Managing Colorectal Cancer from Ethology to Interdisciplinary Treatment: The Gains and Challenges of Modern Medicine
Abstract
1. Introduction
2. The State of Knowledge from Symptoms to Novel Molecular Biology Achievements
2.1. Incidence of Colorectal Cancer and Its Geographic Variations
2.2. Temporal Trends in Colorectal Cancer Incidence, Mortality and Survival Rates
2.3. Symptoms of Colorectal Cancer
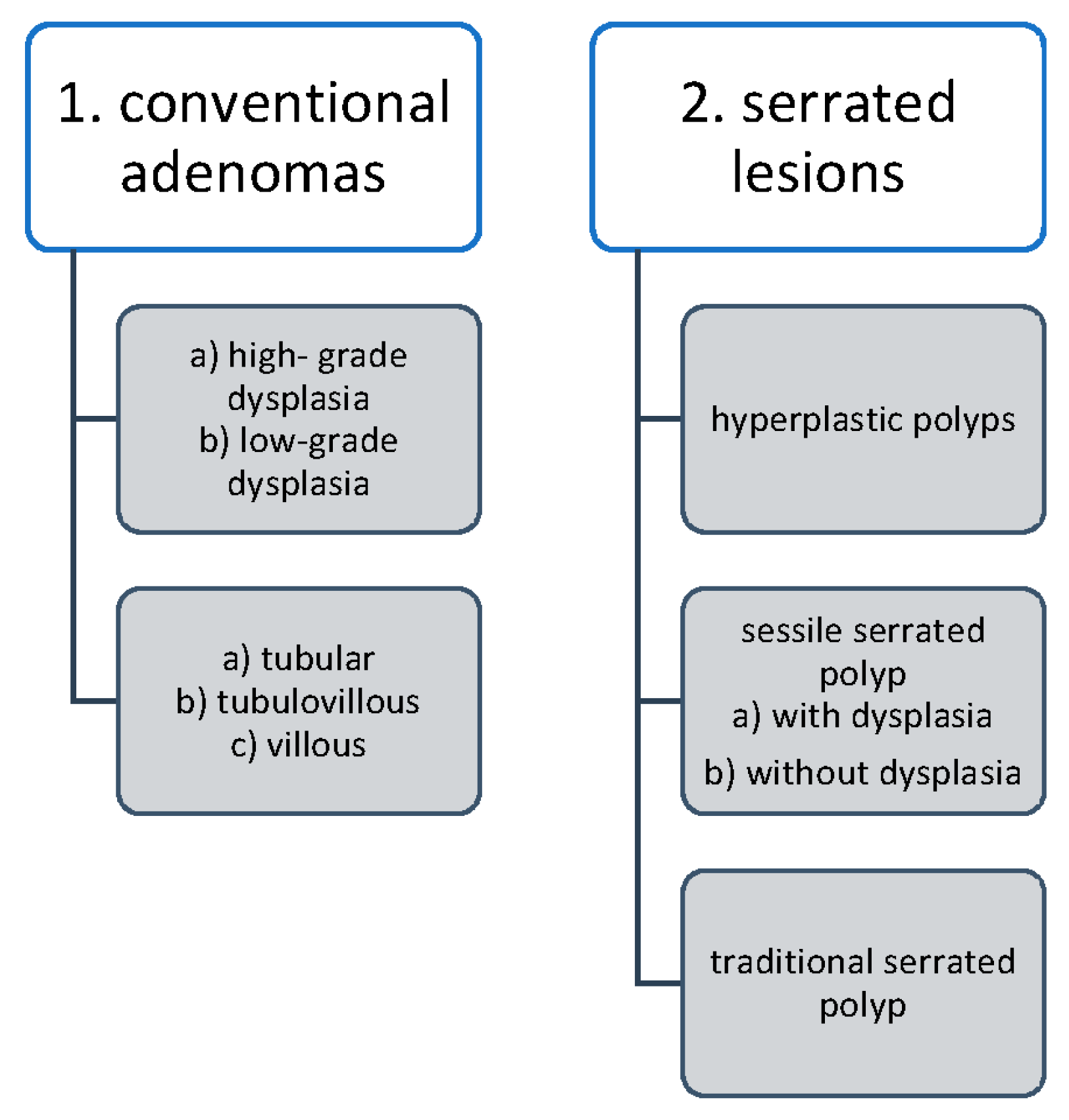
2.4. Pathological Evaluation of Colorectal Carcinogenesis
- (i)
- In 80% of cases, mutations of the APC suppressor gene are inactivated;
- (ii)
- In 15–20% of cases, there are DNA-repair gene mutations (e.g., MLH-1 promoter hypermethylation);
- (iii)
2.5. Epigenetics of CRC
- ❖
- Chromosomal instability is known as the adenoma–carcinoma pathway. Changes include the activation of proto-oncogenes (K-Ras) and the inactivation of three tumor-suppression genes: the loss of APC (chromosome region 5q21; the most common initial gene mutated in familial/inherited and sporadic colon cancer), the loss of p53 (chromosome region 17p13), and loss of heterozygosity for the long arm of chromosome 18 [18,28].
