Effect of Temperature on the Development of Stages of Spermatogenesis and the Functionality of Sertoli Cells In Vitro
Abstract
1. Introduction
2. Results
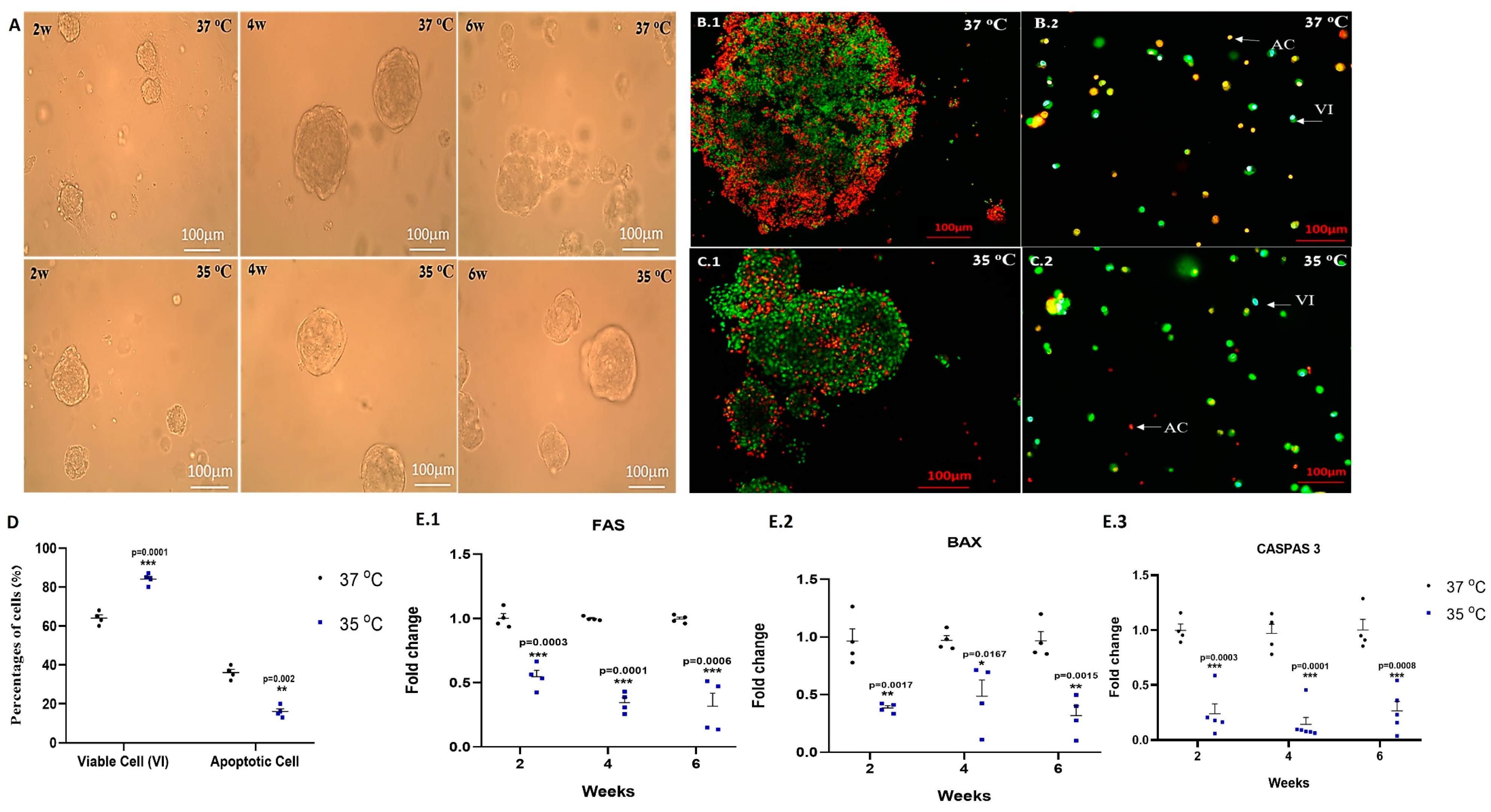
2.1. Effect of Temperature on Apoptosis of the Growing Cells In Vitro
2.2. In Vitro Development of Organoid-like Structure Shows a Cellular Composition Similar to the Seminiferous Tubules
2.3. Effect of Temperature on the Development of Different Stages of Spermatogenesis In Vitro
2.4. Effect of Temperature on the Development of the Pre-Meiotic VASA and GFR-Alpha Positive Cells In Vitro
2.5. Effect of Temperature on the Development of the Meiotic and Post-Meiotic Cells In Vitro
2.6. Effect of Temperature on the Development of Haploid Cells In Vitro
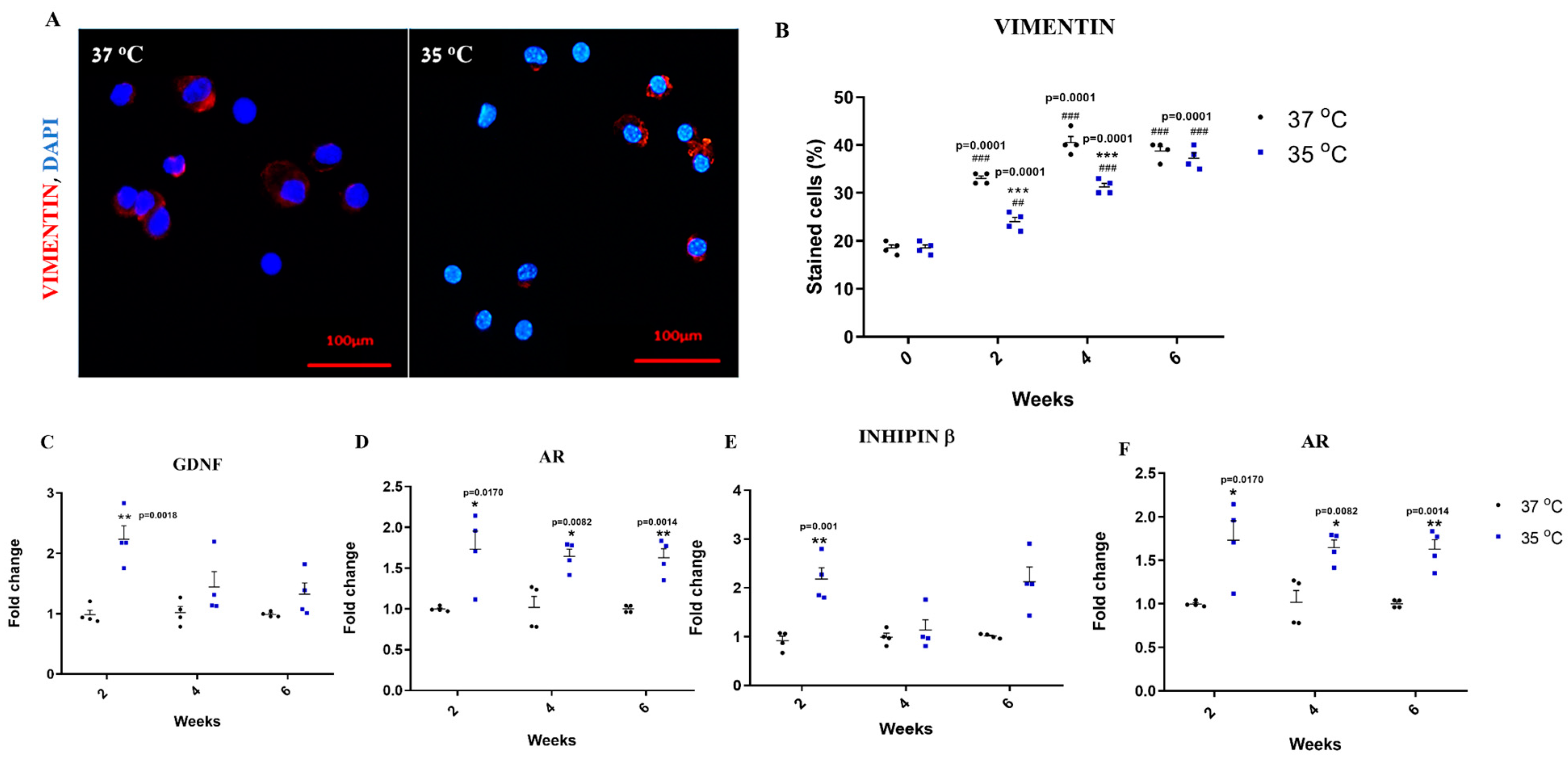
2.7. Effect of Temperature on the Development and Functionality of Sertoli Cells In Vitro
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Isolation of Seminiferous Tubular Cells
4.3. Methylcellulose Culture System (MCS)
4.4. Immunofluorescence Staining of the Cells
4.5. Confocal Microscopy
4.6. Acridine Orange (AO) and Ethidium Bromide (EtBr) Staining
4.7. RNA Extraction and Real-Time PCR Analysis
4.8. DNA Content Analysis
4.9. Data Handling and Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, C.Y.; Mruk, D.D. The Biology of Spermatogenesis: The Past, Present and Future. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Renato de Franca, L. Spermatogenesis and Cycle of the Seminiferous Epithelium. Adv. Exp. Med. Biol. 2008, 636, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oatley, J.M.; Brinster, R.L. The Germline Stem Cell Niche Unit in Mammalian Testes. Physiol. Rev. 2012, 92, 577–595. [Google Scholar] [CrossRef] [PubMed]
- Huhtaniemi, I.; Toppari, J. Endocrine, Paracrine and Autocrine Regulation of Testicular Steroidogenesis. Adv. Exp. Med. Biol. 1995, 377, 33–54. [Google Scholar] [CrossRef] [PubMed]
