The Immunoregulatory and Regenerative Potential of Activated Human Stem Cell Secretome Mitigates Acute-on-Chronic Liver Failure in a Rat Model
Abstract
1. Introduction
2. Results
2.1. MSC-Secretome Administration Increases Survival Rate and Attenuates ACLF Hepatic Injury
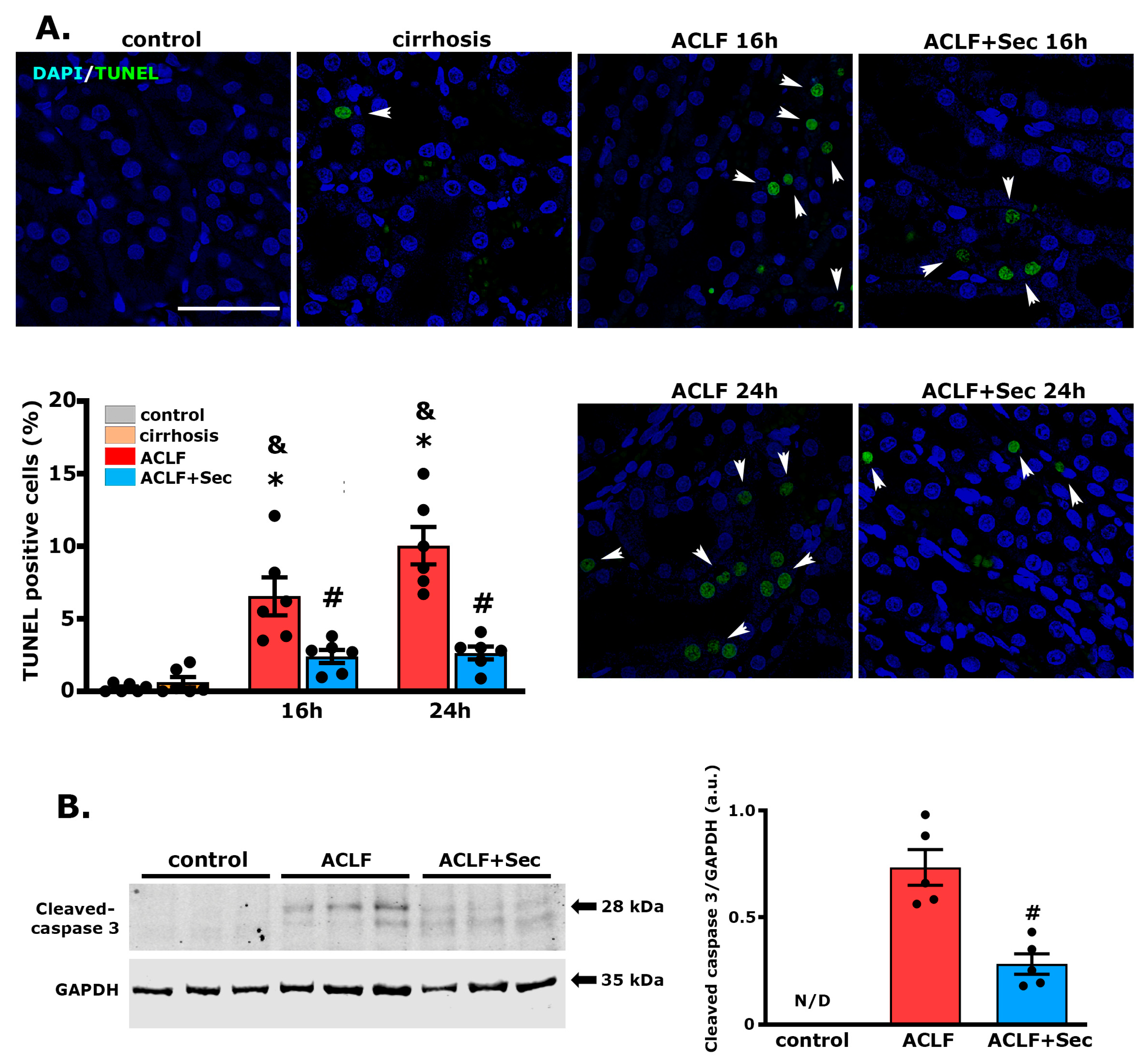
2.2. MSC-Secretome Administration Reduces the Apoptotic Rate and Induces Hepatocyte Proliferation after ACLF Induction
2.3. MSC-Secretome Administration Increases Anti-Inflammatory Cytokine Expression
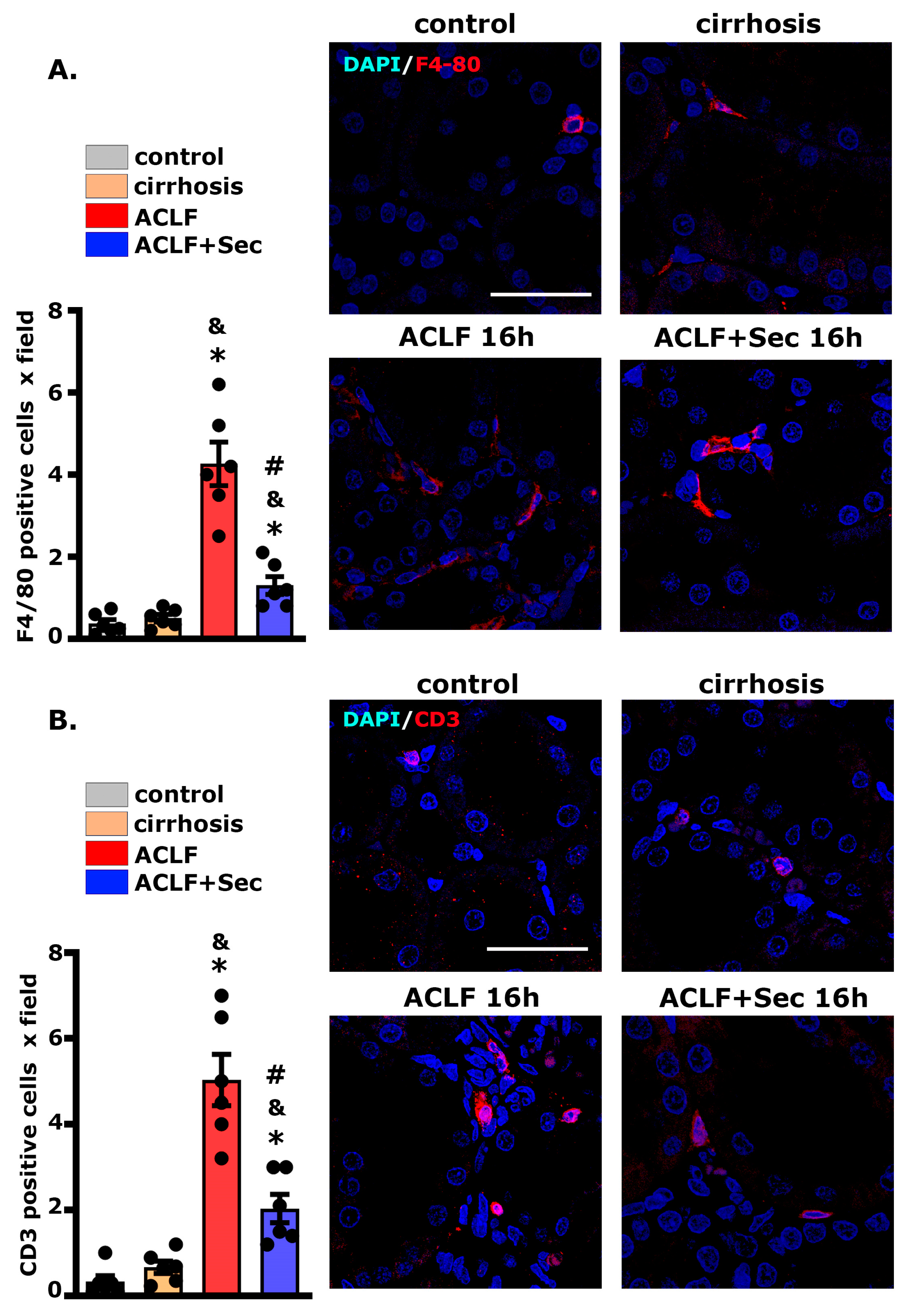
2.4. MSC-Secretome Administration Decreases Hepatic Neutrophil and Macrophage Infiltration
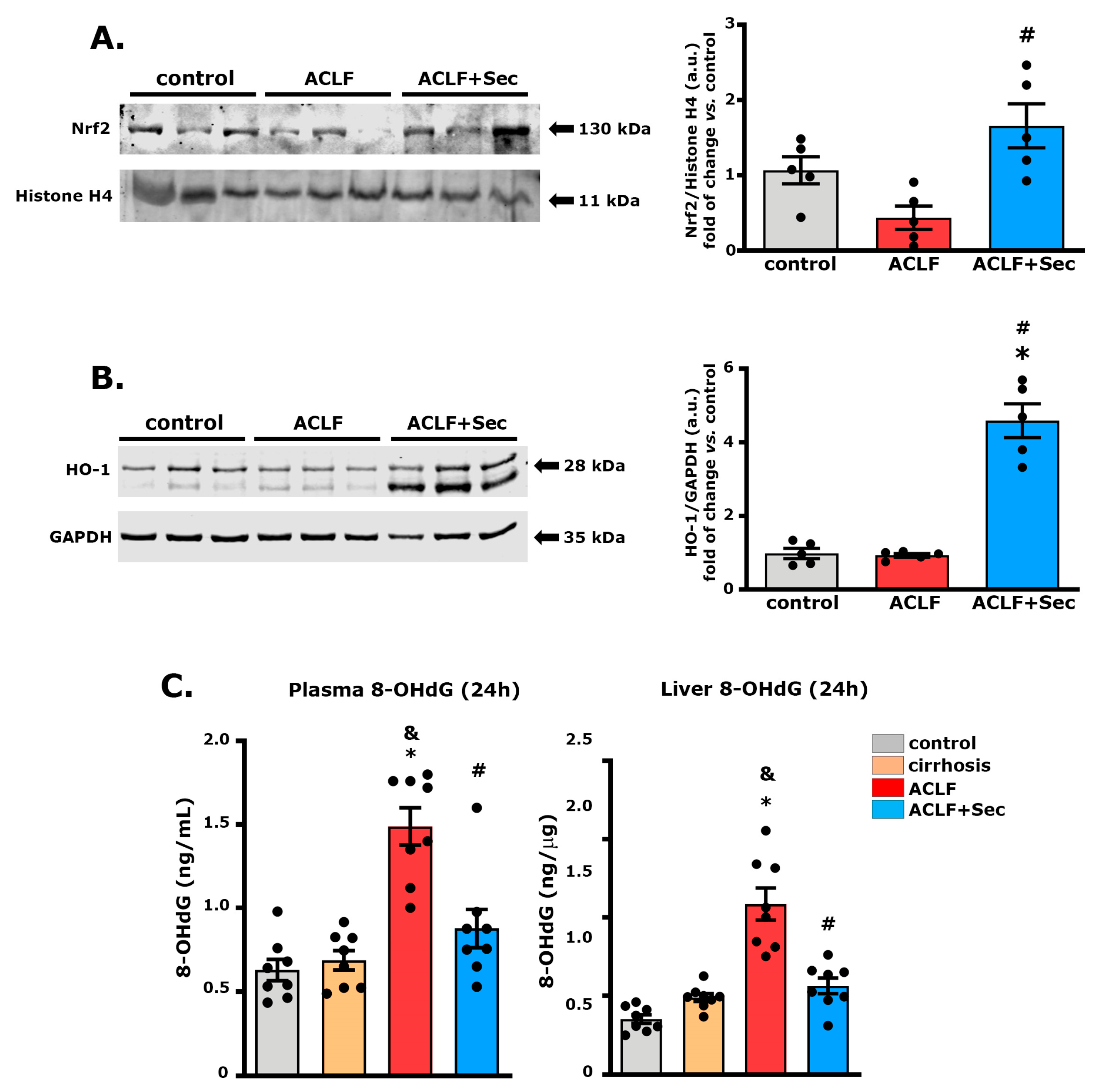
2.5. MSC-Secretome Administration Increases Hepatic Nrf2 and Heme Oxygenase Levels While Decreasing DNA Oxidative Damage
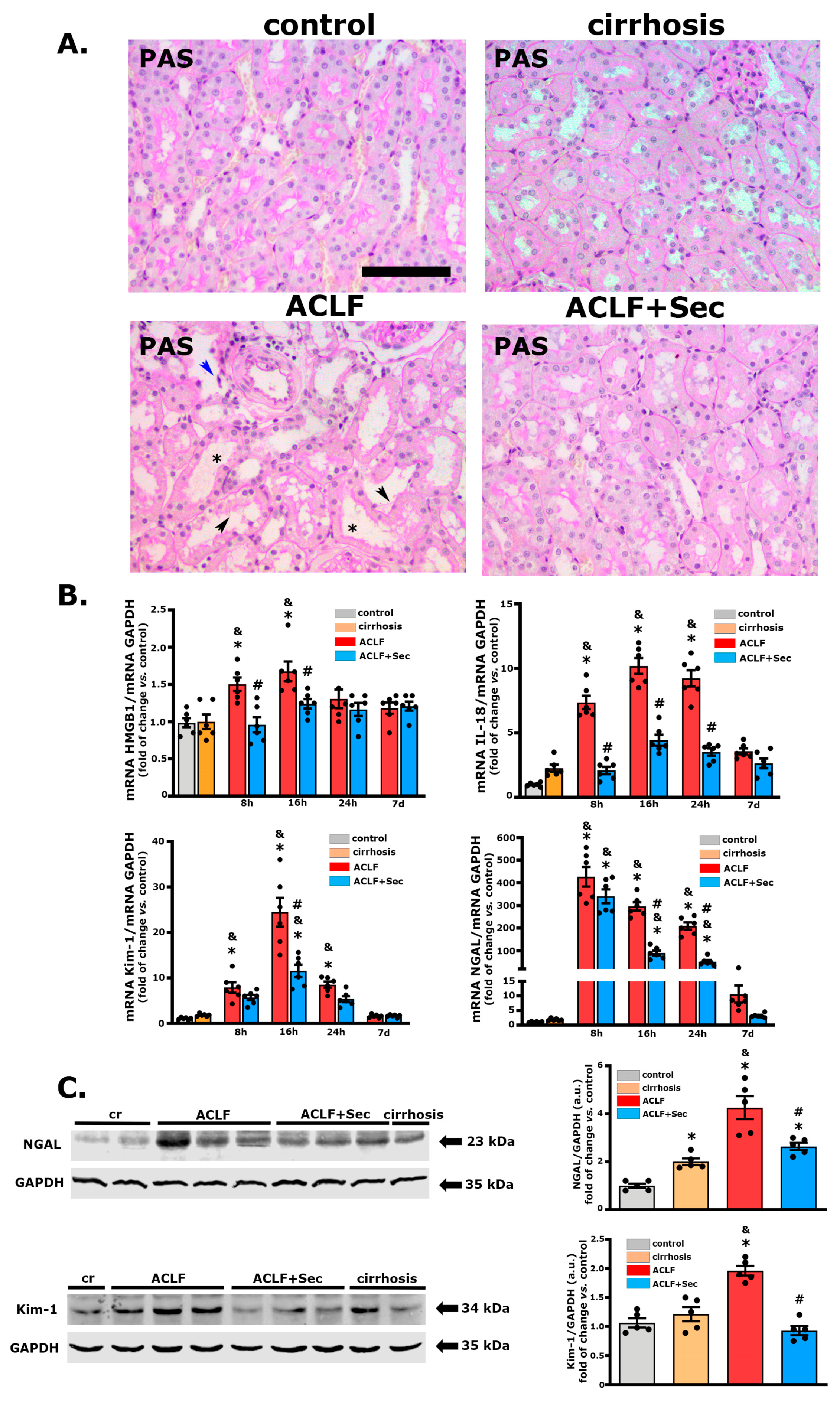
2.6. MSC-Secretome Administration Mitigates Acute Kidney Injury Associated with ACLF
2.7. MSC-Secretome Administration Reduces the Apoptotic Rate of Renal Cells after ACLF Induction
2.8. MSC-Secretome Administration Reduces Renal Macrophage and Lymphocyte Infiltration
3. Discussion
4. Materials and Methods
4.1. Isolation, Expansion, and Characterization of MSCs Derived from Human Adipose Tissue
4.2. MSC Preconditioning and Secretome Generation
4.3. Experimental Animals
4.4. Establishment of the ACLF Model
4.5. Survival Rate and Biochemical Indices
4.6. Hepatic and Renal Histology and Immunofluorescence Analysis
4.7. RNA Isolation and Gene Expression Analysis
4.8. DNA Oxidative Damage Measurement
4.9. Quantification of Systemic and Hepatic Cytokine Levels
4.10. Quantification of Biological Markers by Western Blotting
4.11. Isolation and Flow Cytometric Analysis of Leukocytes
4.12. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, J.; Li, J.; Li, P.; Liang, X.; Hassan, H.M.; Moreau, R.; Li, J. Acute-on-chronic liver failure: Far to go—A review. Crit. Care 2023, 27, 259. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437. [Google Scholar] [CrossRef]
- Sarin, S.K.; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; Devarbhavi, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2018, 67, 1870–1880. [Google Scholar] [CrossRef]
- Yang, L.; Wu, T.; Li, J.; Li, J. Bacterial Infections in Acute-on-Chronic Liver Failure. Semin. Liver Dis. 2018, 38, 121–133. [Google Scholar] [CrossRef]
- Bajaj, J.S.; O’Leary, J.G.; Reddy, K.R.; Wong, F.; Biggins, S.W.; Patton, H.; Fallon, M.B.; Garcia-Tsao, G.; Maliakkal, B.; Malik, R.; et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014, 60, 250–256. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Y.Y.; Chen, C.; Guan, J.; Zhu, H.H.; Chen, Z. The immunological roles in acute-on-chronic liver failure: An update. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 403–411. [Google Scholar] [CrossRef]
- Clària, J.; Arroyo, V.; Moreau, R. Roles of systemic inflammatory and metabolic responses in the pathophysiology of acute-on-chronic liver failure. JHEP Rep. 2023, 5, 100807. [Google Scholar] [CrossRef] [PubMed]
- Laleman, W.; Claria, J.; Van der Merwe, S.; Moreau, R.; Trebicka, J. Systemic Inflammation and Acute-on-Chronic Liver Failure: Too Much, Not Enough. Can. J. Gastroenterol. Hepatol. 2018, 2018, 1027152. [Google Scholar] [CrossRef] [PubMed]
- Zaccherini, G.; Weiss, E.; Moreau, R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment. JHEP Rep. 2021, 3, 100176. [Google Scholar] [CrossRef] [PubMed]
- Shubham, S.; Kumar, D.; Rooge, S.; Maras, J.S.; Maheshwari, D.; Nautiyal, N.; Kumari, R.; Bhat, A.; Kumar, G.; Rastogi, A.; et al. Cellular and functional loss of liver endothelial cells correlates with poor hepatocyte regeneration in acute-on-chronic liver failure. Hepatol. Int. 2019, 13, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Mehta, G.; Tacke, F. Regeneration in acute-on-chronic liver failure—The phantom lost its camouflage. J. Hepatol. 2020, 72, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Gustot, T.; Fernandez, J.; Garcia, E.; Morando, F.; Caraceni, P.; Alessandria, C.; Laleman, W.; Trebicka, J.; Elkrief, L.; Hopf, C.; et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015, 62, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Park, M.Y.; Kim, S.G. Acute kidney injury in patients with acute-on-chronic liver failure: Clinical significance and management. Kidney Res. Clin. Pract. 2023, 42, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Finkenstedt, A.; Nachbaur, K.; Zoller, H.; Joannidis, M.; Pratschke, J.; Graziadei, I.W.; Vogel, W. Acute-on-chronic liver failure: Excellent outcomes after liver transplantation but high mortality on the wait list. Liver Transplant. 2013, 19, 879–886. [Google Scholar] [CrossRef]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Gwam, C.; Mohammed, N.; Ma, X. Stem cell secretome, regeneration, and clinical translation: A narrative review. Ann. Transl. Med. 2021, 9, 70. [Google Scholar] [CrossRef]
