The Effects of Acid on Calcium and Phosphate Metabolism
Abstract
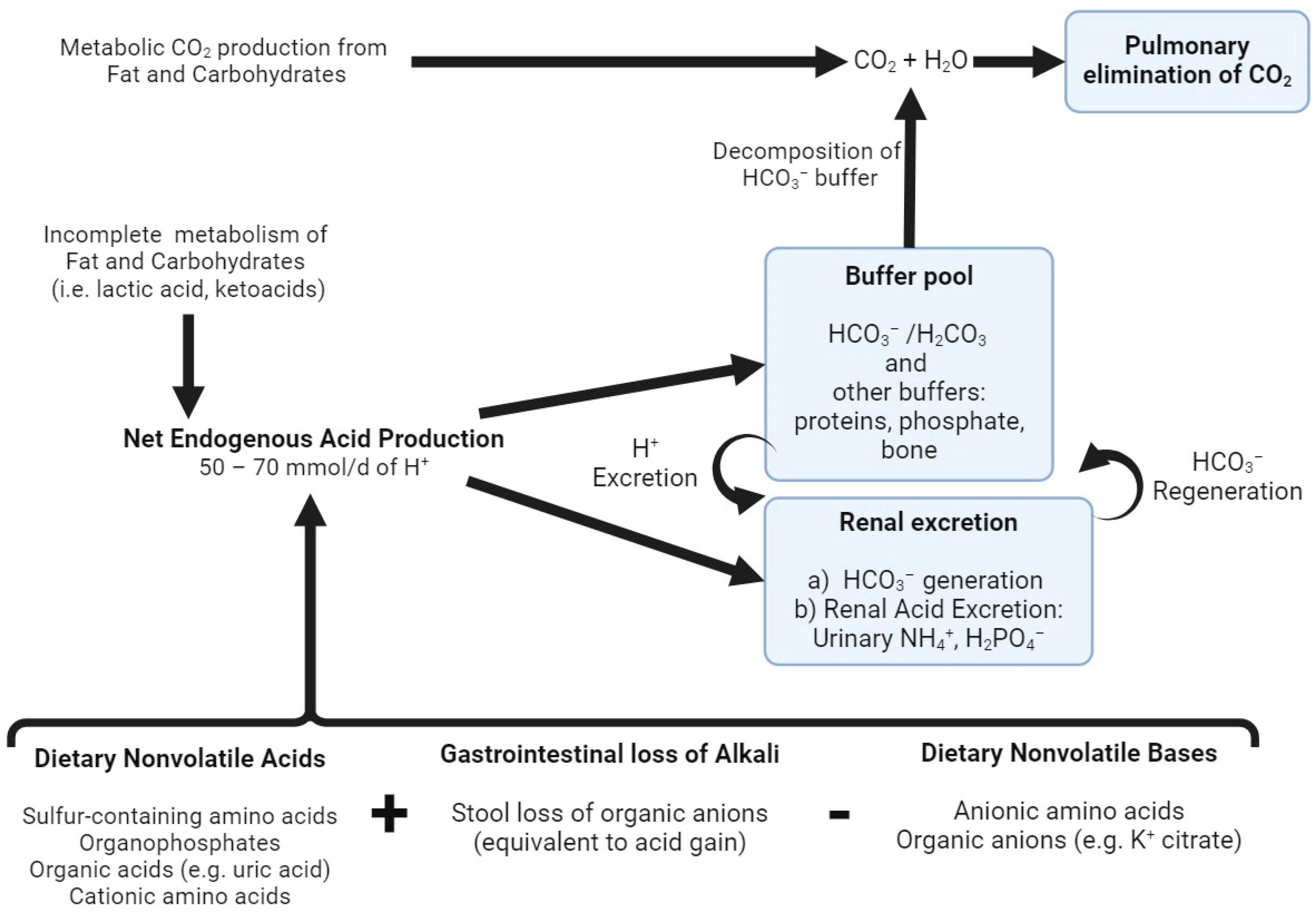
1. Introduction
2. Acid–Base Homeostasis and Gastrointestinal Calcium–Phosphate Handling
2.1. Normal Gastrointestinal Calcium and Phosphate Handling
2.2. Gastrointestinal Calcium Handling in Metabolic Acidosis
2.3. Gastrointestinal Phosphate Handling in Metabolic Acidosis
3. Acid–Base Homeostasis and Renal Calcium–Phosphate Handling
3.1. Normal Renal Calcium and Phosphate Handling
3.2. Renal Calcium Handling in Metabolic Acidosis
3.3. Renal Phosphate Handling in Metabolic Acidosis
4. Acid Effect on Calcium–Phosphate in Bone
4.1. Bone, Acid and Calcium Homeostasis
4.2. Bone, Acid and Phosphate Homeostasis
4.3. Bone, Acid and Citrate Metabolism
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Clancy, J.; McVicar, A. Short-term regulation of acid-base homeostasis of body fluids. Br. J. Nurs. 2007, 16, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Poupin, N.; Calvez, J.; Lassale, C.; Chesneau, C.; Tome, D. Impact of the diet on net endogenous acid production and acid-base balance. Clin. Nutr. 2012, 31, 313–321. [Google Scholar] [CrossRef]
- Bushinsky, D.A. Acid-base imbalance and the skeleton. Eur. J. Nutr. 2001, 40, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Alpern, R.J. Normal Acid-Base Balance. In Comprehensive Clinical Nephrology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 149–154. [Google Scholar] [CrossRef]
- Schiess, W.A.; Ayer, J.L.; Lotspeich, W.D.; Pitts, R.F.; Miner, P. The Renal Regulation of Acid-Base Balance in Man. Ii. Factors Affecting the Excretion of Titratable Acid by the Normal Human Subject. J. Clin. Investig. 1948, 27, 57–64. [Google Scholar] [CrossRef]
- Gennari, F.J.; Weise, W.J. Acid-base disturbances in gastrointestinal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1861–1868. [Google Scholar] [CrossRef]
- Nijenhuis, T.; Hoenderop, J.G.J.; Nilius, B.; Bindels, R.J.M. (Patho)physiological implications of the novel epithelial Ca2+ channels TRPV5 and TRPV6. Pflügers Arch.-Eur. J. Physiol. 2003, 446, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 1257–1272. [Google Scholar] [CrossRef]
- Diaz de Barboza, G.; Guizzardi, S.; Tolosa de Talamoni, N. Molecular aspects of intestinal calcium absorption. World J. Gastroenterol. 2015, 21, 7142–7154. [Google Scholar] [CrossRef]
- Iqbal, T.H.; Lewis, K.O.; Cooper, B.T. Phytase activity in the human and rat small intestine. Gut 1994, 35, 1233–1236. [Google Scholar] [CrossRef]
- Breves, G.; Schroder, B. Comparative aspects of gastrointestinal phosphorus metabolism. Nutr. Res. Rev. 1991, 4, 125–140. [Google Scholar] [CrossRef]
- Hu, M.S.; Kayne, L.H.; Jamgotchian, N.; Ward, H.J.; Lee, D.B. Paracellular phosphate absorption in rat colon: A mechanism for enema-induced hyperphosphatemia. Miner. Electrolyte Metab. 1997, 23, 7–12. [Google Scholar]
- King, A.J.; Siegel, M.; He, Y.; Nie, B.; Wang, J.; Koo-McCoy, S.; Minassian, N.A.; Jafri, Q.; Pan, D.; Kohler, J.; et al. Inhibition of sodium/hydrogen exchanger 3 in the gastrointestinal tract by tenapanor reduces paracellular phosphate permeability. Sci. Transl. Med. 2018, 10, 6474. [Google Scholar] [CrossRef]