- Matthiesson, K.L.; McLachlan, R.I.; O’Donnell, L.; Frydenberg, M.; Robertson, D.M.; Stanton, P.G.; Meachem, S.J. The Relative Roles of Follicle-Stimulating Hormone and Luteinizing Hormone in Maintaining Spermatogonial Maturation and Spermiation in Normal Men. J. Clin. Endocrinol. Metab. 2006, 91, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Testosterone Signaling and the Regulation of Spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine Control of Spermatogenesis: Role of FSH and LH/Testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef]
- Crespo, D.; Assis, L.H.C.; Furmanek, T.; Bogerd, J.; Schulz, R.W. Expression Profiling Identifies Sertoli and Leydig Cell Genes as Fsh Targets in Adult Zebrafish Testis. Mol. Cell. Endocrinol. 2016, 437, 237–251. [Google Scholar] [CrossRef]
- Niederberger, C.S.; Shubhada, S.; Kim, S.J.; Lamb, D.J. Paracrine Factors and the Regulation of Spermatogenesis. World J. Urol. 1993, 11, 120–128. [Google Scholar] [CrossRef]
- Kubota, H.; Avarbock, M.R.; Brinster, R.L. Growth Factors Essential for Self-Renewal and Expansion of Mouse Spermatogonial Stem Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 16489–16494. [Google Scholar] [CrossRef]
- Nakamura, M.; Namiki, M.; Okuyama, A.; Matsui, T.; Doi, Y.; Takeyama, M.; Fujioka, H.; Nishimune, Y.; Matsumoto, K. Sonoda, Temperature Sensitivity of Human Spermatogonia and Spermatocytes in Vitro. Arch. Androl. 1987, 19, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-X. Temperature Control of Spermatogenesis and Prospect of Male Contraception. Front. Biosci. (Schol. Ed.) 2010, 2, 730–755. [Google Scholar] [CrossRef]
- Kandeel, F.R.; Swerdloff, R.S. Role of Temperature in Regulation of Spermatogenesis and the Use of Heating as a Method for Contraception. Fertil. Steril. 1988, 49, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mieusset, R.; Bujan, L. Testicular Heating and Its Possible Contributions to Male Infertility: A Review. Int. J. Androl. 1995, 18, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Abdelhamid, M.; Walschaerts, M.; Ahmad, G.; Mieusset, R.; Bujan, L.; Hamdi, S. Mild Experimental Increase in Testis and Epididymis Temperature in Men: Effects on Sperm Morphology According to Spermatogenesis Stages. Transl. Androl. Urol. 2019, 8, 651–665. [Google Scholar] [CrossRef]