- ❖
- The 18q Loss of Heterozygosity (LOH) is defined as the loss of one of the two copies or alleles of a gene; it is frequently found in the region of 18q21 in advanced CRC. The DCC (Deleted in Colorectal Carcinoma) gene is located on the long arm of chromosome 18 and encodes the transmembrane protein DCC which is called the “conditional tumor suppressor gene”. When the DCC gene is mutated, netrin-1 (ligand produced in the crypts of the colorectal mucosa) does not bind to the DCC transmembrane protein, resulting in abnormal cell survival (apoptosis disorders). DCC loss is related to a worse overall survival [28]. The prevalence of the allele loss of DCC in CRC is reported to range from 33% to 75%. LOH or a decreased DCC expression is associated with CRC liver and lymph node metastasis [29].
- ❖
- Epigenetic Instability and CpG Methylation refer to the covalent attachment of a methyl group to the five positions of cytosine within a CpG dinucleotide in DNA. These methylation reactions are catalyzed by DNA methyltransferases (DNMTs). The regulation via inappropriate methylation of gene promoter regions is common in CRC and as significant as DNA mutations in inactivating tumor suppressor genes. The CIMP–CRC subtype (the result of tumor suppressor genes switch off, CpG island methylator phenotype) is associated with a high frequency of CpGhypermethylation, and is diagnosed based on the methylation status of various genes that participate in the regulation of calcium transport (CACNA1G), proliferation (IGF2), Wnt signaling (NEUROG1), transcription activity (RUNX3), and the suppression of cytokine signaling [28,34].
- ❖
- The TP53 mutation occurs in the transition from adenoma to carcinoma. The tumor suppressor gene p53 is mutated in almost half of all colon cancers. The TP53 gene is involved in the control of the cell cycle and apoptosis (the loss of p53 in colon cancers impacts the cell’s ability to both monitor centrosome integrity and regulate its duplication). Inherited or germline mutations in TP53 are the cause of the Li-Fraumeni syndrome, a syndrome associated with a variety of neoplasms, including soft tissue sarcoma, osteosarcoma, premenopausal breast cancer, brain tumors, and adrenocortical carcinoma. There are investigations that the TP53 gene mutation is present in node-positive CRC patients, and 5-year disease-free survival is almost double the shortness in length (35%) as TP53-negative patients (65%) [28,35].
- ❖
- Microsatellite Instability (MSI) and Mismatch Repair (MMR) Pathways. In total, 12–17% of all CRCs have microsatellite instability, which is the system that checks and repairs the defects overlooked by DNA polymerase during DNA replication. MSI is crucial for Lynch syndrome (Hereditary Non-Polyposis Colorectal Cancer [HNPCC]) and is seen in more than 95% of cases. By contrast, for most cases of sporadic CRC, the mechanisms responsible for chromosomal instability remain elusive, and MSI is responsible for only 15–20% of the cases. MSI tumors typically occur in the proximal colon and display Crohn’s disease-like lymphocytic infiltration. MSI-H tumors are frequently poorly differentiated, but this feature is not considered to be a high-risk category. Patients with MSI-associated CRC are usually younger than 50 years old, and their survival is better than patients with other types of chromosomal alterations [28].The MMR enzymes correct errors that are missed by the proofreading function of DNA polymerase and act as an additional system to preserve genomic integrity. The loss of MLH1 expression potentiates replication errors in microsatellite sequences. Patients with the germline loss of DNA mismatch repair capability typically develop CRC by the age of 40 in 80% of cases [28].
- ❖
- Receptor X (FXR) is a nuclear transcription factor that has been recognized as a tumor suppressor protein in the intestinal mucosa. Compared to healthy colon tissue, the FXR function and expression were decreased in polyps and precancerous lesions, whereas its expression was silenced mostly at later tumor stages (I–IV) [28].
- ❖
- Histone posttranslational modifications (e.g., methylation, acetylation, ubiquitination, phosphorylation) were observed in the context of CRC. The methylation of H3K4, H3K36, and H3K79 is linked to gene expression activation, whereas H3K9me2, H3K9me3, H3K27me3, and H4K20 are associated with gene repression. Wnt family member 5A (Wnt5a) was found to be downregulated in metastatic CRC [33]. The histone trimethylation at H3K4, H3K9, and H4K20 is also associated with better prognosis in early-stage CRC. Butyrate, widely known as a histone deacetylase (HDAC) inhibitor in HT-29 colon cancer cells, prevents the activation of COX-2 transcription [33,34].
- ❖
- Non-Coding RNAs refer to RNA transcripts that are not translated into proteins and are usually classified into the following two groups: (1) short ncRNA (<30 nucleotides) include microRNAs (miRNAs), siRNAs, and piwi-interacting RNAs; (2) long ncRNA (>300 nucleotides) include the long intergenic ncRNA which targets specific loci to regulate expression. The best-classified miRs negatively regulate gene expression at the posttranscriptional level (the loss of miR133a and gain of miR224 are associated with CRC tumorigenesis). Aberrant miR activity has been reported in the traditional “adenoma-carcinoma” and “serrated” models of CRC [34].