- Yee, J.; Rosenbaum, D.; Jacobs, J.W.; Sprague, S.M. Small Intestinal Phosphate Absorption: Novel Therapeutic Implications. Am. J. Nephrol. 2021, 52, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; O’Brien, S.P.; Song, W.; Boulanger, J.H.; Stockmann, A.; Arbeeny, C.; Schiavi, S.C. Intestinal npt2b plays a major role in phosphate absorption and homeostasis. J. Am. Soc. Nephrol. 2009, 20, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, Y.; Giral, H.; Caldas, Y.; Levi, M.; Schiavi, S.C. Intestinal phosphate transport. Adv. Chronic Kidney Dis. 2011, 18, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Segawa, H.; Kaneko, I.; Yamanaka, S.; Ito, M.; Kuwahata, M.; Inoue, Y.; Kato, S.; Miyamoto, K. Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am. J. Physiol. Renal Physiol. 2004, 287, F39–F47. [Google Scholar] [CrossRef] [PubMed]
- Capuano, P.; Radanovic, T.; Wagner, C.A.; Bacic, D.; Kato, S.; Uchiyama, Y.; St-Arnoud, R.; Murer, H.; Biber, J. Intestinal and renal adaptation to a low-Pi diet of type II NaPi cotransporters in vitamin D receptor- and 1alphaOHase-deficient mice. Am. J. Physiol. Cell Physiol. 2005, 288, C429–C434. [Google Scholar] [CrossRef] [PubMed]
- Weinman, E.J.; Light, P.D.; Suki, W.N. Gastrointestinal phosphate handling in CKD and its association with cardiovascular disease. Am. J. Kidney Dis. 2013, 62, 1006–1011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marks, J. The role of SLC34A2 in intestinal phosphate absorption and phosphate homeostasis. Pflugers Arch. 2019, 471, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Katai, K.; Miyamoto, K.; Kishida, S.; Segawa, H.; Nii, T.; Tanaka, H.; Tani, Y.; Arai, H.; Tatsumi, S.; Morita, K.; et al. Regulation of intestinal Na+-dependent phosphate co-transporters by a low-phosphate diet and 1,25-dihydroxyvitamin D3. Biochem. J. 1999, 343 Pt 3, 705–712. [Google Scholar] [CrossRef]
- Bai, L.; Collins, J.F.; Ghishan, F.K. Cloning and characterization of a type III Na-dependent phosphate cotransporter from mouse intestine. Am. J. Physiol. Cell Physiol. 2000, 279, C1135–C1143. [Google Scholar] [CrossRef]
- Kawashima, H.; Kraut, J.A.; Kurokawa, K. Metabolic acidosis suppresses 25-hydroxyvitamin in D3-1alpha-hydroxylase in the rat kidney. Distinct site and mechanism of action. J. Clin. Investig. 1982, 70, 135–140. [Google Scholar] [CrossRef]
- Lee, S.W.; Russell, J.; Avioli, L.V. 25-hydroxycholecalciferol to 1,25-dihydroxycholecalciferol: Conversion impaired by systemic metabolic acidosis. Science 1977, 195, 994–996. [Google Scholar] [CrossRef]
- Adams, N.D.; Gray, R.W.; Lemann, J. The calciuria of increased fixed acid production in humans: Evidence against a role for parathyroid hormone and 1,25(OH)2-vitamin D. Calcif. Tissue Int. 1979, 28, 233–238. [Google Scholar] [CrossRef]
- Gafter, U.; Kraut, J.A.; Lee, D.B.; Silis, V.; Walling, M.W.; Kurokawa, K.; Haussler, M.R.; Coburn, J.W. Effect of metabolic acidosis in intestinal absorption of calcium and phosphorus. Am. J. Physiol. 1980, 239, G480–G484. [Google Scholar] [CrossRef]