- Shahat, A.M.; Rizzoto, G.; Kastelic, J.P. Amelioration of Heat Stress-Induced Damage to Testes and Sperm Quality. Theriogenology 2020, 158, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Thonneau, P.; Bujan, L.; Multigner, L.; Mieusset, R. Occupational Heat Exposure and Male Fertility: A Review. Hum. Reprod. 1998, 13, 2122–2125. [Google Scholar] [CrossRef]
- Jhun, H.; Lee, W.Y.; Park, J.K.; Hwang, S.G.; Park, H.J. Transcriptomic Analysis of Testicular Gene Expression in a Dog Model of Experimentally Induced Cryptorchidism. Cells 2022, 11, 2476. [Google Scholar] [CrossRef]
- Sakib, S.; Lara, N.d.L.e.M.; Huynh, B.C.; Dobrinski, I. Organotypic Rat Testicular Organoids for the Study of Testicular Maturation and Toxicology. Front. Endocrinol. 2022, 13, 892342. [Google Scholar] [CrossRef]
- Gao, W.-J.; Li, H.-X.; Feng, J.; Lu, X.-R.; Yin, P.-L.; Jia, H.; Ma, W.-Z. Transcriptome Analysis in High Temperature Inhibiting Spermatogonial Stem Cell Differentiation In Vitro. Reprod. Sci. 2023, 30, 1938–1951. [Google Scholar] [CrossRef]
- Gan, M.; Jing, Y.; Xie, Z.; Ma, J.; Chen, L.; Zhang, S.; Zhao, Y.; Niu, L.; Wang, Y.; Li, X.; et al. Potential Function of Testicular MicroRNAs in Heat-Stress-Induced Spermatogenesis Disorders. Int. J. Mol. Sci. 2023, 24, 8809. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.; Zeng, Z.; Tang, L.; Cheng, G.; Xia, W.; Zhu, C. Next-Generation Sequencing-Based MicroRNA Profiling of Mice Testis Subjected to Transient Heat Stress. Oncotarget 2017, 8, 111672–111682. [Google Scholar] [CrossRef] [PubMed]
- Vogler, C.J.; Bame, J.H.; Dejarnette, J.M.; McGilliard, M.L.; Saacke, R.G. Effects of Elevated Testicular Temperature on Morphology Characteristics of Ejaculated Spermatozoa in the Bovine. Theriogenology 1993, 40, 1207–1219. [Google Scholar] [CrossRef]
- Hirano, K.; Nonami, Y.; Nakamura, Y.; Sato, T.; Sato, T.; Ishiguro, K.-i.; Ogawa, T.; Yoshida, S. Temperature Sensitivity of DNA Double-Strand Break Repair Underpins Heat-Induced Meiotic Failure in Mouse Spermatogenesis. Commun. Biol. 2022, 5, 504. [Google Scholar] [CrossRef]
- Aldahhan, R.A.; Stanton, P.G. Heat Stress Response of Somatic Cells in the Testis. Mol. Cell. Endocrinol. 2021, 527, 111216. [Google Scholar] [CrossRef]
