Fluoride-Incorporated Apatite Coating on Collagen Sponge as a Carrier for Basic Fibroblast Growth Factor
Abstract
1. Introduction
2. Results
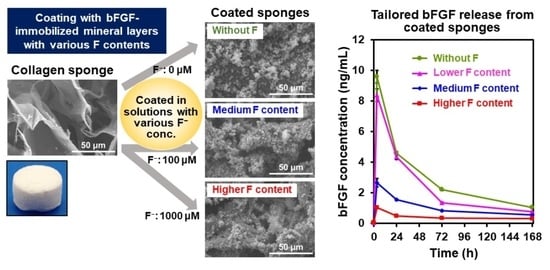
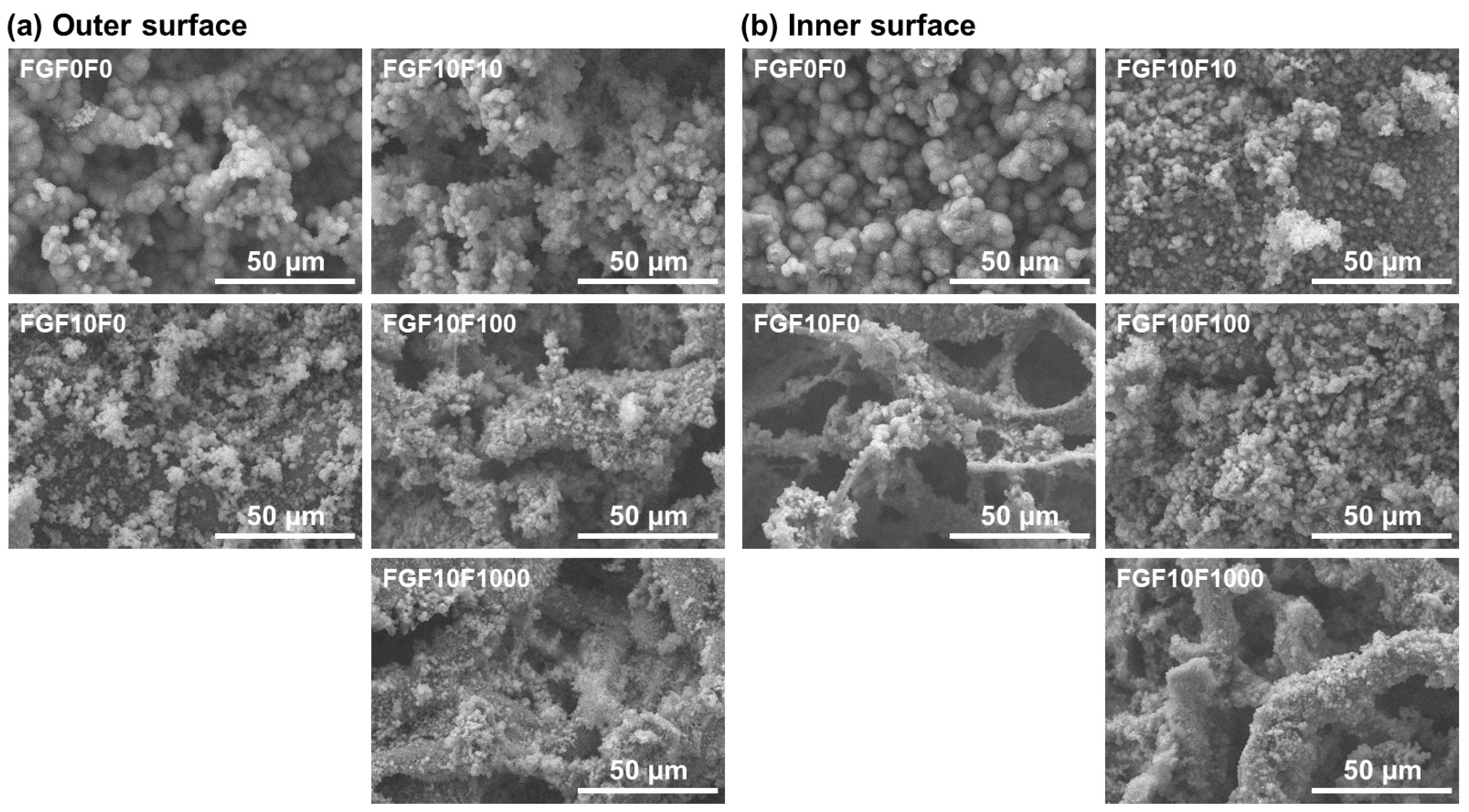
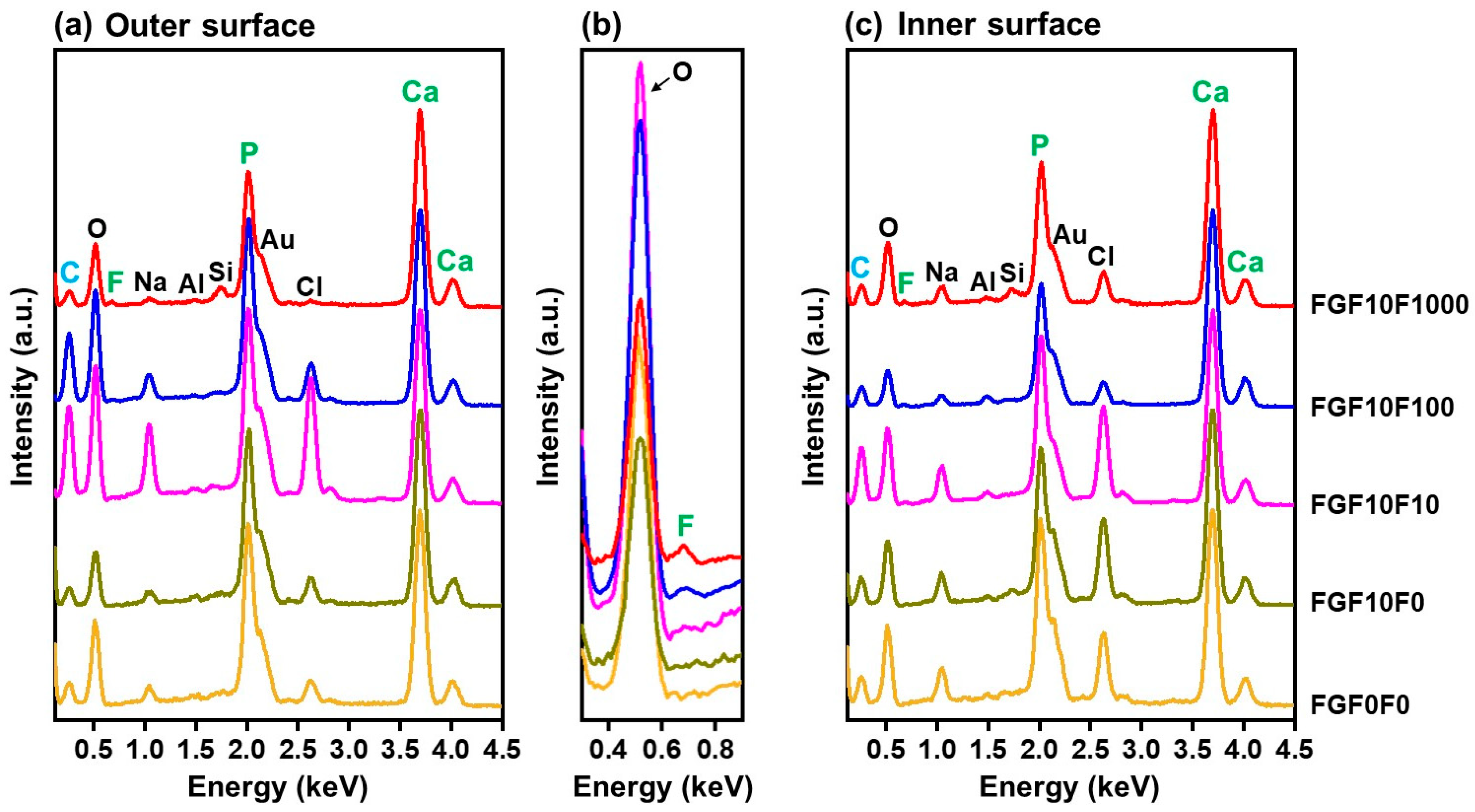
2.1. Surface Characterization
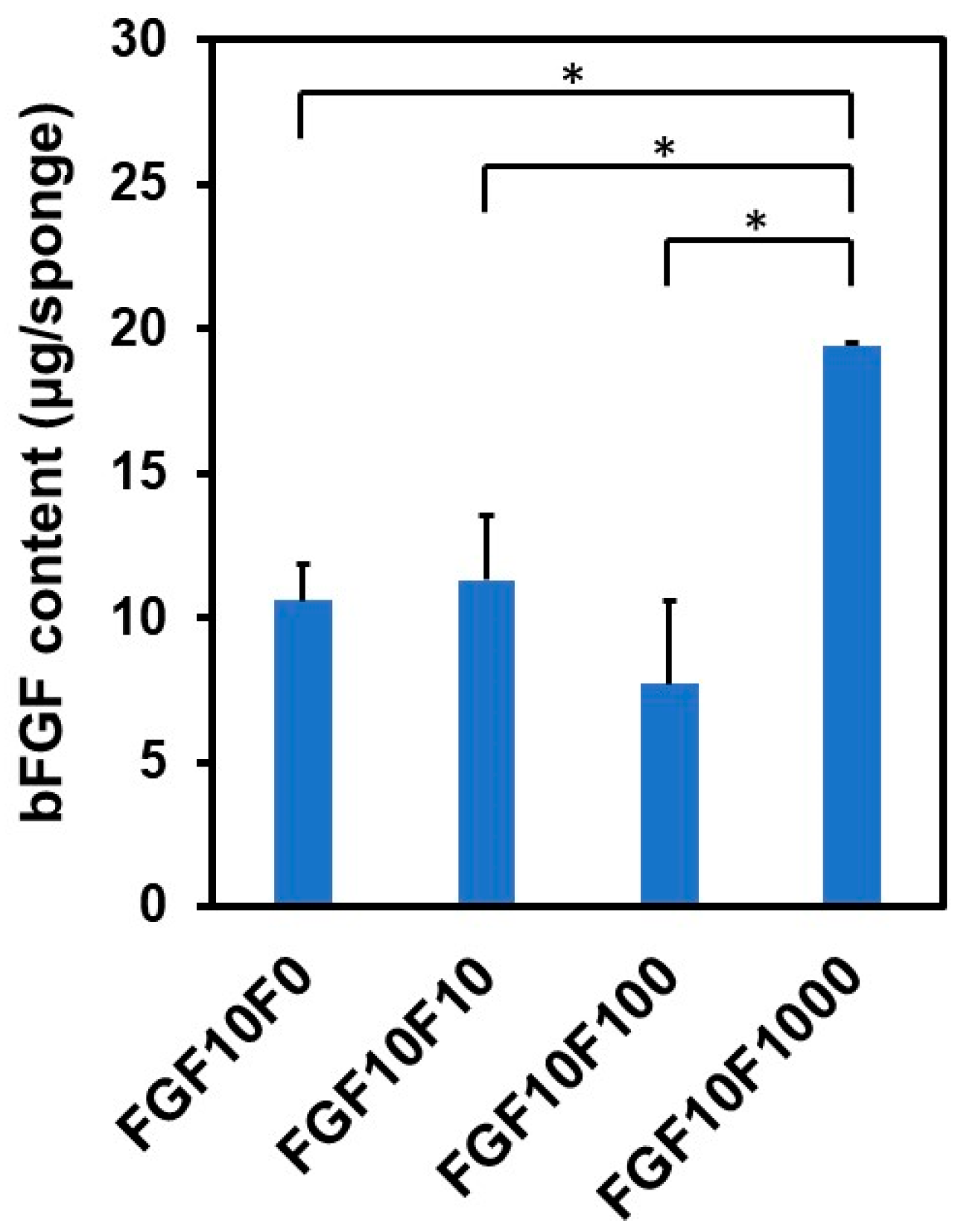
2.2. bFGF Content in the Coated Sponges
2.3. Detailed Analysis of FGF10F0 and FGF10F1000
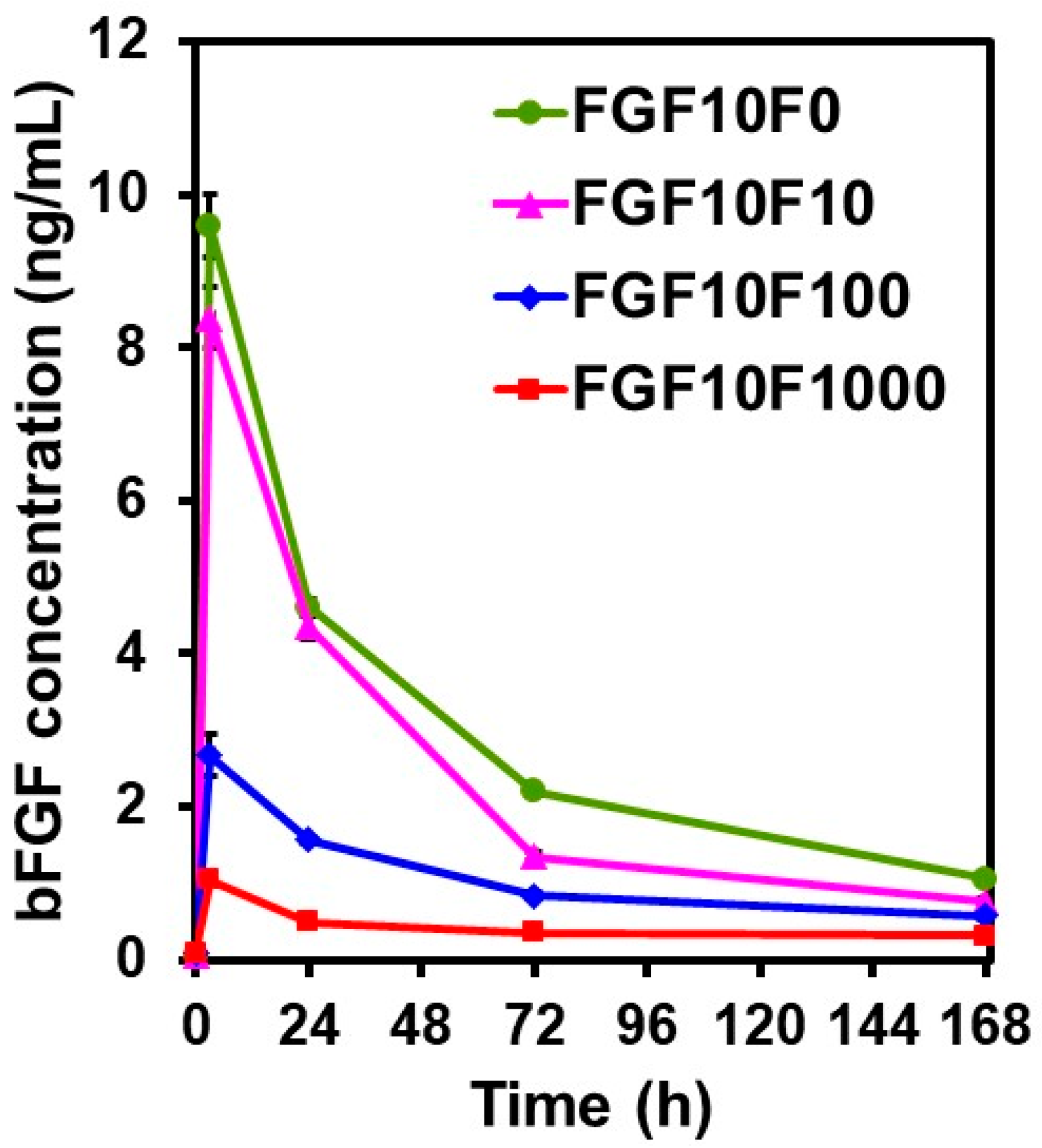
2.4. bFGF-Release Assay
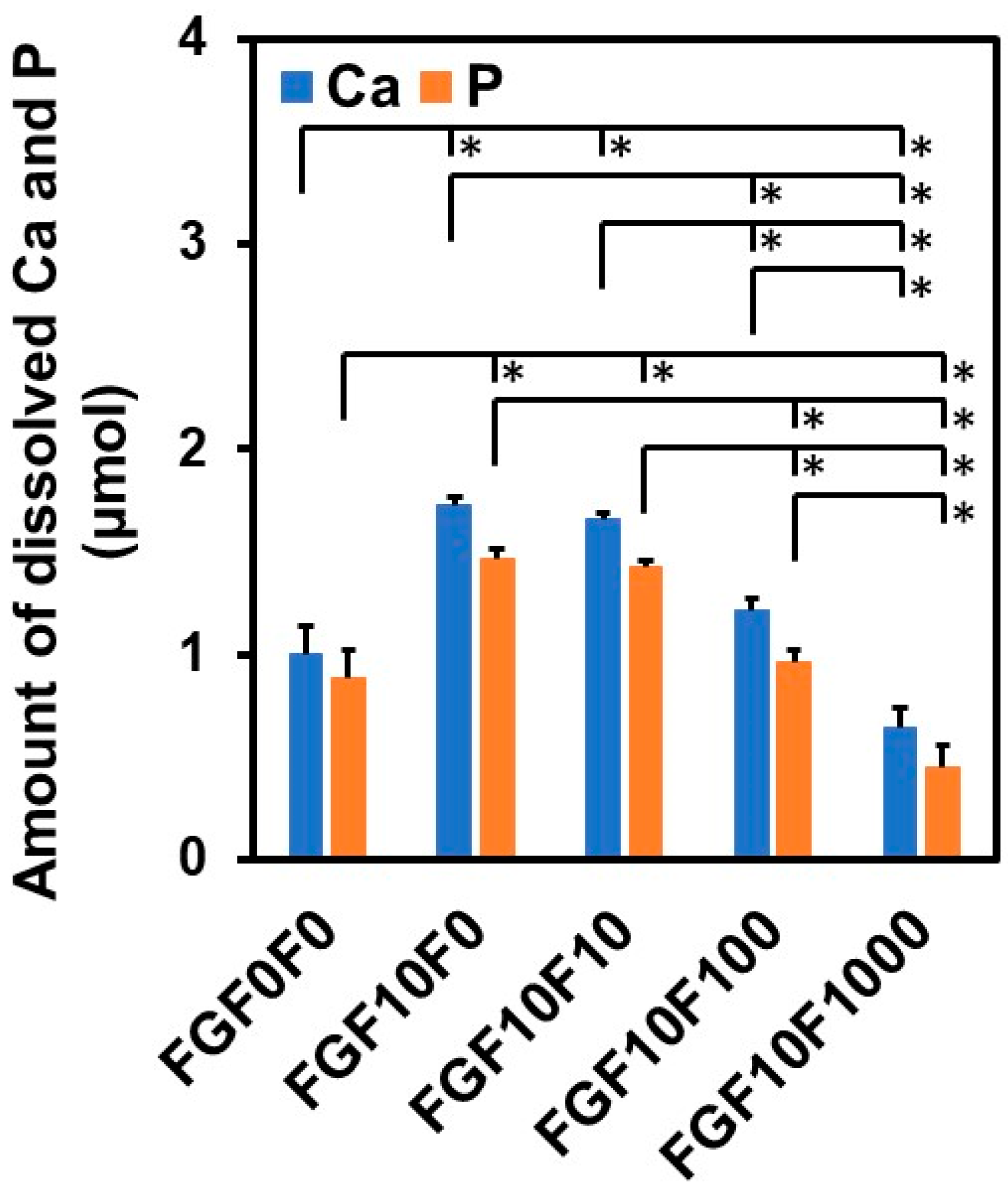
2.5. Solubility Assay of the Coating Layers
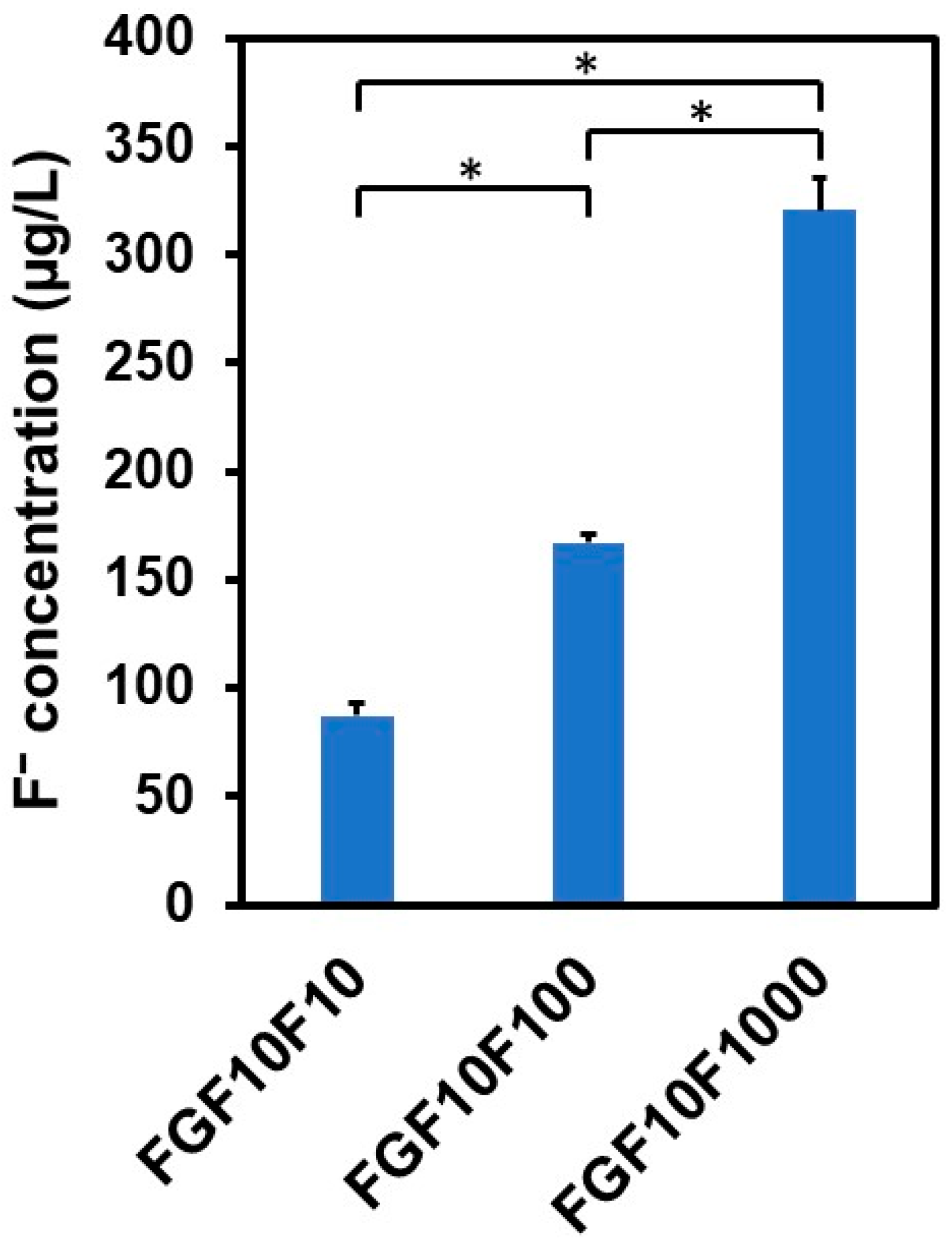
2.6. Fluoride Ion Release in the bFGF-Release Assay
2.7. Mineralization Assay in a Simulated Body Fluid (SBF)
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Supersaturated CaP Solutions
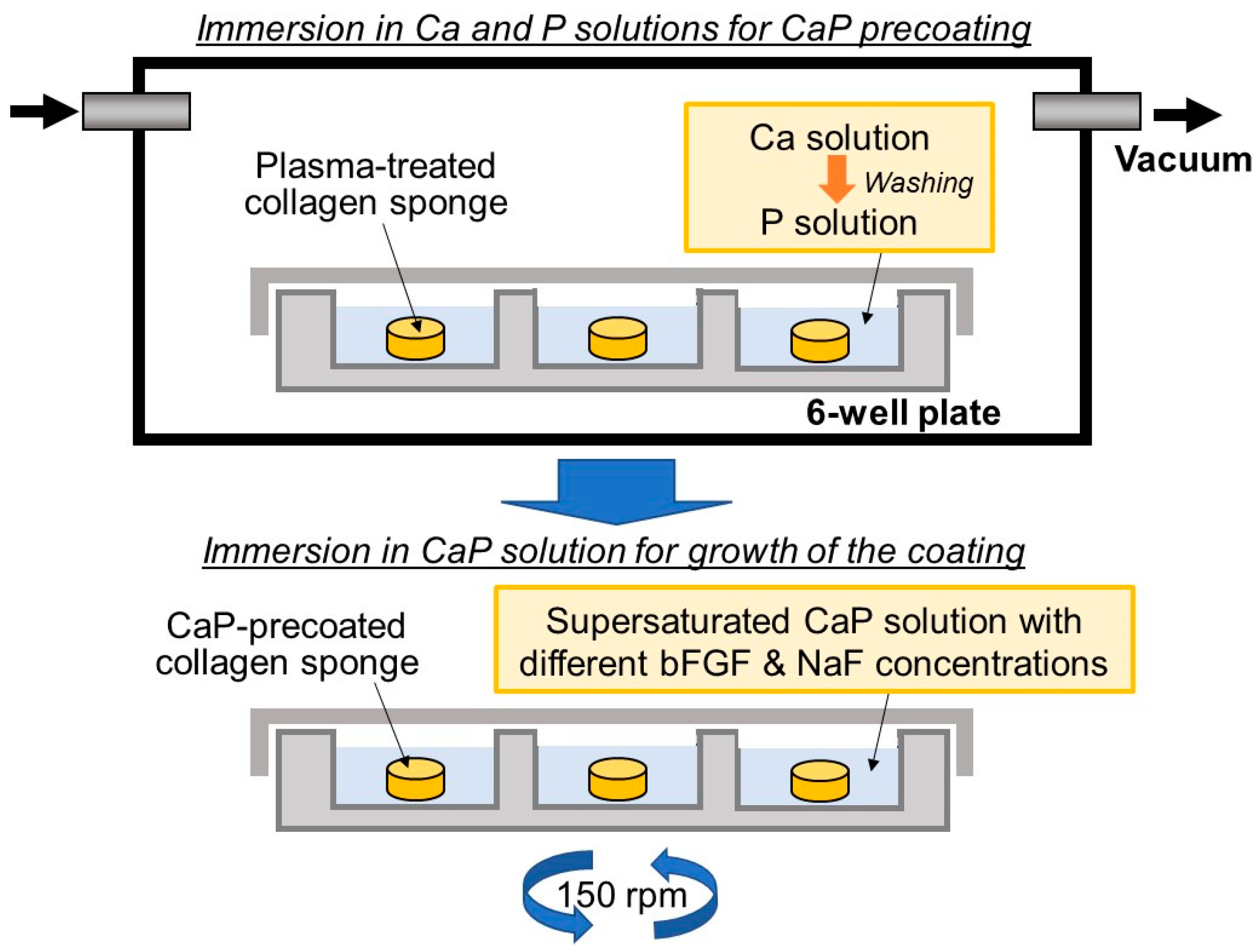
4.3. Precursor-Assisted Biomimetic Process for Coating
4.4. Assessment of Coating Weight and bFGF Content in Coated Sponges
4.5. Surface Characterization
4.6. Ultrastructural Analysis
4.7. bFGF-Release Assay
4.8. Quantification of the Released Ca and P Atoms and Fluoride Ions
4.9. SBF Test
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agocsova, S.V.; Culenova, M.; Birova, I.; Omanikova, L.; Moncmanova, B.; Danisovic, L.; Ziaran, S.; Bakos, D.; Alexy, P. Resorbable biomaterials used for 3D scaffolds in tissue engineering: A review. Materials 2023, 16, 4267. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Pan, H. Bone apatite nanocrystal: Crystalline structure, chemical composition, and architecture. Biomimetics 2023, 8, 90. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Kaflak, A.; Kolmas, J. Biologically inspired collagen/apatite composite biomaterials for potential use in bone tissue regeneration-a review. Materials 2020, 13, 1748. [Google Scholar] [CrossRef] [PubMed]
- Nathanael, A.J.; Oyane, A.; Nakamura, M.; Sakamaki, I.; Nishida, E.; Kanemoto, Y.; Miyaji, H. In vitro and in vivo analysis of mineralized collagen-based sponges prepared by a plasma- and precursor-assisted biomimetic process. ACS Appl. Mater. Interfaces 2017, 9, 22185–22194. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; La, W.G.; Bhang, S.H.; Lee, T.J.; Lee, M.; Kim, B.S. Apatite-coated collagen scaffold for bone morphogenetic protein-2 delivery. Tissue Eng. Part A 2011, 17, 2153–2164. [Google Scholar] [CrossRef]
- Xia, Z.; Villa, M.M.; Wei, M. A biomimetic collagen–apatite scaffold with a multi-level lamellar structure for bone tissue engineering. J. Mater. Chem. B 2014, 2, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, T.; Uezono, M.; Takakuda, K.; Kikuchi, M.; Oshima, S.; Sato, T.; Suzuki, S.; Moriyama, K. Enhanced bone formation onto the bone surface using a hydroxyapatite/collagen bone-like nanocomposite. J. Biomed. Mater. Res. 2020, 108, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Lemler, J.; Sakurai, K.; Yamada, M. Biodegradation property of beta-tricalcium phosphate-collagen composite in accordance with bone formation: A comparative study with Bio-Oss Collagen® in a rat critical-size defect model. Clin. Implant. Dent. Relat. Res. 2014, 16, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Arahira, T.; Todo, M. Development of novel collagen scaffolds with different bioceramic particles for bone tissue engineering. Compos. Commun. 2019, 16, 30–32. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Zhang, Y.; Chen, F.; Zhou, Y.; An, Q. Dehydrothermally crosslinked collagen/hydroxyapatite composite for enhanced in vivo bone repair. Colloids Surf. B 2018, 163, 394–401. [Google Scholar] [CrossRef]
- John, A.; Hong, L.; Ikada, Y.; Tabata, Y. A trial to prepare biodegradable collagen–hydroxyapatite composites for bone repair. J. Biomater. Sci. Polym. Ed. 2001, 12, 689–705. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Low, S.; Choon, A.T.; Kumar, T.S.S.; Ramakrishna, S. Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers. J. Mater. Sci. Mater. Med. 2008, 19, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic mineralization of biomaterials using simulated body fluids for bone tissue engineering and regenerative medicine. Tissue Eng. Part A 2017, 23, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; de Groot, K.; Wang, D.; Hu, Q.; Wismeijer, D.; Liu, Y. A review paper on biomimetic calcium phosphate coatings. Open Biomed. Eng. J. 2015, 9, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic coatings in implant dentistry: A quick update. J. Funct. Biomater. 2024, 15, 15. [Google Scholar] [CrossRef]