- ❖
- Microbiome and CRC. Known risk factors in CRC, such as dietary habits and lifestyle, can modulate the intestine microbiome. Recent investigations suggest that the gut microbiome could be an important factor in both the prevention and etiology of CRC. The Mediterranean diet [33] was associated with changes in the host-microbiome, including increased short-chain fatty acid production and the abundance of Prevotella and fiber-degrading Firmicutes. There is a proven association between red meat consumption and the enrichment of Bacteroidesmassiliensis, Alistipesfinegoldii, and Bilophilawadsworthia bacteria, which may implicate CRC’s etiology [36]. The risk factor for CRC development could be a severe Salmonella infection [37]. Among the Dutch population, an increased risk of CRC in the ascending and transverse parts of the colon in patients with a reported history of Salmonella infection was observed (strongly related to infection with Salmonella enteritidis).
3. Risk Factors for Colorectal Cancer
3.1. Colorectal Cancer Non-Modifiable Risk Factors
3.2. Environmental Risk Factors for Colorectal Cancer
3.3. Inherited Genetic Risk for Colorectal Cancer
- (1)
- Three or more relatives (one of whom is a first-degree relative) with CRC or with HNPCC-s associated cancers (endometrial carcinoma, small bowel adenocarcinoma, ureter or renal pelvis carcinoma);
- (2)
- Two successive generations affected;
- (3)
- FAP excluded;
- (4)
- Tumors confirmed in histology;
- (5)
- One or more HNPCC-related cancers diagnosed before the age of 50 years.
3.4. COX Role in Colorectal Cancer Carcinogenesis
4. Colorectal Cancer Treatment—A Multidisciplinary Approach
4.1. Colorectal Cancer Surgery
- *
- CRC without distant metastases—the resection of the appropriate part of the intestine together with the regional lymph nodes (en-block), depending on tumor localization and intestinal vascularization (segmental resection, hemicolectomy, dilated hemicolectomy, colectomy). Colon segment resection with the maximal length of axial mesentery vessels (containing regional lymph nodes) should be a performer in fascial anatomical spaces known as complete mesocolic excision (CME). It has to evaluate a minimum of 12 lymph nodes—all suspected lymph nodes are also removed.
- *
- CRC with the presence of synchronous resectable liver/lung metastases—the excision of the segmental intestine with the simultaneous/subsequent resection of liver/lung lesions;
- *
- CRC with the presence of synchronous unresectable liver/lung metastases (possible to resect during CTH)—the treatment starts with inductive CTH after 2–3 months of evaluating the possibility of radical surgery performance. Before the beginning of CTH, it is necessary to undergo surgery for a primary tumor if it threatens intestinal stenosis or significant bleeding;
- *
4.2. The Length of the Gut Resection Margins
4.3. Lymph Node Removal in CRC Surgery
4.4. Complete Removal of Mesorectum (TME)
4.5. Colorectal Cancer Systemic Treatment
- (i)
- Short-course radiotherapy: 25 Gy, 5 Gy/fraction FOR 1 week followed by immediate surgery (TME < 10 days from the first radiation fraction); Europe.
- (ii)
- Long-course chemoradiotherapy: 45–50 Gy, 1.8–2 Gy/fraction without or with 5-Fluorouracil (5-FU; bolus injections with leucovorin at 6–10 times during the radiation or continuous infusion or oral capecitabine), followed by radical surgery 6–8 weeks later; United States, Canada.
5. Contemporary Diagnostics and Screening Methods—Guidelines for Colorectal Cancer
5.1. Screening for Colorectal Cancer
5.1.1. Colonoscopy
5.1.2. Fecal Immunochemical Tests (FITs)
5.1.3. FIT-Fecal DNA Test
5.1.4. CT Colonography (Virtual Colonoscopy)
5.1.5. Flexible Sigmoidoscopy (FS)
5.1.6. Colon Capsule Colonoscopy (CCE)
5.2. Per Rectum Examination
5.3. Blood Enzymes Testing
5.4. Recommendations for CRC Screening
6. The Role of General Practitioner and Prophylaxis in Colorectal Cancer Management