- Huleihel, M.; Lunenfeld, E. Approaches and Technologies in Male Fertility Preservation. Int. J. Mol. Sci. 2020, 21, 5471. [Google Scholar] [CrossRef]
- Abu Elhija, M.; Lunenfeld, E.; Schlatt, S.; Huleihel, M. Differentiation of Murine Male Germ Cells to Spermatozoa in a Soft Agar Culture System. Asian J. Androl. 2012, 14, 285–293. [Google Scholar] [CrossRef]
- Abofoul-Azab, M.; AbuMadighem, A.; Lunenfeld, E.; Kapelushnik, J.; Shi, Q.; Pinkas, H.; Huleihel, M. Development of Postmeiotic Cells In Vitro from Spermatogonial Cells of Prepubertal Cancer Patients. Stem Cells Dev. 2018, 27, 1007–1020. [Google Scholar] [CrossRef]
- AbuMadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of Spermatogenesis In Vitro in Three-Dimensional Culture from Spermatogonial Cells of Busulfan-Treated Immature Mice. Int. J. Mol. Sci. 2018, 19, 3804. [Google Scholar] [CrossRef]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of Three-Dimensional Culture Systems to Study Mammalian Spermatogenesis, with an Emphasis on the Rhesus Monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Medrano, J.V.; Vilanova-Pérez, T.; Fornés-Ferrer, V.; Navarro-Gomezlechon, A.; Martínez-Triguero, M.L.; García, S.; Gómez-Chacón, J.; Povo, I.; Pellicer, A.; Andrés, M.M.; et al. Influence of Temperature, Serum, and Gonadotropin Supplementation in Short- and Long-Term Organotypic Culture of Human Immature Testicular Tissue. Fertil. Steril. 2018, 110, 1045–1057.e3. [Google Scholar] [CrossRef] [PubMed]
- Kahsai, T.Z.; Enders, G.C.; Gunwar, S.; Brunmark, C.; Rgen Wieslander, J.; Kalluri, R.; Zhoui, J.; Noelken, M.E.; Hudson, B.G. Seminiferous Tubule Basement Membrane Composition and Organization of Type IV Collagen Chains, and the Linkage of A3(IV) and A5(IV) Chains. J. Biol. Chem. 1997, 272, 17023–17032. [Google Scholar] [CrossRef] [PubMed]
- Abumadighem, A.; Shuchat, S.; Lunenfeld, E.; Yossifon, G.; Huleihel, M. Testis on a Chip—A Microfluidic Three-Dimensional Culture System for the Development of Spermatogenesis in vitro. Biofabrication 2022, 14, 035004. [Google Scholar] [CrossRef] [PubMed]
