SMA20/PMIS2 Is a Rapidly Evolving Sperm Membrane Alloantigen with Possible Species-Divergent Function in Fertilization
Abstract
:1. Introduction
2. Results
2.1. SMA20 cDNA Cloning and Protein Sequence Analysis
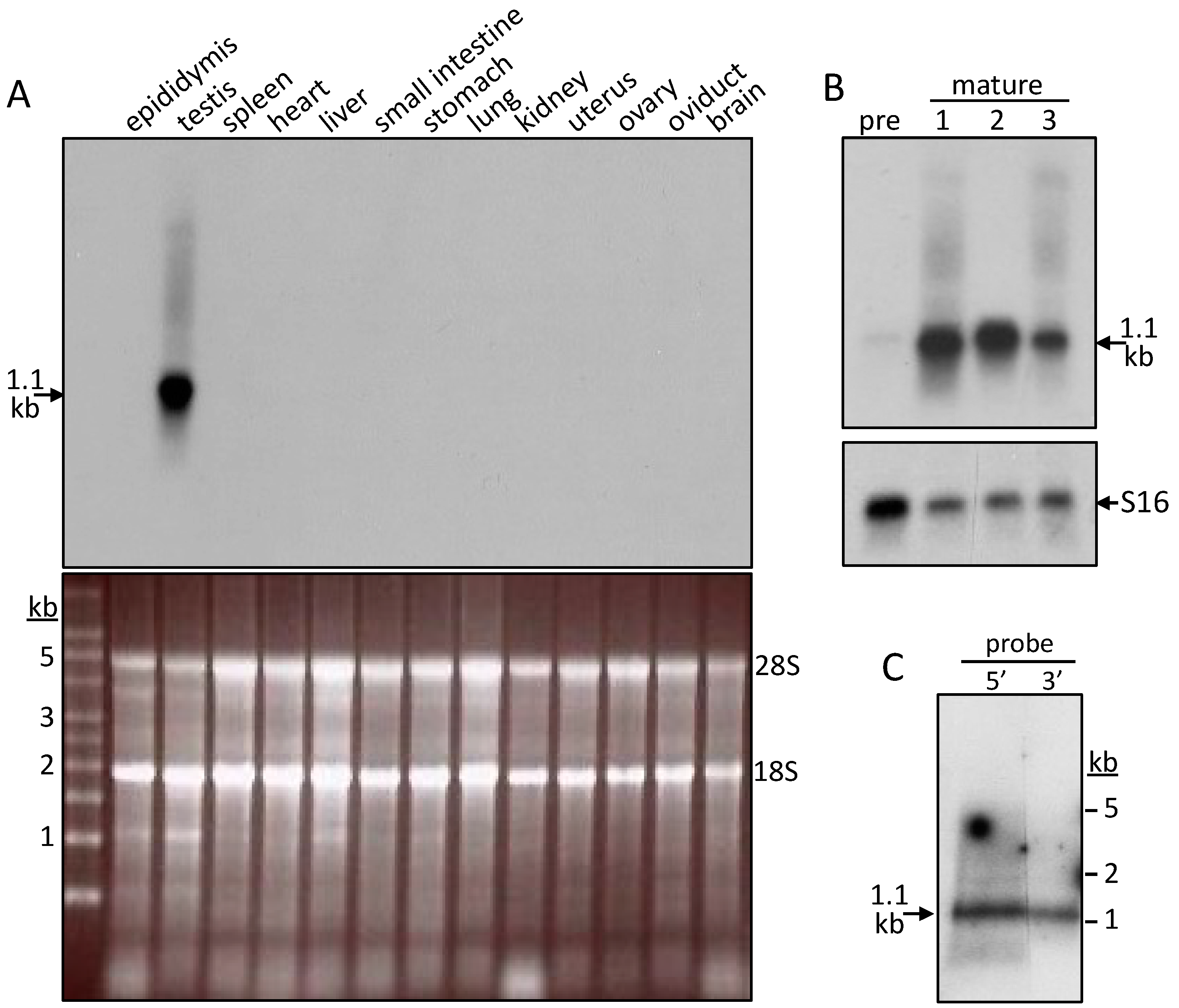
2.2. Tissue Distribution of the SMA20 Transcript
2.3. Characterization of SMA20 in Sperm Membrane Fractions
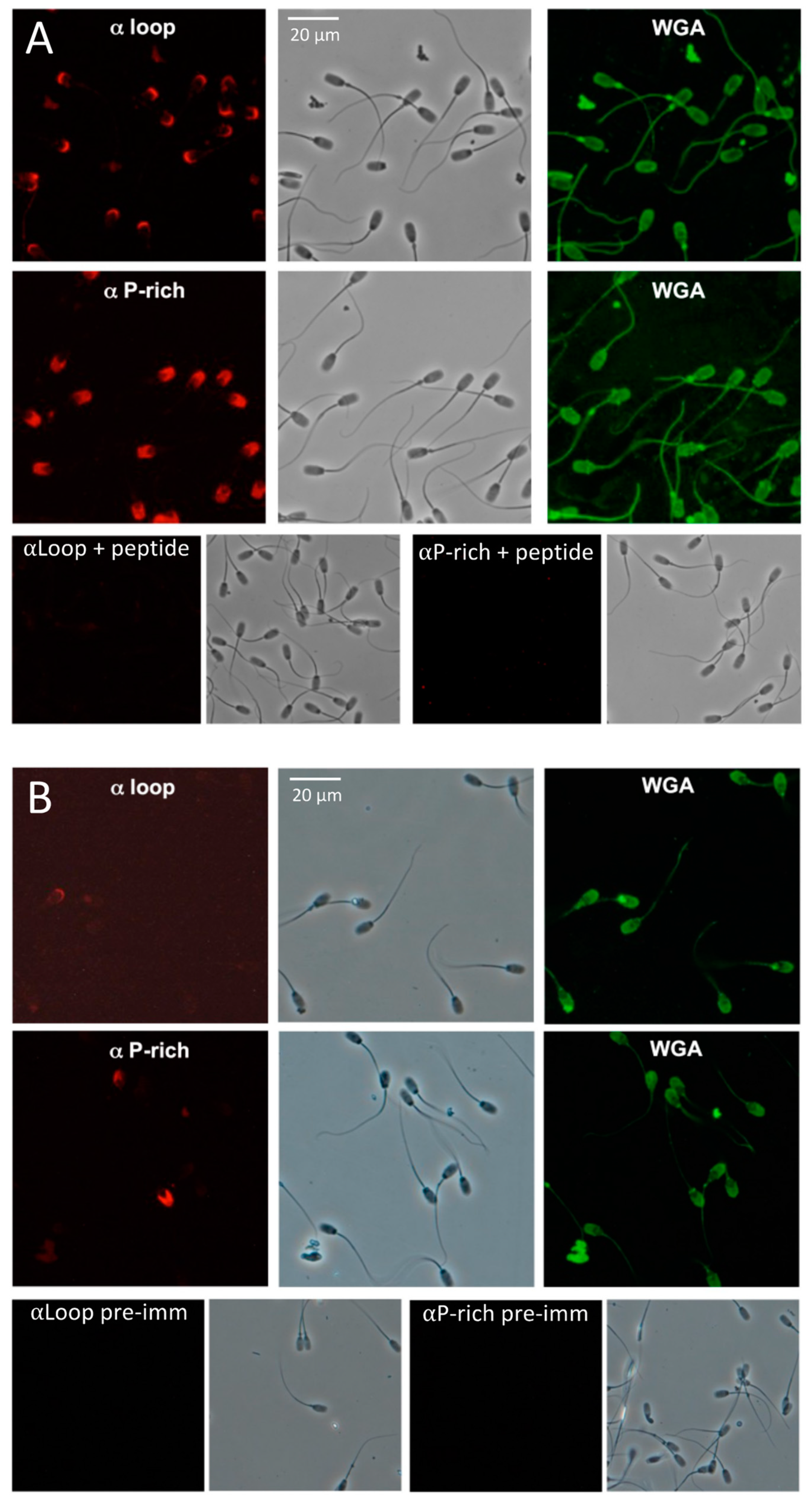
2.4. SMA20 Localization in the Apical Head of Spermatozoa
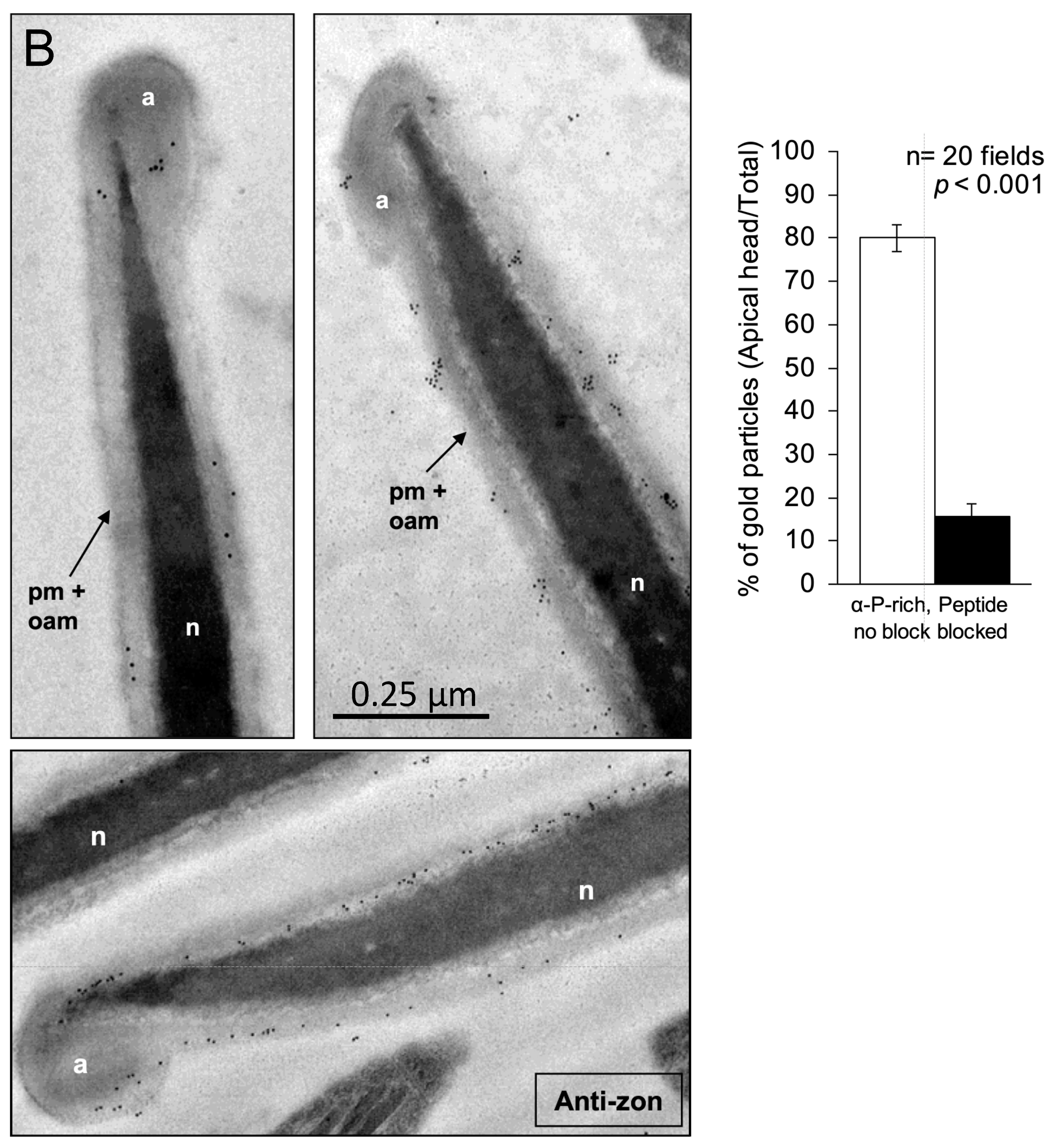
2.5. Ultrastructural Localization of SMA20
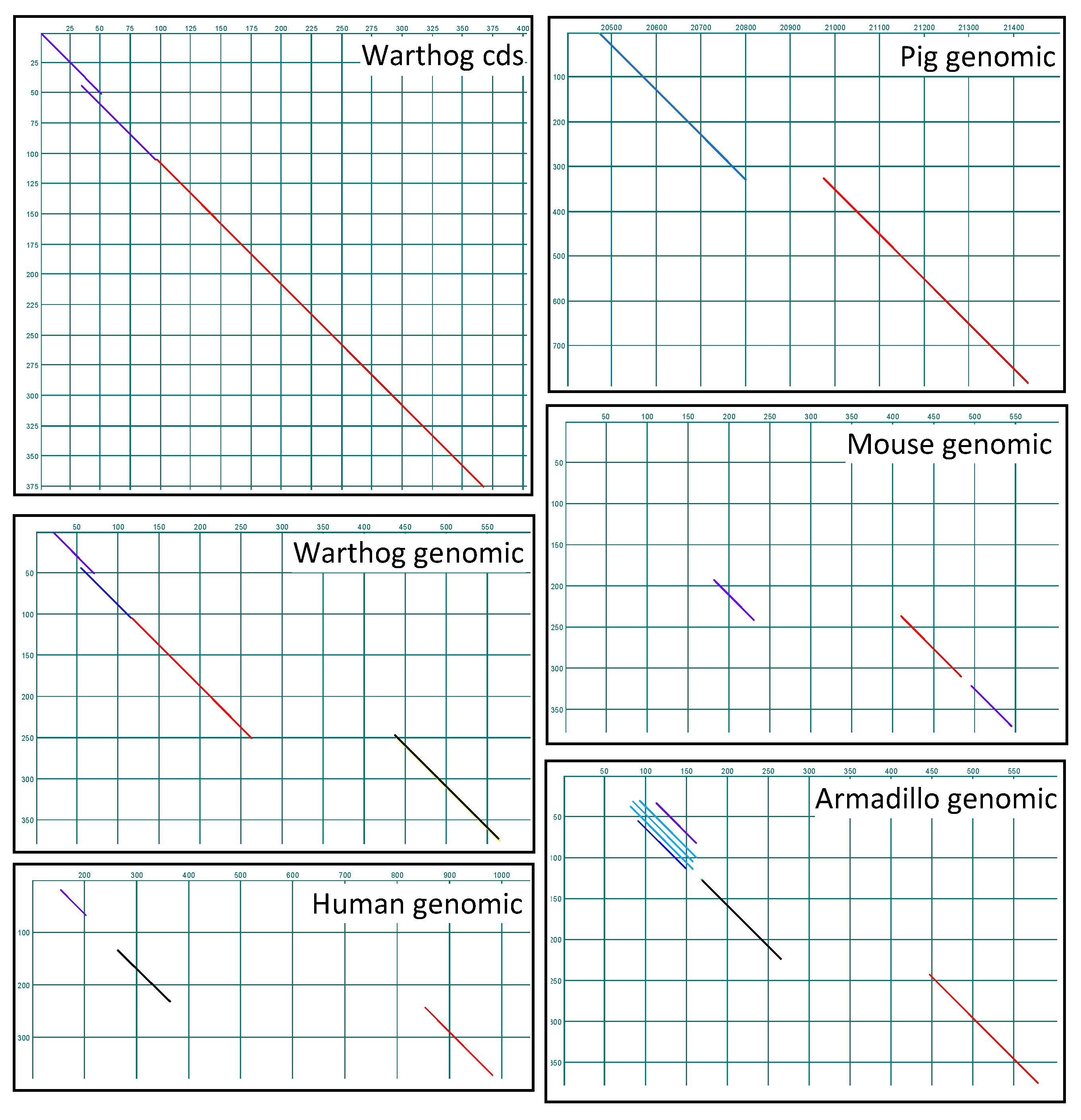
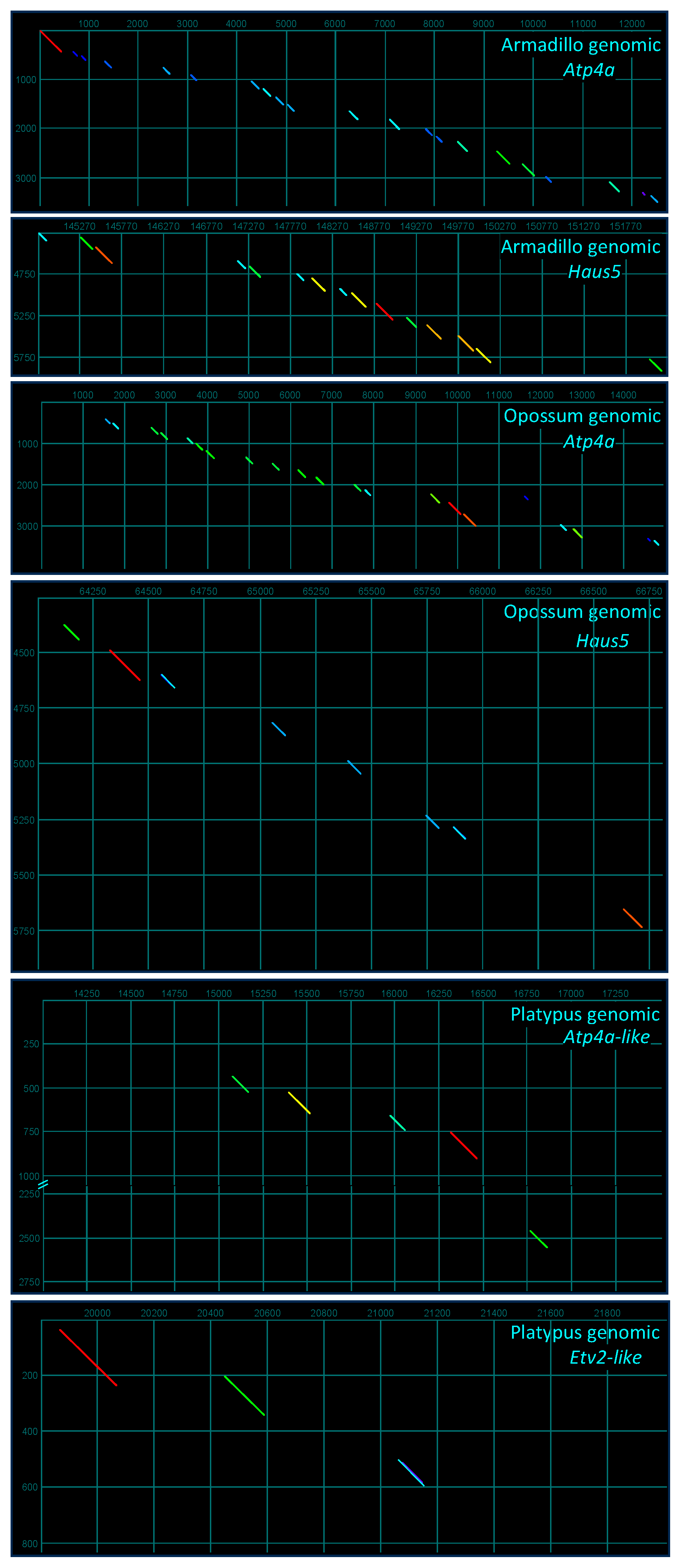
2.6. Identification of SMA20 Orthologs and Genomic Loci
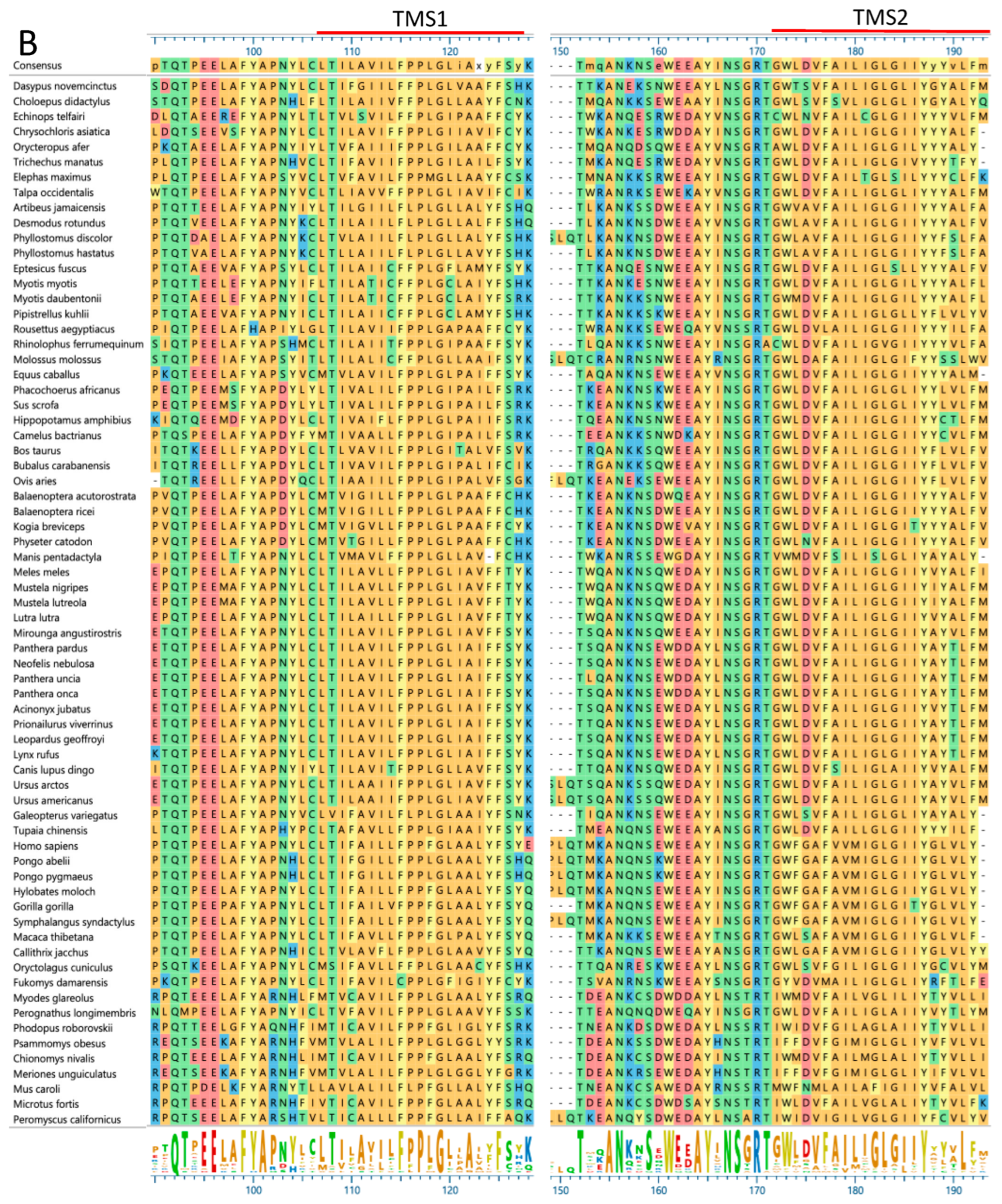
2.7. Rapid Evolution of SMA20/PMIS2
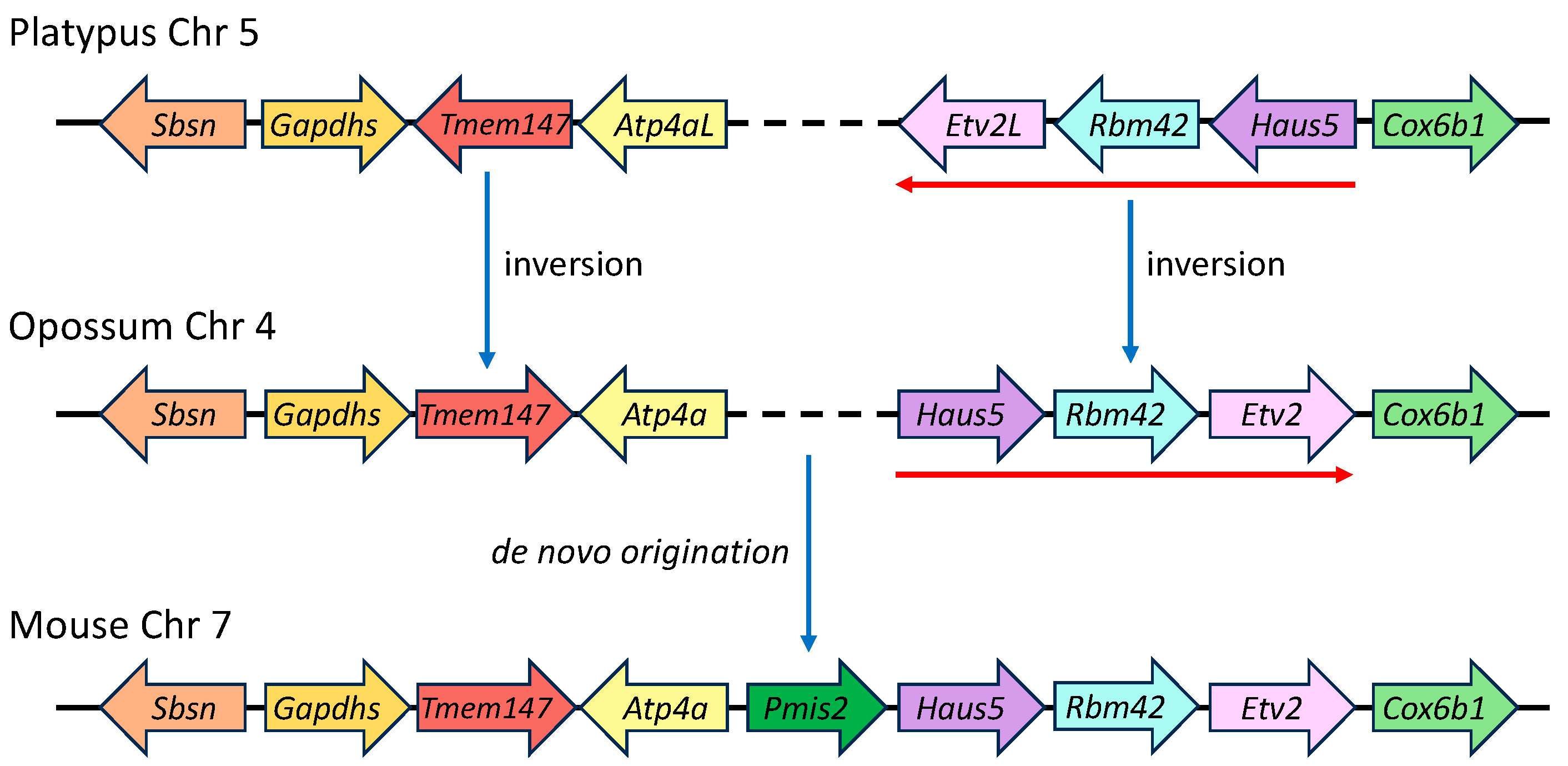
2.8. Genomic Ontogeny of SMA20/PMIS2
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. cDNA Cloning
4.3. Northern Blotting
4.4. Database Queries
4.5. Sperm Preparation and Membrane Isolation
4.6. Antibody Production
4.7. Electrophoresis and Western Blotting
4.8. Immunofluorescence
4.9. Immunoelectron Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven Press: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Yanagimachi, R. Mysteries and unsolved problems of mammalian fertilization and related topics. Biol. Reprod. 2022, 106, 644–675. [Google Scholar] [CrossRef]
- Wassarman, P.M. Mammalian fertilization: Molecular aspects of gamete adhesion, exocytosis, and fusion. Cell 1999, 96, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Okabe, M. The cell biology of mammalian fertilization. Development 2013, 140, 4471–4479. [Google Scholar] [CrossRef]
- Hardy, D.M. (Ed.) Fertilization; Academic Press: San Diego, CA, USA, 2002. [Google Scholar]