7. Conclusions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.D.; Fontham, E.T.H.; Church, T.R.; Flowers, C.R.; Guerra, C.E.; LaMonte, S.J.; Etzioni, R.; McKenna, M.T.; Oeffinger, K.C.; Shih, Y.-C.T.; et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J. Clin. 2018, 68, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.A.; Noujaim, M.; Roper, J. Cause, Epidemiology, and Histology of Polyps and Pathways to Colorectal Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef]
- Mitchell, E.P. Risk Trends in Colorectal Cancer. J. Natl. Med. Assoc. 2020, 112, 445. [Google Scholar] [CrossRef]
- Ellis, L.; Canchola, A.J.; Spiegel, D.; Ladabaum, U.; Haile, R.; Gomez, S.L. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. J. Clin. Oncol. 2018, 36, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Vuik, F.E.; Nieuwenburg, S.A.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, A.; Nakamura, K.; Ukawa, S.; Okada, E.; Hirata, M.; Nagai, A.; Matsuda, K.; Kamatani, Y.; Muto, K.; Kiyohara, Y.; et al. Characteristics and prognosis of Japanese colorectal cancer patients: The BioBank Japan Project. J. Epidemiol. 2017, 27, S36–S42. [Google Scholar] [CrossRef]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, A.B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. JNCI J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef]
- Mahmoud, N.N. Colorectal Cancer: Preoperative Evaluation and Staging. Surg. Oncol. Clin. N. Am. 2022, 31, 127–141. [Google Scholar] [CrossRef]
- Schmoll, H.J.; Van Cutsem, E.; Stein, A.; Valentini, V.; Glimelius, B.; Haustermans, K.; Nordlinger, B.; van de Velde, C.J.; Balmana, J.; Regula, J.; et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann. Oncol. 2012, 23, 2479–2516. [Google Scholar] [CrossRef]
- Böckelman, C.; Engelmann, B.E.; Kaprio, T.; Hansen, T.F.; Glimelius, B. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol. 2014, 54, 5–16. [Google Scholar] [CrossRef]
- Lawler, M.; Alsina, D.; Adams, R.A.; Anderson, A.S.; Brown, G.; Fearnhead, N.S.; Fenwick, S.W.; Halloran, S.P.; Hochhauser, D.; Hull, M.A.; et al. Critical research gaps and recommendations to inform research prioritisation for more effective prevention and improved outcomes in colorectal cancer. Gut 2017, 67, 179–193. [Google Scholar] [CrossRef]
- Guglielmo, A.; Staropoli, N.; Giancotti, M.; Mauro, M. Personalized medicine in colorectal cancer diagnosis and treatment: A systematic review of health economic evaluations. Cost Eff. Resour. Alloc. 2018, 16, 2. [Google Scholar] [CrossRef]
- Holtedahl, K.; Borgquist, L.; Donker, G.A.; Buntinx, F.; Weller, D.; Campbell, C.; Månsson, J.; Hammersley, V.; Braaten, T.; Parajuli, R. Symptoms and signs of colorectal cancer, with differences between proximal and distal colon cancer: A prospective cohort study of diagnostic accuracy in primary care. BMC Fam. Pract. 2021, 22, 148. [Google Scholar] [CrossRef]
- Vega, P.; Valentín, F.; Cubiella, J. Colorectal cancer diagnosis: Pitfalls and opportunities. World J. Gastrointest. Oncol. 2015, 7, 422–433. [Google Scholar] [CrossRef]
- Hamilton, W.; Sharp, D. Diagnosis of colorectal cancer in primary care: The evidence base for guidelines. Fam. Pract. 2004, 21, 99–106. [Google Scholar] [CrossRef]
- Dunne, J.R.; Gannon, C.J.; Osborn, T.M.; Taylor, M.D.; Malone, D.L.; Napolitano, L.M. Preoperative anemia in colon cancer: Assessment of risk factors. Am. Surg. 2002, 68, 582–587. [Google Scholar] [CrossRef]
- Cai, J.; Yuan, Z.; Zhang, S. Abdominal pain, diarrhea, constipation--which symptom is more indispensable to have a colonoscopy? Int. J. Clin. Exp. Pathol. 2015, 8, 938–942. [Google Scholar]
- Chardalias, L.; Papaconstantinou, I.; Gklavas, A.; Politou, M.; Theodosopoulos, T. Iron Deficiency Anemia in Colorectal Cancer Patients: Is Preoperative Intravenous Iron Infusion Indicated? A Narrative Review of the Literature. Cancer Diagn. Progn. 2023, 3, 163–168. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Coupland, C. Symptoms and risk factors to identify men with suspected cancer in primary care: Derivation and validation of an algorithm. Br. J. Gen. Pract. 2013, 63, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, H.; Jia, H.; Huang, D.; Gu, W.; Cai, S.; Zhu, J. Prognosis of three histological subtypes of colorectal adenocarcinoma: A retrospective analysis of 8005 Chinese patients. Cancer Med. 2019, 8, 3411–3419. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, C.R.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017, 153, 307–323. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, D.A.; Rex, D.K.; Winawer, S.J.; Giardiello, F.M.; Johnson, D.A.; Levin, T.R. Guidelines for Colonoscopy Surveillance after Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012, 143, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Chadda, S.D.; Zhao, Z.; Barber, B.L.; Sykes, D.P. A systematic review of treatment guidelines for metastatic colorectal cancer. Color. Dis. 2011, 14, e31–e47. [Google Scholar] [CrossRef][Green Version]
- Armaghany, T.; Wilson, J.D.; Chu, Q.; Mills, G. Genetic Alterations in Colorectal Cancer. Gastrointest. Cancer Res. 2012, 5, 19–27. [Google Scholar]
- Chang, S.-C.; Lin, J.-K.; Lin, T.-C.; Liang, W.-Y. Loss of heterozygosity: An independent prognostic factor of colorectal cancer. World J. Gastroenterol. 2005, 11, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Recillas-Targa, F. Cancer Epigenetics: An Overview. Arch. Med. Res. 2022, 53, 732–740. [Google Scholar] [CrossRef]
- Lugli, A. Towards a Molecular Classification of Colorectal Cancer. Front. Oncol. 2015, 5, 46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Donovan, M.G.; Selmin, O.I.; Doetschman, T.C.; Romagnolo, D.F. Mediterranean Diet: Prevention of Colorectal Cancer. Front. Nutr. 2017, 4, 59. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, M. Epigenetic changes in colorectal cancer. Chin. J. Cancer 2013, 32, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.E.; Bleiler, M.; Giardina, C. A look into centrosome abnormalities in colon cancer cells, how they arise and how they might be targeted therapeutically. Biochem. Pharmacol. 2017, 147, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Saus, E.; Iraola-Guzmán, S.; Willis, J.R.; Brunet-Vega, A.; Gabaldón, T. Microbiome and colorectal cancer: Roles in carcinogenesis and clinical potential. Mol. Asp. Med. 2019, 69, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Mughini-Gras, L.; Schaapveld, M.; Kramers, J.; Mooij, S.; Neefjes-Borst, E.A.; van Pelt, W.; Neefjes, J. Increased colon cancer risk after severe Salmonella infection. PLoS ONE 2018, 13, e0189721. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.A.E.; Simons, C.C.J.M.; van Den Brandt, P.A.; van Engeland, M.; Weijenberg, M.P. Lifestyle, Diet, and Colorectal Cancer Risk According to (Epi)genetic Instability: Current Evidence and Future Directions of Molecular Pathological Epidemiology. Curr. Color. Cancer Rep. 2017, 13, 455–469. [Google Scholar] [CrossRef]
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk Factors for Colorectal Polyps and Cancer. Gastrointest. Endosc. Clin. N. Am. 2022, 32, 195–213. [Google Scholar] [CrossRef]
- Weigl, K.; Chang-Claude, J.; Knebel, P.; Hsu, L.; Hoffmeister, M.; Brenner, H. Strongly enhanced colorectal cancer risk stratification by combining family history and genetic risk score. Clin. Epidemiol. 2018, 10, 143–152. [Google Scholar] [CrossRef]
- Johnson, C.M.; Wei, C.; Ensor, J.E.; Smolenski, D.J.; Amos, C.I.; Levin, B.; Berry, D.A. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013, 24, 1207–1222. [Google Scholar] [CrossRef] [PubMed]
- Sievers, C.K.; Grady, W.M.; Halberg, R.B.; Pickhardt, P.J. New insights into the earliest stages of colorectal tumorigenesis. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 723–729. [Google Scholar] [CrossRef]
- Haggar, F.A.; Boushey, R.P. Colorectal Cancer Epidemiology: Incidence, Mortality, Survival, and Risk Factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Rudzki, G.; Lewandowski, T.; Stryjkowska-Góra, A.; Rudzki, S. Risk Factors for the Diagnosis of Colorectal Cancer. Cancer Control 2022, 29, 1–15. [Google Scholar] [CrossRef]
- Giardiello, F.M.; Allen, J.I.; Axilbund, J.E.; Boland, C.R.; Burke, C.A.; Burt, R.W.; Church, J.M.; Dominitz, J.A.; Johnson, D.A.; Kaltenbach, T.; et al. Guidelines on Genetic Evaluation and Management of Lynch Syndrome: A Consensus Statement by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2014, 147, 502–526. [Google Scholar] [CrossRef]