- Osuru, H.P.; Monroe, J.E.; Chebolu, A.P.; Akamune, J.; Pramoonjago, P.; Ranpura, S.A.; Reddi, P.P. The Acrosomal Protein SP-10 (Acrv1) Is an Ideal Marker for Staging of the Cycle of Seminiferous Epithelium in the Mouse. Mol. Reprod. Dev. 2014, 81, 896–907. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Khan, R.; Yu, C.; Alsheimer, M.; Jiang, X.; Ma, H.; Shi, Q. The Testis-Specific LINC Component SUN3 Is Essential for Sperm Head Shaping during Mouse Spermiogenesis. J. Biol. Chem. 2020, 295, 6289–6298. [Google Scholar] [CrossRef] [PubMed]
- Göb, E.; Schmitt, J.; Benavente, R.; Alsheimer, M. Mammalian Sperm Head Formation Involves Different Polarization of Two Novel LINC Complexes. PLoS ONE 2010, 5, e12072. [Google Scholar] [CrossRef]
- Tang, A.; Yan, Q.; Sun, L.; Diao, R.; Yu, Z.; Zhang, Z.; Gui, Y.; Cai, Z. Developmental Expression of ACRV1 in Humans and Mice. Andrologia 2012, 44, 16–22. [Google Scholar] [CrossRef]
- Shi, J.F.; Li, Y.K.; Ren, K.; Xie, Y.J.; Yin, W.D.; Mo, Z.C. Characterization of Cholesterol Metabolism in Sertoli Cells and Spermatogenesis (Review). Mol. Med. Rep. 2018, 17, 705–713. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. Sertoli-Sertoli and Sertoli-Germ Cell Interactions and Their Significance in Germ Cell Movement in the Seminiferous Epithelium during Spermatogenesis. Endocr. Rev. 2004, 25, 747–806. [Google Scholar] [CrossRef]
- Hagenäs, L.; Ritzén, E.M.; Svensson, J.; Hansson, V.; Purvis, K. Temperature Dependence of Sertoli Cell Function. Int. J. Androl. 1978, 1, 449–458. [Google Scholar] [CrossRef]
- De Alvarenga, É.R.; De França, L.R. Effects of Different Temperatures on Testis Structure and Function, with Emphasis on Somatic Cells, in Sexually Mature Nile Tilapias (Oreochromis niloticus). Biol. Reprod. 2009, 80, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Z.-W.; Fang, T.; Zhang, Y.-Q.; Chen, L.; Du, Z.-Q.; Yang, C.-X. Identification of Internal Reference Genes for Porcine Immature Sertoli Cells under Heat Stress. Reprod. Domest. Anim. 2022, 57, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Rockett, J.C.; Mapp, F.L.; Garges, J.B.; Luft, J.C.; Mori, C.; Dix, D.J. Effects of Hyperthermia on Spermatogenesis, Apoptosis, Gene Expression, and Fertility in Adult Male Mice. Biol. Reprod. 2001, 65, 229–239. [Google Scholar] [CrossRef] [PubMed]