- Ikawa, M.; Inoue, N.; Benham, A.M.; Okabe, M. Fertilization: A sperm’s journey to and interaction with the oocyte. J. Clin. Investig. 2010, 120, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.M.; Huang, T.T.F., Jr.; Driscoll, W.J.; Tung, K.S.K.; Wild, G.C. Purification and characterization of the primary acrosomal autoantigen of guinea pig epididymal spermatozoa. Biol. Reprod. 1988, 38, 423–437. [Google Scholar] [CrossRef]
- Foster, J.A.; Gerton, G.L. Autoantigen 1 of the guinea pig sperm acrosome is the homologue of mouse Tpx-1 and human TPX1 and is a member of the cysteine-rich secretory protein (CRISP) family. Mol. Reprod. Dev. 1996, 44, 221–229. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, Z.; Shen, C. An update of the regulatory factors of sperm migration from the uterus into the oviduct by genetically manipulated mice. Mol. Reprod. Dev. 2019, 86, 935–955. [Google Scholar] [CrossRef]
- Gaikwad, A.S.; Hu, J.; Chapple, D.G.; O’Bryan, M.K. The functions of CAP superfamily proteins in mammalian fertility and disease. Hum. Reprod. Update 2020, 26, 689–723. [Google Scholar] [CrossRef] [PubMed]
- Quill, T.A.; Garbers, D.L. Sperad is a novel sperm-specific plasma membrane protein homologous to a family of cell adhesion proteins. J. Biol. Chem. 1996, 271, 33509–33514. [Google Scholar] [CrossRef]
- Hedrick, J.L.; Urch, U.A.; Hardy, D.M. Chapter 15: Structure-function properties of the sperm enzyme acrosin. In Biocatalysis in Agricultural Biotechnology; Whitaker, J.R., Sonnet, P., Eds.; ACS Symposium Series; CRC Press: Washington, DC, USA, 1989; pp. 215–229. [Google Scholar]