- Bouras, E.; Kim, A.E.; Lin, Y.; Morrison, J.; Du, M.; Albanes, D.; Barry, E.L.; Baurley, J.W.; Berndt, S.I.; Bien, S.A.; et al. Genome-wide interaction analysis of folate for colorectal cancer risk. Am. J. Clin. Nutr. 2023, 118, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Haas, C.B.; Su, Y.-R.; Petersen, P.; Wang, X.; Bien, S.A.; Lin, Y.; Albanes, D.; Weinstein, S.J.; Jenkins, M.A.; Figueiredo, J.C.; et al. Interactions between folate intake and genetic predictors of gene expression levels associated with colorectal cancer risk. Sci. Rep. 2022, 12, 18852. [Google Scholar] [CrossRef]
- DeDecker, L.; Coppedge, B.; Avelar-Barragan, J.; Karnes, W.; Whiteson, K. Microbiome distinctions between the CRC carcinogenic pathways. Gut Microbes 2021, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rebersek, M. Gut microbiome and its role in colorectal cancer. BMC Cancer 2021, 21, 1325. [Google Scholar] [CrossRef]
- Mölzer, C.; Wilson, H.M.; Kuffova, L.; Forrester, J.V. A Role for Folate in Microbiome-Linked Control of Autoimmunity. J. Immunol. Res. 2021, 2021, 9998200. [Google Scholar] [CrossRef]
- Müller, M.F.; Ibrahim, A.E.K.; Arends, M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016, 469, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Brosens, L.A.A.; Offerhaus, G.J.A.; Giardiello, F.M.; De Leng, W.W.J.; Montgomery, E.A. Pathology and genetics of hereditary colorectal cancer. Pathology 2018, 50, 49–59. [Google Scholar] [CrossRef]
- Lindor, N.M. Familial Colorectal Cancer Type X: The Other Half of Hereditary Nonpolyposis Colon Cancer Syndrome. Surg. Oncol. Clin. N. Am. 2009, 18, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Al-Sukhni, W.; Aronson, M.; Gallinger, S. Hereditary Colorectal Cancer Syndromes: Familial Adenomatous Polyposis and Lynch Syndrome. Surg. Clin. N. Am. 2008, 88, 819–844. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, F.; Leoz, M.; Carballal, S.; Moreira, L.; Ocaña, T. The genetic basis of familial adenomatous polyposis and its implications for clinical practice and risk management. Appl. Clin. Genet. 2015, 2015, 95–107. [Google Scholar] [CrossRef]
- Poole, E.M.; Bigler, J.; Whitton, J.; Sibert, J.G.; Potter, J.D.; Ulrich, C.M. Prostacyclin Synthase and Arachidonate 5-Lipoxygenase Polymorphisms and Risk of Colorectal Polyps. Cancer Epidemiol. Biomark. Prev. 2006, 15, 502–508. [Google Scholar] [CrossRef][Green Version]
- Sheng, J.; Sun, H.; Yu, F.-B.; Li, B.; Zhang, Y.; Zhu, Y.-T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef]
- Esquivel, J. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer: Survival outcomes and patient selection. J. Gastrointest. Oncol. 2016, 7, 72–78. [Google Scholar] [CrossRef]
- Rutkowski, A. Surgical treatment of colorectal cancer in Poland. Clin. Gastroenterol. 2013, 5, 152–161. [Google Scholar]
- Kkrzakowski, M. Clinical Oncology Volume II. In Gastrointestinal Cancers; Via Medica: Gdańsk, Poland, 2015; pp. 611–642. [Google Scholar]
- Kozłowska, M.; Głuszek, S. Contemporary methods of treatment of colorectal cancer. Med. Stud. 2015, 4, 307–312. [Google Scholar] [CrossRef]
- Bujko, K.; Rutkowski, A.; Chang, G.J.; Michalski, W.; Chmielik, E.; Kusnierz, J. Is the 1-cm Rule of Distal Bowel Resection Margin in Rectal Cancer Based on Clinical Evidence? A Systematic Review. Indian J. Surg. Oncol. 2012, 3, 139–146. [Google Scholar] [CrossRef]
- Sato, S.; Kato, T.; Tanaka, J.-I. Defining the distal margin of rectal cancer for surgical planning. J. Gastrointest. Oncol. 2017, 8, 194–198. [Google Scholar] [CrossRef]
- Wolpin, B.M.; Mayer, R.J. Systemic Treatment of Colorectal Cancer. Gastroenterology 2008, 134, 1296–1310.e1. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Nordlinger, B.; Cervantes, A. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann. Oncol. 2010, 21, v93–v97. [Google Scholar] [CrossRef]
- Xynos, E.; Gouvas, N.; Triantopoulou, C.; Tekkis, P.; Vini, L.; Tzardi, M.; Boukovinas, I.; Androulakis, N.; Athanasiadis, A.; Christodoulou, C.; et al. Clinical practice guidelines for the surgical management of colon cancer: A consensus statement of the Hel-lenic and Cypriot Colorectal Cancer Study Group by the HeSMO. Ann. Gastroenterol. 2016, 29, 3–17. [Google Scholar] [PubMed]
