Lung Inflammatory Phenotype in Mice Deficient in Fibulin-2 and ADAMTS-12
Abstract
:1. Introduction
2. Results
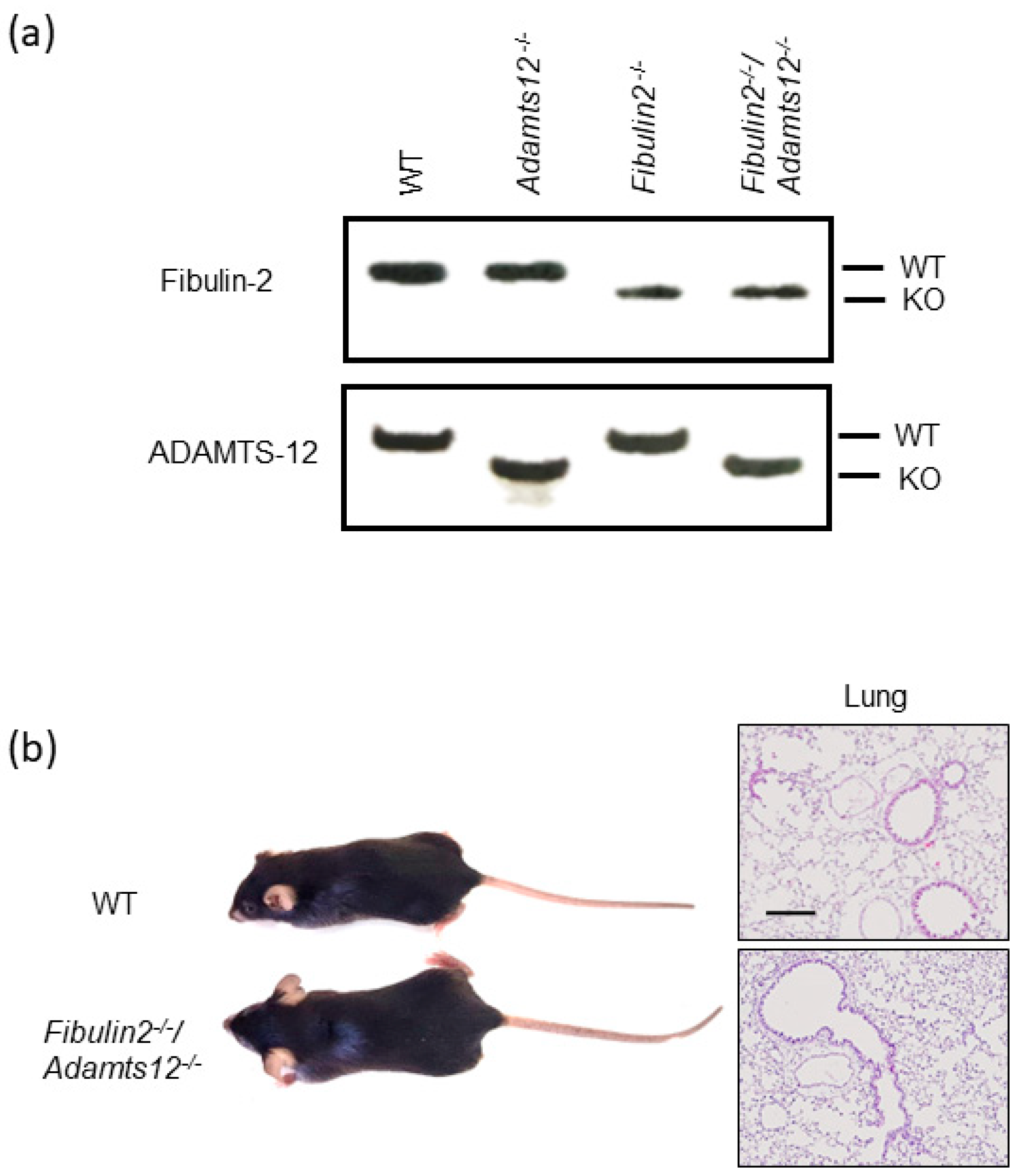
2.1. Double Fibulin-2 and ADAMTS-12-Deficient Mice Are Viable, Fertile and Show No Obvious Macroscopic Phenotype
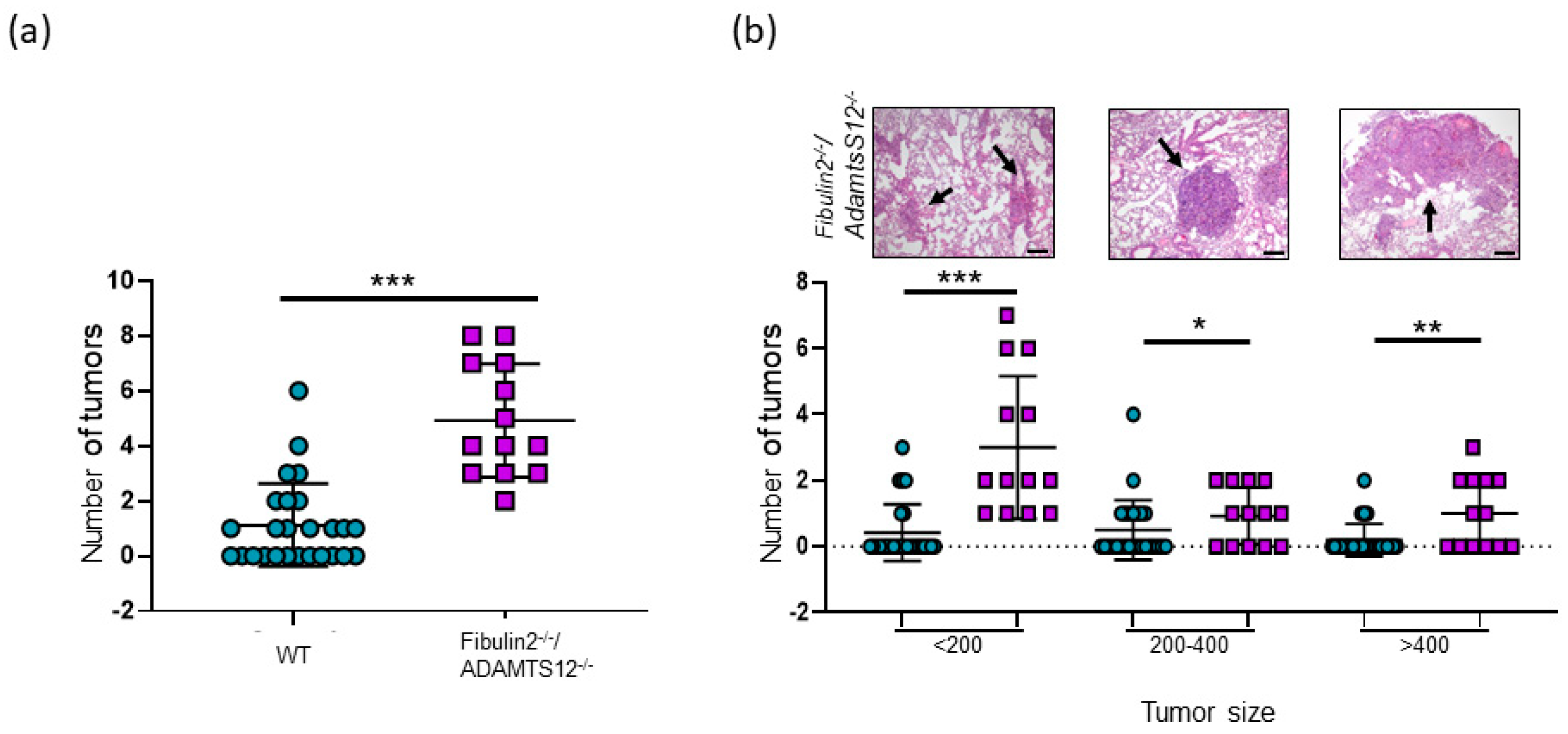
2.2. Analysis of Lung Cancer Susceptibility in Fibulin-2/ADAMTS-12-Deficient Mice
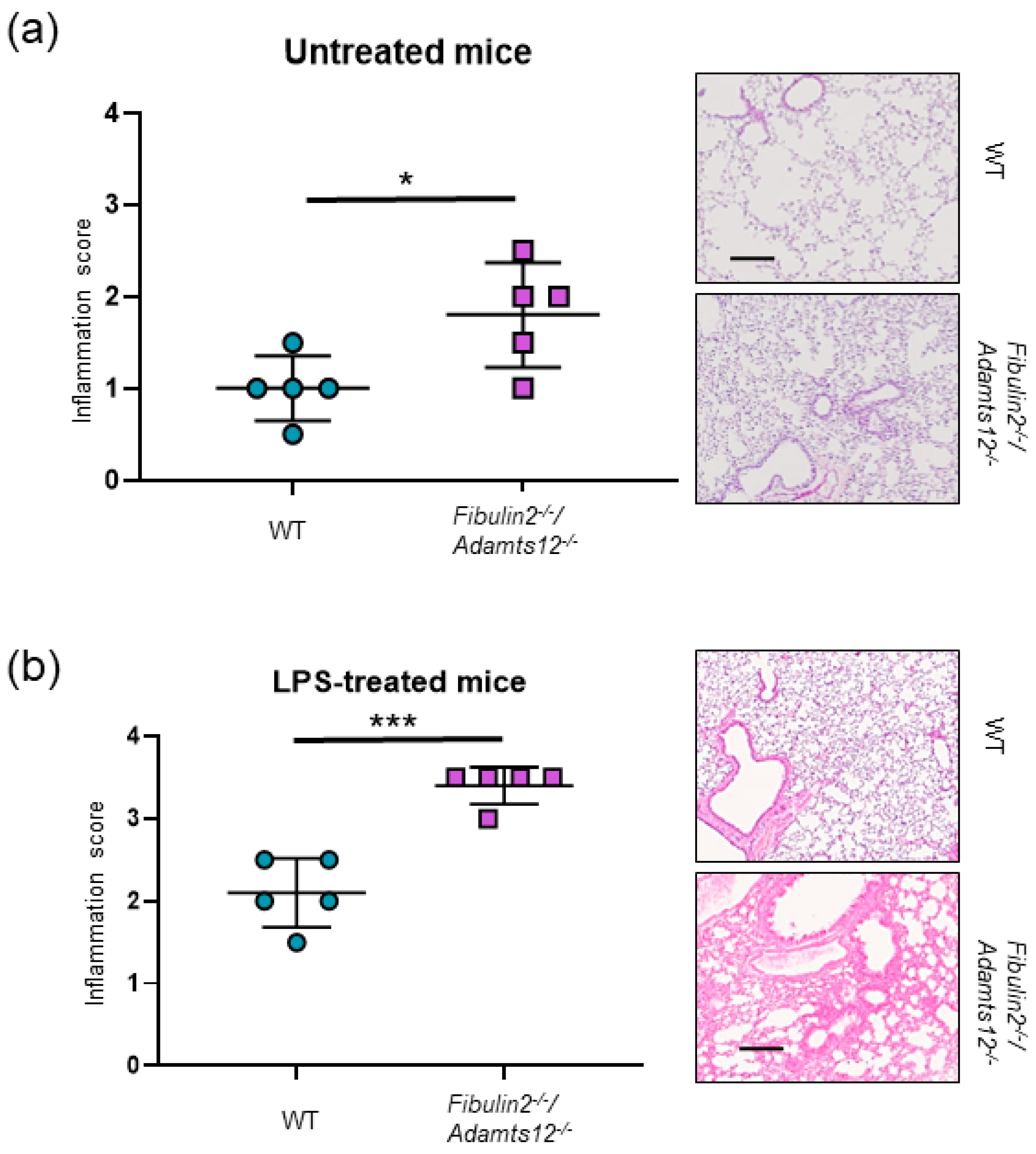
2.3. Fibulin-2 and ADAMTS12 Deficiency Results in Increased Inflammation and Higher Susceptibility to LPS
2.4. Increased Inflammation in Fibulin-2/Adamts12-/- Mice in Lungs Is Associated with Macrophages, Neutrophils and Lymphocytes Accumulation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Lung Carcinogenesis
4.3. Lung Inflammation by LPS Treatment
4.4. Structural and Immunohistochemical Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Caruntu, C.; Dumitru, C.; Surcel, M.; Zurac, S. Inflammation: A key process in skin tumorigenesis. Oncol. Lett. 2019, 17, 4068–4084. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009, 15, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ikwegbue, P.C.; Masamba, P.; Mbatha, L.S.; Oyinloye, B.E.; Kappo, A.P. Interplay between heat shock proteins, inflammation and cancer: A potential cancer therapeutic target. Am. J. Cancer Res. 2019, 9, 242–249. [Google Scholar] [PubMed]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef]
- Sopori, M. Effects of cigarette smoke on the immune system. Nat. Rev. Immunol. 2002, 2, 372–377. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, L.; Yang, H.; Wu, X.; Luo, X.; Shen, J.; Xiao, Z.; Zhao, Y.; Du, F.; Chen, Y.; et al. Dysregulation of immunity by cigarette smoking promotes inflammation and cancer: A review. Environ. Pollut. 2023, 339, 122730. [Google Scholar] [CrossRef]
- Yuan, F.; Fu, X.; Shi, H.; Chen, G.; Dong, P.; Zhang, W. Induction of murine macrophage M2 polarization by cigarette smoke extract via the JAK2/STAT3 pathway. PLoS ONE 2014, 9, e107063. [Google Scholar] [CrossRef] [PubMed]