- Wolfsberg, T.G.; Straight, P.D.; Gerena, R.L.; Huovila, A.P.; Primakoff, P.; Myles, D.G.; White, J.M. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev. Biol. 1995, 169, 378–383. [Google Scholar] [CrossRef]
- Gao, Z.; Garbers, D.L. Species diversity in the structure of zonadhesin, a sperm-specific membrane protein containing multiple cell adhesion molecule-like domains. J. Biol. Chem. 1998, 273, 3415–3421. [Google Scholar] [CrossRef]
- Tardif, S.; Wilson, M.D.; Wagner, R.; Hunt, P.; Gertsenstein, M.; Nagy, A.; Lobe, C.; Koop, B.F.; Hardy, D.M. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J. Biol. Chem. 2010, 285, 24863–24870. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 434, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P. Izumo1 and Juno: The evolutionary origins and coevolution of essential sperm-egg binding partners. R. Soc. Open Sci. 2015, 2, 150296. [Google Scholar] [CrossRef]
- Quill, T.A.; Ren, D.; Clapham, D.E.; Garbers, D.L. A voltage-gated ion channel expressed specifically in spermatozoa. Proc. Natl. Acad. Sci. USA 2001, 98, 12527–12531. [Google Scholar] [CrossRef]
- Ren, D.; Navarro, B.; Perez, G.; Jackson, A.C.; Hsu, S.; Shi, Q.; Tilly, J.L.; Clapham, D.E. A sperm ion channel required for sperm motility and male fertility. Nature 2001, 413, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, H.; Koriyama, J.; Shiraishi, Y.; Hirano, Y.; Suzuki, M.; Koyama, K. Diagnosis and treatment of immunologically infertile women with sperm-immobilizing antibodies in their sera. J. Reprod. Immunol. 2009, 83, 139–144. [Google Scholar] [CrossRef]
- Haden, N.P.; Hickox, J.R.; Whisnant, C.S.; Hardy, D.M. Systematic characterization of sperm-specific membrane proteins in swine. Biol. Reprod. 2000, 63, 1839–1847. [Google Scholar] [CrossRef]
