Abstract
Environmental stress at high altitudes drives the development of distinct adaptive mechanisms in plants. However, studies exploring the genetic adaptive mechanisms of high-altitude plant species are scarce. In the present study, we explored the high-altitude adaptive mechanisms of plants in the Himalayas through whole-genome resequencing. We studied two widespread members of the Himalayan endemic alpine genus Roscoea (Zingiberaceae): R. alpina (a selfing species) and R. purpurea (an outcrossing species). These species are distributed widely in the Himalayas with distinct non-overlapping altitude distributions; R. alpina is distributed at higher elevations, and R. purpurea occurs at lower elevations. Compared to R. purpurea, R. alpina exhibited higher levels of linkage disequilibrium, Tajima’s D, and inbreeding coefficient, as well as lower recombination rates and genetic diversity. Approximately 96.3% of the genes in the reference genome underwent significant genetic divergence (FST ≥ 0.25). We reported 58 completely divergent genes (FST = 1), of which only 17 genes were annotated with specific functions. The functions of these genes were primarily related to adapting to the specific characteristics of high-altitude environments. Our findings provide novel insights into how evolutionary innovations promote the adaptation of mountain alpine species to high altitudes and harsh habitats.
1. Introduction
High-altitude environments are characterized by high ultraviolet radiation, low temperature, hypoxia, and reduced incidence of pathogens [1,2]. To survive and be able to inhabit such harsh environments, local species have evolved effective strategies for the adaptation of genes to specific morphological and physiological traits [3,4,5,6,7]. Plant populations across altitude gradients exhibit genetic differentiation and local adaptation to specific environmental conditions [8,9]. High-altitude plants often exhibit genetic adaptations for cold tolerance to withstand freezing temperatures and frost [10]. They have genetic adaptations to cope with high light intensity and UV radiation [11]. They often possess genetic adaptations for efficient photosynthesis under low-CO2 conditions [12]. They exhibit genetic adaptations to cope with hypoxic conditions, enabling them to maintain energy production and metabolic homeostasis under hypoxic stress [13]. High-altitude plants face water scarcity and drought stress, especially in arid or alpine environments. They possess genetic adaptations for water conservation and drought resistance [14]. For instance, corn cultivated in high-altitude areas frequently accumulates flavonoids within its leaves and filaments to mitigate the effects of high UV-B exposure [15,16]. Genetic adaptations in plants to high-altitude environments are diverse and complex, enabling them to thrive in harsh conditions. Under extreme conditions, such as those in high-altitude regions, natural selection can drive quick alterations in allele frequencies to optimally enhance adaptability [5]. In recent years, there has been a growing interest in assessing genomic variations in natural populations by identifying adaptive loci to understand how organisms adapt to various habitats [17,18]. The development of high-throughput sequencing technology has greatly accelerated genomic research and identification of key genes and promoted the adaptive evolution and ecological research of non-model organisms [19,20]. This technology is also beneficial for further exploring the adaptation of non-model plants to high elevation [8,9,21,22].
The Himalayas, located on the southern margin of the Tibetan Plateau, have an elevation gradient of over 8000 m from south to north within a narrow latitude range [23,24] and are a biodiversity hotspot. Records of endemic species in the Himalayas and recent findings suggest that in situ speciation, especially divergence along the elevational gradient, plays a significant role in the region’s high biodiversity [25,26,27]. High-elevation species in the Himalayas face more rigorous environmental challenges than those experienced by lower-elevation species. Thus, exploring the genetic adaptation and divergence of high-altitude plants can provide important insights into their survival mechanisms in the harsh environments of the high Himalayas.
Roscoea, the only alpine genus in the pantropical family Zingiberaceae, is distributed at elevations ranging from approximately 1200 to 4800 m. Roscoea species have been categorized into two distinct groups: the Himalayas clade and the Hengduan Mountains clade [28,29,30,31]. Species in the Himalayas clade generally exhibit a distribution pattern along the altitude gradient (approximately 1200–4500 m). This distinct altitude divergence among the Himalayas Roscoea species is associated with the rapid uplift of the Himalayas and climate change [27]. R. alpina and R. purpurea are two widely distributed species along the Himalayas from west to east without an overlapping distribution [27,29]. Autonomous selfing is the predominant reproductive mode for R. alpina [32], whereas outcrossing is the reproductive strategy of R. purpurea [32,33]. Among the species in the genus Roscoea, R. alpina has the highest elevation and mainly occurs in alpine meadows, whereas R. purpurea is mainly distributed at lower elevations and found growing under trees [27,29]. Previous phylogenetic reconstruction based on restriction association site DNA (RAD) revealed that R. alpina diverged from the ancestor of R. purpurea approximately 14 million years ago (Ma) [27]. Thus, these two related species provide good models for elucidating the adaptive mechanisms of plants to high-altitude environments in the Himalayas. The aim of the present study was to investigate the adaptive mechanisms of R. alpina to high-altitude environments in the Himalayas by using whole-genome resequencing.