- Peyton, S.R.; Platt, M.O.; Cukierman, E. Challenges and Opportunities Modeling the Dynamic Tumor Matrisome. BME Front. 2023, 4, 0006. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Garcia-Hernandez, A.A.; Ramos, C. Matrix Metalloproteinases’ Role in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 97–131. [Google Scholar] [PubMed]
- Kobayashi, N.; Kostka, G.; Garbe, J.H.; Keene, D.R.; Bachinger, H.P.; Hanisch, F.G.; Markova, D.; Tsuda, T.; Timpl, R.; Chu, M.L.; et al. A comparative analysis of the fibulin protein family. Biochemical characterization, binding interactions, and tissue localization. J. Biol. Chem. 2007, 282, 11805–11816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hui, D.; Fu, X. Roles of Fibulin-2 in Carcinogenesis. Med. Sci. Monit. 2020, 26, e918099. [Google Scholar] [CrossRef]
- Whiteaker, J.R.; Zhang, H.; Zhao, L.; Wang, P.; Kelly-Spratt, K.S.; Ivey, R.G.; Piening, B.D.; Feng, L.C.; Kasarda, E.; Gurley, K.E.; et al. Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J. Proteome. Res. 2007, 6, 3962–3975. [Google Scholar] [CrossRef]
- Yi, C.H.; Smith, D.J.; West, W.W.; Hollingsworth, M.A. Loss of fibulin-2 expression is associated with breast cancer progression. Am. J. Pathol. 2007, 170, 1535–1545. [Google Scholar] [CrossRef]
- Ma, H.; Lian, C.; Song, Y. Fibulin-2 inhibits development of gastric cancer by downregulating beta-catenin. Oncol. Lett. 2019, 18, 2799–2804. [Google Scholar]
- Hu, X.; Liu, T.; Li, L.; Gan, H.; Wang, T.; Pang, P.; Mao, J. Fibulin-2 Facilitates Malignant Progression of Hepatocellular Carcinoma. Turk. J. Gastroenterol. 2023, 34, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Baird, B.N.; Schliekelman, M.J.; Ahn, Y.H.; Chen, Y.; Roybal, J.D.; Gill, B.J.; Mishra, D.K.; Erez, B.; O’Reilly, M.; Yang, Y.; et al. Fibulin-2 is a driver of malignant progression in lung adenocarcinoma. PLoS ONE 2013, 8, e67054. [Google Scholar] [CrossRef] [PubMed]