- Greenberg, A.J.; McNamara, H.; McCrory, W.W. Metabolic balance studies in primary renal tubular acidosis: Effects of acidosis on external calcium and phosphorus balances. J. Pediatr. 1966, 69, 610–618. [Google Scholar] [CrossRef]
- Litzow, J.R.; Lemann, J., Jr.; Lennon, E.J. The effect of treatment of acidosis on calcium balance in patients with chronic azotemic renal disease. J. Clin. Investig. 1967, 46, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Rumenapf, G.; Schwille, P.O. The influence of oral alkali citrate on intestinal calcium absorption in healthy man. Clin. Sci. 1987, 73, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Gafter, U.; Edelstein, S.; Hirsh, J.; Levi, J. Metabolic acidosis enhances 1,25(OH)2D3-induced intestinal absorption of calcium and phosphorus in rats. Miner. Electrolyte Metab. 1986, 12, 213–217. [Google Scholar] [PubMed]
- Borowitz, S.M.; Said, H.M.; Ghishan, F.K. The effects of metabolic acidosis on jejunal phosphate and glucose transport in weanling rats. Pediatr. Res. 1986, 20, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Stauber, A.; Radanovic, T.; Stange, G.; Murer, H.; Wagner, C.A.; Biber, J. Regulation of intestinal phosphate transport. II. Metabolic acidosis stimulates Na(+)-dependent phosphate absorption and expression of the Na(+)-P(i) cotransporter NaPi-IIb in small intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G501–G506. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Moe, S.M. CKD-mineral and bone disorder: Core curriculum 2011. Am. J. Kidney Dis. 2011, 58, 1022–1036. [Google Scholar] [CrossRef]
- Moe, S.M. Disorders of calcium, phosphorus, and magnesium. Am. J. Kidney Dis. 2005, 45, 213–218. [Google Scholar] [CrossRef]
- Baker, S.B.; Worthley, L.I.G. The Essentials of Calcium, Magnesium and Phosphate Metabolism: Part I. Physiology. Crit. Care Resusc. 2002, 4, 301–306. [Google Scholar] [CrossRef]
- O’Dwyer, L. Disorders of Phosphorus. In Acid-Base and Electrolyte Handbook for Veterinary Technicians; Wiley: Hoboken, NJ, USA, 2016; pp. 66–78. [Google Scholar] [CrossRef]
- Alizadeh Naderi, A.S.; Reilly, R.F. Hereditary disorders of renal phosphate wasting. Nat. Rev. Nephrol. 2010, 6, 657–665. [Google Scholar] [CrossRef]
- Berndt, T.J.; Knox, F.G. Renal Regulation of Phosphate Excretion; Seldin, D.G., Giebisch, G., Eds.; Raven Press: New York, NY, USA, 1992; pp. 2511–2532. [Google Scholar]
- Wrong, O.; Davies, H.E. The excretion of acid in renal disease. Q. J. Med. 1959, 28, 259–313. [Google Scholar] [CrossRef]
- Halperin, M.L.; Kamel, K.S.; Goldstein, M.B. Fluid, Electrolyte and Acid-Base Physiology; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Nowik, M.; Picard, N.; Stange, G.; Capuano, P.; Tenenhouse, H.S.; Biber, J.; Murer, H.; Wagner, C.A. Renal phosphaturia during metabolic acidosis revisited: Molecular mechanisms for decreased renal phosphate reabsorption. Pflugers Arch. 2008, 457, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Karim-Jimenez, Z.; Hernando, N.; Biber, J.; Murer, H. A dibasic motif involved in parathyroid hormone-induced down-regulation of the type IIa NaPi cotransporter. Proc. Natl. Acad. Sci. USA 2000, 97, 12896–12901. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Ito, M.; Kuwahata, M.; Kato, S.; Segawa, H. Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther. Apher. Dial. 2005, 9, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ohkido, I.; Segawa, H.; Yanagida, R.; Nakamura, M.; Miyamoto, K. Cloning, gene structure and dietary regulation of the type-IIc Na/Pi cotransporter in the mouse kidney. Pflugers Arch. 2003, 446, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Alon, U. Tubular disorders of acid-base and phosphate metabolism. Nephron 1985, 40, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Centeno, P.P.; Herberger, A.; Mun, H.C.; Tu, C.; Nemeth, E.F.; Chang, W.; Conigrave, A.D.; Ward, D.T. Phosphate acts directly on the calcium-sensing receptor to stimulate parathyroid hormone secretion. Nat. Commun. 2019, 10, 4693. [Google Scholar] [CrossRef] [PubMed]
- Moe, O.W.; Huang, C.L. Hypercalciuria from acid load: Renal mechanisms. J. Nephrol. 2006, 19 (Suppl. S9), S53–S61. [Google Scholar] [PubMed]
- Kim, G.H. Renal Mechanisms for Hypercalciuria Induced by Metabolic Acidosis. Am. J. Nephrol. 2022, 53, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Ambuhl, P.M.; Zajicek, H.K.; Wang, H.; Puttaparthi, K.; Levi, M. Regulation of renal phosphate transport by acute and chronic metabolic acidosis in the rat. Kidney Int. 1998, 53, 1288–1298. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J. Metabolic Acidosis in Chronic Kidney Disease: Pathogenesis, Clinical Consequences, and Treatment. Electrolyte Blood Press 2021, 19, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, P.; Isakova, T.; Asplin, J.; Hamm, L.; Dobre, M.; Rahman, M.; Sharma, K.; Leonard, M.; Miller, E., 3rd; Jaar, B.; et al. Acid Load and Phosphorus Homeostasis in CKD. Am. J. Kidney Dis. 2017, 70, 541–550. [Google Scholar] [CrossRef]
- Villa-Bellosta, R.; Sorribas, V. Compensatory regulation of the sodium/phosphate cotransporters NaPi-IIc (SCL34A3) and Pit-2 (SLC20A2) during Pi deprivation and acidosis. Pflugers Arch. 2010, 459, 499–508. [Google Scholar] [CrossRef]
- Krieger, N.S.; Culbertson, C.D.; Kyker-Snowman, K.; Bushinsky, D.A. Metabolic acidosis increases fibroblast growth factor 23 in neonatal mouse bone. Am. J. Physiol. Renal Physiol. 2012, 303, F431–F436. [Google Scholar] [CrossRef]
- Campion, K.L.; McCormick, W.D.; Warwicker, J.; Khayat, M.E.; Atkinson-Dell, R.; Steward, M.C.; Delbridge, L.W.; Mun, H.C.; Conigrave, A.D.; Ward, D.T. Pathophysiologic changes in extracellular pH modulate parathyroid calcium-sensing receptor activity and secretion via a histidine-independent mechanism. J. Am. Soc. Nephrol. 2015, 26, 2163–2171. [Google Scholar] [CrossRef]
- Disthabanchong, S.; Martin, K.J.; McConkey, C.L.; Gonzalez, E.A. Metabolic acidosis up-regulates PTH/PTHrP receptors in UMR 106-01 osteoblast-like cells. Kidney Int. 2002, 62, 1171–1177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bushinsky, D.A.; Nilsson, E.L. Additive effects of acidosis and parathyroid hormone on mouse osteoblastic and osteoclastic function. Am. J. Physiol. 1995, 269, C1364–C1370. [Google Scholar] [CrossRef] [PubMed]
