Nanotechnology as a Promising Method in the Treatment of Skin Cancer
Abstract
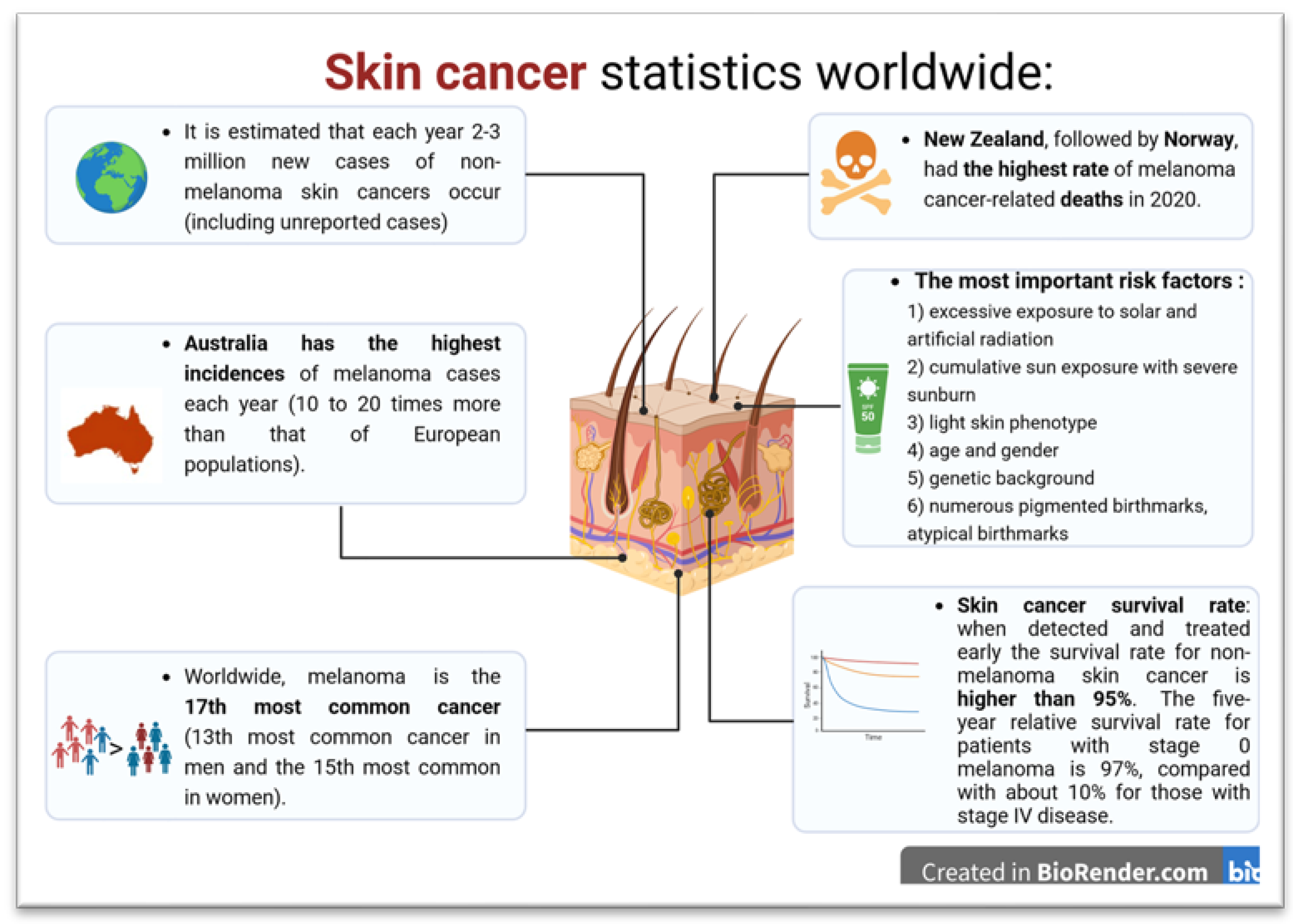
:1. Introduction
2. Skin Cancers
3. Photoaging as a Risk Factor for Skin Cancer Development
4. Molecular Basis of Melanoma Development
5. Nanotechnology as an Innovative Approach to Diagnosing and Treating Skin Cancers
5.1. Lipid-Based Nanoparticles in Skin Cancer
5.1.1. Liposomes
5.1.2. Ethosomes
5.1.3. Solid Lipid Nanoparticles
5.2. Inorganic Nanoparticles
5.2.1. Functionalized Metal Nanoparticles
5.2.2. Carbon Nanotubes
5.2.3. Nanofibers
5.3. Polymer-Based Nanoparticles
5.3.1. Functionalized Polymeric Nanoparticles
5.3.2. Dendrimers
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: Regional Office for Europe. World Cancer Report: Cancer Research for Cancer Development; IARC: Lyon, France, 2020; ISBN 978-92-832-0447-3. [Google Scholar]
- Khazaei, Z.; Ghorat, F.; Jarrahi, A.M.; Adineh, H.A.; Sohrabivafa, M.; Goodarzi, E. Global Incidence and Mortality of Skin Cancer by Histological Subtype and Its Relationship with the Human Development Index (HDI)—An Ecology Study in 2018. WCRJ 2019, 6, 14. [Google Scholar]
- Skin Cancer Statistics | World Cancer Research Fund International. WCRF Int. Available online: https://www.wcrf.org/cancer-trends/skin-cancer-statistics/ (accessed on 18 October 2023).
- Radiation: Ultraviolet (UV) Radiation and Skin Cancer. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-ultraviolet-(uv)-radiation-and-skin-cancer (accessed on 18 October 2023).
- Cancer Facts & Figures 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 18 October 2023).