- Wu, M.C.; Meng, Q.H. Current understanding of mesenchymal stem cells in liver diseases. World J. Stem Cells 2021, 13, 1349–1359. [Google Scholar] [CrossRef]
- Liu, W.; Song, F.; Ren, L.; Guo, W.; Wang, T.; Feng, Y.; Tang, L.; Li, K. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J. Cell. Mol. Med. 2015, 19, 511–520. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; De Gregorio, C.; Silva, V.; Elorza, A.; Léniz, P.; Aliaga-Tobar, V.; Maracaja-Coutinho, V.; Budini, M.; Ezquer, F.; Ezquer, M. Administration of Secretome Derived from Human Mesenchymal Stem Cells Induces Hepatoprotective Effects in Models of Idiosyncratic Drug-Induced Liver Injury Caused by Amiodarone or Tamoxifen. Cells 2023, 12, 636. [Google Scholar] [CrossRef]
- Ryu, K.H.; Kim, S.Y.; Kim, Y.R.; Woo, S.Y.; Sung, S.H.; Kim, H.S.; Jung, S.C.; Jo, I.; Park, J.W. Tonsil-derived mesenchymal stem cells alleviate concanavalin A-induced acute liver injury. Exp. Cell Res. 2014, 326, 143–154. [Google Scholar] [CrossRef]
- Jang, Y.O.; Cho, M.Y.; Yun, C.O.; Baik, S.K.; Park, K.S.; Cha, S.K.; Chang, S.J.; Kim, M.Y.; Lim, Y.L.; Kwon, S.O. Effect of Function-Enhanced Mesenchymal Stem Cells Infected with Decorin-Expressing Adenovirus on Hepatic Fibrosis. Stem Cells Transl. Med. 2016, 5, 1247–1256. [Google Scholar] [CrossRef]
- Zagoura, D.S.; Roubelakis, M.G.; Bitsika, V.; Trohatou, O.; Pappa, K.I.; Kapelouzou, A.; Antsaklis, A.; Anagnou, N.P. Therapeutic potential of a distinct population of human amniotic fluid mesenchymal stem cells and their secreted molecules in mice with acute hepatic failure. Gut 2012, 61, 894–906. [Google Scholar] [CrossRef]
- Peng, L.; Xie, D.Y.; Lin, B.L.; Liu, J.; Zhu, H.P.; Xie, C.; Zheng, Y.B.; Gao, Z.L. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: Short-term and long-term outcomes. Hepatology 2011, 54, 820–828. [Google Scholar] [CrossRef]
- Khan, S.; Khan, R.S.; Newsome, P.N. Cell Therapy for Liver Disease: From Promise to Reality. Semin. Liver Dis. 2020, 40, 411–426. [Google Scholar] [CrossRef]
- Lin, B.L.; Chen, J.F.; Qiu, W.H.; Wang, K.; Xie, D.; Chen, X.; Liu, Q.; Peng, L.; Li, J.; Mei, Y.; et al. Allogeneic bone marrow–derived mesenchymal stromal cells for hepatitis B virus–related acute-on-chronic liver failure: A randomized controlled trial. Hepatology 2017, 66, 209–219. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, H.; Shi, M.; Xu, R.; Fu, J.; Lv, J.; Chen, L.; Lv, S.; Li, Y.; Yu, S.; et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J. Gastroenterol. Hepatol. 2012, 27 (Suppl. S2), 112–120. [Google Scholar] [CrossRef]
- Mohamadnejad, M.; Alimoghaddam, K.; Bagheri, M.; Ashrafi, M.; Abdollahzadeh, L.; Akhlaghpoor, S.; Bashtar, M.; Ghavamzadeh, A.; Malekzadeh, R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013, 33, 1490–1496. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Wu, X.; Xu, X.; Niu, J. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: A systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res. Ther. 2022, 13, 204. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Li, Y.Y.; Xu, R.N.; Meng, F.P.; Yu, S.J.; Fu, J.L.; Hu, J.H.; Li, J.X.; Wang, L.F.; Jin, L.; et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: A long-term follow-up analysis of the randomized controlled clinical trial. Hepatol. Int. 2021, 15, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J. Cell. Mol. Med. 2018, 22, 1428–1442. [Google Scholar] [CrossRef]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, H.; Cao, H.; Jia, Y.; Shu, X.; Cao, H.; Zhang, Y.; Yang, X. The impact of recipient age on the effects of umbilical cord mesenchymal stem cells on HBV-related acute-on-chronic liver failure and liver cirrhosis. Stem Cell Res. Ther. 2021, 12, 466. [Google Scholar]
- Zheng, Y.; Zhu, S.; Zheng, X.; Xu, W.; Li, X.; Li, J.; Gao, Z.; Xie, C.; Peng, L. Serum from Acute-on-chronic Liver Failure Patients May Affect Mesenchymal Stem Cells Transplantation by Impairing the Immunosuppressive Function of Cells. J. Clin. Transl. Hepatol. 2021, 9, 503. [Google Scholar] [CrossRef]