- Bevan, R.; Rutter, M.D. Colorectal Cancer Screening—Who, How, and When? Clin. Endosc. 2018, 51, 37–49. [Google Scholar] [CrossRef]
- Świderska, M.; Choromańska, B.; Dąbrowska, E.; Konarzewska-Duchnowska, E.; Choromańska, K.; Szczurko, G.; Myśliwiec, P.; Dadan, J.; Ładny, J.R.; Zwierz, K. Review The diagnostics of colorectal cancer. Contemp. Oncol. 2014, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Issa, I.A.; Noureddine, M. Colorectal cancer screening: An updated review of the available options. World J. Gastroenterol. 2017, 23, 5086–5096. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef]
- Hanoglu, S.B.; Man, E.; Harmanci, D.; Ruzgar, S.T.; Sanli, S.; Keles, N.A.; Ayden, A.; Tuna, B.G.; Duzgun, O.; Ozkan, O.F.; et al. Magnetic Nanoparticle-Based Electrochemical Sensing Platform Using Ferrocene-Labelled Peptide Nucleic Acid for the Early Diagnosis of Colorectal Cancer. Biosensors 2022, 12, 736. [Google Scholar] [CrossRef]
- Ma, Z.Y.; Law, W.L.; Ng, E.K.O.; Chan, C.S.Y.; Lau, K.S.; Cheng, Y.Y.; Shin, V.Y.; Kwong, A.; Leung, W.K. Methylated Septin 9 and Carcinoembryonic Antigen for Serological Diagnosis and Monitoring of Patients with Colorectal Cancer after Surgery. Sci. Rep. 2019, 9, 10326. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Lee, M.Y.; Park, J.H.; Park, D.I.; Sohn, C.I.; Choi, K.; Jung, Y.S. Serum CEA and CA 19-9 Levels are Associated with the Presence and Severity of Colorectal Neoplasia. Yonsei Med. J. 2017, 58, 918–924. [Google Scholar] [CrossRef]
- Navarro, M.; Nicolas, A.; Ferrandez, A.; Lanas, A. Colorectal cancer population screening programs worldwide in 2016: An update. World J. Gastroenterol. 2017, 23, 3632–3642. [Google Scholar] [CrossRef]
- Cheung, W.Y.; Neville, B.A.; Cameron, D.B.; Cook, E.F.; Earle, C.C. Comparisons of Patient and Physician Expectations for Cancer Survivorship Care. J. Clin. Oncol. 2009, 27, 2489–2495. [Google Scholar] [CrossRef]
- Perfors, I.A.A.; Helsper, C.W.; Noteboom, E.A.; van der Wall, E.; de Wit, N.J.; May, A.M. Randomised controlled trial protocol (GRIP study): Examining the effect of involvement of a general practitioner and home care oncology nurse after a cancer diagnosis on patient reported outcomes and healthcare utilization. BMC Cancer 2018, 18, 132. [Google Scholar] [CrossRef] [PubMed]
- Benton, S.C.; Butler, P.; Allen, K.; Chesters, M.; Rickard, S.; Stanley, S.; Roope, R.; Vulkan, D.; Duffy, S.W. GP participation in increasing uptake in a national bowel cancer screening programme: The PEARL project. Br. J. Cancer 2017, 116, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
| Right-Sided Colon Cancer | Left-Sided Colon Cancer | Rectal Cancer | Anal Cancer |
|---|---|---|---|
| non-characteristic, dull abdominal pain | flatulence, colic pain | pain in the crotch area | bleeding from the anus; itching; enlarged inguinal lymph nodes |
| the dark color of the stool; blood in the stool | fresh blood in the stool | fresh blood covering the stool; painful pressure on the stool | fresh blood in the stool/covering the stool; bleeding from the anal canal |
| iron-deficiency anemia | bowel movement disorder (constipation/diarrhea) | intestinal obstruction symptoms (vomiting, nausea) | incontinence of gases and stools |
| perceptible tumor | constipation; “pencil-shaped” stools | perceptible/visible tumor (per rectum examination) | perceptible/visible tumor (per rectum examination) |
| Family History | Screening Recommendations |
|---|---|
| Lynch Syndrome | Colonoscopy every 1–2 years beginning at age 20–25 years or 2–5 years younger than the youngest age at diagnosis of CRC in a family if diagnosed before age 25 years |
| Family Colon Cancer Syndrome X | Colonoscopy every 3–5 years beginning 10 years before the age at diagnosis of the youngest affected relative |
| CRC/advanced adenoma in 2 first-degree relatives diagnosed at any age OR CRC/advanced adenoma in a single first-degree relative at age < 60 years | Colonoscopy every 5 years beginning 10 years before the age at diagnosis of the youngest affected interval or age 40 (whichever is earlier) |