2. Results
2.1. Genomic Feature Investigation
To obtain insight into natural selection patterns and historical aspects of population growth, genome-wide patterns of linkage disequilibrium (LD) were determined. The average r square (r2) in the LD tended to decrease with increasing distances between pairwise single-nucleotide polymorphisms (SNPs), with a rapidly declining trend observed over the first 0.2 kb. The LD decay of R. alpina and R. purpurea revealed a similar declining trend (Figure 1a). However, a significant difference in r2 was observed over the same distance between the two species (p < 0.05). The highest r2 of R. alpina (approximately 0.8) was in the short-distance bin <300 bp, and it declined to the lowest r2 (approximately 0.45) at the longest-distance of approximately 0.7 kb. The highest r2 of R. purpurea (approximately 0.56) was in the short-distance bin <300 bp, and it declined to the lowest r2 (approximately 0.26) in the longest-distance bin of approximately 0.65 kb. The t-test results of the mean r2 in each 1 kb bin showed a significant difference between the two species (Figure 1a). The population recombination rates corresponding to LD decay indicated a higher recombination rate in R. purpurea on 12 chromosomes than in R. alpina (Figure 1b).
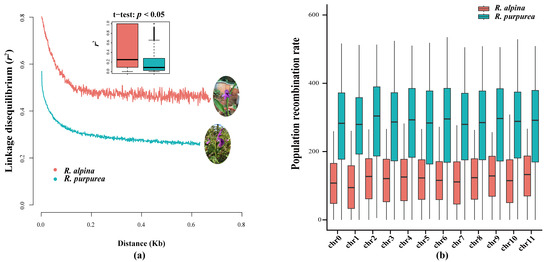
Figure 1.
Linkage disequilibrium (LD) and population recombination rate of R. alpina and R. purpurea: (a) LD decay of R. alpina and R. purpurea. The boxplot of LD decay is average r2 estimates with 1 kb bin; (b) population recombination rate boxplot of R. alpina and R. purpurea across 12 chromosomes.
Tajima’s D, π, FIS, and genome heterozygosity (HE) were used as indicators of genetic diversity; they were compared between R. purpurea and R. alpina. The results indicate that R. alpina has lower genetic variation than R. purpurea. Tajima’s D and FIS of R. alpina were significantly higher than those of R. purpurea (Figure 2a,b), whereas π and HE of R. alpina were significantly lower than those of R. alpina (Figure 2c,d).
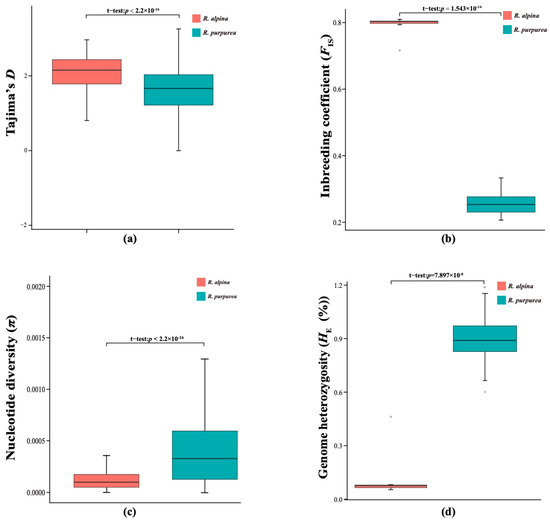
Figure 2.
Genetic diversity parameters of R. alpina and R. purpurea: (a) Tajima’s D between two species; (b) FIS between two species; (c) π between two species; (d) HE between two species.
To eliminate the effects of sampling size on comparisons of the parameters between the two species, random sampling analysis was conducted. Although Tajima’s D and π varied with sampling size, Tajima’s D of all random sampling in R. alpina was significantly higher than that of all random sampling in R. purpurea (Table S1), and π of all random sampling in R. alpina was significantly lower than π of all random sampling in R. purpurea (Table S2). In addition, the variation tended to be stable when the sampling size was seven, indicating that seven individuals had sufficient SNPs for estimating Tajima’s D and π in the present study (Figure S1).
2.2. Difference in Demographic History
Different individuals of each species had a similar demographic history, but it was significantly different between R. purpurea and R. alpina (Figure 3). During the 1.6–1.0 Ma period, the effective population size of R. alpina was greater than that of R. purpurea. The effective population size of R. purpurea began to decline around 1.5 Ma, and the decline lasted until approximately 50,000 years ago. The effective population size of R. alpina started to decline sharply at approximately 1.0 Ma and declined until approximately 15,000 years ago. Approximately 10,000 years ago, the effective population size of R. purpurea was twice that of R. alpina.
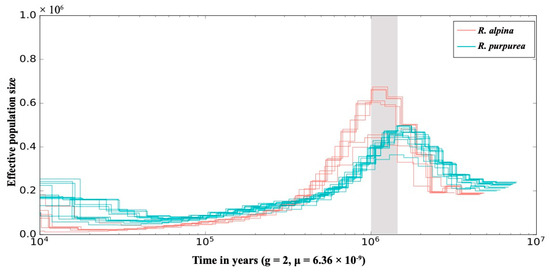
Figure 3.
Demographic history of each individual inferred based on the pairwise sequential Markov coalescent (PSMC) model, colored by species (see legend). The X-axis shows the time in years, and the Y-axis shows the effective population size. Light-gray-colored shading marks the interval of significant decrease in effective population size at ~1.5–1.0 Ma. g, generation time; μ, mutation rate.
2.3. Candidate Genes Associated with High-Altitude Adaptation
Generally, the functions of divergent genes selected by FST are potentially related to adaptability [34,35,36]. We used FST to investigate the genes related to high-altitude adaptation in R. alpina. The random sampling size for FST was analyzed to eliminate the effects of sampling size on FST estimation and the subsequent search for potentially adaptive genes. The random sampling showed similar results; most windows and genes of all random sampling were highly divergent. R. purpurea sample size did not influence the FST distribution landscape (Tables S3 and S4, Figure S2). A total of 17,108 highly genetically divergent windows (FST > 0.25) were identified (including 96.3% of the genes in the reference genome) (Figure 4).