- Duijvestein, M.; Wildenberg, M.E.; Welling, M.M.; Vos, A.C.W.; Hennink, S.; Bosse, T.; Muller, E.S.d.J.; Roelofs, H.; Verspaget, H.W.; Fibbe, W.E.; et al. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011, 29, 1549–1558. [Google Scholar] [CrossRef]
- Schafer, R.; Spohn, G.; Baer, P.C. Mesenchymal Stem/Stromal Cells in Regenerative Medicine: Can Preconditioning Strategies Improve Therapeutic Efficacy? Transfus. Med. Hemother. 2016, 43, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Ezquer, F.; Huang, Y.L.; Ezquer, M. New Perspectives to Improve Mesenchymal Stem Cell Therapies for Drug-Induced Liver Injury. Int. J. Mol. Sci. 2022, 23, 2669. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Tsuchiya, A.; Terai, S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin. Mol. Hepatol. 2021, 27, 70–80. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Ezquer, F.; Quintanilla, M.E.; Morales, P.; Ezquer, M.; Lespay-Rebolledo, C.; Herrera-Marschitz, M.; Israel, Y. Activated mesenchymal stem cell administration inhibits chronic alcohol drinking and suppresses relapse-like drinking in high-alcohol drinker rats. Addict. Biol. 2019, 24, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Oses, C.; Olivares, B.; Ezquer, M.; Acosta, C.; Bosch, P.; Donoso, M.; Leniz, P.; Ezquer, F. Preconditioning of adipose tissue-derived mesenchymal stem cells with deferoxamine increases the production of pro-angiogenic, neuroprotective and anti-inflammatory factors: Potential application in the treatment of diabetic neuropathy. PLoS ONE 2017, 12, e0178011. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, J.; Patel, T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J. Gastroenterol. 2019, 54, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhao, L.; Li, L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res. Ther. 2019, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Temnov, A.; Rogov, K.; Zhalimov, V.; Igor, P.; Pekov, S.; Bader, A.; Sklifas, A.; Giri, S. The effect of a mesenchymal stem cell conditioned medium fraction on morphological characteristics of hepatocytes in acetaminophen-induced acute liver failure: A preliminary study. Hepatic Med. Évid. Res. 2019, 11, 89–96. [Google Scholar] [CrossRef]
- Driscoll, J.; Yan, I.K.; Patel, T. Development of a Lyophilized off-the-Shelf Mesenchymal Stem Cell-Derived Acellular Therapeutic. Pharmaceutics 2022, 14, 849. [Google Scholar] [CrossRef]
- Van Poll, D.; Parekkadan, B.; Cho, C.H.; Berthiaume, F.; Nahmias, Y.; Tilles, A.W.; Yarmush, M.L. Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo. Hepatology 2008, 47, 1634–1643. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Li, F.; Miao, L.; Sun, H.; Zhang, Y.; Bao, X.; Zhang, D. Establishment of a new acute-on-chronic liver failure model. Acta Pharm. Sin. B 2017, 7, 326–333. [Google Scholar] [CrossRef]
- He, Y.; Guo, X.; Lan, T.; Xia, J.; Wang, J.; Li, B.; Peng, C.; Chen, Y.; Hu, X.; Meng, Z. Human umbilical cord-derived mesenchymal stem cells improve the function of liver in rats with acute-on-chronic liver failure via downregulating Notch and Stat1/Stat3 signaling. Stem Cell Res. Ther. 2021, 12, 396. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Gao, L.; Du, Y.; Chen, Y. Efficacy of decoction from Jieduan Niwan formula on rat model of acute-on-chronic liver failure induced by porcine serum. J. Tradit. Chin. Med. 2020, 40, 602–612. [Google Scholar] [CrossRef]
- Wang, S.Y.; Li, M.; Miao, L.Y.; Wu, S.; Tong, Y.F.; Zhang, W.X.; Zhang, Y.Y.; Sun, H. Protective effects of a novel water-soluble biphenyl compound WLP-S-14 against acute-on-chronic liver failure in rats. J. Asian Nat. Prod. Res. 2019, 21, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Liu, N.; Liu, W.; Li, M.; Zhang, F.; Dong, Z.; Zhang, J.L.; Sun, H. Role of dihydroceramides in the progression of acute-on-chronic liver failure in rats. Chin. Med. J. 2020, 133, 198–204. [Google Scholar] [CrossRef]
- Hassan, H.M.; Cai, Q.; Liang, X.; Xin, J.; Ren, K.; Jiang, J.; Shi, D.; Lu, Y.; Li, T.; Shang, Y.; et al. Transcriptomics reveals immune-metabolism disorder in acute-on-chronic liver failure in rats. Life Sci. Alliance 2022, 5, e202101189. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kim, J.O.; Kim, S.J. Secretome from human adipose-derived stem cells protects mouse liver from hepatic ischemia–reperfusion injury. Surgery 2015, 157, 934–943. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, W.; Ren, H.Z.; Zhao, X.; Wang, S.; Ma, H.C.; Shi, X.L. Mesenchymal stem cells increase expression of heme oxygenase-1 leading to anti-inflammatory activity in treatment of acute liver failure. Stem Cell Res. Ther. 2017, 8, 70. [Google Scholar] [CrossRef]
- Assy, N.; Gong, Y.; Zhang, M.; Pettigrew, N.M.; Pashniak, D.; Minuk, G.Y. Use of proliferating cell nuclear antigen as a marker of liver regeneration after partial hepatectomy in rats. J. Lab. Clin. Med. 1998, 131, 251–256. [Google Scholar] [CrossRef]
- Ezquer, F.; Bahamonde, J.; Huang, Y.L.; Ezquer, M. Administration of multipotent mesenchymal stromal cells restores liver regeneration and improves liver function in obese mice with hepatic steatosis after partial hepatectomy. Stem Cell Res. Ther. 2017, 8, 20. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, D.; Hwang, S.; Ren, T.; Wang, X.; Trojnar, E.; Matyas, C.; Mo, R.; Shang, D.; He, Y.; et al. Interleukin-22 ameliorates acute-on-chronic liver failure by reprogramming impaired regeneration pathways in mice. J. Hepatol. 2020, 72, 736–745. [Google Scholar] [CrossRef]
- Jaeschke, H.; Hasegawa, T. Role of neutrophils in acute inflammatory liver injury. Liver Int. 2006, 26, 912–919. [Google Scholar] [CrossRef]
- Saha, R.; Pradhan, S.S.; Shalimar; Das, P.; Mishra, P.; Singh, R.; Sivaramakrishnan, V.; Acharya, P. Inflammatory signature in acute-on-chronic liver failure includes increased expression of granulocyte genes ELANE, MPO and CD177. Sci. Rep. 2021, 11, 18849. [Google Scholar] [CrossRef]
- Metzler, K.D.; Fuchs, T.A.; Nauseef, W.M.; Reumaux, D.; Roesler, J.; Schulze, I.; Wahn, V.; Papayannopoulos, V.; Zychlinsky, A. Myeloperoxidase is required for neutrophil extracellular trap formation: Implications for innate immunity. Blood 2011, 117, 953–959. [Google Scholar] [CrossRef]
- Farfán, N.; Carril, J.; Redel, M.; Zamorano, M.; Araya, M.; Monzón, E.; Alvarado, R.; Contreras, N.; Tapia-Bustos, A.; Quintanilla, M.E.; et al. Intranasal Administration of Mesenchymal Stem Cell Secretome Reduces Hippocampal Oxidative Stress, Neuroinflammation and Cell Death, Improving the Behavioral Outcome Following Perinatal Asphyxia. Int. J. Mol. Sci. 2020, 21, 7800. [Google Scholar] [CrossRef]
- Gao, J.; Yu, Z.; Jing, S.; Jiang, W.; Liu, C.; Yu, C.; Sun, J.; Wang, C.; Chen, J.; Li, H. Protective effect of Anwulignan against D-galactose-induced hepatic injury through activating p38 MAPK–Nrf2–HO-1 pathway in mice. Clin. Interv. Aging 2018, 13, 1859–1869. [Google Scholar] [CrossRef]
- Linillos-Pradillo, B.; Rancan, L.; Paredes, S.D.; Schlumpf, M.; Lichtensteiger, W.; Vara, E.; Tresguerres, J.Á. Low Dose of BPA Induces Liver Injury through Oxidative Stress, Inflammation and Apoptosis in Long-Evans Lactating Rats and Its Perinatal Effect on Female PND6 Offspring. Int. J. Mol. Sci. 2023, 24, 4585. [Google Scholar] [CrossRef]
- Ishizakai, M.; Yoshida, K.; Nishimoto, N.; Saleh, A.M.W.H.; Ishii, C.; Handa, H.; Ogawara, H.; Nagamine, T.; Murakami, M.; Murakami, H. Urinary 8-hydroxy-2’-deoxyguanosin (8-OHdG) in patients with chronic liver diseases. Rinsho Byori. 2004, 52, 732–736. [Google Scholar]
- Liu, C.; Hu, J.; Mao, Z.; Kang, H.; Liu, H.; Fu, W.; Lv, Y.; Zhou, F. Acute kidney injury and inflammatory response of sepsis following cecal ligation and puncture in D-galactose-induced aging rats. Clin. Interv. Aging 2017, 12, 593–602. [Google Scholar] [CrossRef]
- Zhou, F.; Peng, Z.Y.; Bishop, J.V.; Cove, M.E.; Singbartl, K.; Kellum, J.A.M. Effects of fluid resuscitation with 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis. Crit. Care Med. 2014, 42, e270–e278. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.K.; Wang, L.W.; Han, X.Q.; Yang, F.; Gong, Z.J. Blockade of high-mobility group box-1 ameliorates acute on chronic liver failure in rats. Inflamm. Res. 2013, 62, 703–709. [Google Scholar] [CrossRef]
- Thomas, J.M.; Ling, Y.H.; Huuskes, B.; Jelinic, M.; Sharma, P.; Saini, N.; Ferens, D.M.; Diep, H.; Krishnan, S.M.; Kemp-Harper, B.K.; et al. IL-18 (Interleukin-18) Produced by Renal Tubular Epithelial Cells Promotes Renal Inflammation and Injury During Deoxycorticosterone/Salt-Induced Hypertension in Mice. Hypertension 2021, 78, 1296–1309. [Google Scholar] [CrossRef]
- Romejko, K.; Markowska, M.; Niemczyk, S. The Review of Current Knowledge on Neutrophil Gelatinase-Associated Lipocalin (NGAL). Int. J. Mol. Sci. 2023, 24, 10470. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.; Coelho, S.; Rodrigues, B.; Martins, H.; Frade, F.; Lopes, S.; Cunha, L.; Papoila, A.L.; Devarajan, P. Cystatin C as a marker of acute kidney injury in the emergency department. Clin. J. Am. Soc. Nephrol. 2010, 5, 1745–1754. [Google Scholar] [CrossRef]
- Yong, Z.; Pei, X.; Zhu, B.; Yuan, H.; Zhao, W. Predictive value of serum cystatin C for acute kidney injury in adults: A meta-analysis of prospective cohort trials. Sci. Rep. 2017, 7, 41012. [Google Scholar] [CrossRef]
- Ni, S.; Li, S.; Yang, N.; Tang, X.; Zhang, S.; Hu, D.; Lu, M. Deregulation of Regulatory T Cells in Acute-on-Chronic Liver Failure: A Rat Model. Mediat. Inflamm. 2017, 2017, 1390458. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chen, Y.X.; Zhang, L.J.; Chen, Z.X.; Wang, X.Z. Hydrodynamics-based transfection of rat interleukin-10 gene attenuates porcine serum-induced liver fibrosis in rats by inhibiting the activation of hepatic stellate cells. Int. J. Mol. Med. 2014, 34, 677–686. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Zhang, W.; Dong, S.; Song, L.; Zhao, S.; Wu, C.; Wang, X.; Liu, F.; Xie, J.; Wang, J.; et al. Protective effects of sea buckthorn polysaccharide extracts against LPS/d-GalN-induced acute liver failure in mice via suppressing TLR4-NF-kappaB signaling. J. Ethnopharmacol. 2015, 176, 69–78. [Google Scholar] [CrossRef]
- Chen, B.; Wang, Y.H.; Qian, J.Q.; Wu, D.B.; Chen, E.Q.; Tang, H. Human mesenchymal stem cells for hepatitis B virus-related acute-on-chronic liver failure: A systematic review with meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 1224–1229. [Google Scholar] [CrossRef]
- Li, Y.H.; Xu, Y.; Wu, H.M.; Yang, J.; Yang, L.H.; Yue-Meng, W. Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation in Hepatitis B Virus Related Acute-on-Chronic Liver Failure Treated with Plasma Exchange and Entecavir: A 24-Month Prospective Study. Stem Cell Rev. Rep. 2016, 12, 645–653. [Google Scholar] [CrossRef]
- Xue, R.; Meng, Q.; Dong, J.; Li, J.; Yao, Q.; Zhu, Y.; Yu, H. Clinical performance of stem cell therapy in patients with acute-on-chronic liver failure: A systematic review and meta-analysis. J. Transl. Med. 2018, 16, 126. [Google Scholar] [CrossRef]
- Fonteneau, G.; Bony, C.; Goulabchand, R.; Maria, A.T.J.; Le Quellec, A.; Rivière, S.; Jorgensen, C.; Guilpain, P.; Noël, D. Serum-Mediated Oxidative Stress from Systemic Sclerosis Patients Affects Mesenchymal Stem Cell Function. Front. Immunol. 2017, 8, 988. [Google Scholar] [CrossRef]
- Sui, B.D.; Hu, C.H.; Zheng, C.X.; Jin, Y. Microenvironmental Views on Mesenchymal Stem Cell Differentiation in Aging. J. Dent. Res. 2016, 95, 1333–1340. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Sgodda, M.; Aurich, H.; Kleist, S.; Aurich, I.; König, S.; Dollinger, M.M.; Fleig, W.E.; Christ, B. Hepatocyte differentiation of mesenchymal stem cells from rat peritoneal adipose tissue in vitro and in vivo. Exp. Cell Res. 2007, 313, 2875–2886. [Google Scholar] [CrossRef]
- Pacifico, L.; Ferraro, F.; Bonci, E.; Anania, C.; Romaggioli, S.; Chiesa, C. Upper limit of normal for alanine aminotransferase: Quo vadis? Clin. Chim. Acta 2013, 422, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, R.; Fujikawa, M.; Yamamoto, T.; Hamada, Y.; Yamada, H.; Horii, I. In vivo hepatotoxicity study of rats in comparison with in vitro hepatotoxicity screening system. J. Toxicol. Sci. 2006, 31, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Pratt, D.S.; Kaplan, M.M. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N. Engl. J. Med. 2000, 342, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Zhuang, X.; Xu, Q.; Wu, Z.; Zhou, Y.; Li, Y.; Lu, Y.; Zhang, B.; Talbot, P.; Liao, J.; et al. Peripheral infusion of human umbilical cord mesenchymal stem cells rescues acute liver failure lethality in monkeys. Stem Cell Res. Ther. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.M.; Maumus, M.; Bony, C.; Jorgensen, C.; Noël, D. Contribution of microRNAs to the immunosuppressive function of mesenchymal stem cells. Biochimie 2018, 155, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Fouser, L.A.; Artis, D. Border patrol: Regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 2011, 12, 383–390. [Google Scholar] [CrossRef]
- Khawar, M.B.; Azam, F.; Sheikh, N.; Abdul, M.K. How Does Interleukin-22 Mediate Liver Regeneration and Prevent Injury and Fibrosis? J. Immunol. Res. 2016, 2016, 2148129. [Google Scholar] [CrossRef]
- Schwarzkopf, K.M.; Eberle, L.; Uschner, F.E.; Klein, S.; Schierwagen, R.; Mücke, M.M.; Schaefer, L.; Clària, J.; Zeuzem, S.; Hintermann, E.; et al. Interleukin-22 in acute-on-chronic liver failure: A matter of ineffective levels, receptor dysregulation or defective signalling? J. Hepatol. 2020, 73, 980–982. [Google Scholar] [CrossRef]
- Zenewicz, L.A.; Yancopoulos, G.D.; Valenzuela, D.M.; Murphy, A.J.; Karow, M.; Flavell, R.A. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 2007, 27, 647–659. [Google Scholar] [CrossRef]
- Radaeva, S.; Sun, R.; Pan, H.N.; Hong, F.; Gao, B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology 2004, 39, 1332–1342. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Jang, Y.J.; Lim, H.J.; Han, J.; Lee, J.; Lee, G.; Park, J.Y.; Park, S.Y.; Kim, J.H.; Do, B.R.; et al. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology 2017, 152, 1174–1186. [Google Scholar] [CrossRef]
- Damania, A.; Jaiman, D.; Teotia, A.K.; Kumar, A. Mesenchymal stromal cell-derived exosome-rich fractionated secretome confers a hepatoprotective effect in liver injury. Stem Cell Res. Ther. 2018, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Lin, L.; Choi, A.M.; Ryter, S.W. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free. Radic. Biol. Med. 2009, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Beyer, T.A.; Xu, W.; Teupser, D.; Keller, U.A.D.; Bugnon, P.; Hildt, E.; Thiery, J.; Kan, Y.W.; Werner, S. Impaired liver regeneration in Nrf2 knockout mice: Role of ROS-mediated insulin/IGF-1 resistance. EMBO J. 2008, 27, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Fuertes-Agudo, M.; Luque-Tévar, M.; Cucarella, C.; Martín-Sanz, P.; Casado, M. Advances in Understanding the Role of NRF2 in Liver Pathophysiology and Its Relationship with Hepatic-Specific Cyclooxygenase-2 Expression. Antioxidants 2023, 12, 1491. [Google Scholar] [CrossRef]
- Sala, E.; Genua, M.; Petti, L.; Anselmo, A.; Arena, V.; Cibella, J.; Zanotti, L.; D’alessio, S.; Scaldaferri, F.; Luca, G.; et al. Mesenchymal Stem Cells Reduce Colitis in Mice via Release of TSG6, Independently of Their Localization to the Intestine. Gastroenterology 2015, 149, 163–176. [Google Scholar] [CrossRef]
- Taylor, N.J.; Nishtala, A.; Vijay, G.K.M.; Abeles, R.D.; Auzinger, G.; Bernal, W.; Ma, Y.; Wendon, J.A.; Shawcross, D.L. Circulating neutrophil dysfunction in acute liver failure. Hepatology 2013, 57, 1142–1152. [Google Scholar] [CrossRef]
- Ramaiah, S.K.; Jaeschke, H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol. Pathol. 2007, 35, 757–766. [Google Scholar] [CrossRef]
- Kirino, Y.; Takeno, M.; Murakami, S.; Kobayashi, M.; Kobayashi, H.; Miura, K.; Ideguchi, H.; Ohno, S.; Ueda, A.; Ishigatsubo, Y. Tumor necrosis factor alpha acceleration of inflammatory responses by down-regulating heme oxygenase 1 in human peripheral monocytes. Arthritis Rheum. 2007, 56, 464–475. [Google Scholar] [CrossRef]
- Wan, Y.M.; Li, Z.Q.; Liu, C.; He, Y.F.; Wang, M.J.; Wu, X.N.; Zhang, Y.; Li, Y.H. Mesenchymal stem cells reduce alcoholic hepatitis in mice via suppression of hepatic neutrophil and macrophage infiltration, and of oxidative stress. PLoS ONE 2020, 15, e0228889. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Mohamed, F.E.; Jover-Cobos, M.; Macnaughtan, J.; Davies, N.; Moreau, R.; Paradis, V.; Moore, K.; Mookerjee, R.; Jalan, R. Increased renal expression and urinary excretion of TLR4 in acute kidney injury associated with cirrhosis. Liver Int. 2013, 33, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Brune, J.C.; Tormin, A.; Johansson, M.C.; Rissler, P.; Brosjö, O.; Löfvenberg, R.; von Steyern, F.V.; Mertens, F.; Rydholm, A.; Scheding, S. Mesenchymal stromal cells from primary osteosarcoma are non-malignant and strikingly similar to their bone marrow counterparts. Int. J. Cancer 2011, 129, 319–330. [Google Scholar] [CrossRef]
- Rubio, D.; Garcia, S.; Paz, M.F.; De la Cueva, T.; Lopez-Fernandez, L.A.; Lloyd, A.C.; Garcia-Castro, J.; Bernad, A. Molecular characterization of spontaneous mesenchymal stem cell transformation. PLoS ONE 2008, 3, e1398. [Google Scholar] [CrossRef]
- Zhu, W.; Shi, X.L.; Xiao, J.Q.; Gu, G.X.; Ding, Y.T.; Ma, Z.L. Effects of xenogeneic adipose-derived stem cell transplantation on acute-on-chronic liver failure. Hepatobiliary Pancreat. Dis. Int. 2013, 12, 60–67. [Google Scholar] [CrossRef]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Mocchi, M.; Bari, E.; Marrubini, G.; Bonda, A.F.; Perteghella, S.; Tartara, F.; Cofano, F.; di Perna, G.; Giovannelli, L.; Mandracchia, D.; et al. Freeze-Dried Mesenchymal Stem Cell-Secretome Pharmaceuticalization: Optimization of Formulation and Manufacturing Process Robustness. Pharmaceutics 2021, 13, 1129. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, M.E.; Ezquer, F.; Morales, P.; Santapau, D.; Berríos-Cárcamo, P.; Ezquer, M.; Herrera-Marschitz, M.; Israel, Y. Intranasal mesenchymal stem cell secretome administration markedly inhibits alcohol and nicotine self-administration and blocks relapse-intake: Mechanism and translational options. Stem Cell Res. Ther. 2019, 10, 205. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; Mcdevitt, T.C. Pre-conditioning mesenchymal stromal cell spheroids for immunomodulatory paracrine factor secretion. Cytotherapy 2014, 16, 331–345. [Google Scholar] [CrossRef]
- Jin, G.; Qiu, G.; Wu, D.; Hu, Y.; Qiao, P.; Fan, C.; Gao, F. Allogeneic bone marrow-derived mesenchymal stem cells attenuate hepatic ischemia-reperfusion injury by suppressing oxidative stress and inhibiting apoptosis in rats. Int. J. Mol. Med. 2013, 31, 1395–1401. [Google Scholar] [CrossRef]
- Toledo-Pereyra, L.H.; Rodriguez, F.J.; Cejalvo, D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 1993, 55, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A., Jr.; Madden, J.F.; Gao, W.; Selvan, R.S.; Clavien, P.A. Interleukin-6 protects liver against warm ischemia/reperfusion injury and promotes hepatocyte proliferation in the rodent. Hepatology 1997, 26, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, T.; Lee, Y.A.; Fujiwara, N.; Ybanez, M.; Allen, B.; Martins, S.; Fiel, M.I.; Goossens, N.; Chou, H.I.; Hoshida, Y.; et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J. Hepatol. 2018, 69, 385–395. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Ezquer, F.; Giraud-Billoud, M.; Carpio, D.; Cabezas, F.; Conget, P.; Ezquer, M. Proregenerative Microenvironment Triggered by Donor Mesenchymal Stem Cells Preserves Renal Function and Structure in Mice with Severe Diabetes Mellitus. BioMed Res. Int. 2015, 2015, 164703. [Google Scholar] [CrossRef]
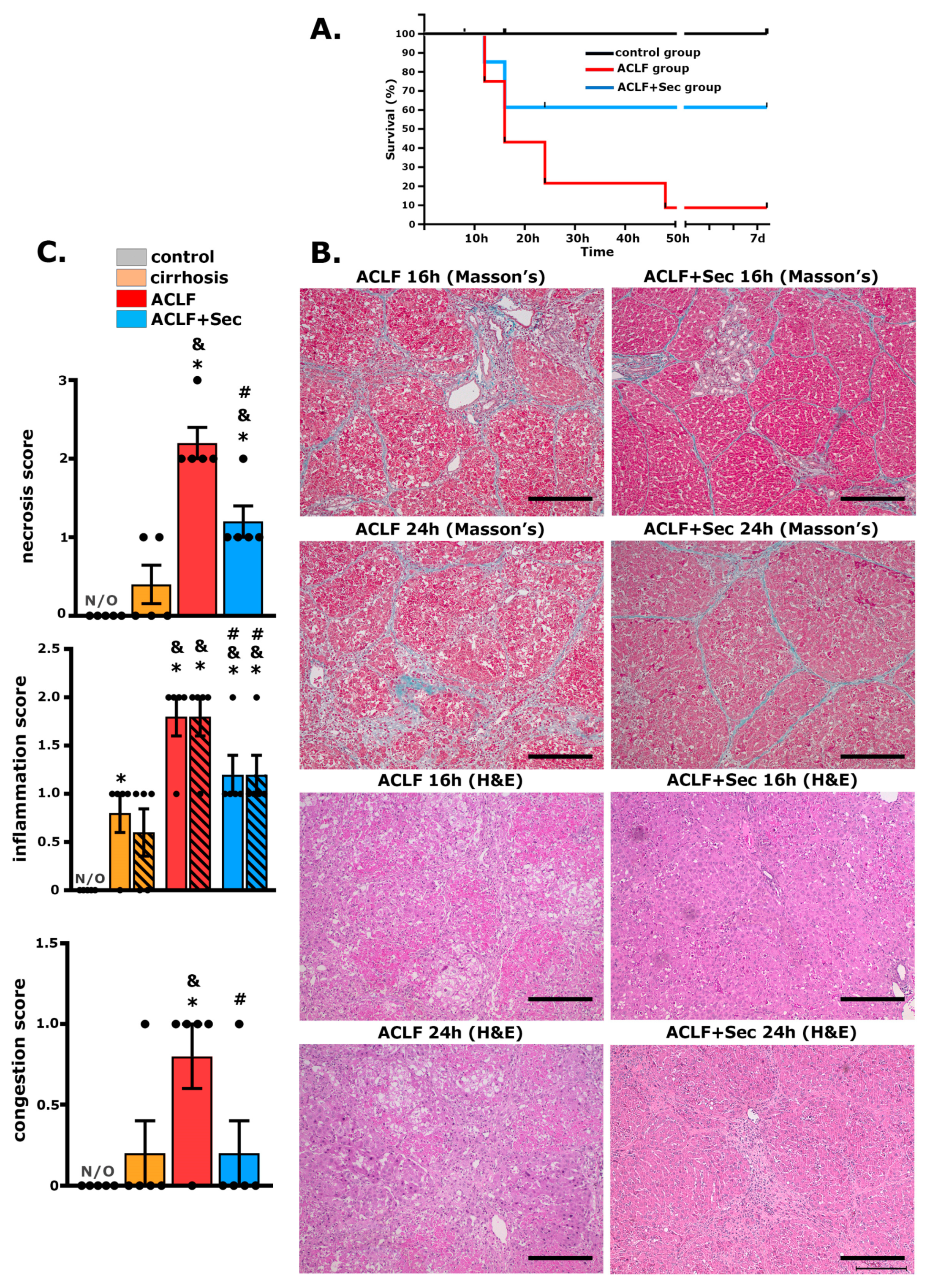
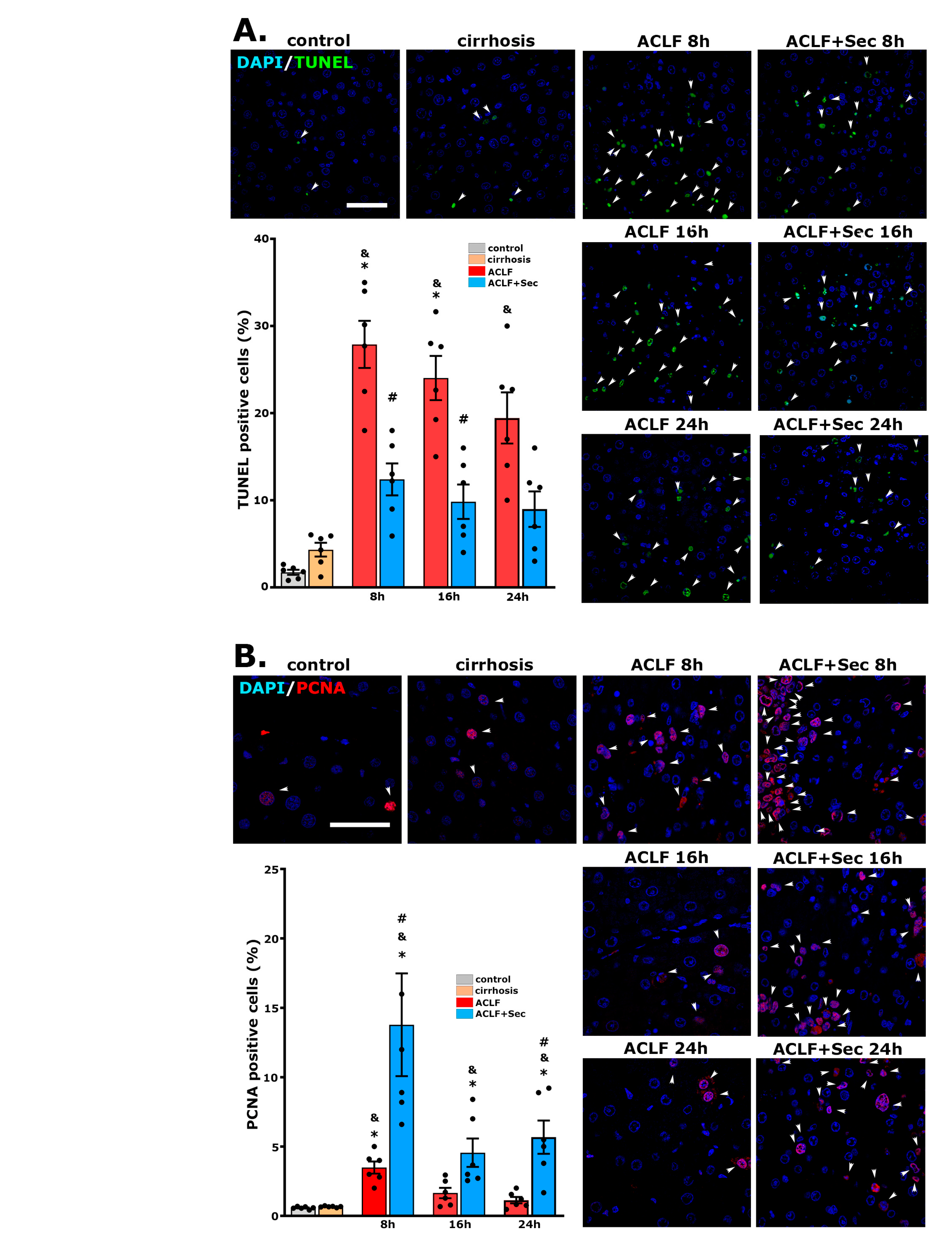

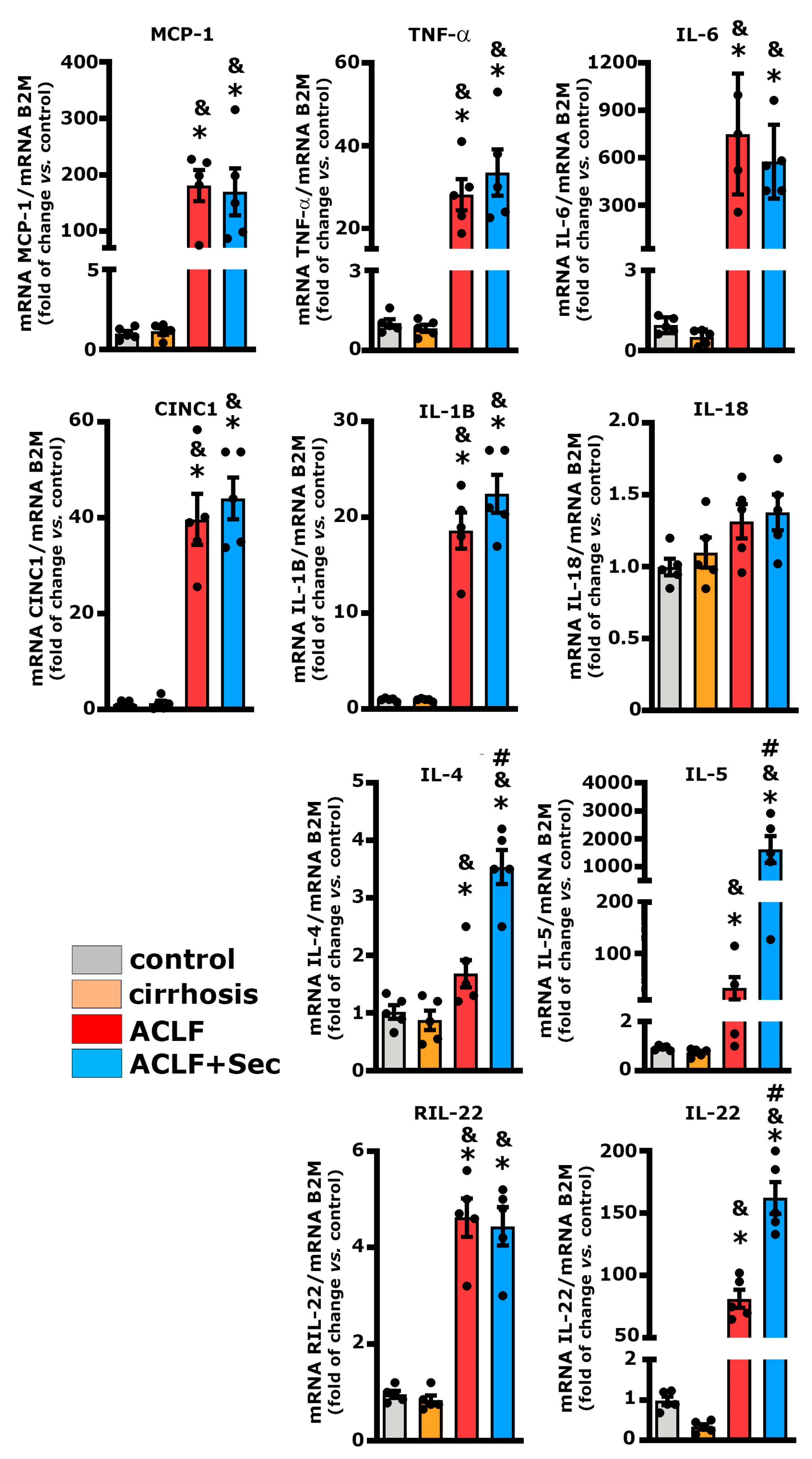
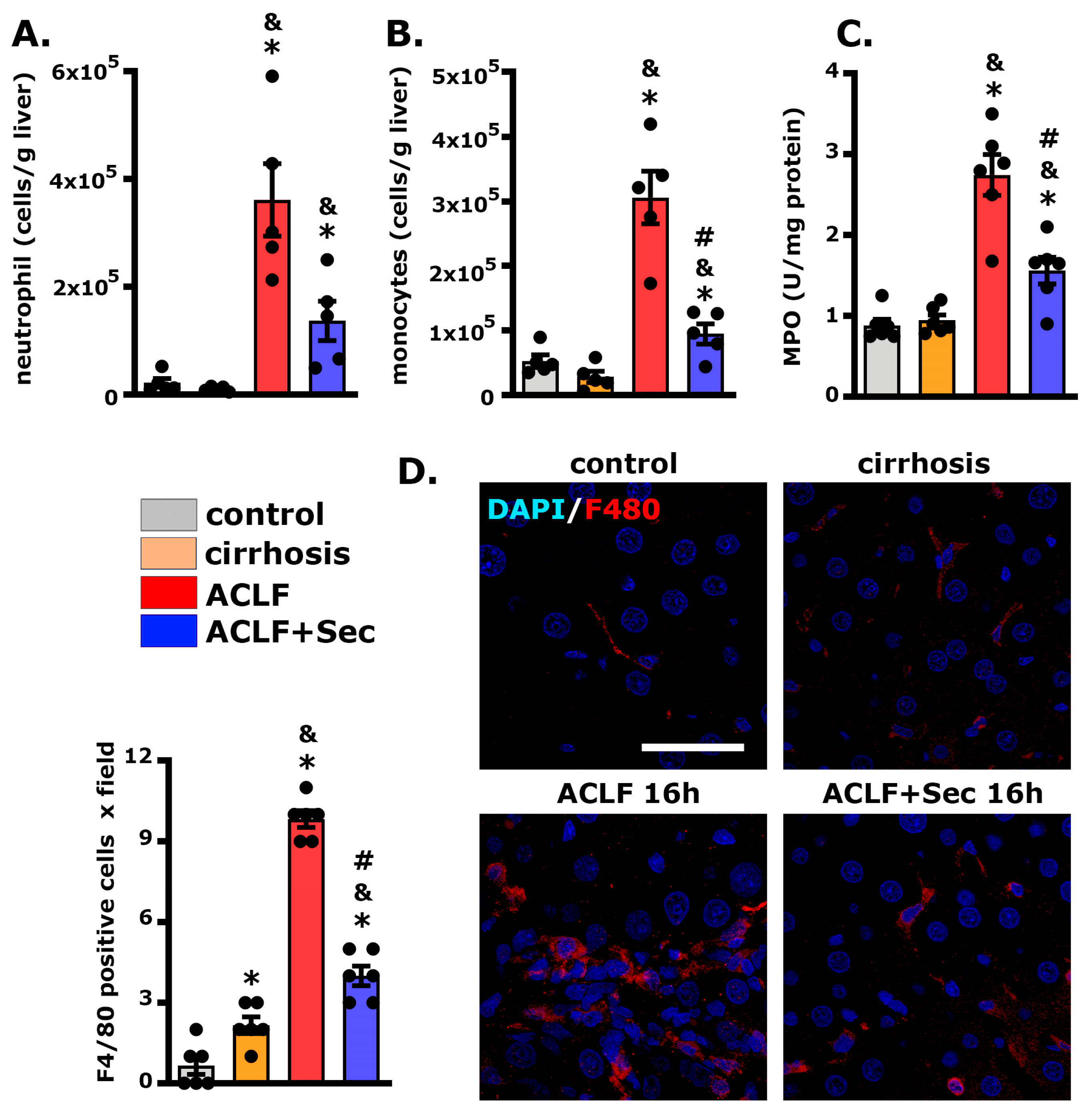
| 8 h | 16 h | 24 h | 7 Days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Chronic | ACLF | ACLF + Sec | ACLF | ACLF + Sec | ACLF | ACLF + Sec | ACLF | ACLF + Sec | |
| liver/body weight ratio (mg/g) | 32.16 ± 0.6 | 35.3 ± 2 | 40.3 ± 2.1 | 37.6 ± 1.5 | 41.6 ± 1.6 a,b | 35.8 ± 1.3 a,c | 37.9 ± 0.8 | 38.1 ± 0.32 | 33.7 ± 0.7 | 31.7 ± 0.8 |
| kidney/body weight ratio (mg/g) | 3.14 ± 0.05 | 3.41 ± 0.13 | 3.49 ± 0.14 | 3.64 ± 0.16 | 3.50 ± 0.21 | 3.60 ± 0.19 | 3.55 ± 0.12 a,b | 3.50 ± 0.8 | 3.38 ± 0.7 | 3.38 ± 0.17 |
| spleen/body weight ratio (mg/g) | 1.69 ± 0.06 | 2.11 ± 0.15 a | 2.89 ± 0.13 a,b | 2.75 ± 0.15 a,b | 3.18 ± 0.24 a,b | 2.5 ± 0.11 a,b,c | 3.21 ± 0.41 a,b | 2.56 ± 0.3 | 2.74 ± 0.03 | 1.91 ± 0.18 c |
| 8 h | 16 h | 24 h | 7 Days | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Cirrhosis | ACLF | ACLF + Sec | ACLF | ACLF + Sec | ACLF | ACLF + Sec | ACLF | ACLF + Sec | |
| AST (U/L) | 148 ± 42 | 243 ± 53 | 7450 ± 984 a,b | 7317 ± 3920 a,b | 28,700 ± 3700 a,b | 5245 ± 4505 a,b,c | 3700 ± 3150 a,b | 5233 ± 2648 a,b | 112 ± 20 | 120 ± 35 |
| ALT (U/L) | 64 ± 4 | 86 ± 13 | 5563 ± 874 a,b | 6350 ± 3242 a,b | 13,850 ± 3750 a,b | 14,071 ± 5862 a,b | 5461 ± 5370 a,b | 7233 ± 4638 a,b | 79 ± 10 | 73 ± 16 |
| alkaline phosphatase (U/L) | 288.8 ± 16 | 350.2 ± 25 | 362 ± 39.7 | 434 ± 31.1 | 463.8 ± 57.9 a,b | 498.3 ± 52.4 a,b | 580 ± 98.9 a,b | 512 ± 48 a,b | 278 ± 28 | 290.2 ± 41.3 |
| direct bilirubin (mg/dL) | 0.06 ± 0.01 | 0.09 ± 0.01 | 0.51 ± 0.09 a,b | 0.47 ± 0.12 a,b | 1.51 ± 0.33 a,b | 0.71 ± 0.23 a,b,c | 1.81 ± 0.72 a,b | 0.42 ± 0.2 a,b,c | 0.08 ± 0.02 | 0.10 ± 0.01 |
| albumin (g/dL) | 3.27 ± 0.04 | 3.20 ± 0.07 | 3.03 ± 0.05 | 2.90 ± 0.09 | 3.40 ± 0.14 | 3.28 ± 0.11 | 3.13 ± 0.03 | 3.07 ± 0.15 | 3.08 ± 0.06 | 3.20 ± 0.08 |
| Hepatic Cytokine Levels | ||||||||
|---|---|---|---|---|---|---|---|---|
| 16 h | 24 h | 7 Days | ||||||
| Control | Cirrhosis | ACLF | Sec | ACLF | Sec | ACLF | Sec | |
| TNFα (pg/mg) | 5.1 ± 1.6 | 5.3 ± 0.9 | 11.1 ± 0.4 a,b | 15.4 ± 1.5 a,b,c | 9.9 ± 1.7 a | 7.2 ± 1.9 | 4.8 ± 1.6 | 4.1 ± 1 |
| CINC-1 (pg/mg) | N/D | 170 ± 30 | 309 ± 110 a | 154 ± 33 a,c | 140 ± 5 a | 129 ± 13 a | 160 ± 15 a | 130 ± 18 a |
| IL-6 (pg/mg) | 1217 ± 38 | 790 ± 160 | 3329 ± 1000 a,b | 1271 ± 60 c | 853 ± 85 | 576 ± 51 | 630 ± 70 | 680 ± 62 |
| MCP-1 (pg/mg) | 44.3 ± 3 | 36.4 ± 3.3 | 158.1 ± 32 | 136.3 ± 25.8 | 108.9 ± 34.4 | 83.2 ± 20.5 | 63.8 ± 15 | 37.5 ± 8 |
| IL-18 (pg/mg) | 471 ± 52 | 779 ± 200 | 690 ± 199 | 946 ± 150 | 1378 ± 180 a,b | 18,822 ± 200 a,b | 670 ± 150 | 638 ± 80 |
| IL-1b (pg/mg) | 164 ± 7 | 183 ± 3 | 1456 ± 452 a,b | 1816 ± 475 a,b | 547 ± 151 | 691 ± 60 | 200 ± 40 | 230 ± 18 |
| IL-2 (pg/mg) | 45.2 ± 3.5 | 33.8 ± 6.4 | 13.4 ± 0.8 | 39.2 ± 3 c | 13.2 ± 2.6 | 34 ± 5.7 c | 25 ± 8 | 35 ± 7 |
| IL-10 (pg/mg) | 133 ± 8 | 116 ± 16 | 95 ± 12 | 111 ± 17 | 94 ± 16 | 116 ± 18 | 78 ± 14 | 120 ± 18 |
| IL-13 (pg/mg) | 5.6 ± 0.1 | 4.9 ± 0.5 | 4.3 ± 0.4 | 7.5 ± 0.5 a,b,c | 4.9 ± 0.6 | 7.3 ± 0.4 a,b,c | 3.8 ± 0.4 | 5.6 ± 0.7 |
| IL-4 (pg/mg) | 53 ± 3.5 | 40 ± 3.7 | 35 ± 3 | 52 ± 0.7 b,c | 33.5 ± 2.1 | 55.5 ± 4 b,c | 30 ± 1.5 | 38 ± 8 |
| IL-5 (pg/mg) | 24.3 ± 2.9 | 22.9 ± 1 | 33.6 ± 2.5 | 65.1 ± 4 a,b,c | 30.4 ± 4.4 | 58.4 ± 3.8 a,b,c | 18 ± 1 | 25 ± 3 |
| Plasma Cytokine Levels | ||||||||
| 16 h | 24 h | 7 Days | ||||||
| Control | Cirrhosis | ACLF | Sec | ACLF | Sec | ACLF | Sec | |
| TNFα (pg/mL) | 1.2 ± 0.2 | 1.4 ± 0.1 | 76.3 ± 14.3 a,b | 59 ± 22.7 a,b | 9.13 ± 5.7 a,b | 15 ± 6.2 a,b | 6.1 ± 2.7 | 1.8 ± 0.5 |
| CINC-1 (ng/mL) | N/D | N/D | 4 ± 0.3 a,b | 3.6 ± 0.2 a,b | 2.1 ± 1 a,b | 1.8 ± 1 a,b | N/D | N/D |
| IL-6 (ng/mL) | 0.06 ± 0.001 | 0.07 ± 0.001 | 11.1 ± 6 a,b | 28.6 ± 15.6 a,b | 17.2 ± 1 a,b | 14 ± 1 a,b | 0.1 ± 0.002 | 0.08 ± 0.025 |
| MCP-1 (ng/mL) | 0.032 ± 0.001 | 0.3 ± 0.09 a | 192.5 ± 36.2 a,b | 90 ± 23.1 a,b | 27.5 ± 10 a,b | 41.5 ± 14 a,b | 0.2 ± 0.04 a | 0.3 ± 0.05 a |
| IL-17 (pg/mL) | 4.6 ± 1 | 6.7 ± 0.8 | 59.3 ± 15 a,b | 74.6 ± 20 a,b | 17.8 ± 12 a,b | 29 ± 8 a,b | 24 ± 4.7 a,b | 18 ± 3.8 a,b |
| IL-2 (pg/mL) | 200 ± 59 | 50 ± 16 a | 226 ± 130 | 1160 ± 400 a,b,c | 160 ± 55 | 1475 ± 738 a,b,c | 83 ± 30 | 53 ± 15 |
| IL-10 (pg/mL) | 236 ± 115 | 10 ± 0.1 a | 591 ± 270 b | 1880 ± 620 a,b,c | 909 ± 465 a,b | 1024 ± 127 a,b | 20 ± 0.5 | 160 ± 32 b |
| IL-13 (pg/mL) | 2.38 ± 0.1 | 2.38 ± 0.1 | 5.2 ± 1.8 | 30.2 ± 12.8 a,b,c | 12.3 ± 5.4 a,b | 14.3 ± 6 a,b | 3 ± 0.2 | 3.5 ± 0.2 |
| IL-4 (pg/mL) | 3.9 ± 0.01 | 3.9 ± 0.01 | 5.1 ± 1.1 | 51.5 ± 2.1 a,b,c | 7.8 ± 2.5 | 4.9 ± 1 | 3.7 ± 0.1 | 4.2 ± 0.1 |
| IL-5 (pg/mL) | 21 ± 9 | 19 ± 5 | 104 ± 3 a,b | 194 ± 35 a,b,c | 62 ± 30 | 85 ± 28 a,b | 20 ± 6 | 23 ± 5 |
| 24 h | 7 days | |||||
|---|---|---|---|---|---|---|
| Control | Cirrhosis | ACLF | Sec | ACLF | Sec | |
| plasma urea (ng/µg) | 0.551 ± 0.039 | 0.651 ± 0.069 | 0.993 ± 0.088 a,b | 1.1 ± 0.108 a,b | 0.731 ± 0.07 | 0.515 ± 0.009 |
| plasma cystatin (ng/mL) | 17.3 ± 1.6 | 16.2 ± 2.8 | 70.0 ± 18.4 a,b | 35.1 ± 7.1 c | 42.2 ± 15.5 | 14.7 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuadra, B.; Silva, V.; Huang, Y.-L.; Diaz, Y.; Rivas, C.; Molina, C.; Simon, V.; Bono, M.R.; Morales, B.; Rosemblatt, M.; et al. The Immunoregulatory and Regenerative Potential of Activated Human Stem Cell Secretome Mitigates Acute-on-Chronic Liver Failure in a Rat Model. Int. J. Mol. Sci. 2024, 25, 2073. https://doi.org/10.3390/ijms25042073
Cuadra B, Silva V, Huang Y-L, Diaz Y, Rivas C, Molina C, Simon V, Bono MR, Morales B, Rosemblatt M, et al. The Immunoregulatory and Regenerative Potential of Activated Human Stem Cell Secretome Mitigates Acute-on-Chronic Liver Failure in a Rat Model. International Journal of Molecular Sciences. 2024; 25(4):2073. https://doi.org/10.3390/ijms25042073
Chicago/Turabian StyleCuadra, Barbara, Veronica Silva, Ya-Lin Huang, Yael Diaz, Claudio Rivas, Cristobal Molina, Valeska Simon, Maria Rosa Bono, Bernardo Morales, Mario Rosemblatt, and et al. 2024. "The Immunoregulatory and Regenerative Potential of Activated Human Stem Cell Secretome Mitigates Acute-on-Chronic Liver Failure in a Rat Model" International Journal of Molecular Sciences 25, no. 4: 2073. https://doi.org/10.3390/ijms25042073
APA StyleCuadra, B., Silva, V., Huang, Y.-L., Diaz, Y., Rivas, C., Molina, C., Simon, V., Bono, M. R., Morales, B., Rosemblatt, M., Silva, S., Acuña, R., Ezquer, F., & Ezquer, M. (2024). The Immunoregulatory and Regenerative Potential of Activated Human Stem Cell Secretome Mitigates Acute-on-Chronic Liver Failure in a Rat Model. International Journal of Molecular Sciences, 25(4), 2073. https://doi.org/10.3390/ijms25042073