| CRC/advanced adenoma in a single first-degree relative diagnosed at age ≥ 60 years | Begin screening at the age of 40 (test and intervals as per the average-risk screening recommendations) |
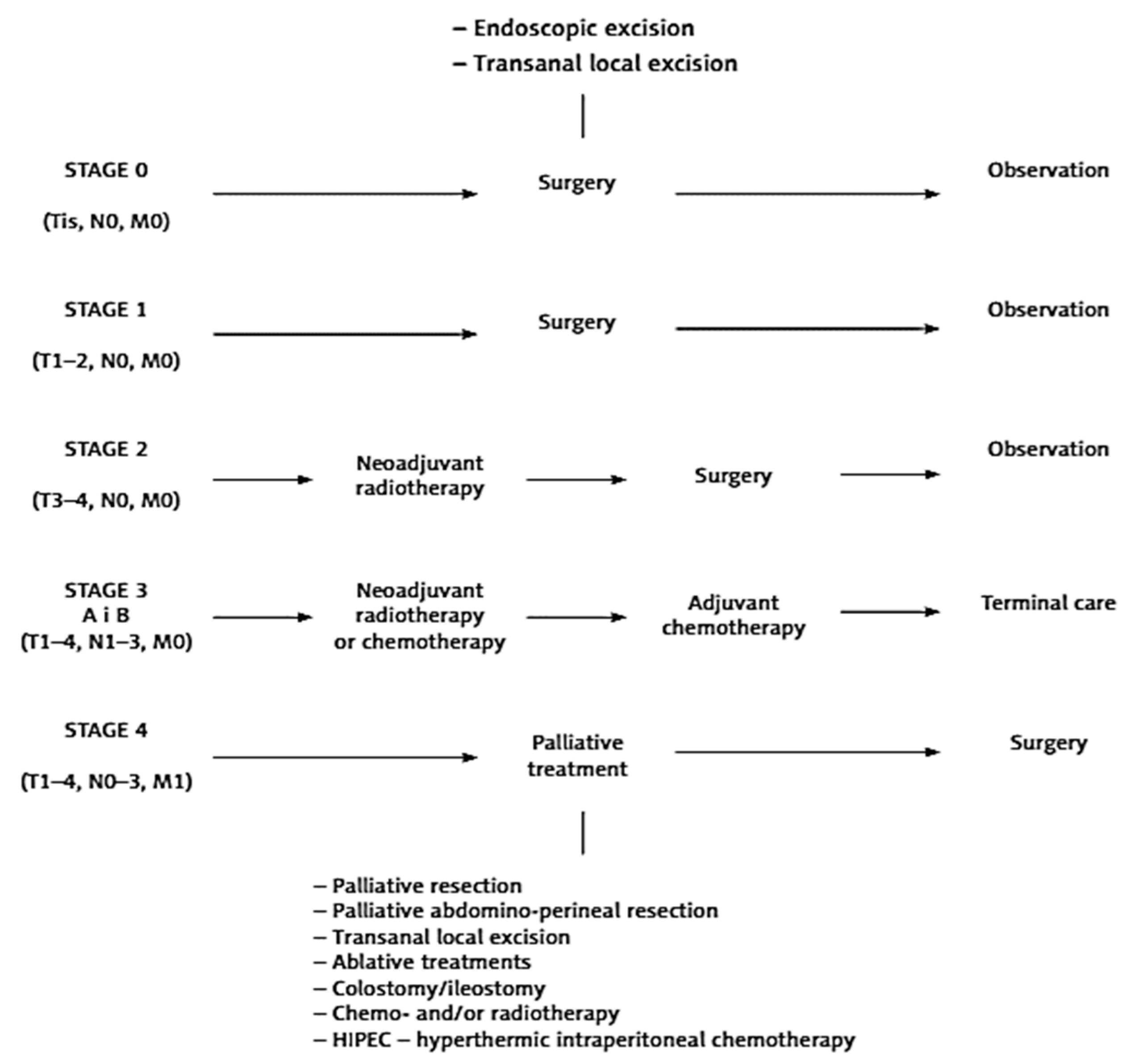
| Stage | Colon and Upper Part of the Rectum | Middle and Lower Part of the Rectum | Comment |
|---|---|---|---|
| Stage 0 (infiltration does not exceed the plaque muscular mucosa = high-grade dysplasia) | Endoscopy | Endoscopy | Surgical treatment in particular cases in which endoscopic treatment is not possible (too large a change, difficult location) |
| Stage I (cancer does not exceed full wall thickness of the intestine with lymph nodes free from cancer, without distant metastases) | Surgery | Surgery | In the case of low-located rectal cancer without infiltration sphincters can perform possible local excision from access via the anus (TEM, transanal endoscopic microsurgery) |
| Stage II (cancer crosses the intestinal wall and infiltrating adjacent organs; free lymph nodes with no distant metastases) | Surgery + CHTH (only in the case of bad prognostic factors (microvilli in blood vessels, lymphatic vessels or perineural vessels, low-grade tumor differentiation (G3), tumor perforation, or a low number (<12) of removed lymph nodes)) | RTH or RTH/CHTH+ surgery | Surgery—the range of resection depends on the location of the tumor. Colon cancer—possible laparoscopic treatment. |
| Stage III (regional lymph node metastases without distant metastases) | Surgery + CHTH | RTH or RTH/CHTH+ surgery | Surgery—the range of resection depends on the location of the tumor, Colon cancer—possible laparoscopic treatment. |
| Stage IV (distantmetastases) | CHIR or CHTH or RTH (depending on the cancer symptoms and patient’s general condition) | CHIR or CHTH or RTH (depending on the cancer symptoms and patient’s general condition) | Palliative surgery: resection of the primary tumor or in the case of bowel obstruction (stoma or bypassing). In certain cases, cytoreductive surgery combined with the HIPEC procedure. If possible, one should strive for the radical removal of the primary tumor and metastases. |
| Polish Recommendation for CRC Screening | |
|---|---|
| Patients without CRC family history | |
| Age ≥ 50 years | Standard screening protocol |
| Patients with CRC family history | |
| CRC in a single first-degree relative diagnosed at age ≥60 years | Standard screening protocol started at age 40 years |
| 2 or more first-degree relatives diagnosed with CRC at age ≥60 OR 1 first-degree relative diagnosed at age <60 years | Standard screening protocol started at age 40 years or 10 years before the diagnosis age of the youngest affect |
| Lynch Syndrome family history | Colonoscopy every 1–2 years beginning at age 20–25 years; colonoscopy with polyps removal every 1–2 years |
| FAP | Colonoscopy every 1–2 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berbecka, M.; Berbecki, M.; Gliwa, A.M.; Szewc, M.; Sitarz, R. Managing Colorectal Cancer from Ethology to Interdisciplinary Treatment: The Gains and Challenges of Modern Medicine. Int. J. Mol. Sci. 2024, 25, 2032. https://doi.org/10.3390/ijms25042032
Berbecka M, Berbecki M, Gliwa AM, Szewc M, Sitarz R. Managing Colorectal Cancer from Ethology to Interdisciplinary Treatment: The Gains and Challenges of Modern Medicine. International Journal of Molecular Sciences. 2024; 25(4):2032. https://doi.org/10.3390/ijms25042032
Chicago/Turabian StyleBerbecka, Monika, Maciej Berbecki, Anna Maria Gliwa, Monika Szewc, and Robert Sitarz. 2024. "Managing Colorectal Cancer from Ethology to Interdisciplinary Treatment: The Gains and Challenges of Modern Medicine" International Journal of Molecular Sciences 25, no. 4: 2032. https://doi.org/10.3390/ijms25042032
APA StyleBerbecka, M., Berbecki, M., Gliwa, A. M., Szewc, M., & Sitarz, R. (2024). Managing Colorectal Cancer from Ethology to Interdisciplinary Treatment: The Gains and Challenges of Modern Medicine. International Journal of Molecular Sciences, 25(4), 2032. https://doi.org/10.3390/ijms25042032