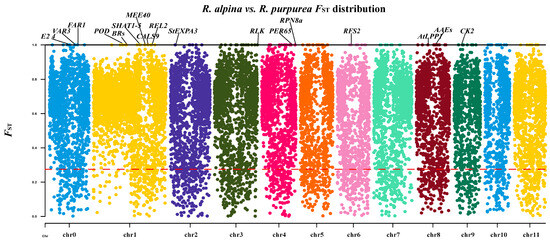
Figure 4.
Manhattan plot of genome-wide FST between R. alpina and R. purpurea on each of the 12 chromosomes. The red dashed line indicates FST = 0.25 and the black solid line indicates FST = 1.
The genes with FST = 1 have the highest degree of divergence in the genome. Such a high degree of divergence suggests that the genes may play an important role in environmental adaptation. To eliminate the influence of sample size bias and extract more reliable environmental adaptive genes, genes in FST = 1 windows were extracted by all random sampling and non-random sampling strategies. There were 76 common windows across all sampling strategies (Table S3), and 58 genes were annotated in the windows (Table S4). Among the 58 genes, 17 genes were annotated with specific functions. Most of the gene functions were associated with responses to environmental stress, DNA repair, and photosynthesis (Table 1). However, 41 of the 58 genes’ functions were unknown (Table S5), and we speculated that their functions may be related to high-altitude adaptation.

Table 1.
Completely divergent (FST = 1) genes and function list for the comparison of R. alpina and R. purpurea.
3. Discussion
3.1. Genomic Features for Adaptive Evolution in the High Himalayas
We speculated that different reproductive strategies and selection pressures may lead to differences in LD decay and population recombination rate. Self-fertilization is an adaptive strategy for plants in harsh environments, such as those where pollinators are absent [36,53,54]. Self-fertilization provides reproductive assurance under low levels of insect diversity in alpine ecosystems [55,56,57,58]. Autonomous selfing in R. alpina has been proposed as an evolutionary strategy for reproductive success in the alpine zone of the Himalayas [32]. Inbreeding and selfing have been observed to increase the correlation between alleles at different loci, contributing to increased LD and decreased recombination rate [36,54,59,60,61]. Contrasting LD and recombination rates between these two species are likely associated with their different mating systems, namely autonomous selfing in R. alpina [62] and outcrossing in R. purpurea [32,33]. Similar results were observed in maize and Arabidopsis [63]. In some cases, selection can increase the LD [64]. When interacting loci are closely linked or selection is strong, the recombination rate is likely to decrease [65,66]. The lower recombination rate and higher LD levels in R. alpina suggest that it may have undergone strong natural selection.
Long-term selfing may decrease genetic diversity and enhance linkage effects in the genome [61,67], consistent with our findings of lower π and HE and higher FIS in R. alpina than in R. purpurea. High LD is predicted to decrease the polymorphism of the linked loci, which may eventually lead to a significant decrease in the genetic diversity of R. alpina. Positive Tajima’s D values were observed in both R. alpina and R. purpurea, which, combined with the demographic results (Figure 4), suggest that the two species have undergone genetic bottlenecks [68,69]. However, higher Tajima’s D values suggest that R. alpina has undergone a stronger genetic bottleneck in comparison with R. purpurea. A strong genetic bottleneck has also been observed in other alpine plants [70,71]. Therefore, the lower genetic diversity of R alpina could be the consequence of adaptive evolution to the higher elevation in the Himalayas [71,72].
3.2. Difference in Demographic History within the Himalayas
The difference in demographic history suggests that R. alpina and R. purpurea in the Himalayas may respond differently to climate change. Under the influence of changing climate, the habitable area for the species shifts toward mountain tops and thus becomes narrower, with the habitats becoming harsher [73,74,75]. Consequently, colonization of higher mountain elevations should result in stronger genetic bottlenecks/drift and a sharp decrease in effective population size, as indicated by our findings in R. alpina. When the effective population size of R. alpina increased to the maximum from ~1.6–1.2 Ma, temperature variation between glaciations and interglaciations was relatively stable, not reaching full glacial values [76]. However, the maximum increase in the effective population size of R. purpurea occurred from ~1.8–1.5 Ma, and its population size began to decline at the onset of the Ice Age (~1.5 Ma). The decline in the effective population size of R. alpina was delayed by about 0.5 Ma compared to that of R. purpurea. We speculated that R. alpina may have long-term adaptations to low-temperature environments at higher altitudes, which is why its survival would have been largely unaffected until the temperature dropped to full glacial values.
Recombination rate is positively correlated with effective population size and genetic diversity [77,78,79]. The lower genetic diversity (Figure 2c) and recombination rate (Figure 1b) related to the selfing characteristics of R. alpina could have lagged behind R. purpurea in restoring the effective population size. The higher genetic diversity (Figure 2c) and recombination rate (Figure 1b) of R. purpurea could have improved its ability to restore the effective population size because, after genetic bottlenecks, outcrossing species with higher recombination rates and genetic diversity possess a greater ability to increase their effective population size [77,80,81].