- Cormier, N.; McGlone, J.J.; Leszyk, J.; Hardy, D.M. Immunocontraceptive target repertoire defined by systematic identification of sperm membrane alloantigens in a single species. PLoS ONE 2018, 13, e0190891. [Google Scholar] [CrossRef]
- Kozak, M. The scanning model for translation: An update. J. Cell Biol. 1989, 108, 229–241. [Google Scholar] [CrossRef]
- von Heijne, G. Patterns of amino acids near signal-sequence cleavage sites. Eur. J. Biochem. 1983, 133, 17–21. [Google Scholar] [CrossRef]
- Barnes, W.M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from L bacteriophage templates. Proc. Natl. Acad. Sci. USA 1994, 91, 2216–2220. [Google Scholar] [CrossRef]
- Bi, M.; Hickox, J.R.; Winfrey, V.P.; Olson, G.E.; Hardy, D.M. Processing, localization and binding activity of zonadhesin suggest a function in sperm adhesion to the zona pellucida during exocytosis of the acrosome. Biochem. J. 2003, 375, 477–488. [Google Scholar] [CrossRef]
- Welch, J.E.; Brown, P.L.; O’Brien, D.A.; Magyar, P.L.; Bunch, D.O.; Mori, C.; Eddy, E.M. Human glyceraldehyde 3-phosphate dehydrogenase-2 gene is expressed specifically in spermatogenic cells. J. Androl. 2000, 21, 328–338. [Google Scholar] [CrossRef]
- Bininda-Emonds, O.R.; Cardillo, M.; Jones, K.E.; MacPhee, R.D.; Beck, R.M.; Grenyer, R.; Price, S.A.; Vos, R.A.; Gittleman, J.L.; Purvis, A. The delayed rise of present-day mammals. Nature 2007, 446, 507–512. [Google Scholar] [CrossRef]
- Roberts, E.K.; Tardif, S.; Wright, E.A.; Platt II, R.N.; Bradley, R.D.; Hardy, D.M. Rapid divergence of a gamete recognition gene promoted macroevolution of Eutheria. Genome Biol. 2022, 23, 155. [Google Scholar] [CrossRef]