| Antibody | Dilution | Catalog Number | Company | |
|---|---|---|---|---|
| Collagen (rabbit) | Rabbit polyclonal anti-collagen IV | 0.1111111 | ab-6586 | Abcam, Cambridge, UK |
| Vimentin (rabbit) | Rabbit polyclonal anti-vimentin | 0.1111111 | sc-7557 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| Vimentin (mouse) | Mouse monoclonal anti-vimentin | 0.1805556 | sc-6260 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| SOX9 | Rabbit polyclonal anti-SOX9 | 1:50 | ab185966 | Abcam, Cambridge, UK |
| 3βHSD | Goat polyclonal anti-3βHSD | 1:50 | sc30820 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| Alpha smooth muscle actin (ASMA) | Goat polyclonal anti-ASMA | 1:50 | ab21027 | Abcam, Cambridge, UK |
| VASA | Rabbit Polyclonal anti VASA | 0.1805556 | NBP2-24558 | NOVUS biologicals, Littleton, Centennial, CO, USA |
| GFR-α | Mouse monoclonal anti GFR-α | 1:50 | sc-271546 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| BOULE | Mouse monoclonal anti boule | 0.1111111 | sc-166660 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| CREM (rabbit) | Rabbit polyclonal anti CREM | 0.1805556 | 12131-1-AP | ProteinTech Group, Chicago, IL, USA |
| CREM (mouse) | Mouse monoclonal anti CREM | 1:50 | sc-390426 | Santa Cruz Biotechnology, Santa Cruz, CA, USA |
| ACROSIN | Rabbit polyclonal anti Acrosin | 0.7361111 | NBP-14260 | NOVUS biologicals Littleton, Centennial, CO, USA |
| Cy3 | Cy™3 AffiniPure F(ab’)₂ Fragment Donkey Anti-mouse\rabbit\goat IgG (H + L) | Mouse 1:1000 | 715-006-150 | Jackson ImmunoResearch Laboratories, West Grove, PA, USA |
| Rabbit 1:700 | 711-006-152 | |||
| Goat 1:1000 | 705-546-147 | |||
| Alexa-flour 488 | Alexa Fluor® 488 AffiniPure F(ab’)₂ Fragment Donkey Anti-mouse\rabbit\goat IgG (H + L) | Mouse 1:100 | 715-006-150 | |
| Rabbit 1:200 | 711-006-152 | |||
| Goat 1:200 | 705-546-147 | |||
| Primer | Gene Full Name | Primer Sequence |
|---|---|---|
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | Fw-5′-ACCACAGTCCATGCCATCAC |
| Rw-5′-CACCACCCTGTTGCTGTAGCC | ||
| VASA | ATP-dependent RNA helicase DDX4 | Fw-5′-AGTATTCATGGTGATCGGGAGCAG |
| Rw-5′-GCAACAAGAACTGGGCACTTTCCA | ||
| GFR-α | GFR-α | Fw-5′-CAGTTTTCGTCTGCTGAGGTTG |