3.3. Candidate Genes Associated with High-Altitude Adaptation
At high altitudes in the Himalayas, the most severe environmental stresses include extreme cold, low oxygen levels, high UV radiation, pathogens, and other biotic and abiotic stressors [82,83]. Plants that inhabit the Himalayas have evolved in their morphological structure, physiology, and metabolism to adapt to the extreme ecological conditions of this region. Their evolutionary genetic changes often adhere to certain patterns, evident in factors such as cold tolerance, efficiency of photosynthesis, hypoxia tolerance, antioxidant defense mechanisms, stress response, and drought resistance. We found several completely divergent genes that were likely associated with alpine adaptations in R. alpina (Table 1). AAEs and VAR3 genes can help species adjust their secondary metabolite production to cope with harsh environments. The RFS2, RLK, and PER65 genes were also related to stress responses, and SHAT1-5, BRs, REL2, E2, CALS9, StEXPA3, RPN8a, and MEE40 play important roles in the response to biotic and abiotic stress at high altitudes. Based on our observations, budding and flowering times differed between R. alpina and R. purpurea. The CK2 gene may regulate the circadian clock to adjust to the unpredictable climate between higher and lower altitudes in the Himalayas. The FAR1 and POD genes can improve the photosynthetic rate by regulating the synthesis of sucrose and starch. The AtLPP1 gene can repair the DNA damage caused by high UV and solar radiation. Notably, among these key genes, E2, RLK, and FAR1 have been proposed to facilitate the adaptation of alpine plants to high-altitude environments through convergent evolution [9,84]. These genes could have facilitated R. alpina adaptation to higher elevation, with extensive distribution along the Himalayas.
4. Materials and Methods
4.1. Resequencing and Variant Discovery
Seven individuals of R. alpina and thirteen individuals of R. purpurea were collected from wild populations in the Himalayas (Figure 5, Table S6). To obtain a sufficient number of SNPs, a whole-genome resequencing depth greater than 30× was adopted for SNP extraction. For genome sequencing, at least 5 μg of genomic DNA was extracted from fresh leaves by using the cetyltrimethylammonium bromide (CTAB) method [85]. DNA libraries were constructed and barcoded by using the DNA Library Prep Reference Guide (Illumina, Inc., San Diego, CA, USA). After sequencing on the Illumina Hiseq X Ten platform, 150 paired-end whole-genome sequencing reads with an insert size of 350 bp were obtained. The average sequencing depth was >30×, and 1072 Gb of raw sequencing data were obtained, with an average of 53.60 Gb per sample (Table S6).
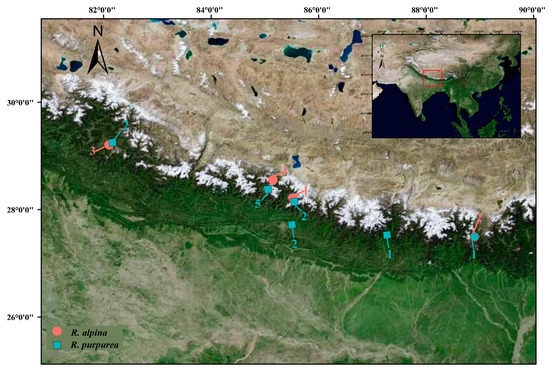
Figure 5.
Sampling sites of Roscoea alpina and R. purpurea. The numbers beside the dots are the sample sizes of the sampling sites.
FastQC v.0.11.9 was used to assess the quality of raw data (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 8 January 2019)). Subsequently, Trimmomatic v.0.36 [86] was used to filter the sequences. First, the first 15 bp potential adaptor sequences evaluated by FastQC were removed. Second, low-quality paired reads with more than 10% unrecognized bases were eliminated. Third, low-quality bases with Phred quality scores <30 were trimmed. After filtering, the reads were aligned to the reference genome of Roscoea schneideriana (unpublished) by using BWA-MEM v.0.7.17 [87] with the default parameters. SAMtools v.1.12 (https://sourceforge.net/projects/samtools/ (accessed on 17 March 2021)) [88] was used to convert the mapping results into the BAM format and filter the unaligned and non-unique aligned reads. Duplicated reads were marked and filtered by using Picard v.2.1.1 (picard.sourceforge.net (accessed on 4 March 2016)). After mapping, the reads were realigned by using the Genome Analysis Toolkit (GATK) v.3.8 (https://hub.docker.com/r/broadinstitute/gatk3/tags/ (accessed on 28 July 2017)) [89] in two steps. In the first step, the “RealignerTargetCreator” package was used to identify regions where realignment was required. In the second step, the “IndelRealigner” package was used to realign the regions found in the first step to produce a realigned BAM file for each sample.
Variation detection with the realigned BAM file followed the best-practice workflow recommended by GATK [89]. Briefly, the variants were called for each individual by using the GATK HaplotypeCaller. A joint genotyping step for a comprehensive variation union was performed by using the gVCF files. In the hard filtering step, the SNP filter expression was set as “QD < 2.0 || MQ < 40.0 || FS > 60.0 || SOR > 3.0 || MQRankSum < −12.5 || ReadPosRankSum < −8.0 || QUAL < 30”. PLINK v.1.90 (https://www.cog-genomics.org/plink/ (accessed on 15 May 2014)) was used to further filter the SNPs with the parameters “--geno 0.1 --maf 0.01”. Finally, 1,776,773 filtered SNPs were obtained for subsequent analysis.