- Lopez, I.; Aguilera-Tejero, E.; Felsenfeld, A.J.; Estepa, J.C.; Rodriguez, M. Direct effect of acute metabolic and respiratory acidosis on parathyroid hormone secretion in the dog. J. Bone Miner. Res. 2002, 17, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Hamm, L.L.; Simon, E.E. Roles and mechanisms of urinary buffer excretion. Am. J. Physiol. 1987, 253, F595–F605. [Google Scholar] [CrossRef] [PubMed]
- Emmett, M.; Seldin, D.W. Disturbances in acid-base balance during hypophosphatemia and phosphate depletion. Adv. Exp. Med. Biol. 1978, 103, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Bonjour, J.P. Calcium and phosphate: A duet of ions playing for bone health. J. Am. Coll Nutr. 2011, 30, 438S–448S. [Google Scholar] [CrossRef]
- Oh, M.S. Irrelevance of bone buffering to acid-base homeostasis in chronic metabolic acidosis. Nephron 1991, 59, 7–10. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Wolbach, W.; Sessler, N.E.; Mogilevsky, R.; Levi-Setti, R. Physicochemical effects of acidosis on bone calcium flux and surface ion composition. J. Bone Miner. Res. 1993, 8, 93–102. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Krieger, N.S. Effects of acid on bone. Kidney Int. 2022, 101, 1160–1170. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Lam, B.C.; Nespeca, R.; Sessler, N.E.; Grynpas, M.D. Decreased bone carbonate content in response to metabolic, but not respiratory, acidosis. Am. J. Physiol. 1993, 265, F530–F536. [Google Scholar] [CrossRef]
- Lemann, J.; Litzow, J.R.; Lennon, E.J. The effects of chronic acid loads in normal man: Further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J. Clin. Investig. 1966, 45, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J.A.; Mishler, D.R.; Kurokawa, K. Effect of colchicine and calcitonin on calcemic response to metabolic acidosis. Kidney Int. 1984, 25, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, E.M.; Mc, C.R.; Spray, C.M. The chemical composition of the human body. Clin. Sci. 1951, 10, 113–125. [Google Scholar] [PubMed]
- Lemann, J., Jr.; Bushinsky, D.A.; Hamm, L.L. Bone buffering of acid and base in humans. Am. J. Physiol. Renal Physiol. 2003, 285, F811–F832. [Google Scholar] [CrossRef] [PubMed]
- Guntupalli, J.; Eby, B.; Lau, K. Mechanism for the phosphaturia of NH4Cl: Dependence on acidemia but not on diet PO4 or PTH. Am. J. Physiol. 1982, 242, F552–F560. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.A.; Wong, N.L.; Dirks, J.H. Effects of metabolic acidosis and alkalosis on sodium and calcium transport in the dog kidney. Kidney Int. 1979, 15, 520–533. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.D.; Lemann, J., Jr.; Lennon, E.J.; Relman, A.S. Production, Excretion, and Net Balance of Fixed Acid in Patients with Renal Acidosis. J. Clin. Investig. 1965, 44, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Lemann, J., Jr. Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: A review. Nephron 1999, 81 (Suppl. S1), 18–25. [Google Scholar] [CrossRef]
- Morris, R.C., Jr.; Frassetto, L.A.; Schmidlin, O.; Forman, A.; Sebastian, A. Expression of osteoporosis as determined by diet-disordered electrolyte and acid-base metabolism. In Nutritional Aspects of Osteoporosis; Burckhardt, P.D.-H., Heaney, R.P., Eds.; Academic Press: San Diego, CA, USA, 2001; pp. 357–378. [Google Scholar]
- Lemann, J., Jr.; Adams, N.D.; Wilz, D.R.; Brenes, L.G. Acid and mineral balances and bone in familial proximal renal tubular acidosis. Kidney Int. 2000, 58, 1267–1277. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Chabala, J.M.; Gavrilov, K.L.; Levi-Setti, R. Effects of in vivo metabolic acidosis on midcortical bone ion composition. Am. J. Physiol. 1999, 277, F813–F819. [Google Scholar] [CrossRef]
- Bushinsky, D.A.; Smith, S.B.; Gavrilov, K.L.; Gavrilov, L.F.; Li, J.; Levi-Setti, R. Acute acidosis-induced alteration in bone bicarbonate and phosphate. Am. J. Physiol. Renal Physiol. 2002, 283, F1091–F1097. [Google Scholar] [CrossRef] [PubMed]
- Bushinsky, D.A.; Smith, S.B.; Gavrilov, K.L.; Gavrilov, L.F.; Li, J.; Levi-Setti, R. Chronic acidosis-induced alteration in bone bicarbonate and phosphate. Am. J. Physiol. Renal Physiol. 2003, 285, F532–F539. [Google Scholar] [CrossRef] [PubMed]
- Kaye, M. Hypocalcemia after an acute phosphate load is secondary to reduced calcium efflux from bone: Studies in patients with minimal renal function and varying parathyroid activity. J. Am. Soc. Nephrol. 1995, 6, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Dickens, F. The citric acid content of animal tissues, with reference to its occurrence in bone and tumour. Biochem. J. 1941, 35, 1011–1023. [Google Scholar] [CrossRef]
- Costello, L.C.; Chellaiah, M.; Zou, J.; Franklin, R.B.; Reynolds, M.A. The status of citrate in the hydroxyapatite/collagen complex of bone; and Its role in bone formation. J. Regen. Med. Tissue Eng. 2014, 3, 4. [Google Scholar] [CrossRef]
- Moe, O.W.; Maalouf, N.M.; Sakhaee, K.; Lederer, E. Preclinical and Clinical Evidence of Effect of Acid on Bone Health. Adv. Chronic. Kidney Dis. 2022, 29, 381–394. [Google Scholar] [CrossRef]
- Apata, I.W.; Bailey, J.L.; Franch, H.A. Metabolic and nutritional responses to acidemia and alkalemia. In Nutritional Management of Renal Disease; Kopple, J.D., Massry, S.G., Kalantar-Zadeh, K., Fouque, D., Eds.; Academic Press: San Diego, CA, USA, 2022; pp. 127–145. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salcedo-Betancourt, J.D.; Moe, O.W. The Effects of Acid on Calcium and Phosphate Metabolism. Int. J. Mol. Sci. 2024, 25, 2081. https://doi.org/10.3390/ijms25042081
Salcedo-Betancourt JD, Moe OW. The Effects of Acid on Calcium and Phosphate Metabolism. International Journal of Molecular Sciences. 2024; 25(4):2081. https://doi.org/10.3390/ijms25042081
Chicago/Turabian StyleSalcedo-Betancourt, Juan D., and Orson W. Moe. 2024. "The Effects of Acid on Calcium and Phosphate Metabolism" International Journal of Molecular Sciences 25, no. 4: 2081. https://doi.org/10.3390/ijms25042081
APA StyleSalcedo-Betancourt, J. D., & Moe, O. W. (2024). The Effects of Acid on Calcium and Phosphate Metabolism. International Journal of Molecular Sciences, 25(4), 2081. https://doi.org/10.3390/ijms25042081