- Foley, N.M.; Springer, M.S.; Teeling, E.C. Mammal madness: Is the mammal tree of life not yet resolved? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150140. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Fujihara, Y.; Ikawa, M.; Okabe, M. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa. Mol. Biol. Cell 2012, 23, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, M.; Wada, I.; Kominami, K.; Watanabe, D.; Toshimori, K.; Nishimune, Y.; Okabe, M. The putative chaperone calmegin is required for sperm fertility. Nature 1997, 387, 607–611. [Google Scholar] [CrossRef]
- Bedford, J.M. Site of the mammalian sperm physiological acrosome reaction. Proc. Natl. Acad. Sci. USA 2011, 108, 4703–4704. [Google Scholar] [CrossRef]
- Jin, M.; Fujiwara, E.; Kakiuchi, Y.; Okabe, M.; Satouh, Y.; Baba, S.A.; Chiba, K.; Hirohashi, N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 2011, 108, 4892–4896. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, M.K.; Miller, S.C.; Tikhonova, E.B.; Karamyshev, A.L. SRPassing Co-translational Targeting: The Role of the Signal Recognition Particle in Protein Targeting and mRNA Protection. Int. J. Mol. Sci. 2021, 22, 6284. [Google Scholar] [CrossRef]
- Tirincsi, A.; Sicking, M.; Hadzibeganovic, D.; Haßdenteufel, S.; Lang, S. The molecular biodiversity of protein targeting and protein transport related to the endoplasmic reticulum. Int. J. Mol. Sci. 2021, 23, 143. [Google Scholar] [CrossRef]
- Farkas, Á.; Bohnsack, K.E. Capture and delivery of tail-anchored proteins to the endoplasmic reticulum. J. Cell Biol. 2021, 220, e202105004. [Google Scholar] [CrossRef]
- Wessels, H.P.; Spiess, M. Insertion of a multispanning membrane protein occurs sequentially and requires only one signal sequence. Cell 1988, 55, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xiong, X.; Helm, A.; Kimani, K.; Bragin, A.; Skach, W.R. Co- and posttranslational translocation mechanisms direct cystic fibrosis transmembrane conductance regulator N terminus transmembrane assembly. J. Biol. Chem. 1998, 273, 568–576. [Google Scholar] [CrossRef]
- Tu, L.; Wang, J.; Helm, A.; Skach, W.R.; Deutsch, C. Transmembrane biogenesis of Kv1.3. Biochemistry 2000, 39, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Spiess, M. Heads or tails-what determines the orientation of proteins in the membrane. FEBS Lett. 1995, 369, 76–79. [Google Scholar] [CrossRef]
- Goder, V.; Spiess, M. Topogenesis of membrane proteins: Determinants and dynamics. FEBS Lett. 2001, 504, 87–93. [Google Scholar] [CrossRef]
- von Heijne, G. Membrane-protein topology. Nat. Rev. Mol. Cell Biol. 2006, 7, 909–918. [Google Scholar] [CrossRef]
- Hartmann, E.; Rapoport, T.A.; Lodish, H.F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc. Natl. Acad. Sci. USA 1989, 86, 5786–5790. [Google Scholar] [CrossRef]
- Hartmann, E.; Sommer, T.; Prehn, S.; Gorlich, D.; Jentsch, S.; Rapoport, T.A. Evolutionary conservation of components of the protein translocation complex. Nature 1994, 367, 654–657. [Google Scholar] [CrossRef]
- Picot, D.; Loll, P.J.; Garavito, R.M. The X-ray crystal structure of the membrane protein prostaglandin H2 synthase-1. Nature 1994, 367, 243–249. [Google Scholar] [CrossRef]
- Saraste, M.; Musacchio, A. Backwards and forwards binding. Nat. Struct. Biol. 1994, 1, 835–837. [Google Scholar] [CrossRef]
- Kay, B.K.; Williamson, M.P.; Sudol, M. The importance of being proline: The interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000, 14, 231–241. [Google Scholar] [CrossRef]