4.2. Genomic Feature Investigation
LD was calculated based on the correlation coefficient (r2) statistics for genome-wide filtered SNPs by using PopLDdecay v.3.29 (https://github.com/BGI-shenzhen/PopLDdecay (accessed on 11 September 2018)) with the default parameters. An LD decay plot was made by using Perl script Plot_MultiPop.pl, with the parameters set as “-bin1 1 -bin2 10 -maxX 0.8”. To conduct statistical tests for LD differences, geno-r2 of LD statistics in VCFtools v.0.1.13 (https://vcftools.sourceforge.net (accessed on 3 August 2015)) [90] was used to calculate the average r2 of each 1 kb bin. Subsequently, a paired t-test for r2 between R. alpina and R. purpurea was performed by using the R program (https://www.R-project.org/ (accessed on 21 April 2023)).
To estimate the population recombination rate (ρ), Beagle v.5.2 (https://faculty.washington.edu/browning/beagle/b5_2.html (accessed on 28 January 2021)) [91] was used to phase the filtered SNPs, and the phased data were then input into the FastEPRR_VCF_step1 function in FastEPRR v.2.0 (https://www.picb.ac.cn/evolgen/softwares/download/FastEPRR/FastEPRR2.0/ (accessed on 10 January 2021)) [92] to scan the sequences and store the required information in files for each 50 kb window with the parameters winLength = 50,000 and winDXThreshold = 10. Subsequently, FastEPRR_VCF_step2 was used to estimate the recombination rate for each window. Finally, FastEPRR_VCF_step3 was used to merge the files generated by step 2 for each chromosome.
Tajima’s D and π of R. alpina and R. purpurea were computed by using VCFtools. FIS of the two species was calculated by using PLINK. KmerGenie v.1.7048 (http://kmergenie.bx.psu.edu (accessed on 14 March 2018)) [93] was used to estimate the optimal k-mer length for the de novo genome assembly. GenomeScope v.1.0 (https://github.com/schatzlab/genomescope/ (accessed on 15 January 2017)) [94] was used to estimate genome heterozygosity (HE). The t-test for the four genetic diversity parameters between R. alpina and R. purpurea was performed using the R program.
Although our sample size is small, other studies have shown that a small sample size (as small as n = 4–6) with a sufficient number of SNPs (at least 3000 SNPs) can estimate parameters of population genomics accurately, including genetic diversity [95,96]. In addition, to test whether our genetic diversity results could be affected by sample size, random sampling strategies were adopted for the calculation of genetic diversity. Four, five, six, and seven individuals of each species were selected randomly to calculate genetic diversity. We adopted ten times random sampling for each sample size. The Tajima’s D and π of each random sampling were calculated for both species. The parameters between species under different sample sizes were compared to test the impact of sample size on the two values.
4.3. Demographic Inferences
Consensus sequences were used to estimate demographic history. Individual paired-end whole-genome sequencing reads were mapped to reference genomes by using the subscripts mpileup and the call format of BCFtools v.0.1.17 (https://www.htslib.org/doc/1.0/bcftools.html (accessed on 7 July 2017)), and vcf files of consensus sequences were generated. Subsequently, vcfutils.pl was used to convert the consensus sequence vcf files into consensus FASTQ sequence files, and then the FASTQ files were converted to consensus FASTA sequences by using SeqTK v.1.3 (https://github.com/lh3/seqtk/ (accessed on 18 June 2018)). The PSMC v.0.6.5 (https://github.com/lh3/psmc/ (accessed on 30 April 2015)) model was used to examine the demographic history of each individual [97]. It was run for a total of 25 iterations with the parameters “-t15 -r5 -p ‘4 + 25 × 2 + 4 + 6’”, a generation time of two years, and a mutation rate of 6.36 × 10−9 per site per generation.
4.4. Identification of Candidate Genes Associated with High-Altitude Adaptation
Several researchers have used FST = 1 to search adaptive candidate genes between related species [98,99,100]. We used the same strategy to obtain the genes potentially related to high-altitude adaptation. The FST values across the Roscoea genome (window size = 50,000 bp) were used to identify the candidate genomic regions. The genomic windows with FST = 1 were treated as candidate high-altitude adaptation windows.
The sample size of R. purpurea is nearly twice that of R. alpina. The imbalance in sample size may skew the FST results. To exclude the bias and evaluate the effect of sample size on FST, seven, eight, nine, ten, eleven, and twelve individuals of R. purpurea were selected randomly for estimating FST under a stable sample size of R. alpina of seven. The number of times adopted for random sampling of each sample size was ten. Subsequently, the windows with FST = 1 were extracted. The windows shared by 10 random sampling times under the same sample size and all random sampling were extracted. Genes were extracted if the regions overlapped with the final extracted windows. The gene sequences were annotated to the NR database by using BLAST v. 2.11s. Homologous genes in the NR database were retained. The functions of the homologous genes were found in the literature. Finally, the genes with functional annotations were identified as candidate genes.
5. Conclusions
In the present study, we compared the genetic differentiation between the high-altitude R. alpina and low-altitude R. purpurea in the Himalayas and explored whether their genetic variation is associated with adaptation to high-altitude environments. Our results suggested that perhaps under the influence of selective pressure and autonomous selfing characteristics, the high-altitude R. alpina exhibited a high level of LD, low population recombination rate, delayed decrease in effective population size, and smaller effective population size. Additionally, it had high values of Tajima’s D and FIS while displaying low π and HE. Founder effect and lower recombination rate (higher LD) correlation with lower genetic diversity of R. alpina and inbreeding may enhance the LD. Furthermore, selection possibly reduced the nucleotide diversity of genomic loci and reduced the diversity of its linkage loci. Due to its long-term survival and propagation in the Himalayas, this species has evolved hereditary genes that protect it from extremely harsh high-altitude environments. Moreover, we identified numerous high-altitude adaptation-related genes that diverged during the adaptation to high-altitude environments. These completely divergent genes may be the evolutionary innovations of R. alpina driven by harsh environmental stresses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms25042265/s1.
Author Contributions
Conceptualization, Y.-L.W. and J.-L.Z.; Methodology, Y.-L.W.; Software, Y.-L.W.; Formal Analysis, Y.-L.W.; Resources, L.L., B.R.P., and J.-L.Z.; Writing, Y.-L.W., J.-L.Z., and B.R.P.; Supervision, B.R.P. and J.-L.Z.; Funding Acquisition, L.L. and J.-L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 41871047) and the Yunnan Revitalization Talent Support Program “Young Talent Project” (YNWR-QNBJ-2019-214).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Singh, S.; Singh, R. High-altitude clear-sky direct solar ultraviolet irradiance at Leh and Hanle in the western Himalayas: Observations and model calculations. J. Geophys. Res. Atmos. 2004, 109, D19201. [Google Scholar] [CrossRef]
- Stres, B.; Sul, W.J.; Murovec, B.; Tiedje, J.M. Recently deglaciated high-altitude soils of the Himalaya: Diverse environments, heterogenous bacterial communities and long-range dust inputs from the upper troposphere. PLoS ONE 2013, 8, e76440. [Google Scholar] [CrossRef]
- Monge, C.; León-Velarde, F. Physiological adaptation to high altitude: Oxygen transport in mammals and birds. Physiol. Rev. 1991, 71, 1135–1172. [Google Scholar] [CrossRef]
- Storz, J.F.; Scott, G.R.; Cheviron, Z.A. Phenotypic plasticity and genetic adaptation to high-altitude hypoxia in vertebrates. J. Exp. Biol. 2010, 213, 4125–4136. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Sgrò, C.M. Climate change and evolutionary adaptation. Nature 2011, 470, 479485. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.R.; Zhang, Z.H.; Zhang, C.L.; Zhang, J.Q.; Ran, A. Ecophysiological responses of three tree species to a high-altitude environment in the southeastern Tibetan Plateau. Forests 2018, 9, 48. [Google Scholar] [CrossRef]
- Gros-Balthazard, M.; Besnard, G.; Sarah, G.; Holtz, Y.; Leclercq, J.; Santoni, S.; Wegmann, D.; Glemin, S.; Khadari, B. Evolutionary transcriptomics reveals the origins of olives and the genomic changes associated with their domestication. Plant J. 2019, 100, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Halbritter, A.H.; Fior, S.; Keller, I.; Billeter, R.; Edwards, P.J.; Holderegger, R.; Karrenberg, S.; Pluess, A.R.; Widmer, A.; Alexander, J.M. Trait differentiation and adaptation of plants along elevation gradients. J. Evol. Biol. 2018, 31, 784–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kuang, T.; Dong, W.; Qian, Z.; Zhang, H.; Landis, J.B.; Feng, T.; Li, L.; Sun, Y.; Huang, J.; et al. Genomic convergence underlying high-altitude adaptation in alpine plants. J. Integr. Plant Biol. 2023, 65, 1620–1635. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, G.K.; Dalrymple, S.E.; Pritchard, H.W. Seed survival at low temperatures: A potential Sselecting factor influencing community level changes in high altitudes under climate change. Crit. Rev. Plant Sci. 2020, 39, 479–492. [Google Scholar] [CrossRef]
- Tyagi, A.; Yadav, A.; Tripathi, A.M.; Roy, S. High light intensity plays a major role in emergence of population level variation in Arabidopsis thaliana along an altitudinal gradient. Sci. Rep. 2016, 23, 26160. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Sun, Y.; Yang, G.; Li, L.; Sun, W.; Wang, Z.; Zhang, H.; Li, Y. Natural variation in stress response induced by low CO2 in Arabidopsis thaliana. Open Life Sci. 2020, 21, 923–938. [Google Scholar] [CrossRef] [PubMed]
- León, J.; Castillo, M.C.; Gayubas, B. The hypoxia-reoxygenation stress in plants. J. Exp. Bot. 2021, 72, 5841–5856. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.F.; Wang, Y.B.; Zhang, J.B.; Maddock, S.; Snook, M.; Peterson, T. A maize QTL for silk maysin levels contains duplicated Myb-homologous genes which jointly regulate flavone biosynthesis. Plant Mol. Biol. 2003, 52, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Casati, P.; Walbot, V. Differential accumulation of maysin and rhamnosylisoorientin in leaves of high-altitude landraces of maize after UV-B exposure. Plant Cell Environ. 2005, 28, 788–799. [Google Scholar] [CrossRef]
- Flood, P.J.; Hancock, A.M. The genomic basis of adaptation in plants. Curr. Opin. Plant Biol. 2017, 36, 88–94. [Google Scholar] [CrossRef]
- Tiffin, P.; Ross-Ibarra, J. Advances and limits of using population genetics to understand local adaptation. Trends Ecol. Evol. 2014, 29, 673–780. [Google Scholar] [CrossRef]
- Stapley, J.; Reger, J.; Feulner, P.G.D.; Smadja, C.; Galindo, J.; Ekblom, R.; Bennison, C.; Ball, A.D.; Beckerman, A.P.; Slate, J. Adaptation genomics: The next generation. Trends Ecol. Evol. 2010, 25, 705–712. [Google Scholar] [CrossRef]
- Ekblom, R.; Galindo, J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 2011, 107, 1–15. [Google Scholar] [CrossRef]
- Zaborowska, J.; Labiszak, B.; Perry, A.; Cavers, S.; Wachowiak, W. Candidate genes for the high-altitude adaptations of two mountain pine taxa. Int. J. Mol. Sci. 2021, 22, 3477. [Google Scholar] [CrossRef]
- Abbas, M.; Sharma, G.; Dambire, C.; Marquez, J.; Alonso-Blanco, C.; Proano, K.; Holdsworth, M.J. An oxygen-sensing mechanism for angiosperm adaptation to altitude. Nature 2022, 606, 565–569. [Google Scholar] [CrossRef]
- Wu, Z.Y. Origin and Evolution of the Flora of Tibet; Science Press: Beijing, China, 1988. [Google Scholar]
- Chang, H.T.; Kong, Y.C.; But, P.H. The origin and its affinity of the Nepalese flora. Acta Sci. Nat. Univ. Sunyatseni 1988, 2, 1–12. [Google Scholar]
- Singh, J.S.; Singh, S.P. Forest vegetation of the Himalaya. Bot. Rev. 1987, 53, 80–192. [Google Scholar] [CrossRef]
- Vetaas, O.R.; Grytnes, J.A. Distribution of vascular plant species richness and endemic richness along the Himalayan elevation gradient in Nepal. Glob. Ecol. Biogeogr. 2002, 11, 291–301. [Google Scholar] [CrossRef]
- Zhao, J.L.; Paudel, B.R.; Yu, X.Q.; Zhang, J.; Li, Q.J. Speciation along the elevation gradient: Divergence of Roscoea species within the south slope of the Himalayas. Mol. Phylogenet. Evol. 2021, 164, 107292. [Google Scholar] [CrossRef]
- Zhao, J.L.; Zhong, J.S.; Fan, Y.L.; Xia, Y.M.; Li, Q.J. A preliminary species-level phylogeny of the alpine ginger Roscoea: Implications for speciation. J. Syst. Evol. 2017, 55, 215–224. [Google Scholar] [CrossRef]
- Cowley, E. The Genus Roscoea; The Royal Botanic Gardens: Kew, UK, 2007. [Google Scholar]
- Zhao, J.L.; Xia, Y.M.; Cannon, C.H.; Kress, W.J.; Li, Q.J. Evolutionary diversification of alpine ginger reflects the early uplift of the Himalayan–Tibetan Plateau and rapid extrusion of Indochina. Gondwana Res. 2016, 32, 232–241. [Google Scholar] [CrossRef]
- Ngamriabsakul, C.; Newman, M.; Cronk, Q. Phylogeny and disjunction in Roscoea (Zingiberaceae). Edinb. J. Bot. 2000, 57, 39–61. [Google Scholar] [CrossRef]
- Paudel, B.R.; Dyer, A.G.; Garcia, J.E.; Shrestha, M. The effect of elevational gradient on alpine gingers (Roscoea alpina and R. purpurea) in the Himalayas. PeerJ 2019, 7, e7503. [Google Scholar] [CrossRef]
- Paudel, B.R.; Shrestha, M.; Burd, M.; Adhikari, S.; Sun, Y.S.; Li, Q.J. Coevolutionary elaboration of pollination-related traits in an alpine ginger (Roscoea purpurea) and a tabanid fly in the Nepalese Himalayas. New Phytol. 2016, 211, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Liu, B.G.; Li, Y.; Liu, Y.; He, Y.X.; Qian, Z.Q.; Li, J.X. Landscape genetics reveals that adaptive genetic divergence in Pinus bungeana (Pinaceae) is driven by environmental variables relating to ecological habitats. BMC Evol. Biol. 2019, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Hartfield, M.; Bataillon, T.; Glemin, S. The evolutionary interplay between adaptation and self-fertilization. Trends Genet. 2017, 33, 420–431. [Google Scholar] [CrossRef]
- Walter, G.M.; Clark, J.; Cristaudo, A.; Terranova, D.; Nevado, B.; Catara, S.; Paunov, M.; Velikova, V.; Filatov, D.; Cozzolino, S.; et al. Adaptive divergence generates distinct plastic responses in two closely related Senecio species. Evolution 2022, 76, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Shockey, J.M.; Fulda, M.S.; Browse, J.A. Arabidopsis contains nine long-chain acyl-coenzyme a synthetase genes that participate in fatty acid and glycerolipid metabolism. Plant Physiol. 2002, 129, 1710–1722. [Google Scholar] [CrossRef]
- Naested, H.; Holm, A.; Jenkins, T.; Nielsen, H.B.; Harris, C.A.; Beale, M.H.; Andersen, M.; Mant, A.; Scheller, H.; Camara, B.; et al. Arabidopsis VARIEGATED 3 encodes a chloroplast-targeted, zinc-finger protein required for chloroplast and palisade cell development. J. Cell Sci. 2004, 117, 4807–4818. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, X.; Liu, J.; Wang, B.H.; Liu, B.L.; Wang, Y.Z. Pod shattering resistance associated with domestication is mediated by a NAC gene in soybean. Nat. Commun. 2014, 5, 3352. [Google Scholar] [CrossRef]
- Ohnishi, T. Recent advances in brassinosteroid biosynthetic pathway: Insight into novel brassinosteroid shortcut pathway. J. Pestic. Sci. 2018, 43, 159–167. [Google Scholar] [CrossRef]
- Yang, S.Q.; Li, W.Q.; Miao, H.; Gan, P.F.; Qiao, L.; Chang, Y.L.; Shi, C.H.; Chen, K.M. REL2, a gene encoding an unknown function protein which contains DUF630 and DUF632 domains controls leaf rolling in rice. Rice 2016, 9, 37. [Google Scholar] [CrossRef]