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Gruber, P.; Zito, P.M. Skin Cancer. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma. J. Am. Acad. Dermatol. 2018, 80, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Shalin, S.C.; Tackett, A.J. Current state of melanoma diagnosis and treatment. Cancer Biol. Ther. 2019, 20, 1366–1379. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.; Wernham, A.; Patel, A. When to suspect a non-melanoma skin cancer. BMJ 2020, 368, m692. [Google Scholar] [CrossRef]
- Sreekantaswamy, S.; Endo, J.; Chen, A.; Butler, D.; Morrison, L.; Linos, E. Aging and the treatment of basal cell carcinoma. Clin. Dermatol. 2019, 37, 373–378. [Google Scholar] [CrossRef]
- Martens, M.C.; Seebode, C.; Lehmann, J.; Emmert, S. Photocarcinogenesis and Skin Cancer Prevention Strategies: An Update. Anticancer Res. 2018, 38, 1153–1158. [Google Scholar] [CrossRef]
- Feldman, S.R.; Fleischer, A.B. Progression of actinic keratosis to squamous cell carcinoma revisited: Clinical and treatment implications. Cutis 2011, 87, 201–207. [Google Scholar]
- Zhao, J.; Chen, S.; Zhou, H.; Zhang, T.; Liu, Y.; He, J.; Zhu, J.; Ruan, J. XPG rs17655 G > C polymorphism associated with cancer risk: Evidence from 60 studies. Aging 2018, 10, 1073–1088. [Google Scholar] [CrossRef]
- Ghartimagar, D.; Ghosh, A.; Shrestha, S.R.; Shrestha, S.; Thapa, S.; Narasimhan, R.; Talwar, O.P. Basal Cell Carcinoma in Cases with or without Xeroderma Pigmentosum. JNMA J. Nepal. Med. Assoc. 2017, 56, 432–437. [Google Scholar] [CrossRef]
- Lim, K.-R.; Cho, K.-H.; Hwang, S.-M.; Jung, Y.-H.; Song, J.K. Basal Cell Carcinoma Presenting as a Hypertrophic Scar. Arch. Plast. Surg. 2013, 40, 289–291. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef]
- Ramirez, C.C.; Federman, D.G.; Kirsner, R.S. Skin cancer as an occupational disease: The effect of ultraviolet and other forms of radiation. Int. J. Dermatol. 2005, 44, 95–100. [Google Scholar] [CrossRef]
- Sánchez, G.; Nova, J.; Rodriguez-Hernandez, A.E.; Medina, R.D.; Solorzano-Restrepo, C.; Gonzalez, J.; Olmos, M.; Godfrey, K.; Arevalo-Rodriguez, I. Sun protection for preventing basal cell and squamous cell skin cancers. Emergencias 2016, 2016, CD011161. [Google Scholar] [CrossRef]
- Kong, M.-F.; Jogia, R.; Nayyar, V.; Berrington, R.; Jackson, S. Squamous Cell Carcinoma in a Heel Ulcer in a Patient with Diabetes. Diabetes Care 2008, 31, e57. [Google Scholar] [CrossRef]
- Rebecca, V.W.; Sondak, V.K.; Smalley, K.S. A brief history of melanoma. Melanoma Res. 2012, 22, 114–122. [Google Scholar] [CrossRef]
- Von Thaler, A.; Kamenisch, Y.; Berneburg, M. The role of ultraviolet radiation in melanomagenesis. Exp. Dermatol. 2010, 19, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Leiter, U.; Eigentler, T.; Garbe, C. Epidemiology of Skin Cancer. In Sunlight, Vitamin D and Skin Cancer; Springer: New York, NY, USA, 2014; pp. 120–140. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davó, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mech. Insights. Front. Pharmacol. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Brash, D.E. Roles of the transcription factor p53 in keratinocyte carcinomas. Br. J. Dermatol. 2006, 154, 8–10. [Google Scholar] [CrossRef]
- Makrantonaki, E.; Zouboulis, C.C. Molecular Mechanisms of Skin Aging. Ann. N. Y. Acad. Sci. 2007, 1119, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Choquet, H.; Ashrafzadeh, S.; Kim, Y.; Asgari, M.M.; Jorgenson, E. Genetic and environmental factors underlying keratinocyte carcinoma risk. JCI Insight 2020, 5, e134783. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I Through VI. Arch. Dermatol. 1988, 124, 869. [Google Scholar] [CrossRef] [PubMed]
- Zakhem, G.A.; Pulavarty, A.N.; Lester, J.C.; Stevenson, M.L. Skin Cancer in People of Color: A Systematic Review. Am. J. Clin. Dermatol. 2021, 23, 137–151. [Google Scholar] [CrossRef] [PubMed]
- de Gruijl, F. Skin cancer and solar UV radiation. Eur. J. Cancer 1999, 35, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Sunlight, Ultraviolet Radiation, Vitamin D and Skin Cancer: How Much Sunlight Do We Need? Adv. Exp. Med. Biol. 2014, 810, 1–16. [Google Scholar]
- Balk, S.J. Section on the Council on Environmental Health and Section on Dermatology Ultraviolet Radiation: A Hazard to Children and Adolescents. Pediatrics 2011, 127, e791–e817. [Google Scholar] [CrossRef] [PubMed]
- Greinert, R.; de Vries, E.; Erdmann, F.; Espina, C.; Auvinen, A.; Kesminiene, A.; Schüz, J. European Code against Cancer 4th Edition: Ultraviolet radiation and cancer. Cancer Epidemiol. 2015, 39, S75–S83. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The Protective Role of Melanin Against UV Damage in Human Skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Diffey, B.L. Sources and measurement of ultraviolet radiation. Methods 2002, 28, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Beani, J.-C. [Ultraviolet A-induced DNA damage: Role in skin cancer]. Bull. Acad. Natl. Med. 2014, 198, 273–295. [Google Scholar] [PubMed]
- Chomiczewska-Skóra, D.; Adamus, A.; Trznadel-Grodzka, E.; Rotsztejn, H. Effects of ultraviolet radiation on Langerhans cells. Cent. Eur. J. Immunol. 2013, 3, 393–398. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Agar, N.S.; Halliday, G.M.; Barnetson, R.S.; Ananthaswamy, H.N.; Wheeler, M.; Jones, A.M. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 4954–4959. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T. Formation of UV-induced DNA damage contributing to skin cancer development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef] [PubMed]
- Rodust, P.; Stockfleth, E.; Ulrich, C.; Leverkus, M.; Eberle, J. UV-induced squamous cell carcinoma—A role for antiapoptotic signalling pathways. Br. J. Dermatol. 2009, 161, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.D.; Tessman, I. Identification of the altered bases in mutated single-stranded DNA: III. Mutagenesis by ultraviolet light. J. Mol. Biol. 1964, 9, 372–375. [Google Scholar] [CrossRef]
- Miller, J.H. Mutagenic specificity of ultraviolet light. J. Mol. Biol. 1985, 182, 45–65. [Google Scholar] [CrossRef]
- Soura, E.; Eliades, P.J.; Shannon, K.; Stratigos, A.J.; Tsao, H. Hereditary melanoma: Update on syndromes and management. J. Am. Acad. Dermatol. 2016, 74, 395–407. [Google Scholar] [CrossRef]
- Toussi, A.; Mans, N.; Welborn, J.; Kiuru, M. Germline mutations predisposing to melanoma. J. Cutan. Pathol. 2020, 47, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Hussussian, C.J.; Struewing, J.P.; Goldstein, A.M.; Higgins, P.A.T.; Ally, D.S.; Sheahan, M.D.; Clark, W.H.; Tucker, M.A.; Dracopoli, N.C. Germline p16 mutations in familial melanoma. Nat. Genet. 1994, 8, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bishop, D.T.; Ghiorzo, P. Geographical Variation in the Penetrance of CDKN2A Mutations for Melanoma. JNCI J. Natl. Cancer Inst. 2002, 94, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Berwick, M.; Orlow, I.; Mahabir, S.; Myskowski, P.; Coit, D.; Brady, M.S.; Roy, P.; Song, Y.; Canchola, R.; Barz, A.; et al. Estimating the relative risk of developing melanoma in INK4A carriers. Eur. J. Cancer Prev. 2004, 13, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Piepkorn, M. Melanoma genetics: An update with focus on the CDKN2A(p16)/ARF tumor suppressors. J. Am. Acad. Dermatol. 2000, 42, 705–726. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Garinis, G.A.; Mitchell, J.R.; Moorhouse, M.J.; Hanada, K.; de Waard, H.; Vandeputte, D.; Jans, J.; Brand, K.; Smid, M.; van der Spek, P.J.; et al. Transcriptome analysis reveals cyclobutane pyrimidine dimers as a major source of UV-induced DNA breaks. EMBO J. 2005, 24, 3952–3962. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, D.J.; Timchenko, N.A.; Shibahara, S.; Medrano, E.E. Dynamic regulation of the human dopachrome tautomerase promoter by MITF, ER-α and chromatin remodelers during proliferation and senescence of human melanocytes. Pigment. Cell Res. 2005, 18, 203–213. [Google Scholar] [CrossRef]
- McGill, G.G.; Horstmann, M.; Widlund, H.R.; Du, J.; Motyckova, G.; Nishimura, E.K.; Lin, Y.-L.; Ramaswamy, S.; Avery, W.; Ding, H.-F.; et al. Bcl2 Regulation by the Melanocyte Master Regulator Mitf Modulates Lineage Survival and Melanoma Cell Viability. Cell 2002, 109, 707–718. [Google Scholar] [CrossRef]
- Fotedar, R.; Bendjennat, M.; Fotedar, A. Functional Analysis of CDK Inhibitor p21WAF1. In Checkpoint Controls and Cancer. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2004; Volume 281, pp. 55–71. [Google Scholar] [CrossRef]
- Yang, Q.; Manicone, A.; Coursen, J.D.; Linke, S.P.; Nagashima, M.; Forgues, M.; Wang, X.W. Identification of a Functional Domain in a GADD45-mediated G2/M Checkpoint. J. Biol. Chem. 2000, 275, 36892–36898. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Antinore, M.J.; Lung, F.-D.T.; Dong, X.; Zhao, H.; Fan, F.; Colchagie, A.B.; Blanck, P.; Roller, P.P.; Fornace, A.J.; et al. The GADD45 Inhibition of Cdc2 Kinase Correlates with GADD45-mediated Growth Suppression. J. Biol. Chem. 2000, 275, 16602–16608. [Google Scholar] [CrossRef] [PubMed]
- Bonitsis, N.; Batistatou, A.; Karantima, S.; Charalabopoulos, K. The role of cadherin/catenin complex in malignant melanoma. Exp. Oncol. 2006, 28, 187–193. [Google Scholar] [PubMed]
- Jamal, S.; Schneider, R.J. UV-induction of keratinocyte endothelin-1 downregulates E-cadherin in melanocytes and melanoma cells. J. Clin. Investig. 2002, 110, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Sample, A.; He, Y. Mechanisms and prevention of UV-induced melanoma. Photodermatol. Photoimmunol. Photomed. 2017, 34, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, X.; Liu, W.; Yang, X.; An, N.; Yang, F.; Sun, J.; Xing, Y.; Shang, H. Advances in the application of nanotechnology in reducing cardiotoxicity induced by cancer chemotherapy. Semin. Cancer Biol. 2021, 86, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Hamid, L.; Bader, G.N.; Shoaib, A.; Rahamathulla, M.; Alshahrani, M.Y.; Alam, P.; Shakeel, F. Role of Nanotechnology in Overcoming the Multidrug Resistance in Cancer Therapy: A Review. Molecules 2022, 27, 6608. [Google Scholar] [CrossRef] [PubMed]
- Avula, L.R.; Grodzinski, P. Nanotechnology-aided advancement in the combating of cancer metastasis. Cancer Metastasis Rev. 2022, 41, 383–404. [Google Scholar] [CrossRef]
- Khan, N.H.; Mir, M.; Qian, L.; Baloch, M.; Khan, M.F.A.; Rehman, A.-U.; Ngowi, E.E.; Wu, D.-D.; Ji, X.-Y. Skin cancer biology and barriers to treatment: Recent applications of polymeric micro/nanostructures. J. Adv. Res. 2022, 36, 223–247. [Google Scholar] [CrossRef]
- Battaglia, L.; Scomparin, A.; Dianzani, C.; Milla, P.; Muntoni, E.; Arpicco, S.; Cavalli, R. Nanotechnology Addressing Cutaneous Melanoma: The Italian Landscape. Pharmaceutics 2021, 13, 1617. [Google Scholar] [CrossRef]
- Mishra, H.; Mishra, P.K.; Ekielski, A.; Jaggi, M.; Iqbal, Z.; Talegaonkar, S. Melanoma treatment: From conventional to nanotechnology. J. Cancer Res. Clin. Oncol. 2018, 144, 2283–2302. [Google Scholar] [CrossRef] [PubMed]
- Gmeiner, W.H.; Ghosh, S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2014, 3, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Toderascu, L.I.; Sima, L.E.; Orobeti, S.; Florian, P.E.; Icriverzi, M.; Maraloiu, V.-A.; Comanescu, C.; Iacob, N.; Kuncser, V.; Antohe, I.; et al. Synthesis and Anti-Melanoma Activity of L-Cysteine-Coated Iron Oxide Nanoparticles Loaded with Doxorubicin. Nanomaterials 2023, 13, 621. [Google Scholar] [CrossRef] [PubMed]
- Borgheti-Cardoso, L.N.; Viegas, J.S.R.; Silvestrini, A.V.P.; Caron, A.L.; Praça, F.G.; Kravicz, M.; Bentley, M.V.L.B. Nanotechnology approaches in the current therapy of skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 109–136. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhu, Y.; Bai, S.; He, C.; Du, G.; Zhang, Y.; Zhong, Y.; Chen, W.; Wang, H.; Sun, X. Nanoparticles with rough surface improve the therapeutic effect of photothermal immunotherapy against melanoma. Acta Pharm. Sin. B 2021, 12, 2934–2949. [Google Scholar] [CrossRef] [PubMed]
- Mello, V.C.; Araújo, V.H.S.; de Paiva, K.L.R.; Simões, M.M.; Marques, D.C.; Costa, N.R.d.S.; de Souza, I.F.; da Silva, P.B.; Santos, I.; Almeida, R.; et al. Development of New Natural Lipid-Based Nanoparticles Loaded with Aluminum-Phthalocyanine for Photodynamic Therapy against Melanoma. Nanomaterials 2022, 12, 3547. [Google Scholar] [CrossRef]
- Akanda, M.; Mithu, S.H.; Douroumis, D. Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment. J. Drug Deliv. Sci. Technol. 2023, 86. [Google Scholar] [CrossRef]
- Aziz, A.; Rehman, U.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Lipid-based nanocarrier mediated CRISPR/Cas9 delivery for cancer therapy. J. Biomater. Sci. Polym. Ed. 2022, 34, 398–418. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-Based Nanoparticles: Application and Recent Advances in Cancer Treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Pachauri, A.; Chitme, H.; Visht, S.; Chidrawar, V.; Mohammed, N.; Abdel-Wahab, B.A.; Khateeb, M.M.; Habeeb, M.S.; Orabi, M.A.A.; Bakir, M.B. Permeability-Enhanced Liposomal Emulgel Formulation of 5-Fluorouracil for the Treatment of Skin Cancer. Gels 2023, 9, 209. [Google Scholar] [CrossRef] [PubMed]
- Frías, E.A.; Vega, D.M.; Calienni, M.; Lillo, C.; Vazquez, D.; Alonso, S.; Montanari, J. Enhanced skin delivery of vismodegib-loaded rigid liposomes combined with ethosomes. OpenNano 2023, 14, 100186. [Google Scholar] [CrossRef]
- Obeid, M.A.; Ogah, C.A.; Ogah, C.O.; Ajala, O.S.; Aldea, M.R.; Gray, A.I.; Igoli, J.I.; Ferro, V.A. Formulation and evaluation of nanosized hippadine-loaded niosome: Extraction and isolation, physicochemical properties, and in vitro cytotoxicity against human ovarian and skin cancer cell lines. J. Drug Deliv. Sci. Technol. 2023, 87, 104766. [Google Scholar] [CrossRef]
- Cassano, R.; Mellace, S.; Marrelli, M.; Conforti, F.; Trombino, S. α-Tocopheryl linolenate solid lipid nanoparticles for the encapsulation, protection, and release of the omega-3 polyunsaturated fatty acid: In vitro anti-melanoma activity evaluation. Colloids Surf. B Biointerfaces 2017, 151, 128–133. [Google Scholar] [CrossRef]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in liposomal drug delivery to cancer: An overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal drug delivery systems and anticancer drugs. Molecules 2018, 23, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S.S.H. Liposomes for oral delivery of protein and peptide-based therapeutics: Challenges, formulation strategies, and advances. J. Mater. Chem. B 2021, 9, 4773–4792. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.E.M.; Corvo, M.L.; Martins, M.B.; Simões, S.; Gaspar, M.M. Liposomes as Tools to Improve Therapeutic Enzyme Performance. Pharmaceutics 2022, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Petrilli, R.; Eloy, J.O.; Saggioro, F.P.; Chesca, D.L.; de Souza, M.C.; Dias, M.V.; Dasilva, L.L.; Lee, R.J.; Lopez, R.F. Skin cancer treatment effectiveness is improved by iontophoresis of EGFR-targeted liposomes containing 5-FU compared with subcutaneous injection. J. Control. Release 2018, 283, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. Liposome encapsulation of doxorubicin and celecoxib in combination inhibits progression of human skin cancer cells. Int. J. Nanomed. 2018, 13, 11–13. [Google Scholar] [CrossRef]
- Cadinoiu, A.N.; Rata, D.M.; Atanase, L.I.; Daraba, O.M.; Gherghel, D.; Vochita, G.; Popa, M. Aptamer-Functionalized Liposomes as a Potential Treatment for Basal Cell Carcinoma. Polymers 2019, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Marwah, M.; Perrie, Y.; Badhan, R.K.S.; Lowry, D. Intracellular uptake of EGCG-loaded deformable controlled release liposomes for skin cancer. J. Liposome Res. 2019, 30, 136–149. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Labala, S.; Venuganti, V.V.K. Co-delivery of curcumin and STAT3 siRNA using deformable cationic liposomes to treat skin cancer. J. Drug Target. 2016, 25, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; Labala, S.; Ninave, K.M.; Gade, S.K.; Venuganti, V.V.K. Effective Skin Cancer Treatment by Topical Co-delivery of Curcumin and STAT3 siRNA Using Cationic Liposomes. Aaps Pharmscitech 2017, 19, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.J.; Lowery, A.R.; Thompson, P.A.; Blaney, S.M.; West, J.L. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: An in vitro evaluation using human cell lines. J. Neuro. Oncol. 2007, 86, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhang, J.; Kong, L.; Yu, Y.; Hu, Q.; Yang, T.; Wang, Y.; Tu, K.; Qiao, Q.; Qin, X.; et al. Immunogenic Hybrid Nanovesicles of Liposomes and Tumor-Derived Nanovesicles for Cancer Immunochemotherapy. ACS Nano 2021, 15, 3123–3138. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-B.; Yu, Z.-C.; He, Y.-N.; Zhang, T.; Du, L.-B.; Dong, Y.-M.; Chen, H.-W.; Zhang, Y.-Y.; Wang, W.-Q. Salinomycin-loaded lipid-polymer nanoparticles with anti-CD20 aptamers selectively suppress human CD20+ melanoma stem cells. Acta Pharmacol. Sin. 2017, 39, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.A.; Watts, R.J.; Robertson, G.P. Use of liposomes as drug delivery vehicles for treatment of melanoma. Pigment. Cell Melanoma Res. 2009, 22, 388–399. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Azzazy, H.M.E.-S.; Schaefer, J. Liposome Photosensitizer Formulations for Effective Cancer Photodynamic Therapy. Pharmaceutics 2021, 13, 1345. [Google Scholar] [CrossRef]
- Feng, L.; Tao, D.; Dong, Z.; Chen, Q.; Chao, Y.; Liu, Z.; Chen, M. Near-infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection. Biomaterials 2017, 127, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.P.; Ferreira, Q.; Vieira, T.; Silva, J.C.; Simões, S.; Ribeiro, P.A.; Raposo, M. Liposomes Encapsulating Methylene Blue and Acridine Orange: An Approach for Phototherapy of Skin Cancer. Colloids Surf. B-Biointerfaces 2022, 220. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kubo, H.; Murata, H.; Uhara, H.; Takata, M.; Shibata, S.; Yasue, S.; Sakakibara, A.; Tomita, Y.; Kageshita, T.; et al. A Pilot Study of Human Interferon Gene Therapy for Patients with Advanced Melanoma by in vivo Transduction Using Cationic Liposomes. Ultrasound Med. Biol. 2008, 38, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R.; Suresh, P. Preparation and characterization of solid lipid nanoparticles—A review. Curr. Cancer Drug Targets 2012, 9, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Gargett, T.; Abbas, M.N.; Rolan, P.; Price, J.D.; Gosling, K.M.; Ferrante, A.; Ruszkiewicz, A.; Atmosukarto, I.I.C.; Altin, J.; Parish, C.R.; et al. Phase I trial of Lipovaxin-MM, a novel dendritic cell-targeted liposomal vaccine for malignant melanoma. Cancer Immunol. Immunother. 2018, 67, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Das, M.; Liu, Y.; Huang, L. Targeted drug delivery to melanoma. Adv. Drug Deliv. Rev. 2017, 127, 208–221. [Google Scholar] [CrossRef] [PubMed]
- Janowitz, T.; Lu, L.; Yan, H.; Shyam-Sundar, V. Cross-sectional and longitudinal analysis of cancer vaccination trials registered on the US Clinical Trials Database demonstrates paucity of immunological trial endpoints and decline in registration since 2008. Drug Des. Dev. Ther. 2014, 8, 1539–1553. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Singh, V.; Singh, M.; Singh, D.; Singh, T.; Piplani, M.; Singh, R. Ethosomes: Novel Vesicular Carriers for Effective Transdermal Delivery of Natural Therapeutics. Lett. Drug Des. Discov. 2024, 21, 665–683. [Google Scholar] [CrossRef]
- Ma, L.; Wang, X.; Wu, J.; Zhang, D.; Zhang, L.; Song, X.; Hong, H.; He, C.; Mo, X.; Wu, S.; et al. Polyethylenimine and sodium cholate-modified ethosomes complex as multidrug carriers for the treatment of melanoma through transdermal delivery. Nanomedicine 2019, 14, 2395–2408. [Google Scholar] [CrossRef]
- Lin, H.; Lin, L.; Choi, Y.; Michniak-Kohn, B. Development and in-vitro evaluation of co-loaded berberine chloride and evodiamine ethosomes for treatment of melanoma. Int. J. Pharm. 2020, 581, 119278. [Google Scholar] [CrossRef]
- Ismail, T.A.; Shehata, T.M.; Mohamed, D.I.; Elsewedy, H.S.; Soliman, W.E. Quality by Design for Development, Optimization and Characterization of Brucine Ethosomal Gel for Skin Cancer Delivery. Molecules 2021, 26, 3454. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, M.; Arafa, M.G.; Nasr, M.; Sammour, O.A. Nobiletin-loaded composite penetration enhancer vesicles restore the normal miRNA expression and the chief defence antioxidant levels in skin cancer. Sci. Rep. 2021, 11, 20197. [Google Scholar] [CrossRef] [PubMed]
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (-)-epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: A comprehensive experimental study with preclinical investigation. Eur. J. Pharm. Sci. 2019, 137, 104972. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.R.; Wong, T.W. 5-Fluorouracil ethosomes—Skin deposition and melanoma permeation synergism with microwave. Artif. Cells Nanomed. Biotechnol. 2018, 46, 568–577. [Google Scholar] [CrossRef]
- Yu, X.; Du, L.; Li, Y.; Fu, G.; Jin, Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed. Pharmacother. 2015, 73, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Mousa, I.A.; Hammady, T.M.; Gad, S.; Zaitone, S.A.; El-Sherbiny, M.; Sayed, O.M. Formulation and Characterization of Metformin-Loaded Ethosomes for Topical Application to Experimentally Induced Skin Cancer in Mice. Pharmaceuticals 2022, 15, 657. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin loaded binary ethosomes for management of skin cancer by dermal application on UV exposed mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Kumar, N.S.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Kim, K.S.; Youn, Y.S.; Bae, Y.H. Immune-triggered cancer treatment by intestinal lymphatic delivery of docetaxel-loaded nanoparticle. J. Control. Release 2019, 311–312, 85–95. [Google Scholar] [CrossRef]
- Khallaf, R.A.; Salem, H.F.; Abdelbary, A. 5-Fluorouracil shell-enriched solid lipid nanoparticles (SLN) for effective skin carcinoma treatment. Drug Deliv. 2016, 23, 3452–3460. [Google Scholar] [CrossRef]
- Ali, A.; Madni, A.; Shah, H.; Jamshaid, T.; Jan, N.; Khan, S.; Khan, M.M.; Mahmood, M.A. Solid lipid-based nanoparticulate system for sustained release and enhanced in-vitro cytotoxic effect of 5-fluorouracil on skin Melanoma and squamous cell carcinoma. PLoS ONE 2023, 18, e0281004. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sharma, S. Dacarbazine-encapsulated solid lipid nanoparticles for skin cancer: Physical characterization, stability, in-vivo activity, histopathology, and immunohistochemistry. Front. Oncol. 2023, 13, 1102269. [Google Scholar] [CrossRef] [PubMed]
- Saweres-Argüelles, C.; Ramírez-Novillo, I.; Vergara-Barberán, M.; Carrasco-Correa, E.J.; Lerma-García, M.J.; Simó-Alfonso, E.F. Skin absorption of inorganic nanoparticles and their toxicity: A review. Eur. J. Pharm. Biopharm. 2023, 182, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Gold Nanoparticles for Drug Delivery and Cancer Therapy. Appl. Sci. 2020, 10, 3824. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential Theranostics Application of Bio-Synthesized Silver Nanoparticles (4-in-1 System). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.S.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C.R. Green Synthesis and Characterization of Monodispersed Gold Nanoparticles: Toxicity Study, Delivery of Doxorubicin and Its Bio-Distribution in Mouse Model. J. Biomed. Nanotechnol. 2016, 12, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Kotcherlakota, R.; Nimushakavi, S.; Roy, A.; Yadavalli, H.C.; Mukherjee, S.; Haque, S.; Patra, C.R. Biosynthesized Gold Nanoparticles: In Vivo Study of Near-Infrared Fluorescence (NIR)-Based Bio-imaging and Cell Labeling Applications. ACS Biomater. Sci. Eng. 2019, 5, 5439–5452. [Google Scholar] [CrossRef] [PubMed]
- Meka, R.R.; Mukherjee, S.; Patra, C.R.; Chaudhuri, A. Shikimoyl-ligand decorated gold nanoparticles for use in ex vivo engineered dendritic cell based DNA vaccination. Nanoscale 2019, 11, 7931–7943. [Google Scholar] [CrossRef]
- Katoozi, D.; Clayton, A.H.A.; Moss, D.J.; Chon, J.W.M. Uptake quantification of gold nanoparticles inside of cancer cells using high order image correlation spectroscopy. Biomed. Opt. Express 2020, 12, 539–552. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X. Gold nanoparticles for skin drug delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Fluorouracil-Loaded Gold Nanoparticles for the Treatment of Skin Cancer: Development, In Vitro Characterization, and In Vivo Evaluation in a Mouse Skin Cancer Xenograft Model. Mol. Pharm. 2018, 15, 2194–2205. [Google Scholar] [CrossRef]
- Rana, K.; Pandey, S.K.; Chauhan, S.; Preet, S. Anticancer therapeutic potential of 5-fluorouracil and nisin co-loaded chitosan coated silver nanoparticles against murine skin cancer. Int. J. Pharm. 2022, 620, 121744. [Google Scholar] [CrossRef]
- Preet, S.; Pandey, S.K.; Kaur, K.; Chauhan, S.; Saini, A. Gold nanoparticles assisted co-delivery of nisin and doxorubicin against murine skin cancer. J. Drug Deliv. Sci. Technol. 2019, 53, 101147. [Google Scholar] [CrossRef]
- Fratoddi, I.; Benassi, L.; Botti, E.; Vaschieri, C.; Venditti, I.; Bessar, H.; Samir, M.A.; Azzoni, P.; Magnoni, C.; Costanzo, A.; et al. Effects of topical methotrexate loaded gold nanoparticle in cutaneous inflammatory mouse model. Nanomedicine: Nanotechnology. Biol. Med. 2019, 17, 276–286. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold Nanoparticles in Diagnostics and Therapeutics for Human Cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef] [PubMed]
- Nirmala, J.G.; Akila, S.; Narendhirakannan, R.; Chatterjee, S. Vitis vinifera peel polyphenols stabilized gold nanoparticles induce cytotoxicity and apoptotic cell death in A431 skin cancer cell lines. Adv. Powder Technol. 2017, 28, 1170–1184. [Google Scholar] [CrossRef]
- Wu, F.; Zhu, J.; Li, G.; Wang, J.; Veeraraghavan, V.P.; Mohan, S.K.; Zhang, Q. Biologically synthesized green gold nanoparticles from Siberian ginseng induce growth-inhibitory effect on melanoma cells (B16). Artif. Cells Nanomed. Biotechnol. 2019, 47, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.M.; Mirtajani, S.B.; Karimzadeh, K. Green synthesis of silver nanoparticles using Trapa natans extract and their anticancer activity against A431 human skin cancer cells. J. Drug Deliv. Sci. Technol. 2018, 47, 375–379. [Google Scholar] [CrossRef]
- Dhanalekshmi, K.I.; Sangeetha, K.; Magesan, P.; Johnson, J.; Zhang, X.; Jayamoorthy, K. Photodynamic cancer therapy: Role of Ag- and Au-based hybrid nano-photosensitizers. J. Biomol. Struct. Dyn. 2020, 40, 4766–4773. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liang, R.; Li, Q.; Wang, K.; Hussain, M.; Dong, L.; Shen, C.; Li, H.; Shen, G.; Zhu, J.; et al. Photosensitizer-loaded gold nanocages for immunogenic phototherapy of aggressive melanoma. Acta Biomater. 2022, 142, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Montaseri, H.; Nkune, N.W.; Abrahamse, H. Active targeted photodynamic therapeutic effect of silver-based nanohybrids on melanoma cancer cells. J. Photochem. Photobiol. 2022, 11, 100136. [Google Scholar] [CrossRef]
- Farahavar, G.; Abolmaali, S.S.; Nejatollahi, F.; Safaie, A.; Javanmardi, S.; Zadeh, H.K.; Yousefi, R.; Nadgaran, H.; Mohammadi-Samani, S.; Tamaddon, A.M.; et al. Single-chain antibody-decorated Au nanocages@liposomal layer nanoprobes for targeted SERS imaging and remote-controlled photothermal therapy of melanoma cancer cells. Mater. Sci. Eng. C 2021, 124, 112086. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Miao, X.; Fan, G.-C.; Xu, T.; Jiang, L.-P.; Wu, P.; Cai, C.; Zhu, J.-J. Aptamer-Conjugated Au Nanocage/SiO2 Core–Shell Bifunctional Nanoprobes with High Stability and Biocompatibility for Cellular SERS Imaging and Near-Infrared Photothermal Therapy. ACS Sens. 2019, 4, 301–308. [Google Scholar] [CrossRef]
- Loh, K.J.; Hou, T.-C.; Lynch, J.P.; Kotov, N.A. Carbon Nanotube Sensing Skins for Spatial Strain and Impact Damage Identification. J. Nondestruct. Eval. 2009, 28, 9–25. [Google Scholar] [CrossRef]
- Akhtar, N.; Pathak, K. Carbon Nanotubes in the Treatment of Skin Cancers: Safety and Toxic ological Aspects. Pharm. Nanotechnol. 2017, 5, 95–110. [Google Scholar] [CrossRef]
- Lima, E.N.d.C.; Piqueira, J.R.C.; Maria, D.A. Advances in Carbon Nanotubes for Malignant Melanoma: A Chance for Treatment. Mol. Diagn. Ther. 2018, 22, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Hesabi, M.; Hesabi, M. The interaction between carbon nanotube and skin anti-cancer drugs: A DFT and NBO approach. J. Nanostructure Chem. 2013, 3, 22. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Bao, H.; Pan, Y.; Pal, M.; Kakran, M.; Cheng, H.K.F.; Li, L.; Tan, L.P. Functionalized carbon nanomaterials as nanocarriers for loading and delivery of a poorly water-soluble anticancer drug: A comparative study. Chem. Commun. 2011, 47, 5235–5237. [Google Scholar] [CrossRef]
- Mehan, N.; Kumar, M.; Bhatt, S.; Saini, V. A Current Review on Drug Loaded Nanofibers: Interesting and Valuable Platform for Skin Cancer Treatment. Pharm. Nanotechnol. 2020, 8, 191–206. [Google Scholar] [CrossRef]
- Rengifo, A.F.C.; Stefanes, N.M.; Toigo, J.; Mendes, C.; Argenta, D.F.; Dotto, M.E.R.; da Silva, M.C.S.; Nunes, R.J.; Caon, T.; Parize, A.L.; et al. PEO-chitosan nanofibers containing carboxymethyl-hexanoyl chitosan/dodecyl sulfate nanoparticles loaded with pyrazoline for skin cancer treatment. Eur. Polym. J. 2019, 119, 335–343. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Sucharithra, G.; Mary, S.A.; Selvi, A.T. Efficacy of biopolymeric PVA-AuNPs and PCL-Curcumin loaded electrospun nanofibers and their anticancer activity against A431 skin cancer cell line. Mater. Today Commun. 2020, 25, 101276. [Google Scholar] [CrossRef]
- Nurani, M.; Akbari, V.; Taheri, A. Preparation and characterization of metformin surface modified cellulose nanofiber gel and evaluation of its anti-metastatic potentials. Carbohydr. Polym. 2017, 165, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-F.; Zheng, Y.; Fan, J.; Yao, Y.; Ahmad, Z.; Chang, M.-W. A novel core-shell nanofiber drug delivery system intended for the synergistic treatment of melanoma. Eur. J. Pharm. Sci. 2019, 137, 105002. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, A.; Tahami, S.; Nejad, P.A. Preparation and characterization of Fe3O4–Ag2O quantum dots decorated cellulose nanofibers as a carrier of anticancer drugs for skin cancer. J. Photochem. Photobiol. B Biol. 2017, 175, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Janrao, C.; Khopade, S.; Bavaskar, A.; Gomte, S.S.; Agnihotri, T.G.; Jain, A. Recent advances of polymer based nanosystems in cancer management. J. Biomater. Sci. Polym. Ed. 2023, 34, 1274–1335. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Targeted Delivery of Combination Therapeutics Using Monoclonal Antibody 2C5-Modified Immunoliposomes for Cancer Therapy. Pharm. Res. 2021, 38, 429–450. [Google Scholar] [CrossRef]
- Zhang, P.; Meng, J.; Li, Y.; Yang, C.; Hou, Y.; Tang, W.; McHugh, K.J.; Jing, L. Nanotechnology-enhanced immunotherapy for metastatic cancer. Innov. 2021, 2, 100174. [Google Scholar] [CrossRef]
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent Advances in the Structural Design of Photosensitive Agent Formulations Using “Soft” Colloidal Nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef]
- Zhang, Z.; Tsai, P.-C.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric nanoparticles-based topical delivery systems for the treatment of dermatological diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef]
- Zou, Y.; Wei, Y.; Sun, Y.; Bao, J.; Yao, F.; Li, Z.; Meng, F.; Hu, C.; Storm, G.; Zhong, Z. Cyclic RGD-Functionalized and Disulfide-Crosslinked Iodine-Rich Polymersomes as a Robust and Smart Theranostic Agent for Targeted CT Imaging and Chemotherapy of Tumor. Theranostics 2019, 9, 8061–8072. [Google Scholar] [CrossRef]
- Bungau, S. How to Face Skin Cancer with Nanomaterials: A Review. Biointerface Res. Appl. Chem. 2020, 11, 11931–11955. [Google Scholar] [CrossRef]
- Cavallaro, G.; Sardo, C.; Craparo, E.F.; Porsio, B.; Giammona, G. Polymeric nanoparticles for siRNA delivery: Production and applications. Int. J. Pharm. 2017, 525, 313–333. [Google Scholar] [CrossRef] [PubMed]
- Scopel, R.; Falcão, M.A.; Cappellari, A.R.; Morrone, F.B.; Guterres, S.S.; Cassel, E.; Kasko, A.M.; Vargas, R.M.F. Lipid-polymer hybrid nanoparticles as a targeted drug delivery system for melanoma treatment. Int. J. Polym. Mater. Polym. Biomater. 2020, 71, 127–138. [Google Scholar] [CrossRef]
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. Int. J. Nanomed. 2017, 12, 4117–4127. [Google Scholar] [CrossRef]
- Gamal-Eldeen, A.M.; El-Daly, S.M.; Borai, I.H.; Wafay, H.A.; Abdel-Ghaffar, A.-R.B. Photodynamic therapeutic effect of indocyanine green entrapped in polymeric nanoparticles and their anti-EGFR-conjugate in skin cancer in CD1 mice. Photodiagnosis Photodyn. Ther. 2013, 10, 446–459. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, J.; Zhao, S.; Guo, B.; Meng, F.; Klumperman, B.; Zhong, Z. Systemic administration of polymersomal oncolytic peptide LTX-315 combining with CpG adjuvant and anti-PD-1 antibody boosts immunotherapy of melanoma. J. Control. Release 2021, 336, 262–273. [Google Scholar] [CrossRef]
- Ybarra, D.E.; Calienni, M.N.; Ramirez, L.F.B.; Frias, E.T.A.; Lillo, C.; Alonso, S.d.V.; Montanari, J.; Alvira, F.C. Vismodegib in PAMAM-dendrimers for potential theragnosis in skin cancer. OpenNano 2022, 7, 100053. [Google Scholar] [CrossRef]
- Xia, C.; Yin, S.; Xu, S.; Ran, G.; Deng, M.; Mei, L.; Tang, X.; Rao, J.; Li, M.; Zhang, Z.; et al. Low Molecular Weight Heparin-Coated and Dendrimer-Based Core-Shell Nanoplatform with Enhanced Immune Activation and Multiple Anti-Metastatic Effects for Melanoma Treatment. Theranostics 2019, 9, 337–354. [Google Scholar] [CrossRef]



| Type | Therapeutic Agent | In Vitro Cytotoxicity Study | In Vivo Animal Model | References |
|---|---|---|---|---|
| Lipid-based nanoparticles | ||||
| EGFR-targeted liposomes | 5-FU | A431 and B16F10 cell lines | Immunosuppressed Swiss nude mice | [85] |
| Liposomes | Doxorubicin and celecoxib | A431 cell line | - | [86] |
| Aptamer liposomes | 5-FU | TE 354.T cell line | - | [87] |
| Liposomes | Epigallocatechin gallatein | HDFa and HaCat cell lines | - | [88] |
| Liposomes | Quercetin and resveratrol | HDFa cell line | - | [89] |
| Cationic liposomes | Curcumin and STAT3 siRNA | A431, B16F10 cell lines | - | [91,92] |
| Liposomes | 5-FU | B16-F10 cell line | - | [77] |
| Cubosomes | Paclitaxel | A431 cell line | Mice (female Balb/c nu/nu) | [166] |
| Liposomes | Doxorubicin | B16F10-OVA cell line | 16F10 tumor- bearing mouse model | [95] |
| Lipid–polymer nanoparticles | Salinomycin | WM266-4 and A375 cell lines | Immunodeficient (SCID) mice | [96] |
| Liposomes | chlorin e61, 1’-dioctadecyl-3,3,3’,3’-tetramethylindotricarbocyanine iodide (DiR) | 4T1 cell line | Balb/c mice | [99] |
| Liposomes | Methylene Blue and Acridine Orange | MET1 cell line | - | [100] |
| Liposomes | Human interferon b (HuIFNb) gene (IAB-1) | - | Stage IV or III melanoma patients | [108] |
| Liposomes | Lipovaxin-MM | - | Patient cohorts | [103] |
| Ethosomes | Doxorubicin and curcumin | B16 cell line | SD rats and C57BL/6 | [107] |
| Ethosomes | Berberine chloride and evodiamine | B16 cell line | - | [108] |
| Ethosomes | Brucine | A375 cell line | - | [109] |
| Ethosomes | Nobiletin | A431 cells | Male Balb/C mice | [110] |
| Ethosomes | (−)-Epigallocatechin-3-gallate | A431 cells | Male Balb/C nude mice | [111] |
| Ethosomes | 5-FU | SKMEL-2 cell line | Male Sprague Dawley rats | [112] |
| Ethosomes | Mitoxantrone | B16 cell line | Balb/C nude mice | [113] |
| Ethosomes | Metformin | - | Swiss albino mice | [114] |
| Ethosomes | Fisetin | - | Mice | [115] |
| Solid lipid nanoparticles | Docetaxel | SK-BR3, CT26 and 4T1 cell lines | Male C57BL/6 mouse and Sprague-Dawley rats | [117] |
| Solid lipid nanoparticles | 5-FU | - | Male balb/C mice | [118] |
| Solid lipid nanoparticles | 5-FU | B16F10 and A431 cell lines | - | [119] |
| Solid lipid nanoparticles | Dacarbazine | - | Wistar rats | [120] |
| Inorganic nanoparticles–metal nanoparticles | ||||
| Gold nanoparticles | Doxorubicine | A549 and B16F10 cell lines | C57BL6/J mice | [123] |
| Gold nanoparticles | Zinnia elegans plant extract | SK-OV-3, A549, and MCF-7 cell lines | C57BL6/J mice | [125] |
| Gold nanoparticles | Shikimoyl ligand | B16F10 cell line | C57BL6/J mice | [126] |
| Gold nanoparticles | 5-FU | A431 cell line | C57BL6/J mice | [129] |
| Silver nanoparticles | 5-FU | A431 cell line | C57BL6/J mice | [130] |
| Gold nanoparticles | Doxorubicin and nisin | - | BALB/c mice | [131] |
| Gold nanoparticles | Methotrexate | Human skin equivalents (HSEs) | - | [132] |
| Gold nanoparticles | Vitis vinifera peel polyphenols | A431 cell line | - | [134] |
| Gold nanoparticles | Siberian ginseng | B16 cell line | - | [135] |
| Silver nanoparticles | Trapa natans extract | A431 cell line | - | [136] |
| Gold nanocages | Monophosphoryl lipid and indocyanine green | B16-F10 cell line | C57BL/6 mice | [138] |
| Silver based nanohybrids | Zinc phthalocyanine tetrasulfonate (ZnPcS4) and folic acid | A375 cell line | - | [139] |
| Gold nanocages | anti-MUC18 single-chain antibod | A375 cell line | - | [140] |
| Gold nanocages/SiO2 | Aptamer | Mcf-7 and NIH 3T3 cell lines | - | [141] |
| Nanotubes and nanofibers | ||||
| Carbon nanotubes | Camptothecin | MDA-MB-231 cell line | - | [146] |
| Chitosan/dodecyl sulfate nanofibers | Pyrazoline H3TM04 | B16-F10 cell line | - | [148] |
| Nanofibers | AuNPs and curcumin | 3 T3 and A431 cell lines | - | [149] |
| Nanofibers | 5-FU | L929 and B16-F10 cell lines | - | [151] |
| Nanofibers | Etoposide and Methotrexate | SKMEL-3 cell line | - | [152] |
| Polymeric nanoparticles | ||||
| Polymersomes | Doxorubicin | B16 cell line | C57BL/6 | [158] |
| Lipid–polymer hybrid nanoparticles | Vitamin D3 | B16 cell line | - | [161] |
| Polymersomes | Protoporphyrin IX- | A375 cell line | - | [162] |
| Polymeric nanoparticles | Indocyanine green | - | CD1 mice | [163] |
| Polymersomes | Oncolytic peptide LTX-315 | B16-F10 cell line | B16F10 tumor-bearing mice | [164] |
| PAMAM-dendrimers | Vismodegib | HaCaT cell line | - | [165] |
| G4 PAMAM-dendrimers | Doxorubicin | B16-F10 cell line | B16F10 tumor-bearing mice | [166] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamus-Grabicka, A.A.; Hikisz, P.; Sikora, J. Nanotechnology as a Promising Method in the Treatment of Skin Cancer. Int. J. Mol. Sci. 2024, 25, 2165. https://doi.org/10.3390/ijms25042165
Adamus-Grabicka AA, Hikisz P, Sikora J. Nanotechnology as a Promising Method in the Treatment of Skin Cancer. International Journal of Molecular Sciences. 2024; 25(4):2165. https://doi.org/10.3390/ijms25042165
Chicago/Turabian StyleAdamus-Grabicka, Angelika A., Pawel Hikisz, and Joanna Sikora. 2024. "Nanotechnology as a Promising Method in the Treatment of Skin Cancer" International Journal of Molecular Sciences 25, no. 4: 2165. https://doi.org/10.3390/ijms25042165
APA StyleAdamus-Grabicka, A. A., Hikisz, P., & Sikora, J. (2024). Nanotechnology as a Promising Method in the Treatment of Skin Cancer. International Journal of Molecular Sciences, 25(4), 2165. https://doi.org/10.3390/ijms25042165