- Williamson, M.P. The structure and function of proline-rich regions in proteins. Biochem. J. 1994, 297, 249–260. [Google Scholar] [CrossRef]
- Pawson, T.; Nash, P. Assembly of cell regulatory systems through protein interaction domains. Science 2003, 300, 445–452. [Google Scholar] [CrossRef]
- McPherson, P.S. Regulatory role of SH3 domain-mediated protein-protein interactions in synaptic vesicle endocytosis. Cell Signal 1999, 11, 229–238. [Google Scholar] [CrossRef]
- Suarez, S.S.; Pacey, A.A. Sperm transport in the female reproductive tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef]
- Swanson, W.J.; Vacquier, V.D. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 2002, 3, 137–144. [Google Scholar] [CrossRef]
- Clark, N.L.; Gasper, J.; Sekino, M.; Springer, S.A.; Aquadro, C.F.; Swanson, W.J. Coevolution of interacting fertilization proteins. PLoS Genet. 2009, 5, e1000570. [Google Scholar] [CrossRef]
- Hardy, D.M.; Wright, E.A.; Worsham, A.E.; Bradley, R.D.; Roberts, E.K. Zan Til+E Domain Duplication and Divergence Reflect Unique Evolution of a Speciation Gene in Muroid Rodents; Texas Tech University Health Sciences Center: Lubbock, TX, USA, 2024; In Preparation. [Google Scholar]
- Ashton, M.N.; Worsham, A.E.; Cheung, T.L.; Grozdanov, P.N.; Hardy, D.M. Heterogeneous RNA Splicing and Permissive Surveillance in Human Male Germ Cells Drive Individual Variation in Expressed Transcripts of the Speciation Gene ZAN; Texas Tech University Health Sciences Center: Lubbock, TX, USA, 2024; In Preparation. [Google Scholar]
- Long, M.; VanKuren, N.W.; Chen, S.; Vibranovski, M.D. New gene evolution: Little did we know. Annu. Rev. Genet. 2013, 47, 307–333. [Google Scholar] [CrossRef]
- Andersson, D.I.; Jerlström-Hultqvist, J.; Näsvall, J. Evolution of new functions de novo and from preexisting genes. Cold Spring Harb. Perspect. Biol. 2015, 7, a017996. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Church, G.M.; Gilbert, W. Genomic sequencing. Proc. Natl. Acad. Sci. USA 1984, 81, 1991–1995. [Google Scholar] [CrossRef]
- Hickox, J.R.; Bi, M.; Hardy, D.M. Heterogeneous processing and zona pellucida binding activity of pig zonadhesin. J. Biol. Chem. 2001, 276, 41502–41509. [Google Scholar] [CrossRef]
- Olson, G.E.; Winfrey, V.P.; NagDas, S.K. Acrosome biogenesis in the hamster: Ultrastructurally distinct matrix regions are assembled from a common precursor polypeptide. Biol. Reprod. 1998, 58, 361–370. [Google Scholar] [CrossRef]
- Olson, G.E.; Winfrey, V.P.; Nagdas, S.K. Structural modification of the hamster sperm acrosome during posttesticular development in the epididymis. Microsc. Res. Tech. 2003, 61, 46–55. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cormier, N.; Worsham, A.E.; Rich, K.A.; Hardy, D.M. SMA20/PMIS2 Is a Rapidly Evolving Sperm Membrane Alloantigen with Possible Species-Divergent Function in Fertilization. Int. J. Mol. Sci. 2024, 25, 3652. https://doi.org/10.3390/ijms25073652
Cormier N, Worsham AE, Rich KA, Hardy DM. SMA20/PMIS2 Is a Rapidly Evolving Sperm Membrane Alloantigen with Possible Species-Divergent Function in Fertilization. International Journal of Molecular Sciences. 2024; 25(7):3652. https://doi.org/10.3390/ijms25073652
Chicago/Turabian StyleCormier, Nathaly, Asha E. Worsham, Kinsey A. Rich, and Daniel M. Hardy. 2024. "SMA20/PMIS2 Is a Rapidly Evolving Sperm Membrane Alloantigen with Possible Species-Divergent Function in Fertilization" International Journal of Molecular Sciences 25, no. 7: 3652. https://doi.org/10.3390/ijms25073652