| RW-5-TTCTGCTCAAAGTGGCTCCAT | ||
| BOULE | boule homolog, RNA binding protein | Fw-5′-AACCCAACAAGTGGCCCAAGATAC |
| Rw-5′-CTTTGGACACTCCAGCTCTGTCAT | ||
| CREM | cAMP responsive element modulator | Fw-5′-TTCTTTCACGAAGACCCTCA |
| Rw-5′-TGTTAGGTGGTGTCCCTTCT | ||
| PROTAMINE | Protamine 1 | Fw-5′-TCCATCAAAACTCCTGCGTGA |
| Rw-5′-AGGTGGCATTGTTCCTTAGCA | ||
| ACROSIN | Acrosin prepropeptide | Fw-5′-TGTCCGTGGTTGCCAAGGATAACA |
| Rw-5′-AATCCGGGTACCTGCTTGTGAGTT | ||
| ACRV1 | Activin A receptor type I | FW-5′-GCTTCGGTTCAGCAACTTTC |
| RW-5′-ACCACTCAGAGTCTTCTCATCTA | ||
| SUN-3 | SUN domain-containing protein 3 | FW-5′-GAAGCTGGGACCTCAGAAAG |
| RW-5′-TATCCGGAGGCATCTCATAGT | ||
| AR | Androgen receptor | Fw-5′-TTGGGTGTGGAAGCATTGGA |
| Rw-5′-TGGCGTAACCTCCCTTGAAA | ||
| Inhibin-β | Inhibin beta B chain | FW-5′-TCAGCTTTGCAGAGACAGAT |
| RW-5′-TCTTGGAAGTACACCTTGACC | ||
| GDNF | Glial cell line-derived neurotrophic factor | FW-5′-GCCCCTGCTTTCTATCTGCT |
| RW-5′-AGCCTTCTGAATGCGTGGTT | ||
| ABP | sex hormone binding globulin | Fw-5′-GCAGCATGAGGATTGCACTA |
| Rw-5′-CATGAGGCTGGGGAATGTCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorban, A.; Lunenfeld, E.; Huleihel, M. Effect of Temperature on the Development of Stages of Spermatogenesis and the Functionality of Sertoli Cells In Vitro. Int. J. Mol. Sci. 2024, 25, 2160. https://doi.org/10.3390/ijms25042160
Jorban A, Lunenfeld E, Huleihel M. Effect of Temperature on the Development of Stages of Spermatogenesis and the Functionality of Sertoli Cells In Vitro. International Journal of Molecular Sciences. 2024; 25(4):2160. https://doi.org/10.3390/ijms25042160
Chicago/Turabian StyleJorban, Areej, Eitan Lunenfeld, and Mahmoud Huleihel. 2024. "Effect of Temperature on the Development of Stages of Spermatogenesis and the Functionality of Sertoli Cells In Vitro" International Journal of Molecular Sciences 25, no. 4: 2160. https://doi.org/10.3390/ijms25042160
APA StyleJorban, A., Lunenfeld, E., & Huleihel, M. (2024). Effect of Temperature on the Development of Stages of Spermatogenesis and the Functionality of Sertoli Cells In Vitro. International Journal of Molecular Sciences, 25(4), 2160. https://doi.org/10.3390/ijms25042160