- Lin, X.; Chen, J.; Liao, Y.; Pathak, J.L.; Li, H.; Liu, Y. Biomimetic calcium phosphate coating as a drug delivery vehicle for bone tissue engineering: A mini-review. Coatings 2020, 10, 1118. [Google Scholar] [CrossRef]
- Kanemoto, Y.; Miyaji, H.; Nishida, E.; Miyata, S.; Mayumi, K.; Yoshino, Y.; Kato, A.; Sugaya, T.; Akasaka, T.; Nathanael, A.J.; et al. Periodontal tissue engineering using an apatite/collagen scaffold obtained by a plasma- and precursor-assisted biomimetic process. J. Periodont. Res. 2022, 57, 205–218. [Google Scholar] [CrossRef]
- Bikfalvi, A.; Klein, S.; Pintucci, G.; Rifkin, D.B. Biological roles of fibroblast growth factor-2. Endocr. Rev. 1997, 18, 26–45. [Google Scholar] [CrossRef]
- Yun, Y.R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.H.; Shin, U.S.; Kim, H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Novais, A.; Chatzopoulou, E.; Chaussain, C.; Gorin, C. The potential of FGF-2 in craniofacial bone tissue engineering: A review. Cells 2021, 10, 932. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Mutsuzaki, H.; Hara, Y.; Nagashima, K.; Okano, E.; Yanagisawa, Y.; Noguchi, H.; Sankai, T.; Yamazaki, M. Safety and osteointegration of titanium screws coated with a fibroblast growth factor-2-calcium phosphate composite layer in non-human primates: A pilot study. J. Funct. Biomater. 2023, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Oyane, A.; Hyodo, K.; Ito, A.; Sogo, Y.; Kamitakahara, M.; Ioku, K. Preparation and biological evaluation of a fibroblast growth factor-2–apatite composite layer on polymeric material. Biomed. Mater. 2010, 5, 065008. [Google Scholar] [CrossRef] [PubMed]
- Tsurushima, H.; Marushima, A.; Suzuki, K.; Oyane, A.; Sogo, Y.; Nakamura, K.; Matsumura, A.; Ito, A. Enhanced bone formation using hydroxyapatite ceramic coated with fibroblast growth factor-2. Acta Biomater. 2010, 6, 2751–2759. [Google Scholar] [CrossRef]
- Ito, Y.; Tsurushima, H.; Sato, M.; Ito, A.; Oyane, A.; Sogo, Y.; Matsumura, A. Angiogenesis therapy for brain infarction using a slow releasing drug delivery, system for fibroblast growth factor 2. Biochem. Biophys. Res. Commun. 2013, 432, 182–187. [Google Scholar] [CrossRef]
- Yazaki, Y.; Oyane, A.; Sogo, Y.; Ito, A.; Yamazaki, A.; Tsurushima, H. Control of gene transfer on a DNA–fibronectin–apatite composite layer by the incorporation of carbonate and fluoride ions. Biomaterials 2011, 32, 4896–4902. [Google Scholar] [CrossRef]
- Okazaki, M.; Aoba, T.; Doi, Y.; Takahashi, J.; Moriwaki, Y. Solubility and crystallinity in relation to fluoride content of fluoridated hydroxyapatites. J. Dent. Res. 1981, 60, 845–849. [Google Scholar] [CrossRef]
- Aoba, T. The effect of fluoride on apatite structure and growth. Crit. Rev. Oral Biol. Med. 1997, 8, 136–153. [Google Scholar] [CrossRef]
- Hughes, J.M.; Cameron, M.; Crowley, K.D. Structural variations in natural F, OH, and Cl apatites. Am. Mineral. 1989, 74, 870–876. [Google Scholar]
- Kim, J.Y.; Fenton, R.R.; Hunter, B.A.; Kennedy, B.J. Powder diffraction studies of synthetic calcium and lead apatites. Aust. J. Chem. 2000, 53, 679–686. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kozlowska, J. Characterization of collagen/hydroxyapatite composite sponges as a potential bone substitute. Int. J. Biol. Macromol. 2010, 47, 483–487. [Google Scholar] [CrossRef]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details: Holly springs hydroxyapatite. Acta Crystallogr. 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Chen, G.; Gulbranson, D.R.; Yu, P.; Hou, Z.; Thomson, J.A. Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells 2012, 30, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Benington, L.; Rajan, G.; Locher, C.; Lim, L.Y. Fibroblast growth factor 2—A review of stabilisation approaches for clinical applications. Pharmaceutics 2020, 12, 508. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Araki, H.; Nakamura, M.; Aiki, Y.; Higuchi, K.; Pyatenko, A.; Adachi, M.; Ito, Y. Controlled release of basic fibroblast growth factor from a water-floatable polyethylene nonwoven fabric sheet for maintenance culture of iPSCs. RSC Adv. 2020, 10, 95–104. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- ISO 23317:2014; Implants for Surgery, In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. International Organization for Standardization: Geneva, Switzerland, 2014.
- Oyane, A.; Uchida, M.; Onuma, K.; Ito, A. Spontaneous growth of a laminin-apatite nano-composite in a metastable calcium phosphate solution. Biomaterials 2006, 27, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Tiselius, A.; Hjertén, S.; Levin, Ö. Protein chromatography on calcium phosphate columns. Arch. Biochem. Biophys. 1956, 65, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kobayashi, W.; Ikeda, K.; Takahashi, S.; Monma, H. High-performance liquid chromatography using spherical aggregates of hydroxyapatite micro-crystals as adsorbent. Eur. J. Biochem. 1986, 157, 291–295. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.M.; Hart, J.N.; Gross, K.A. Influence of fluorine in the synthesis of apatites. Synthesis of solid solutions of hydroxy–fluorapatite. Biomaterials 2003, 24, 3777–3785. [Google Scholar] [CrossRef]
- Wei, M.; Evans, J.H.; Bostrom, T. Synthesis and characterization of hydroxyapatite, fluoride-substituted hydroxyapatite and fluorapatite. J. Mater. Sci. Mater. Med. 2003, 14, 311–320. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, G.; Liu, X.Y. Will fluoride toughen or weaken our teeth? Understandings based on nucleation, morphology, and structural assembly. J. Phys. Chem. B 2009, 113, 16393–16399. [Google Scholar] [CrossRef]
- Moreno, E.; Kresak, M.; Zahradnik, R. Fluoridated hydroxyapatite solubility and caries formation. Nature 1974, 247, 64–65. [Google Scholar] [CrossRef]
- Qiao, W.; Liu, Q.; Li, Z.; Zhang, H.; Chen, Z. Changes in physicochemical and biological properties of porcine bone derived hydroxyapatite induced by the incorporation of fluoride. Sci. Technol. Adv. Mater. 2017, 18, 110–121. [Google Scholar] [CrossRef]
- Ishimaru, T.; Komura, M.; Komura, H.; Otani, Y.; Komuro, H.; Sugiyma, M.; Terawaki, K.; Suzuki, K.; Tabata, Y.; Iwanaka, T. Slow release of basic fibroblast growth factor (b-FGF) promotes growth of tracheal cartilage. J. Pediatr. Surg. 2013, 48, 288–292. [Google Scholar] [CrossRef]
- Furue, M.K.; Na, J.; Jackson, J.P.; Okamoto, T.; Jones, M.; Baker, D.; Hata, R.I.; Moore, H.D.; Sato, J.D.; Andrews, P.W. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc. Natl. Acad. Sci. USA 2008, 105, 13409–13414. [Google Scholar] [CrossRef] [PubMed]
- Ringe, J.D. Fluoride in Osteoporosis. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press Inc.: Cambridge, UK, 2002; pp. 1387–1399. [Google Scholar]
- Jeng, J.H.; Hsieh, C.C.; Lan, W.H.; Chang, M.C.; Lin, S.K.; Hahn, L.J.; Kuo, M.Y.P. Cytotoxicity of sodium fluoride on human oral mucosal fibroblasts and its mechanisms. Cell Biol. Toxicol. 1998, 14, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Farley, J.R.; Wergedal, J.E.; Baylink, D.J. Fluoride directly stimulates proliferation and alkaline phosphatase activity of bone-forming cells. Science 1983, 222, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.C.; Park, S.S.; Subieta, A.R.; Brennan, T.J. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology 2004, 101, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Zhu, Y.; Yang, F.; Li, J.; Zhu, Z.; Zhu, H. Dissolution and solubility of hydroxylapatite and fluorapatite at 25 °C at different pH. Res. J. Chem. Environ. 2013, 17, 57–61. [Google Scholar]
- Onuma, K.; Pal, A.; Makino, M.; Sakamaki, I.; Nakamura, M.; Tanaka, S.; Miyaji, H.; Oyane, A. Rapid regeneration of artificial enamel using laser-assisted biomineralization: With and without mesoscale hierarchy in hydroxyapatite rods. Mater. Today Commun. 2024, 38, 108030. [Google Scholar] [CrossRef]


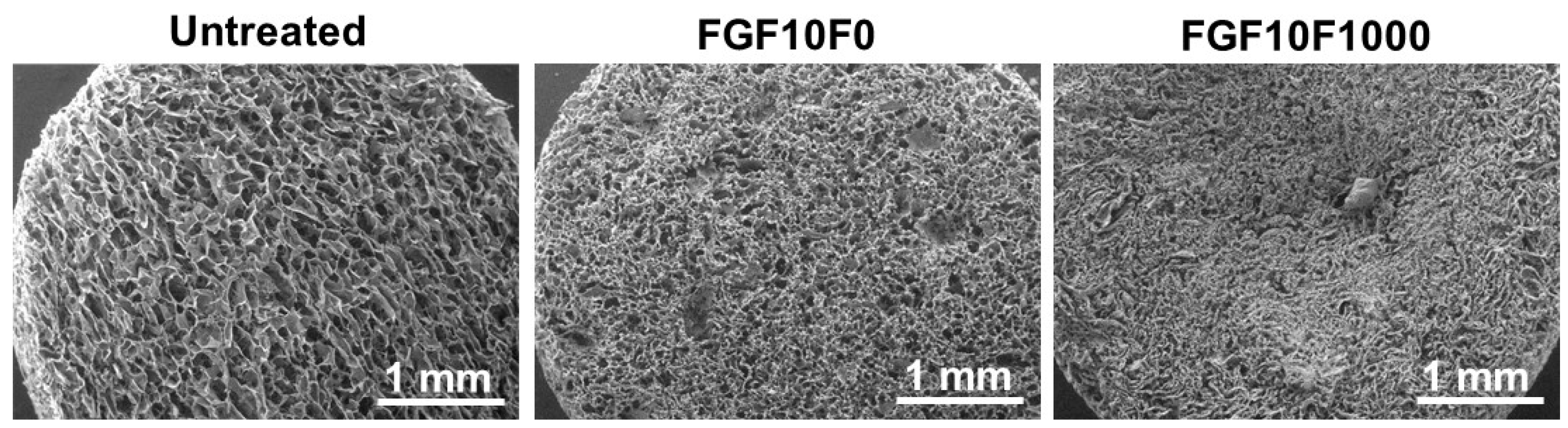


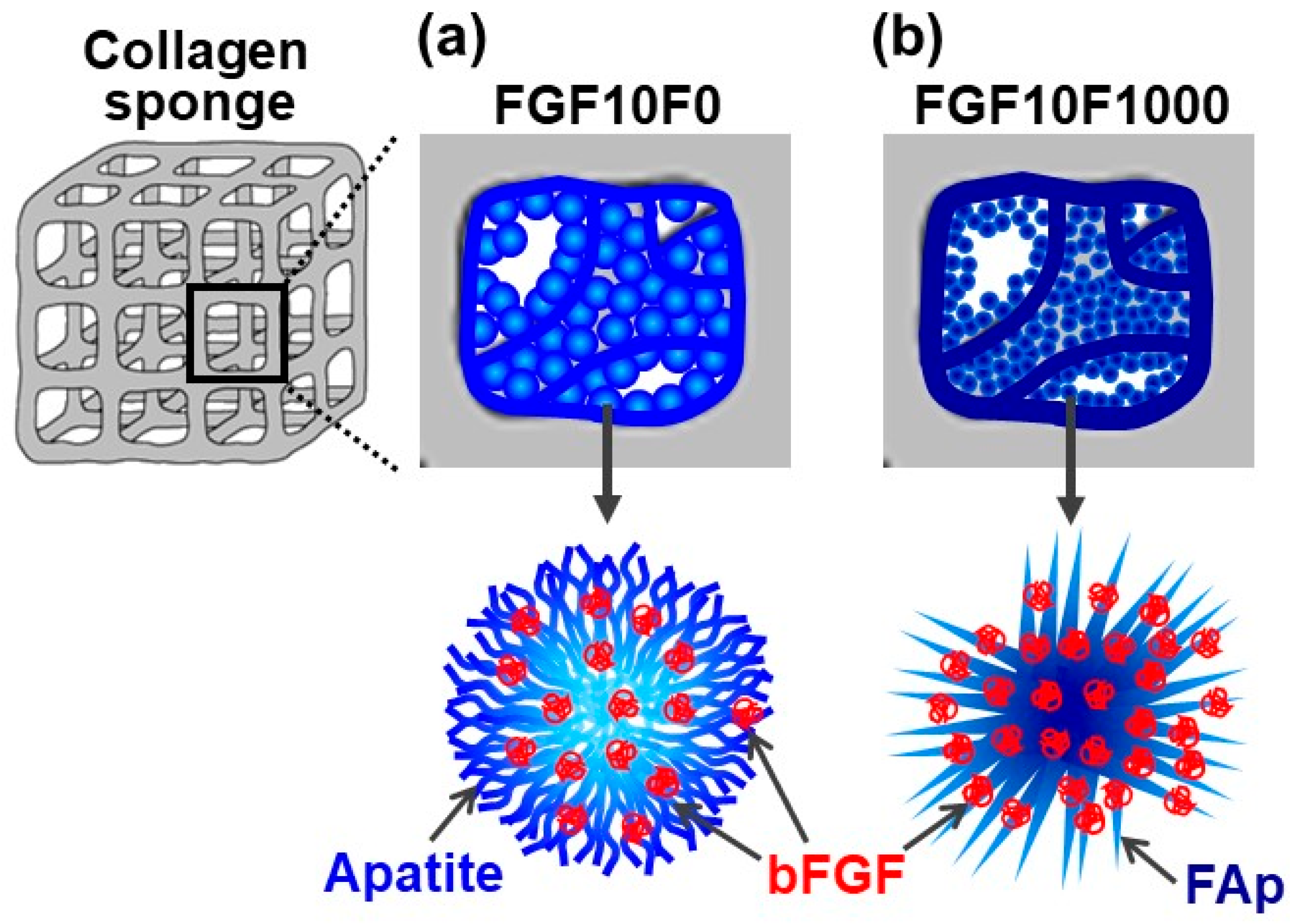
| Untreated | FGF10F0 | FGF10F1000 | |
|---|---|---|---|
| 2D porosity (%) | 58 ± 10 | 44 ± 6 | 38 ± 6 |
| Coating weight (mg/mg of sponge) | − | 2.08 ± 0.09 | 2.68 ± 0.22 |
| Ca/P molar ratio of the coating | − | 1.54 ± 0.02 | 1.65 ± 0.03 |
| F/P molar ratio of the coating | − | − | 0.14 ± 0.04 |
| bFGF (μg/mL) | NaF (μM) | Sample Name |
|---|---|---|
| 0 | 0 | FGF0F0 |
| 10 | 0 | FGF10F0 |
| 10 | 10 | FGF10F10 |
| 10 | 100 | FGF10F100 |
| 10 | 1000 | FGF10F1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pal, A.; Oyane, A.; Nakamura, M.; Koga, K.; Nishida, E.; Miyaji, H. Fluoride-Incorporated Apatite Coating on Collagen Sponge as a Carrier for Basic Fibroblast Growth Factor. Int. J. Mol. Sci. 2024, 25, 1495. https://doi.org/10.3390/ijms25031495
Pal A, Oyane A, Nakamura M, Koga K, Nishida E, Miyaji H. Fluoride-Incorporated Apatite Coating on Collagen Sponge as a Carrier for Basic Fibroblast Growth Factor. International Journal of Molecular Sciences. 2024; 25(3):1495. https://doi.org/10.3390/ijms25031495
Chicago/Turabian StylePal, Aniruddha, Ayako Oyane, Maki Nakamura, Kenji Koga, Erika Nishida, and Hirofumi Miyaji. 2024. "Fluoride-Incorporated Apatite Coating on Collagen Sponge as a Carrier for Basic Fibroblast Growth Factor" International Journal of Molecular Sciences 25, no. 3: 1495. https://doi.org/10.3390/ijms25031495
APA StylePal, A., Oyane, A., Nakamura, M., Koga, K., Nishida, E., & Miyaji, H. (2024). Fluoride-Incorporated Apatite Coating on Collagen Sponge as a Carrier for Basic Fibroblast Growth Factor. International Journal of Molecular Sciences, 25(3), 1495. https://doi.org/10.3390/ijms25031495