- Zhou, B.; Zeng, L. Elucidating the role of highly homologous Nicotiana benthamiana ubiquitin E2 gene family members in plant immunity through an improved virus-induced gene silencing approach. Plant Methods 2017, 13, 59. [Google Scholar] [CrossRef]
- Toller, A.; Brownfield, L.; Neu, C.; Twell, D.; Schulze-Lefert, P. Dual function of Arabidopsis glucan synthase-like genes GSL8 and GSL10 in male gametophyte development and plant growth. Plant J. 2008, 54, 911–923. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, B.; Li, C.; Lei, C.; Kong, C.; Yang, Y.; Gong, M. A comprehensive expression analysis of the expansin gene family in potato (Solanum tuberosum) discloses stress-responsive expansin-like B genes for drought and heat tolerances. PLoS ONE 2019, 14, e0219837. [Google Scholar] [CrossRef]
- Huang, W.; Pi, L.; Liang, W.; Xu, B.; Wang, H.; Cai, R.; Huang, H. The proteolytic function of the Arabidopsis 26S proteasome is required for specifying leaf adaxial identity. Plant Cell 2006, 18, 2479–2492. [Google Scholar] [CrossRef][Green Version]
- Pagnussat, G.C.; Yu, H.J.; Ngo, Q.A.; Rajani, S.; Mayalagu, S.; Johnson, C.S.; Capron, A.; Xie, L.F.; Ye, D.; Sundaresan, V. Genetic and molecular identification of genes required for female gametophyte development and function in Arabidopsis. Development 2005, 132, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Sugano, S.; Andronis, C.; Ong, M.; Green, R.; Tobin, E. The protein kinase CK2 is involved in regulation of circadian rhythms in Arabidopsis. Proc. Natl. Acad. Sci. USA 1999, 96, 12362–12366. [Google Scholar] [CrossRef]
- Triwitayakorn, K.; Njiti, V.N.; Iqbal, M.J.; Yaegashi, S.; Town, C.; Lightfoot, D.A. Genomic analysis of a region encompassing QRfs1 and QRfs2: Genes that underlie soybean resistance to sudden death syndrome. Genome 2005, 48, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Restrepo-Montoya, D.; Brueggeman, R.; McClean, P.E.; Osorno, J.M. Computational identification of receptor-like kinases “RLK” and receptor-like proteins “RLP” in legumes. BMC Genom. 2020, 21, 459. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xue, N.; Fu, X.; Zhang, H.; Li, G. Arabidopsis thaliana FAR-RED ELONGATED HYPOCOTYLS3 (FHY3) and FAR-RED-IMPAIRED RESPONSE1 (FAR1) modulate starch synthesis in response to light and sugar. New Phytol. 2017, 213, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Li, B.; He, W.; Yin, X.; Liu, S.; Lian, L.; Zhang, Y.; Liang, W.; Zhang, P. Physiological analysis of the effect of altitudinal gradients on Leymus secalinus on the Qinghai-Tibetan Plateau. PLoS ONE 2018, 13, e0202881. [Google Scholar] [CrossRef] [PubMed]
- Pierrugues, O.; Brutesco, C.; Oshiro, J.; Gouy, M.; Deveaux, Y.; Carman, G.M.; Thuriaux, P.; Kazmaier, M. Lipid phosphate phosphatases in Arabidopsis. Regulation of the AtLPP1 gene in response to stress. J. Biol. Chem. 2001, 276, 20300–20308. [Google Scholar] [CrossRef]
- Wright, S.I.; Kalisz, S.; Slotte, T. Evolutionary consequences of self-fertilization in plants. Proc. Biol. Sci. 2013, 280, 20130133. [Google Scholar] [CrossRef]
- van Ginkel, M.; Flipphi, R.C.H. Why self-fertilizing plants still exist in wild populations: Diversity assurance through stress-induced male sterility may promote selective outcrossing and recombination. Agronomy 2020, 10, 349. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Li, Q.J. Autonomous selfing provides reproductive assurance in an alpine ginger Roscoea schneideriana (Zingiberaceae). Ann. Bot. 2008, 102, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bliss, L.C. Adaptations of arctic and alpine plants to environmental conditions. Arctic 1962, 15, 117–144. [Google Scholar] [CrossRef]
- García-Camacho, R.; Totland, Ø. Pollen limitation in the alpine: A meta-analysis. Arct. Antarctic. Alp. Res. 2009, 41, 103–111. [Google Scholar] [CrossRef]
- Bingham, R.A.; Orthner, A.R. Efficient pollination of alpine plants. Nature 1998, 391, 238–239. [Google Scholar] [CrossRef]
- Golding, G.B.; Strobeck, C. Linkage disequilibrium in a finite population that is partially selfing. Genetics 1980, 94, 777–789. [Google Scholar] [CrossRef]
- Pollak, E. On the theory of partially inbreeding finite populations. I. Partial selfing. Genetics 1987, 117, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Gaut, B.S.; Long, A.D. The lowdown on linkage disequilibrium. Plant Cell 2003, 15, 1502–1506. [Google Scholar] [CrossRef]
- Paudel, B.R.; Shrestha, M.; Dyer, A.G.; Li, Q.J. Ginger and the beetle: Evidence of primitive pollination system in a Himalayan endemic alpine ginger (Roscoea alpina, