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Atherosclerotic plaque formation. Rev. Prat. 1999, 49, 2081–2086. [Google Scholar]
- Park, K.H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.V.; Harrison, D.G.; Olbrych, M.T.; Alexander, R.W.; Medford, R.M. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 9114–9119. [Google Scholar] [CrossRef] [PubMed]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.J. Macrophages in Atherosclerosis Regression. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 20–33. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Wang, B.; Tang, X.; Yao, L.; Wang, Y.; Chen, Z.; Li, M.; Wu, N.; Wu, D.; Dai, X.; Jiang, H.; et al. Disruption of USP9X in macrophages promotes foam cell formation and atherosclerosis. J. Clin. Investig. 2022, 132, e154217. [Google Scholar] [CrossRef]
- Malekmohammad, K.; Sewell, R.D.E.; Rafieian-Kopaei, M. Antioxidants and Atherosclerosis: Mechanistic Aspects. Biomolecules 2019, 9, 301. [Google Scholar] [CrossRef]
- Tall, A.R.; Thomas, D.G.; Gonzalez-Cabodevilla, A.G.; Goldberg, I.J. Addressing dyslipidemic risk beyond LDL-cholesterol. J. Clin. Investig. 2022, 132, e148559. [Google Scholar] [CrossRef]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic Insights into the Oxidized Low-Density Lipoprotein-Induced Atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Mussbacher, M.; Salzmann, M.; Brostjan, C.; Hoesel, B.; Schoergenhofer, C.; Datler, H.; Hohensinner, P.; Basílio, J.; Petzelbauer, P.; Assinger, A.; et al. Cell Type-Specific Roles of NF-κB Linking Inflammation and Thrombosis. Front. Immunol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Jaipersad, A.S.; Lip, G.Y.; Silverman, S.; Shantsila, E. The role of monocytes in angiogenesis and atherosclerosis. J. Am. Coll. Cardiol. 2014, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxidative Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Vilahur, G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J. Intern. Med. 2014, 276, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Frostegård, J. Immunity, atherosclerosis and cardiovascular disease. BMC Med. 2013, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Zhang, N.; Li, J.S.; Yang, Z.H.; Huang, X.L.; Yang, X.F. Purinergic receptors mediate endothelial dysfunction and participate in atherosclerosis. Purinergic Signal. 2023, 19, 265–272. [Google Scholar] [CrossRef]
- Dong, Y.; Fernandes, C.; Liu, Y.; Wu, Y.; Wu, H.; Brophy, M.L.; Deng, L.; Song, K.; Wen, A.; Wong, S.; et al. Role of endoplasmic reticulum stress signalling in diabetic endothelial dysfunction and atherosclerosis. Diabetes Vasc. Dis. Res. 2017, 14, 14–23. [Google Scholar] [CrossRef]
- Chatzizisis, Y.S.; Coskun, A.U.; Jonas, M.; Edelman, E.R.; Feldman, C.L.; Stone, P.H. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 2007, 49, 2379–2393. [Google Scholar] [CrossRef]
- Harman, J.L.; Jørgensen, H.F. The role of smooth muscle cells in plaque stability: Therapeutic targeting potential. Br. J. Pharmacol. 2019, 176, 3741–3753. [Google Scholar] [CrossRef]
- Puylaert, P.; Zurek, M.; Rayner, K.J.; De Meyer, G.R.Y.; Martinet, W. Regulated Necrosis in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 1283–1306. [Google Scholar] [CrossRef]
- Libby, P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef]
- Babaniamansour, P.; Mohammadi, M.; Babaniamansour, S.; Aliniagerdroudbari, E. The Relation between Atherosclerosis Plaque Composition and Plaque Rupture. J. Med. Signals Sens. 2020, 10, 267–273. [Google Scholar] [PubMed]
- Chiorescu, R.M.; Mocan, M.; Inceu, A.I.; Buda, A.P.; Blendea, D.; Vlaicu, S.I. Vulnerable Atherosclerotic Plaque: Is There a Molecular Signature? Int. J. Mol. Sci. 2022, 23, 13638. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, Y.; Daghem, M.; Tzolos, E.; Meah, M.N.; Doris, M.K.; Moss, A.J.; Kwiecinski, J.; Kroon, J.; Nurmohamed, N.S.; van der Harst, P.; et al. Association of Lipoprotein(a) With Atherosclerotic Plaque Progression. J. Am. Coll. Cardiol. 2022, 79, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yu, Y.; Chen, R.; Liu, X.; Hu, Y.; Ma, Z.; Gao, L.; Jian, W.; Wang, L. Wall shear stress and its role in atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1083547. [Google Scholar] [CrossRef] [PubMed]
- Eshtehardi, P.; Brown, A.J.; Bhargava, A.; Costopoulos, C.; Hung, O.Y.; Corban, M.T.; Hosseini, H.; Gogas, B.D.; Giddens, D.P.; Samady, H. High wall shear stress and high-risk plaque: An emerging concept. Int. J. Cardiovasc. Imaging 2017, 33, 1089–1099. [Google Scholar] [CrossRef]
- Badimon, L.; Padró, T.; Vilahur, G. Atherosclerosis, platelets and thrombosis in acute ischaemic heart disease. Eur. Heart J. Acute Cardiovasc. Care 2012, 1, 60–74. [Google Scholar] [CrossRef]
- Shah, P.K. Mechanisms of plaque vulnerability and rupture. J. Am. Coll. Cardiol. 2003, 41, 15S–22S. [Google Scholar] [CrossRef]
- Bobryshev, Y.V.; Ivanova, E.A.; Chistiakov, D.A.; Nikiforov, N.G.; Orekhov, A.N. Macrophages and Their Role in Atherosclerosis: Pathophysiology and Transcriptome Analysis. Biomed. Res. Int. 2016, 2016, 9582430. [Google Scholar] [CrossRef]
- Dhawan, U.K.; Singhal, A.; Subramanian, M. Dead cell and debris clearance in the atherosclerotic plaque: Mechanisms and therapeutic opportunities to promote inflammation resolution. Pharmacol. Res. 2021, 170, 105699. [Google Scholar] [CrossRef]
- Hong, B.V.; Agus, J.K.; Tang, X.; Zheng, J.J.; Romo, E.Z.; Lei, S.; Zivkovic, A.M. Precision Nutrition and Cardiovascular Disease Risk Reduction: The Promise of High-Density Lipoproteins. Curr. Atheroscler. Rep. 2023, 25, 663–677. [Google Scholar] [CrossRef]
- Zarkasi, K.A.; Abdullah, N.; Abdul Murad, N.A.; Ahmad, N.; Jama, L.R. Genetic Factors for Coronary Heart Disease and Their Mechanisms: A Meta-Analysis and Comprehensive Review of Common Variants from Genome-Wide Association Studies. Diagnostics 2022, 12, 2561. [Google Scholar] [CrossRef]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and Inflammation: Insights from the Theory of General Pathological Processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Aboouf, M.A.; Gorr, T.A.; Hamdy, N.M.; Gassmann, M.; Thiersch, M. Myoglobin in Brown Adipose Tissue: A Multifaceted Player in Thermogenesis. Cells 2023, 12, 2240. [Google Scholar] [CrossRef]
- Green, G.B.; Skarbek-Borowski, G.W.; Chan, D.W.; Kelen, G.D. Myoglobin for early risk stratification of emergency department patients with possible myocardial ischemia. Acad. Emerg. Med. 2000, 7, 625–636. [Google Scholar] [CrossRef]
- Vatansever, S.; Akkaya, V.; Erk, O.; Oztürk, S.; Karan, M.A.; Salmayenli, N.; Taşçioğlu, C.; Güler, K. The diagnostic value of troponin T and myoglobin levels in acute myocardial infarction: A study in Turkish patients. J. Int. Med. Res. 2003, 31, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Scrivner, O.; Fletcher, E.; Hoffmann, C.; Li, F.; Wilkinson, T.; Miserlis, D.; Smith, R.S.; Bohannon, W.T.; Sutliff, R.; Jordan, W.D.; et al. Myoglobinemia, Peripheral Arterial Disease, and Patient Mortality. J. Am. Coll. Surg. 2023, 236, 588–598. [Google Scholar] [CrossRef]
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, M.S. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 2017, 113, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Welsh, P.; Preiss, D.; Hayward, C.; Shah, A.S.V.; McAllister, D.; Briggs, A.; Boachie, C.; McConnachie, A.; Padmanabhan, S.; Welsh, C.; et al. Cardiac Troponin T and Troponin I in the General Population. Circulation 2019, 139, 2754–2764. [Google Scholar] [CrossRef]
- Wei, B.; Jin, J.P. Troponin T isoforms and posttranscriptional modifications: Evolution, regulation and function. Arch. Biochem. Biophys. 2011, 505, 144–154. [Google Scholar] [CrossRef]
- Quyyumi, A.A.; Tahhan, A.S. High-Sensitivity Troponin and Coronary Artery Disease Severity: A Bridge Too Far? JACC Cardiovasc. Imaging 2019, 12, 1056–1057. [Google Scholar] [CrossRef]
- Falk, T.; Ljungvall, I.; Zois, N.E.; Höglund, K.; Olsen, L.H.; Pedersen, H.D.; Häggström, J. Cardiac troponin-I concentration, myocardial arteriosclerosis, and fibrosis in dogs with congestive heart failure because of myxomatous mitral valve disease. J. Vet. Intern. Med. 2013, 27, 500–506. [Google Scholar] [CrossRef]
- McLeish, M.J.; Kenyon, G.L. Relating structure to mechanism in creatine kinase. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 1–20. [Google Scholar] [CrossRef]
- Kittipeerapat, N.; Fabian, R.; Bernsen, S.; Weydt, P.; Castro-Gomez, S. Creatine Kinase MB Isoenzyme Is a Complementary Biomarker in Amyotrophic Lateral Sclerosis. Int. J. Mol. Sci. 2023, 24, 11682. [Google Scholar] [CrossRef]
- Darabedian, N.; Ji, W.; Fan, M.; Lin, S.; Seo, H.S.; Vinogradova, E.V.; Yaron, T.M.; Mills, E.L.; Xiao, H.; Senkane, K.; et al. Depletion of creatine phosphagen energetics with a covalent creatine kinase inhibitor. Nat. Chem. Biol. 2023, 19, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Schlattner, U.; Tokarska-Schlattner, M.; Wallimann, T. Mitochondrial creatine kinase in human health and disease. Biochim. Biophys. Acta 2006, 1762, 164–180. [Google Scholar] [CrossRef] [PubMed]
- Wright, R.S.; Ray, K.K.; Raal, F.J.; Kallend, D.G.; Jaros, M.; Koenig, W.; Leiter, L.A.; Landmesser, U.; Schwartz, G.G.; Friedman, A.; et al. Pooled Patient-Level Analysis of Inclisiran Trials in Patients With Familial Hypercholesterolemia or Atherosclerosis. J. Am. Coll. Cardiol. 2021, 77, 1182–1193. [Google Scholar] [CrossRef]
- Frangie, C.; Daher, J. Role of myeloperoxidase in inflammation and atherosclerosis (Review). Biomed. Rep. 2022, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Teng, N.; Maghzal, G.J.; Talib, J.; Rashid, I.; Lau, A.K.; Stocker, R. The roles of myeloperoxidase in coronary artery disease and its potential implication in plaque rupture. Redox Rep. 2017, 22, 51–73. [Google Scholar] [CrossRef]
- Odobasic, D.; Holdsworth, S.R. Emerging Cellular Therapies for Anti-myeloperoxidase Vasculitis and Other Autoimmune Diseases. Front. Immunol. 2021, 12, 642127. [Google Scholar] [CrossRef]
- Senders, M.L.; Mulder, W.J.M. Targeting myeloperoxidase in inflammatory atherosclerosis. Eur. Heart J. 2018, 39, 3311–3313. [Google Scholar] [CrossRef]
- Kjaer-Sorensen, K.; Engholm, D.H.; Kamei, H.; Morch, M.G.; Kristensen, A.O.; Zhou, J.; Conover, C.A.; Duan, C.; Oxvig, C. Pregnancy-associated plasma protein A (PAPP-A) modulates the early developmental rate in zebrafish independently of its proteolytic activity. J. Biol. Chem. 2013, 288, 9982–9992. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, P.; Gurumurthy, P.; Nayar, P.; Rao, G.S.; Babu, R.S.; Sarasabharati, A.; Cherian, K.M. Pregnancy associated plasma protein-A (PAPP-A) as an early marker for the diagnosis of acute coronary syndrome. Indian Heart J. 2012, 64, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Barrios, V.; Chowen, J.A.; Martín-Rivada, Á.; Guerra-Cantera, S.; Pozo, J.; Yakar, S.; Rosenfeld, R.G.; Pérez-Jurado, L.A.; Suárez, J.; Argente, J. Pregnancy-Associated Plasma Protein (PAPP)-A2 in Physiology and Disease. Cells 2021, 10, 3576. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.L.; Zhao, Z.W.; Liu, S.M.; Wang, G.; Yu, X.H.; Zou, J.; Wang, S.Q.; Dai, X.Y.; Fu, M.G.; Zheng, X.L.; et al. Pregnancy-Associated Plasma Protein-A Accelerates Atherosclerosis by Regulating Reverse Cholesterol Transport and Inflammation. Circ. J. 2019, 83, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, H.; Zhou, L.; Wang, Z.; Hua, B. Pregnancy-Associated Plasma Protein a Induces Inflammatory Cytokine Expression by Activating IGF-I/PI3K/Akt Pathways. Mediat. Inflamm. 2019, 2019, 8436985. [Google Scholar] [CrossRef]
- Hou, X.Z.; Liu, E.Q.; Liu, S.Q.; Lv, H.; Cui, H.F.; Han, J. The negative association between serum albumin levels and coronary heart disease risk in adults over 45 years old: A cross-sectional survey. Sci. Rep. 2023, 13, 672. [Google Scholar] [CrossRef]
- Huang, F.; Wang, K.; Shen, J. Lipoprotein-associated phospholipase A2: The story continues. Med. Res. Rev. 2020, 4, 79–134. [Google Scholar] [CrossRef]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-Modified Albumin: Origins and Clinical Implications. Dis. Markers 2021, 2021, 9945424. [Google Scholar] [CrossRef]
- Lin, X.; Ke, F.; Chen, M. Association of albumin levels with the risk of intracranial atherosclerosis. BMC Neurol. 2023, 23, 198. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Niu, J.; Wu, S.; Xin, Z.; Zhao, Z.; Xu, M.; Lu, J.; Wang, T.; Chen, Y.; Wang, S.; et al. Urinary albumin-to-creatinine ratio levels are associated with subclinical atherosclerosis and predict CVD events and all-cause deaths: A prospective analysis. BMJ Open 2021, 11, e040890. [Google Scholar] [CrossRef] [PubMed]
- Colley, K.J.; Wolfert, R.L.; Cobble, M.E. Lipoprotein associated phospholipase A(2): Role in atherosclerosis and utility as a biomarker for cardiovascular risk. EPMA J. 2011, 2, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Corbaz, A.; Scoazec, A.; Besnard, S.; Lesèche, G.; Chvatchko, Y.; Tedgui, A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation 2001, 104, 1598–1603. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Jang, S.Y.; Lee, J.Y.; Kim, J.W.; Jung, Y.; Kim, J.; Seo, J.; Han, T.S.; Jang, E.; Son, H.Y.; et al. The lipoprotein-associated phospholipase A2 inhibitor Darapladib sensitises cancer cells to ferroptosis by remodelling lipid metabolism. Nat. Commun. 2023, 14, 5728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, G.; Song, H.; Cao, C.; Ji, X.; Cao, G. Elevated Lipoprotein-Associated Phospholipase A2 Is Associated With Intracranial Atherosclerosis. Front. Neurol. 2022, 13, 858302. [Google Scholar] [CrossRef]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Gerdes, N.; Sukhova, G.K.; Libby, P.; Reynolds, R.S.; Young, J.L.; Schönbeck, U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for atherogenesis. J. Exp. Med. 2002, 195, 245–257. [Google Scholar] [CrossRef]
- Rezaieyazdi, Z.; AkbariRad, M.; Saadati, N.; Salari, M.; Orang, R.; Sedighi, S.; Esmaily, H.; Azarpazhooh, M.R.; Firoozi, A.; Akbarpour, E. Serum interleukin-18 and its relationship with subclinical atherosclerosis in systemic lupus erythematosus. ARYA Atheroscler. 2021, 17, 1–6. [Google Scholar]
- Tang, X. Analysis of interleukin-17 and interleukin-18 levels in animal models of atherosclerosis. Exp. Ther. Med. 2019, 18, 517–522. [Google Scholar] [CrossRef]
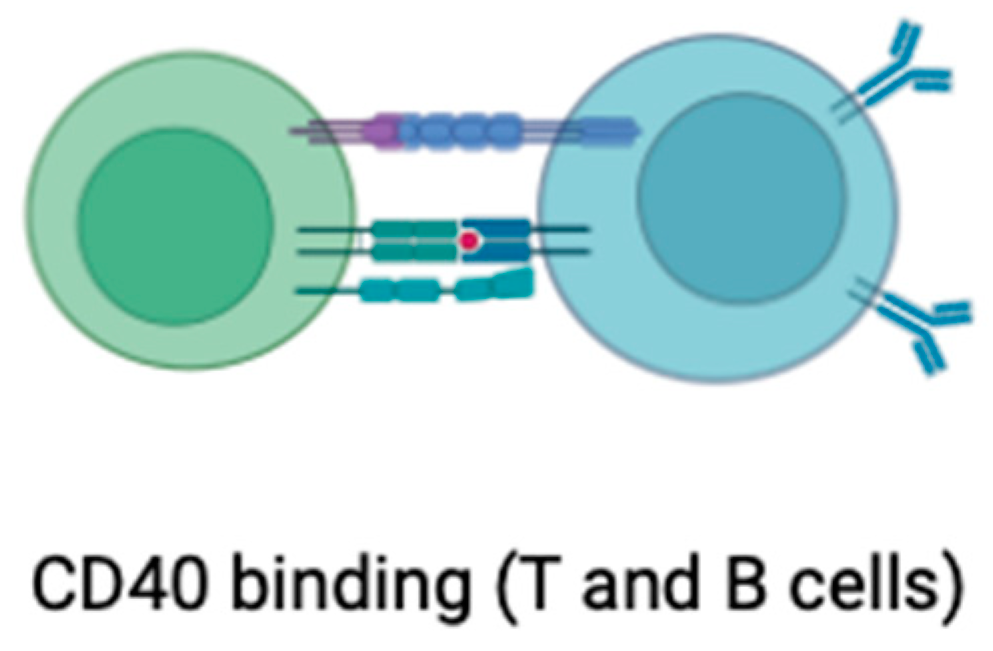
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- Lacy, M.; Bürger, C.; Shami, A.; Ahmadsei, M.; Winkels, H.; Nitz, K.; van Tiel, C.M.; Seijkens, T.T.P.; Kusters, P.J.H.; Karshovka, E.; et al. Cell-specific and divergent roles of the CD40L-CD40 axis in atherosclerotic vascular disease. Nat. Commun. 2021, 12, 3754. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, L.A.; Bosch, L.; Kusters, P.J.H.; Lutgens, E.; Seijkens, T.T.P. The CD40-CD40L Dyad as Immunotherapeutic Target in Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2021, 14, 13–22. [Google Scholar] [CrossRef]
- Bosmans, L.A.; van Tiel, C.M.; Aarts, S.A.B.M.; Willemsen, L.; Baardman, J.; van Os, B.W.; Toom, M.D.; Beckers, L.; Ahern, D.J.; Levels, J.H.M.; et al. Myeloid CD40 deficiency reduces atherosclerosis by impairing macrophages’ transition into a pro-inflammatory state. Cardiovasc. Res. 2023, 119, 1146–1160. [Google Scholar] [CrossRef]
- Seijkens, T.T.P.; van Tiel, C.M.; Kusters, P.J.H.; Atzler, D.; Soehnlein, O.; Zarzycka, B.; Aarts, S.A.B.M.; Lameijer, M.; Gijbels, M.J.; Beckers, L.; et al. Targeting CD40-Induced TRAF6 Signaling in Macrophages Reduces Atherosclerosis. J. Am. Coll. Cardiol. 2018, 71, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Schönbeck, U.; Sukhova, G.K.; Bourcier, T.; Bonnefoy, J.Y.; Pober, J.S.; Libby, P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: Implications for CD40-CD40 ligand signaling in atherosclerosis. Proc. Natl. Acad. Sci. USA 1997, 94, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Schönbeck, U.; Sukhova, G.K.; Shimizu, K.; Mach, F.; Libby, P. Inhibition of CD40 signaling limits evolution of established atherosclerosis in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 7458–7463. [Google Scholar] [CrossRef] [PubMed]
- Kobiyama, K.; Ley, K. Atherosclerosis. Circ. Res. 2018, 123, 1118–1120. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Yu, G.; Zheng, M.; Peng, W.; Li, L. Recent Advances for Dynamic-Based Therapy of Atherosclerosis. Int. J. Nanomed. 2023, 18, 3851–3878. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
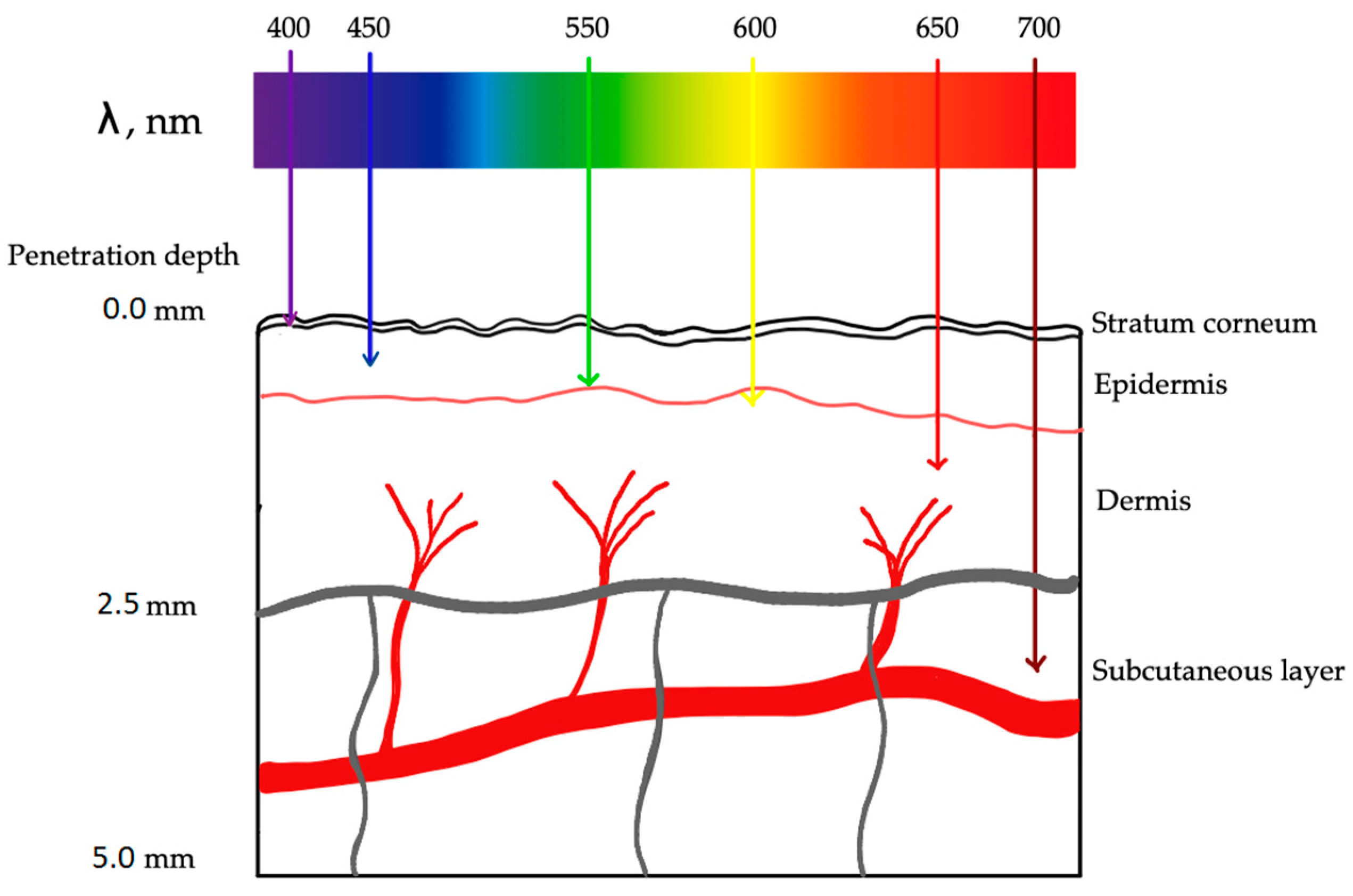
- Algorri, J.F.; López-Higuera, J.M.; Rodríguez-Cobo, L.; Cobo, A. Advanced Light Source Technologies for Photodynamic Therapy of Skin Cancer Lesions. Pharmaceutics 2023, 15, 2075. [Google Scholar] [CrossRef]
- Berezin, M.Y.; Achilefu, S. Fluorescence lifetime measurements and biological imaging. Chem. Rev. 2010, 110, 2641–2684. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, M.A.C.; Prange, K.H.M.; Slenders, L.; Örd, T.; Elbersen, D.; Boltjes, A.; de Jager, S.C.A.; Asselbergs, F.W.; de Borst, G.J.; Aavik, E.; et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ. Res. 2020, 127, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Macías, J.; Flores-Gallegos, A.C.; Nava-Reyna, E.; Morales, I.; Tortella, G.; Solís-Gaona, S.; Benavides-Mendoza, A. Reactive Oxygen, Nitrogen, and Sulfur Species (RONSS) as a Metabolic Cluster for Signaling and Biostimulation of Plants: An Overview. Plants 2022, 11, 3203. [Google Scholar] [CrossRef] [PubMed]
- AbdulSalam, S.F.; Thowfeik, F.S.; Merino, E.J. Excessive Reactive Oxygen Species and Exotic DNA Lesions as an Exploitable Liability. Biochemistry 2016, 55, 5341–5352. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Martindale, J.L.; Holbrook, N.J. Cellular response to oxidative stress: Signaling for suicide and survival. J. Cell. Physiol. 2002, 192, 1–15. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Hou, P.; Fang, J.; Liu, Z.; Shi, Y.; Agostini, M.; Bernassola, F.; Bove, P.; Candi, E.; Rovella, V.; Sica, G.; et al. Macrophage polarization and metabolism in atherosclerosis. Cell Death Dis. 2023, 14, 691. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, B.; Guo, Y.; Zhang, A.; Yang, K.; He, Y.; Wang, J.; Cheng, Y.; Cui, D. SR-A-Targeted Nanoplatform for Sequential Photothermal/Photodynamic Ablation of Activated Macrophages to Alleviate Atherosclerosis. ACS Appl. Mater. Interfaces 2021, 13, 29349–29362. [Google Scholar] [CrossRef]
- Rodriguez, L.; Vallecorsa, P.; Battah, S.; Di Venosa, G.; Calvo, G.; Mamone, L.; Sáenz, D.; Gonzalez, M.C.; Batlle, A.; MacRobert, A.J.; et al. Aminolevulinic acid dendrimers in photodynamic treatment of cancer and atheromatous disease. Photochem. Photobiol. Sci. 2015, 14, 1617–1627. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Vozovikov, I.N.; Andreeva, E.R.; Kuz’min, S.G. Photodynamic approaches to elimination and prevention of atherosclerotic changes in the vessels. Ross. Fiziol. Zh. Im. I M. Sechenova 2004, 90, 569–576. [Google Scholar] [PubMed]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Newman, C.B. Safety of Statins and Nonstatins for Treatment of Dyslipidemia. Endocrinol. Metab. Clin. N. Am. 2022, 51, 655–679. [Google Scholar] [CrossRef] [PubMed]
- Morofuji, Y.; Nakagawa, S.; Ujifuku, K.; Fujimoto, T.; Otsuka, K.; Niwa, M.; Tsutsumi, K. Beyond Lipid-Lowering: Effects of Statins on Cardiovascular and Cerebrovascular Diseases and Cancer. Pharmaceuticals 2022, 15, 151. [Google Scholar] [CrossRef] [PubMed]
- Kowara, M.; Cudnoch-Jedrzejewska, A. Different Approaches in Therapy Aiming to Stabilize an Unstable Atherosclerotic Plaque. Int. J. Mol. Sci. 2021, 22, 4354. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.D.; Litovsky, S.H.; Steinkampf, M.P.; Caulfield, J.B. Statins improve human coronary atherosclerotic plaque morphology. Tex. Heart Inst. J. 2008, 35, 99–103. [Google Scholar] [PubMed]
- Hafiane, A. Vulnerable Plaque, Characteristics, Detection, and Potential Therapies. J. Cardiovasc. Dev. Dis. 2019, 6, 26. [Google Scholar] [CrossRef]
- Diamantis, E.; Kyriakos, G.; Quiles-Sanchez, L.V.; Farmaki, P.; Troupis, T. The Anti-Inflammatory Effects of Statins on Coronary Artery Disease: An Updated Review of the Literature. Curr. Cardiol. Rev. 2017, 13, 209–216. [Google Scholar] [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Mao, Y.; Ren, J.; Yang, L. Advances of nanomedicine in treatment of atherosclerosis and thrombosis. Environ. Res. 2023, 238, 116637. [Google Scholar] [CrossRef]
- Hu, Q.; Fang, Z.; Ge, J.; Li, H. Nanotechnology for cardiovascular diseases. Innovation 2022, 3, 100214. [Google Scholar] [CrossRef]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef]
- Gaytan, S.L.; Beaven, E.; Gadad, S.S.; Nurunnabi, M. Progress and prospect of nanotechnology for cardiac fibrosis treatment. Interdiscip. Med. 2023, 1, e20230018. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Millican, R.; Creutzmann, J.E.; Martin, S.; Jun, H.W. High density lipoprotein mimicking nanoparticles for atherosclerosis. Nano Converg. 2020, 7, 6. [Google Scholar] [CrossRef]
- Palekar, R.U.; Jallouk, A.P.; Lanza, G.M.; Pan, H.; Wickline, S.A. Molecular imaging of atherosclerosis with nanoparticle-based fluorinated MRI contrast agents. Nanomedicine 2015, 10, 1817–1832. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Puricelli, C.; Gigliotti, C.L.; Stoppa, I.; Sacchetti, S.; Pantham, D.; Scomparin, A.; Rolla, R.; Pizzimenti, S.; Dianzani, U.; Boggio, E.; et al. Use of Poly Lactic-co-glycolic Acid Nano and Micro Particles in the Delivery of Drugs Modulating Different Phases of Inflammation. Pharmaceutics 2023, 15, 1772. [Google Scholar] [CrossRef]
- Chen, H.; Chen, L.; Liang, R.; Wei, J. Ultrasound and magnetic resonance molecular imaging of atherosclerotic neovasculature with perfluorocarbon magnetic nanocapsules targeted against vascular endothelial growth factor receptor 2 in rats. Mol. Med. Rep. 2017, 16, 5986–5996. [Google Scholar] [CrossRef]
- Kirla, H.; Henry, D.J.; Jansen, S.; Thompson, P.L.; Hamzah, J. Use of Silica Nanoparticles for Drug Delivery in Cardiovascular Disease. Clin. Ther. 2023, 45, 1060–1068. [Google Scholar] [CrossRef]
- Prilepskii, A.Y.; Serov, N.S.; Kladko, D.V.; Vinogradov, V.V. Nanoparticle-Based Approaches towards the Treatment of Atherosclerosis. Pharmaceutics 2020, 12, 1056. [Google Scholar] [CrossRef]
- Chung, E.J. Targeting and therapeutic peptides in nanomedicine for atherosclerosis. Exp. Biol. Med. 2016, 241, 891–898. [Google Scholar] [CrossRef]
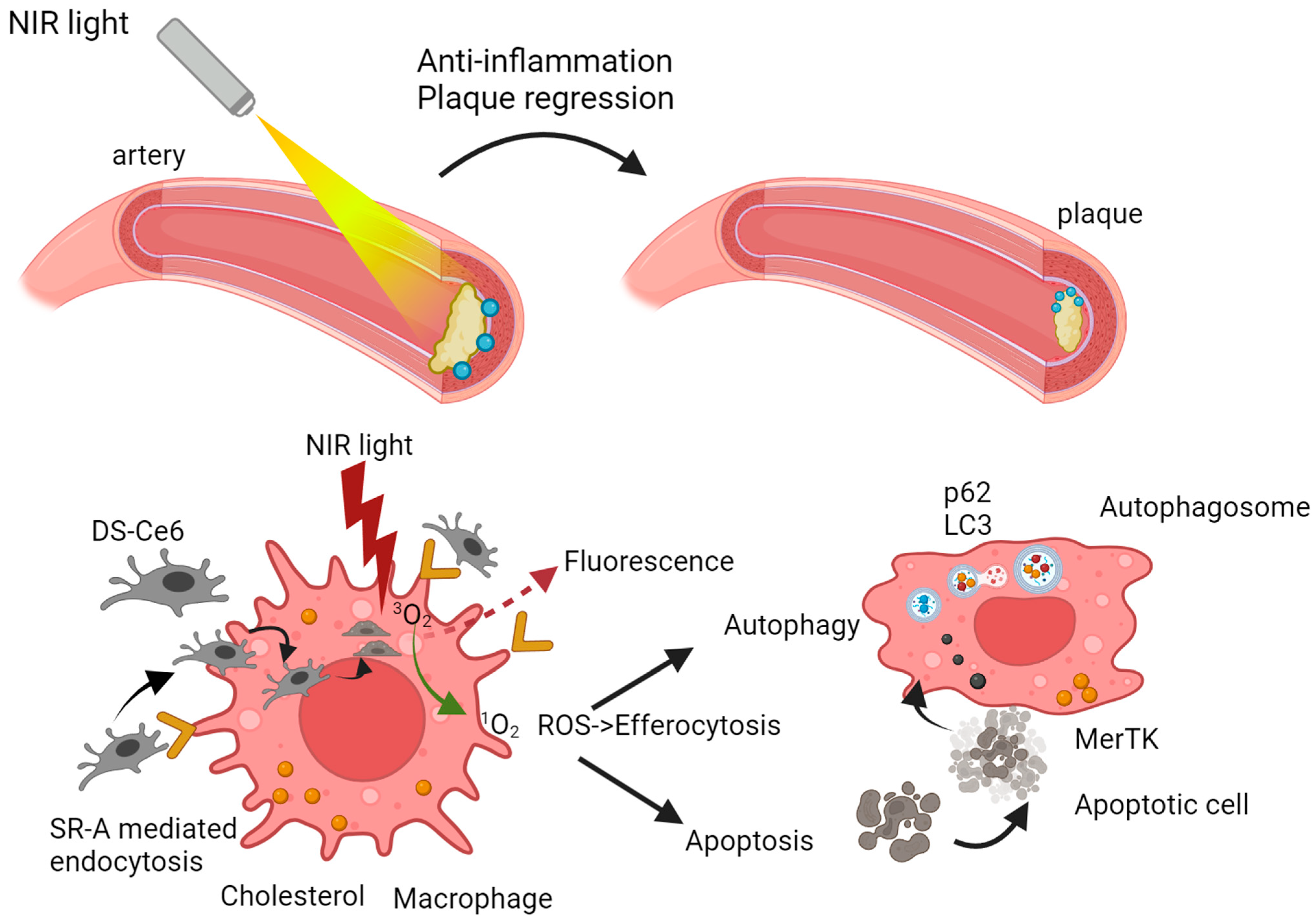
- Han, X.B.; Li, H.X.; Jiang, Y.Q.; Wang, H.; Li, X.S.; Kou, J.Y.; Zheng, Y.H.; Liu, Z.N.; Li, H.; Li, J.; et al. Upconversion nanoparticle-mediated photodynamic therapy induces autophagy and cholesterol efflux of macrophage-derived foam cells via ROS generation. Cell Death Dis. 2017, 8, e2864. [Google Scholar] [CrossRef]
- Dai, T.; He, W.; Tu, S.; Han, J.; Yuan, B.; Yao, C.; Ren, W.; Wu, A. Black TiO2 nanoprobe-mediated mild phototherapy reduces intracellular lipid levels in atherosclerotic foam cells via cholesterol regulation pathways instead of apoptosis. Bioact. Mater. 2022, 17, 18–28. [Google Scholar] [CrossRef]
- Spyropoulos-Antonakakis, N.; Sarantopoulou, E.; Trohopoulos, P.N.; Stefi, A.L.; Kollia, Z.; Gavriil, V.E.; Bourkoula, A.; Petrou, P.S.; Kakabakos, S.; Semashko, V.V.; et al. Selective aggregation of PAMAM dendrimer nanocarriers and PAMAM/ZnPc nanodrugs on human atheromatous carotid tissues: A photodynamic therapy for atherosclerosis. Nanoscale Res. Lett. 2015, 10, 210. [Google Scholar] [CrossRef]
- Liu, Q.; Hamblin, M.R. Macrophage-targeted photodynamic therapy: Scavenger receptor expression and activation state. Int. J. Immunopathol. Pharmacol. 2005, 18, 391–402. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, Y.; Li, K.; Liao, B.; Wang, F.; Shao, L.; Huang, L.; Bai, D. Curcumin-mediated Photodynamic Therapy Inhibits the Phenotypic Transformation, Migration, and Foaming of Oxidized Low-density Lipoprotein-treated Vascular Smooth Muscle Cells by Promoting Autophagy. J. Cardiovasc. Pharmacol. 2021, 78, 308–318. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Zheng, L.; Zhong, Z.; Li, X.; Zhao, J.; Kou, J.; Jiang, Y.; Zheng, X.; Liu, Z.; et al. Upconversion nanoparticle-mediated photodynamic therapy induces THP-1 macrophage apoptosis via ROS bursts and activation of the mitochondrial caspase pathway. Int. J. Nanomed. 2015, 10, 3719–3736. [Google Scholar]
- Granville, D.J.; Cassidy, B.A.; Ruehlmann, D.O.; Choy, J.C.; Brenner, C.; Kroemer, G.; van Breemen, C.; Margaron, P.; Hunt, D.W.; McManus, B.M. Mitochondrial release of apoptosis-inducing factor and cytochrome c during smooth muscle cell apoptosis. Am. J. Pathol. 2001, 159, 305–311. [Google Scholar] [CrossRef]
- de Vries, H.E.; Moor, A.C.; Dubbelman, T.M.; van Berkel, T.J.; Kuiper, J. Oxidized low-density lipoprotein as a delivery system for photosensitizers: Implications for photodynamic therapy of atherosclerosis. J. Pharmacol. Exp. Ther. 1999, 289, 528–534. [Google Scholar]
- Huang, J.; Xu, S.; Liu, L.; Zhang, J.; Xu, J.; Zhang, L.; Zhou, X.; Huang, L.; Peng, J.; Wang, J.; et al. Targeted treatment of atherosclerosis with protein-polysaccharide nanoemulsion co-loaded with photosensitiser and upconversion nanoparticles. J. Drug Target. 2023, 31, 1111–1127. [Google Scholar] [CrossRef]
- Huang, L.; Chen, Q.; Yu, L.; Bai, D. Pyropheophorbide-α methyl ester-mediated photodynamic therapy induces apoptosis and inhibits LPS-induced inflammation in RAW264.7 macrophages. Photodiagnosis Photodyn. Ther. 2019, 25, 148–156. [Google Scholar] [CrossRef]
- Biały, D.; Derkacz, A.; Wawrzyńska, M.; Bednarkiewicz, A.; Ziółkowski, P.; Nowosad, H.; Strek, W. In vitro photodynamic diagnosis of atherosclerotic wall changes with the use of mono-l-aspartyl chlorin eA preliminary report. Kardiol. Pol. 2003, 59, 293–301. [Google Scholar]
- Mu, D.; Wang, X.; Wang, H.; Sun, X.; Dai, Q.; Lv, P.; Liu, R.; Qi, Y.; Xie, J.; Xu, B.; et al. Chemiexcited Photodynamic Therapy Integrated in Polymeric Nanoparticles Capable of MRI Against Atherosclerosis. Int. J. Nanomed. 2022, 17, 2353–2366. [Google Scholar] [CrossRef]
- Xu, M.; Mao, C.; Chen, H.; Liu, L.; Wang, Y.; Hussain, A.; Li, S.; Zhang, X.; Tuguntaev, R.G.; Liang, X.J.; et al. Osteopontin targeted theranostic nanoprobes for laser-induced synergistic regression of vulnerable atherosclerotic plaques. Acta Pharm. Sin. B 2022, 12, 2014–2028. [Google Scholar] [CrossRef]
- Han, X.; Kou, J.; Zheng, Y.; Liu, Z.; Jiang, Y.; Gao, Z.; Cong, L.; Yang, L. ROS Generated by Upconversion Nanoparticle-Mediated Photodynamic Therapy Induces Autophagy via PI3K/AKT/ mTOR Signaling Pathway in M1 Peritoneal Macrophage. Cell. Physiol. Biochem. 2019, 52, 1325–1338. [Google Scholar] [CrossRef]
- Jain, M.; Zellweger, M.; Frobert, A.; Valentin, J.; van den Bergh, H.; Wagnières, G.; Cook, S.; Giraud, M.N. Intra-Arterial Drug and Light Delivery for Photodynamic Therapy Using Visudyne®: Implication for Atherosclerotic Plaque Treatment. Front. Physiol. 2016, 7, 400. [Google Scholar] [CrossRef]
- Wennink, J.W.H.; Liu, Y.; Mäkinen, P.I.; Setaro, F.; de la Escosura, A.; Bourajjaj, M.; Lappalainen, J.P.; Holappa, L.P.; van den Dikkenberg, J.B.; Al Fartousi, M.; et al. Macrophage selective photodynamic therapy by meta-tetra(hydroxyphenyl)chlorin loaded polymeric micelles: A possible treatment for cardiovascular diseases. Eur. J. Pharm. Sci. 2017, 107, 112–125. [Google Scholar] [CrossRef]
- Waksman, R.; McEwan, P.E.; Moore, T.I.; Pakala, R.; Kolodgie, F.D.; Hellinga, D.G.; Seabron, R.C.; Rychnovsky, S.J.; Vasek, J.; Scott, R.W.; et al. PhotoPoint photodynamic therapy promotes stabilization of atherosclerotic plaques and inhibits plaque progression. J. Am. Coll. Cardiol. 2008, 52, 1024–1032. [Google Scholar] [CrossRef]
- Usui, M.; Asahara, T.; Naitoh, Y.; Katoh, T.; Ibukiyama, C. Photodynamic therapy for the prevention of intimal hyperplasia in balloon-injured rabbit arteries. Jpn. Circ. J. 1999, 63, 387–393. [Google Scholar] [CrossRef]
- Ortu, P.; LaMuraglia, G.M.; Roberts, W.G.; Flotte, T.J.; Hasan, T. Photodynamic therapy of arteries. A novel approach for treatment of experimental intimal hyperplasia. Circulation 1992, 85, 1189–1196. [Google Scholar] [CrossRef]
- Hayashi, J.; Saito, T.; Aizawa, K. Photodynamic diagnosis and treatment for atherosclerosis by an endoscopic approach. Diagn. Ther. Endosc. 1999, 5, 191–195. [Google Scholar] [CrossRef]
- Hayase, M.; Woodbum, K.W.; Perlroth, J.; Miller, R.A.; Baumgardner, W.; Yock, P.G.; Yeung, A. Photoangioplasty with local motexafin lutetium delivery reduces macrophages in a rabbit post-balloon injury model. Cardiovasc. Res. 2001, 49, 449–455. [Google Scholar] [CrossRef]
- Woodburn, K.W.; Fan, Q.; Kessel, D.; Wright, M.; Mody, T.D.; Hemmi, G.; Magda, D.; Sessler, J.L.; Dow, W.C.; Miller, R.A.; et al. Phototherapy of cancer and atheromatous plaque with texaphyrins. J. Clin. Laser Med. Surg. 1996, 14, 343–348. [Google Scholar] [CrossRef]
- Spokojny, A.M.; Serur, J.R.; Skillman, J.; Spears, J.R. Uptake of hematoporphyrin derivative by atheromatous plaques: Studies in human in vitro and rabbit in vivo. J. Am. Coll. Cardiol. 1986, 8, 1387–1392. [Google Scholar] [CrossRef]
- Tawakol, A.; Castano, A.P.; Anatelli, F.; Bashian, G.; Stern, J.; Zahra, T.; Gad, F.; Chirico, S.; Ahmadi, A.; Fischman, A.J.; et al. Photosensitizer delivery to vulnerable atherosclerotic plaque: Comparison of macrophage-targeted conjugate versus free chlorin(e6). J. Biomed. Opt. 2006, 11, 021008. [Google Scholar] [CrossRef]
- Peng, C.; Li, Y.; Liang, H.; Cheng, J.; Li, Q.; Sun, X.; Li, Z.; Wang, F.; Guo, Y.; Tian, Z.; et al. Detection and photodynamic therapy of inflamed atherosclerotic plaques in the carotid artery of rabbits. J. Photochem. Photobiol. B 2011, 102, 26–31. [Google Scholar] [CrossRef]
- Pai, M.; Jamal, W.; Mosse, A.; Bishop, C.; Bown, S.; McEwan, J. Inhibition of in-stent restenosis in rabbit iliac arteries with photodynamic therapy. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 573–581. [Google Scholar] [CrossRef]
- Kwon, O.C.; Yoon, H.J.; Kim, K.H.; Kim, H.T.; Yoon, Y.H.; Kim, J.K. Fluorescence kinetics of protoporphyrin-IX induced from 5-ALA compounds in rabbit postballoon injury model for ALA-photoangioplasty. Photochem. Photobiol. 2008, 84, 1209–1214. [Google Scholar] [CrossRef]
- Statius van Eps, R.G.; ChandraSekar, N.R.; Hasan, T.; LaMuraglia, G.M. Importance of the treatment field for the application of vascular photodynamic therapy to inhibit intimal hyperplasia. Photochem. Photobiol. 1998, 67, 337–342. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Woodburn, K.W.; Hayase, M.; Hoyt, G.; Robbins, R.C. Photodynamic therapy with motexafin lutetium (Lu-Tex) reduces experimental graft coronary artery disease. Transplantation 2001, 71, 1526–1532. [Google Scholar] [CrossRef]
- Jenkins, M.P.; Buonaccorsi, G.A.; Mansfield, R.; Bishop, C.C.; Bown, S.G.; McEwan, J.R. Reduction in the response to coronary and iliac artery injury with photodynamic therapy using 5-aminolaevulinic acid. Cardiovasc. Res. 2000, 45, 478–485. [Google Scholar] [CrossRef]
- Jenkins, M.P.; Buonaccorsi, G.; MacRobert, A.; Bishop, C.C.; Bown, S.G.; McEwan, J.R. Intra-arterial photodynamic therapy using 5-ALA in a swine model. Eur. J. Vasc. Endovasc. Surg. 1998, 16, 284–291. [Google Scholar] [CrossRef]
- Jenkins, M.P.; Buonaccorsi, G.A.; Raphael, M.; Nyamekye, I.; McEwan, J.R.; Bown, S.G.; Bishop, C.C. Clinical study of adjuvant photodynamic therapy to reduce restenosis following femoral angioplasty. Br. J. Surg. 1999, 86, 1258–1263. [Google Scholar] [CrossRef]
- Rockson, S.G.; Kramer, P.; Razavi, M.; Szuba, A.; Filardo, S.; Fitzgerald, P.; Cooke, J.P.; Yousuf, S.; DeVault, A.R.; Renschler, M.F.; et al. Photoangioplasty for human peripheral atherosclerosis: Results of a phase I trial of photodynamic therapy with motexafin lutetium (Antrin). Circulation 2000, 102, 2322–2324. [Google Scholar] [CrossRef]
- Hsiang, Y.N.; Crespo, M.T.; Richter, A.M.; Jain, A.K.; Fragoso, M.; Levy, J.G. In vitro and in vivo uptake of benzoporphyrin derivative into human and miniswine atherosclerotic plaque. Photochem. Photobiol. 1993, 57, 670–674. [Google Scholar] [CrossRef]
- Usui, M.; Miyagi, M.; Fukasawa, S.; Hara, T.; Ueyama, N.; Nakajima, H.; Takata, R.; Sasame, A.; Tamura, K.; Naitou, Y.; et al. A first trial in the clinical application of photodynamic therapy for the prevention of restenosis after coronary-stent placement. Lasers Surg. Med. 2004, 34, 235–241. [Google Scholar] [CrossRef]



| Examples of Nanoparticles in Atherosclerosis with a Brief Description of Use | |
|---|---|
| High-Density Lipoprotein (HDL) Mimicking Nanoparticles | Nanoparticles are designed to mimic the structure and function of HDL, often loaded with anti-inflammatory or antioxidant agents. They can target atherosclerotic plaques to deliver therapeutic payloads [137]. |
| Superparamagnetic Iron Oxide Nanoparticles (SPIONs) | Used for imaging purposes in magnetic resonance imaging (MRI). SPIONs can be functionalized with targeting ligands for specific binding to atherosclerotic plaques, enabling non-invasive imaging [138]. |
| Gold Nanorods | Utilized for both imaging and therapy. Gold nanorods can absorb near-infrared light, enabling photothermal therapy to target and treat atherosclerotic plaques [139]. |
| PLGA (Poly(lactic-co-glycolic acid)) Nanoparticles | Biodegradable polymeric nanoparticles that can encapsulate drugs for sustained release. PLGA nanoparticles have been investigated for the targeted delivery of anti-inflammatory drugs to atherosclerotic lesions [140]. |
| Perfluorocarbon Nanobubbles | Used as contrast agents for imaging, nanobubbles can be designed to target atherosclerotic plaques. They have been explored for ultrasound imaging to detect and monitor plaque progression [141]. |
| Mesoporous Silica Nanoparticles | Designed to carry therapeutic agents and deliver them to specific locations. Mesoporous silica nanoparticles can be functionalized for targeted drug delivery to atherosclerotic lesions [142]. |
| Nanoparticle-Mediated Gene Therapy | Nanoparticles can be used to deliver therapeutic genes to cells within atherosclerotic plaques. This approach aims to modulate the expression of specific genes to mitigate inflammation or promote plaque stabilization [143]. |
| Targeting Peptide-Modified Nanoparticles | Nanoparticles functionalized with peptides that have an affinity for molecules overexpressed in atherosclerotic plaques. This enhances the nanoparticles’ ability to target and accumulate at specific sites [144]. |
| PDT | Statin Therapy | |
|---|---|---|
| Costs | Photodynamic therapy involves the administration of photosensitizers and the use of specialized light sources for activation. The costs associated with PDT can be relatively high due to the need for specific equipment, skilled personnel, and the development or acquisition of photosensitizing agents. Additionally, repeated sessions may be required for optimal efficacy, contributing to the overall economic burden. | Statin therapy is a well-established and widely prescribed approach for managing atherosclerosis. The costs associated with statins are generally lower compared to PDT. Statins are available in generic forms, contributing to cost-effectiveness. However, the overall economic impact may vary depending on the specific statin prescribed, patient adherence, and the need for additional cardiovascular medications. |
| Side effects | While PDT is generally considered a localized and targeted therapy, side effects can occur. Common side effects include photosensitivity reactions, skin irritation, and temporary discoloration of the treated area. The specificity of PDT for atherosclerotic plaques minimizes systemic side effects, but the potential for skin reactions remains a consideration. | Statins are generally well-tolerated, with a favorable safety profile. Common side effects include muscle pain or weakness, gastrointestinal disturbances, and liver enzyme abnormalities. Serious side effects, such as rhabdomyolysis, are rare but can occur. Regular monitoring of liver function and muscle health is recommended during statin therapy to mitigate potential adverse effects. |
| Effectiveness | PDT may be more suitable for localized and specific interventions, aimed at the ablation of localized plaques. | Statins provide a broader systemic approach suitable for chronic management, primarily addressing systemic factors, including cholesterol levels and inflammation. |
| Long-term considerations | The long-term safety and efficacy of PDT, especially concerning repeated treatments over extended periods, require further investigation. | Statin therapy has an extensive track record of long-term safety and efficacy, supported by numerous clinical trials and real-world evidence. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mytych, W.; Bartusik-Aebisher, D.; Łoś, A.; Dynarowicz, K.; Myśliwiec, A.; Aebisher, D. Photodynamic Therapy for Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 1958. https://doi.org/10.3390/ijms25041958
Mytych W, Bartusik-Aebisher D, Łoś A, Dynarowicz K, Myśliwiec A, Aebisher D. Photodynamic Therapy for Atherosclerosis. International Journal of Molecular Sciences. 2024; 25(4):1958. https://doi.org/10.3390/ijms25041958
Chicago/Turabian StyleMytych, Wiktoria, Dorota Bartusik-Aebisher, Aleksandra Łoś, Klaudia Dynarowicz, Angelika Myśliwiec, and David Aebisher. 2024. "Photodynamic Therapy for Atherosclerosis" International Journal of Molecular Sciences 25, no. 4: 1958. https://doi.org/10.3390/ijms25041958
APA StyleMytych, W., Bartusik-Aebisher, D., Łoś, A., Dynarowicz, K., Myśliwiec, A., & Aebisher, D. (2024). Photodynamic Therapy for Atherosclerosis. International Journal of Molecular Sciences, 25(4), 1958. https://doi.org/10.3390/ijms25041958