- Sicot, F.X.; Tsuda, T.; Markova, D.; Klement, J.F.; Arita, M.; Zhang, R.Z.; Pan, T.C.; Mecham, R.P.; Birk, D.E.; Chu, M.L. Fibulin-2 is dispensable for mouse development and elastic fiber formation. Mol. Cell Biol. 2008, 28, 1061–1067. [Google Scholar] [CrossRef]
- Lee, N.V.; Rodriguez-Manzaneque, J.C.; Thai, S.N.; Twal, W.O.; Luque, A.; Lyons, K.M.; Argraves, W.S.; Iruela-Arispe, M.L. Fibulin-1 acts as a cofactor for the matrix metalloprotease ADAMTS-1. J. Biol. Chem. 2005, 280, 34796–34804. [Google Scholar] [CrossRef]
- Fontanil, T.; Rua, S.; Llamazares, M.; Moncada-Pazos, A.; Quiros, P.M.; Garcia-Suarez, O.; Vega, J.A.; Sasaki, T.; Mohamedi, Y.; Esteban, M.M.; et al. Interaction between the ADAMTS-12 metalloprotease and fibulin-2 induces tumor-suppressive effects in breast cancer cells. Oncotarget 2014, 5, 1253–1264. [Google Scholar] [CrossRef]
- Fontanil, T.; Alvarez-Teijeiro, S.; Villaronga, M.A.; Mohamedi, Y.; Solares, L.; Moncada-Pazos, A.; Vega, J.A.; Garcia-Suarez, O.; Perez-Basterrechea, M.; Garcia-Pedrero, J.M.; et al. Cleavage of Fibulin-2 by the aggrecanases ADAMTS-4 and ADAMTS-5 contributes to the tumorigenic potential of breast cancer cells. Oncotarget 2017, 8, 13716–13729. [Google Scholar] [CrossRef]
- Wang, Z.; Li, W.; Chen, S.; Tang, X.X. Role of ADAM and ADAMTS proteases in pathological tissue remodeling. Cell Death Discov. 2023, 9, 447. [Google Scholar] [CrossRef]
- Redondo-Garcia, S.; Peris-Torres, C.; Caracuel-Peramos, R.; Rodriguez-Manzaneque, J.C. ADAMTS proteases and the tumor immune microenvironment: Lessons from substrates and pathologies. Matrix Biol. Plus. 2021, 9, 100054. [Google Scholar] [CrossRef]
- Dekky, B.; Azar, F.; Bonnier, D.; Monseur, C.; Kalebic, C.; Arpigny, E.; Colige, A.; Legagneux, V.; Theret, N. ADAMTS12 is a stromal modulator in chronic liver disease. FASEB J. 2023, 37, e23237. [Google Scholar] [CrossRef]
- Wei, J.L.; Fu, W.; Hettinghouse, A.; He, W.J.; Lipson, K.E.; Liu, C.J. Role of ADAMTS-12 in Protecting Against Inflammatory Arthritis in Mice By Interacting With and Inactivating Proinflammatory Connective Tissue Growth Factor. Arthritis. Rheumatol. 2018, 70, 1745–1756. [Google Scholar] [CrossRef]
- He, R.Z.; Zheng, J.H.; Yao, H.F.; Xu, D.P.; Yang, M.W.; Liu, D.J.; Sun, Y.W.; Huo, Y.M. ADAMTS12 promotes migration and epithelial-mesenchymal transition and predicts poor prognosis for pancreatic cancer. Hepatobiliary Pancreat. Dis. Int. 2023, 22, 169–178. [Google Scholar] [CrossRef]
- Yang, S.; Zhou, X.; Jia, Z.; Zhang, M.; Yuan, M.; Zhou, Y.; Wang, J.; Xia, D. Epigenetic regulatory mechanism of ADAMTS12 expression in osteoarthritis. Mol. Med. 2023, 29, 86. [Google Scholar] [CrossRef]
- Nah, S.S.; Lee, S.; Joo, J.; Kim, H.K.; Sohn, D.R.; Kwon, J.T.; Woo, K.M.; Hong, S.J.; Kim, H.J. Association of ADAMTS12 polymorphisms with rheumatoid arthritis. Mol. Med. Rep. 2012, 6, 227–231. [Google Scholar]
- Fontanil, T.; Mohamedi, Y.; Moncada-Pazos, A.; Cobo, T.; Vega, J.A.; Cobo, J.L.; Garcia-Suarez, O.; Cobo, J.; Obaya, A.J.; Cal, S. Neurocan is a New Substrate for the ADAMTS12 Metalloprotease: Potential Implications in Neuropathies. Cell Physiol. Biochem. 2019, 52, 1003–1016. [Google Scholar]
- Rabadan, R.; Mohamedi, Y.; Rubin, U.; Chu, T.; Alghalith, A.N.; Elliott, O.; Arnes, L.; Cal, S.; Obaya, A.J.; Levine, A.J.; et al. Identification of relevant genetic alterations in cancer using topological data analysis. Nat. Commun. 2020, 11, 3808. [Google Scholar] [CrossRef]
- Moncada-Pazos, A.; Obaya, A.J.; Llamazares, M.; Heljasvaara, R.; Suarez, M.F.; Colado, E.; Noel, A.; Cal, S.; Lopez-Otin, C. ADAMTS-12 metalloprotease is necessary for normal inflammatory response. J. Biol. Chem. 2018, 293, 11648. [Google Scholar] [CrossRef]
- Paulissen, G.; El Hour, M.; Rocks, N.; Gueders, M.M.; Bureau, F.; Foidart, J.M.; Lopez-Otin, C.; Noel, A.; Cataldo, D.D. Control of allergen-induced inflammation and hyperresponsiveness by the metalloproteinase ADAMTS-12. J. Immunol. 2012, 189, 4135–4143. [Google Scholar] [CrossRef]
- Paulissen, G.; El Hour, M.; Rocks, N.; Gueders, M.M.; Bureau, F.; Foidart, J.M.; Lopez-Otin, C.; Noel, A.; Cataldo, D.D. Correction: Control of Allergen-Induced Inflammation and Hyperresponsiveness by the Metalloproteinase ADAMTS-12. J. Immunol. 2021, 207, 2388–2389. [Google Scholar] [CrossRef]
- Llamazares, M.; Obaya, A.J.; Moncada-Pazos, A.; Heljasvaara, R.; Espada, J.; Lopez-Otin, C.; Cal, S. The ADAMTS12 metalloproteinase exhibits anti-tumorigenic properties through modulation of the Ras-dependent ERK signalling pathway. J. Cell Sci. 2007, 120 Pt 20, 3544–3552. [Google Scholar] [CrossRef]
- Zheng, S.; Lin, F.; Zhang, M.; Fu, J.; Ge, X.; Mu, N. AK001058 promotes the proliferation and migration of colorectal cancer cells by regulating methylation of ADAMTS12. Am. J. Transl. Res. 2019, 11, 5869–5878. [Google Scholar]
- Moncada-Pazos, A.; Obaya, A.J.; Fraga, M.F.; Viloria, C.G.; Capella, G.; Gausachs, M.; Esteller, M.; Lopez-Otin, C.; Cal, S. The ADAMTS12 metalloprotease gene is epigenetically silenced in tumor cells and transcriptionally activated in the stroma during progression of colon cancer. J. Cell Sci. 2009, 122 Pt 16, 2906–2913. [Google Scholar] [CrossRef]
- Ho, T.H.; Serie, D.J.; Parasramka, M.; Cheville, J.C.; Bot, B.M.; Tan, W.; Wang, L.; Joseph, R.W.; Hilton, T.; Leibovich, B.C.; et al. Differential gene expression profiling of matched primary renal cell carcinoma and metastases reveals upregulation of extracellular matrix genes. Ann. Oncol. 2017, 28, 604–610. [Google Scholar] [CrossRef]
- Liang, L.; Zhu, J.H.; Chen, G.; Qin, X.G.; Chen, J.Q. Prognostic Values for the mRNA Expression of the ADAMTS Family of Genes in Gastric Cancer. J. Oncol. 2020, 2020, 9431560. [Google Scholar] [CrossRef]
- Li, X.; Xiao, X.; Chang, R.; Zhang, C. Comprehensive bioinformatics analysis identifies lncRNA HCG22 as a migration inhibitor in esophageal squamous cell carcinoma. J. Cell Biochem. 2020, 121, 468–481. [Google Scholar] [CrossRef]
- El Hour, M.; Moncada-Pazos, A.; Blacher, S.; Masset, A.; Cal, S.; Berndt, S.; Detilleux, J.; Host, L.; Obaya, A.J.; Maillard, C.; et al. Higher sensitivity of Adamts12-deficient mice to tumor growth and angiogenesis. Oncogene 2010, 29, 3025–3032. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Ma, Y.; Kroemer, G. The cancer-immune dialogue in the context of stress. Nat. Rev. Immunol. 2023, 1–18. [Google Scholar] [CrossRef]
- Attiq, A.; Afzal, S. Trinity of inflammation, innate immune cells and cross-talk of signalling pathways in tumour microenvironment. Front. Pharmacol. 2023, 14, 1255727. [Google Scholar] [CrossRef]
- Nikitovic, D.; Corsini, E.; Kouretas, D.; Tsatsakis, A.; Tzanakakis, G. ROS-major mediators of extracellular matrix remodeling during tumor progression. Food Chem. Toxicol. 2013, 61, 178–186. [Google Scholar] [CrossRef]
- Kennel, K.B.; Bozlar, M.; De Valk, A.F.; Greten, F.R. Cancer-Associated Fibroblasts in Inflammation and Antitumor Immunity. Clin. Cancer Res. 2023, 29, 1009–1016. [Google Scholar] [CrossRef]
- Senapati, S.; Gnanapragassam, V.S.; Moniaux, N.; Momi, N.; Batra, S.K. Role of MUC4-NIDO domain in the MUC4-mediated metastasis of pancreatic cancer cells. Oncogene 2012, 31, 3346–3356. [Google Scholar] [CrossRef]
- Avsar, M.; Tambas, M.; Yalniz, Z.; Akdeniz, D.; Tuncer, S.B.; Kilic, S.; Sukruoglu Erdogan, O.; Ciftci, R.; Dagoglu, N.; Vatansever, S.; et al. The expression level of fibulin-2 in the circulating RNA (ctRNA) of epithelial tumor cells of peripheral blood and tumor tissue of patients with metastatic lung cancer. Mol. Biol. Rep. 2019, 46, 4001–4008. [Google Scholar] [CrossRef]
- Ma, Y.; Nenkov, M.; Schroder, D.C.; Abubrig, M.; Gassler, N.; Chen, Y. Fibulin 2 Is Hypermethylated and Suppresses Tumor Cell Proliferation through Inhibition of Cell Adhesion and Extracellular Matrix Genes in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11834. [Google Scholar] [CrossRef]
- Hill, V.K.; Hesson, L.B.; Dansranjavin, T.; Dallol, A.; Bieche, I.; Vacher, S.; Tommasi, S.; Dobbins, T.; Gentle, D.; Euhus, D.; et al. Identification of 5 novel genes methylated in breast and other epithelial cancers. Mol. Cancer 2010, 9, 51. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Dong, H.; Khan, S.A.; Chu, M.L.; Tsuda, T. Fibulin-2 deficiency attenuates angiotensin II-induced cardiac hypertrophy by reducing transforming growth factor-beta signalling. Clin. Sci. (Lond) 2014, 126, 275–288. [Google Scholar] [CrossRef]
- Tsuda, T.; Wu, J.; Gao, E.; Joyce, J.; Markova, D.; Dong, H.; Liu, Y.; Zhang, H.; Zou, Y.; Gao, F.; et al. Loss of fibulin-2 protects against progressive ventricular dysfunction after myocardial infarction. J. Mol. Cell Cardiol. 2012, 52, 273–282. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Zhang, R.; Xia, Y. Knockdown of FBLN2 suppresses TGF-beta1-induced MRC-5 cell migration and fibrosis by downregulating VTN. Tissue Cell 2023, 81, 102005. [Google Scholar] [CrossRef]
- Olijnyk, D.; Ibrahim, A.M.; Ferrier, R.K.; Tsuda, T.; Chu, M.L.; Gusterson, B.A.; Stein, T.; Morris, J.S. Fibulin-2 is involved in early extracellular matrix development of the outgrowing mouse mammary epithelium. Cell Mol. Life Sci. 2014, 71, 3811–3828. [Google Scholar] [CrossRef]
- Li, S.; Jiang, H.; Wang, S.; Li, Y.; Guo, D.; Zhan, J.; Li, Q.; Meng, H.; Chen, A.; Chen, L.; et al. Fibulin-2: A potential regulator of immune dysfunction after bone trauma. Immun. Inflamm. Dis. 2023, 11, e846. [Google Scholar] [CrossRef]
- Li, S.D.; Xing, W.; Wang, S.C.; Li, Y.B.; Jiang, H.; Zheng, H.X.; Li, X.M.; Yang, J.; Guo, D.B.; Xie, X.Y.; et al. Fibulin2: A negative regulator of BMSC osteogenic differentiation in infected bone fracture healing. Exp. Mol. Med. 2023, 55, 443–456. [Google Scholar] [CrossRef]
- Folgueras, A.R.; Freitas-Rodriguez, S.; Espanol, Y.; Velasco, G. Cancer Susceptibility Models in Protease-Deficient Mice. Methods Mol. Biol. 2018, 1731, 235–245. [Google Scholar]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 295, L379–L399. [Google Scholar] [CrossRef]
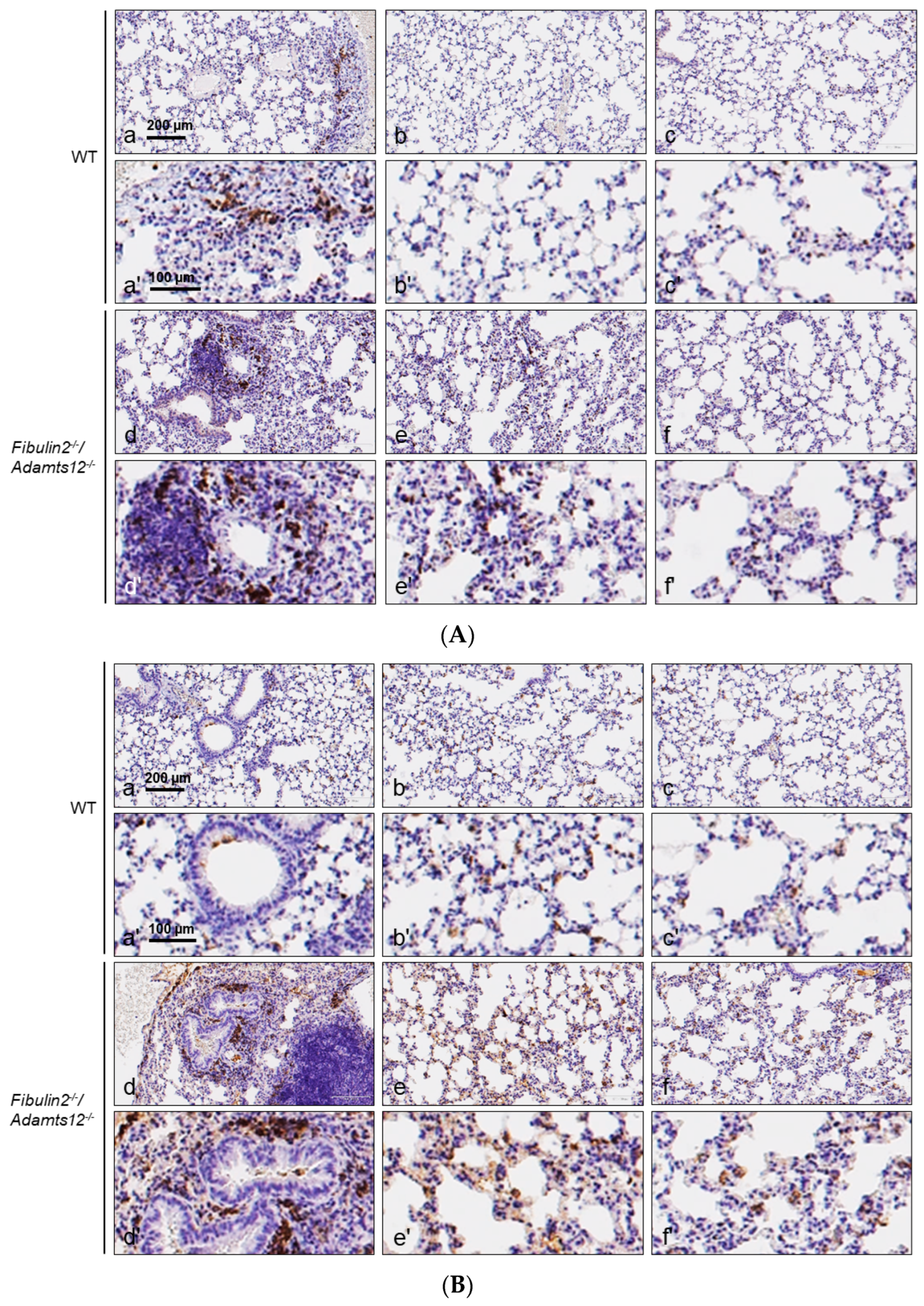
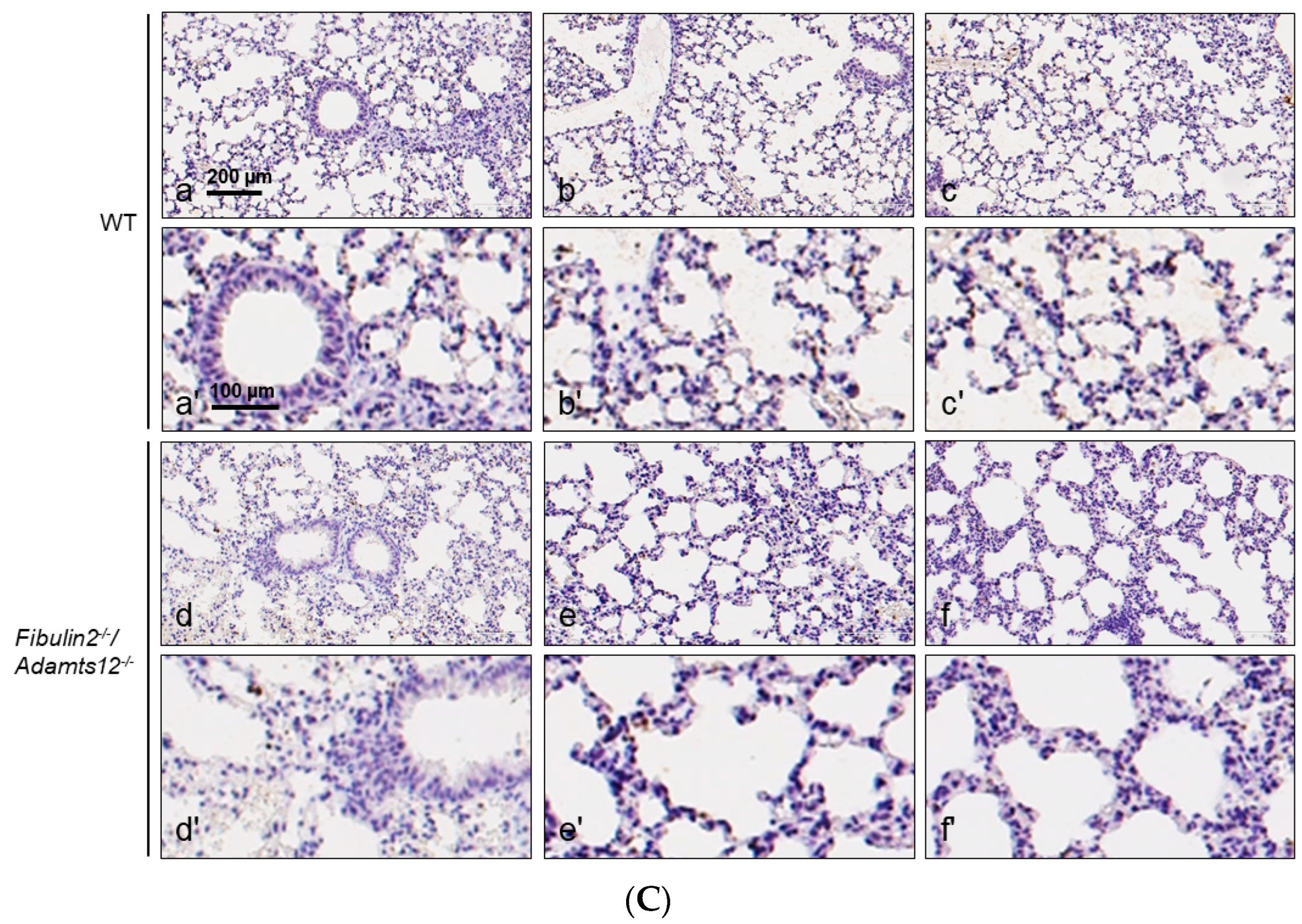
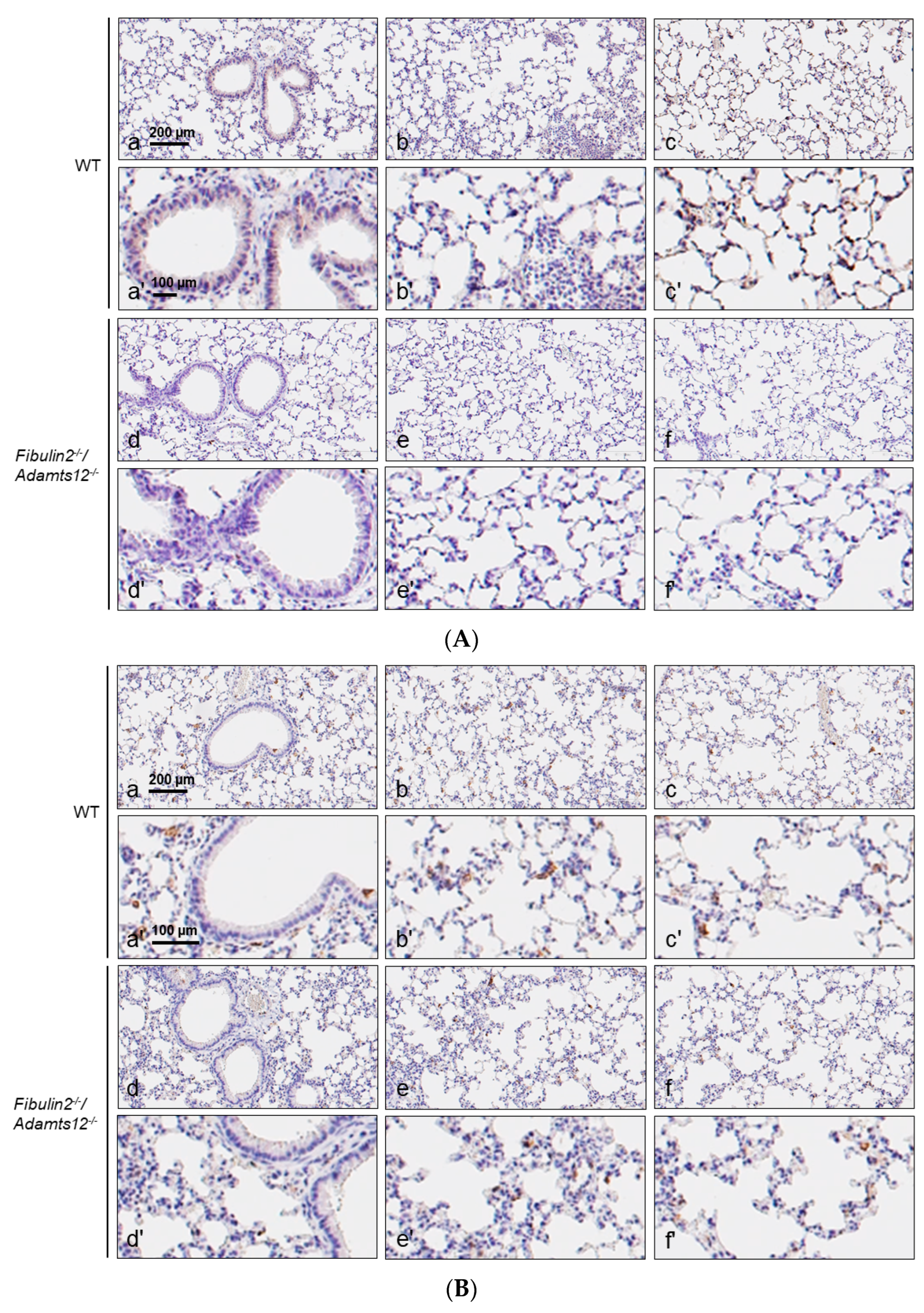
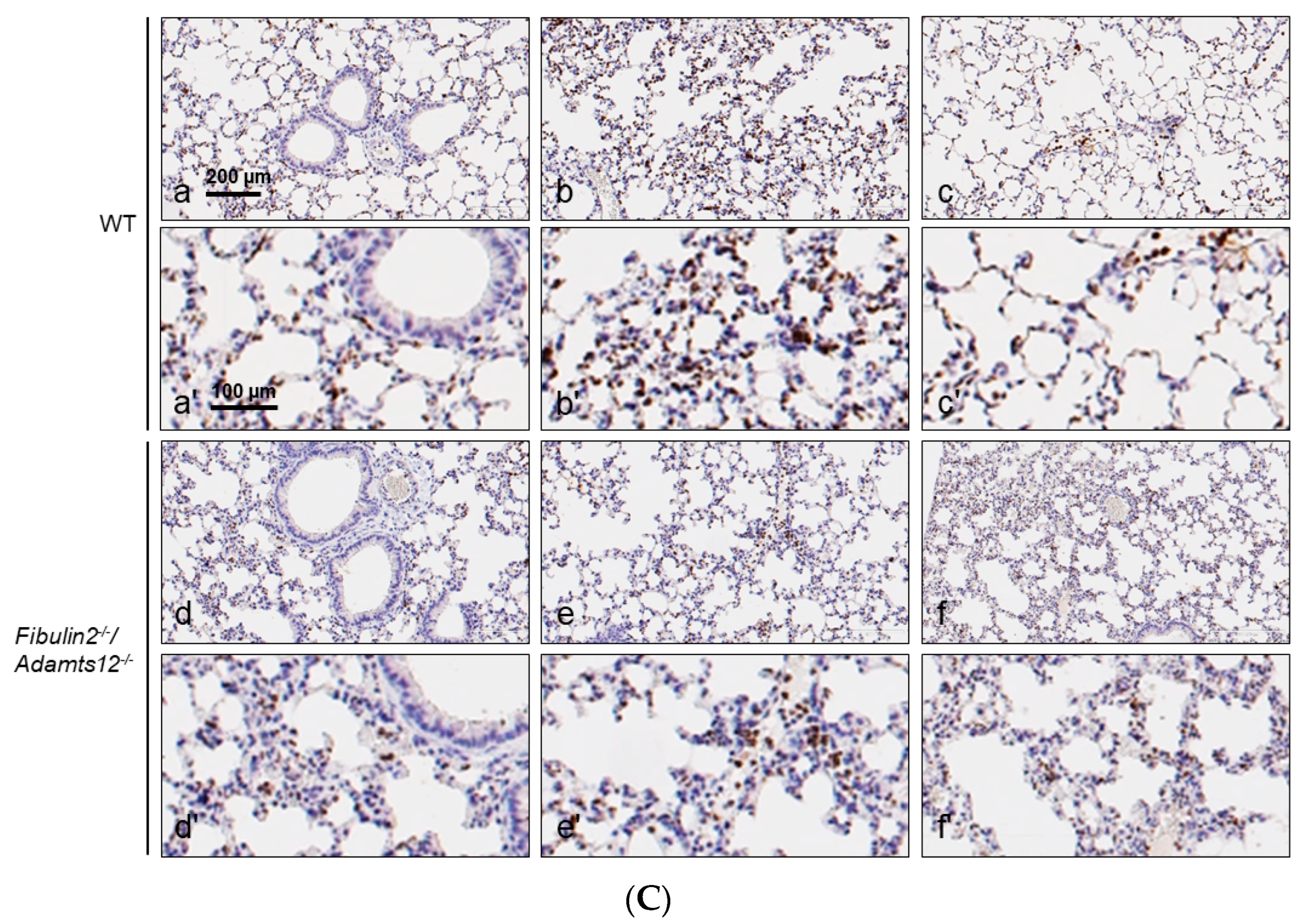
| Parabronchial/Perivascular | Intermediate | Peripheral | Antigen | |
|---|---|---|---|---|
| WT | 0/+ | 0 | 0 | CD3 |
| 0/++ | 0/+ | 0/+ | F4/80 | |
| 0 | 0 | 0 | MPO | |
| Fibulin2-/-/Adamts12-/- | 0/++ | 0 | 0 | CD3 |
| ++/+++ | +/++ | +/++ | F4/80 | |
| 0/++ | 0/+ | 0/+ | MPO | |
| WT after LPS treatment | +/0 | +/++ | 0/++ | CD3 |
| 0/++ | +/++ | +/++ | F4/80 | |
| +/+ | +/++ | 0/++ | MPO | |
| Fibulin2-/-/Adamts12-/- after LPS treatment | 0/+ | 0/+ | 0/+ | CD3 |
| 0/+ | +/++ | +/++ | F4/80 | |
| 0/+ | +/++ | +/++ | MPO |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamedi, Y.; Fontanil, T.; Vega, J.A.; Cobo, T.; Cal, S.; Obaya, Á.J. Lung Inflammatory Phenotype in Mice Deficient in Fibulin-2 and ADAMTS-12. Int. J. Mol. Sci. 2024, 25, 2024. https://doi.org/10.3390/ijms25042024
Mohamedi Y, Fontanil T, Vega JA, Cobo T, Cal S, Obaya ÁJ. Lung Inflammatory Phenotype in Mice Deficient in Fibulin-2 and ADAMTS-12. International Journal of Molecular Sciences. 2024; 25(4):2024. https://doi.org/10.3390/ijms25042024
Chicago/Turabian StyleMohamedi, Yamina, Tania Fontanil, José A. Vega, Teresa Cobo, Santiago Cal, and Álvaro J. Obaya. 2024. "Lung Inflammatory Phenotype in Mice Deficient in Fibulin-2 and ADAMTS-12" International Journal of Molecular Sciences 25, no. 4: 2024. https://doi.org/10.3390/ijms25042024
APA StyleMohamedi, Y., Fontanil, T., Vega, J. A., Cobo, T., Cal, S., & Obaya, Á. J. (2024). Lung Inflammatory Phenotype in Mice Deficient in Fibulin-2 and ADAMTS-12. International Journal of Molecular Sciences, 25(4), 2024. https://doi.org/10.3390/ijms25042024







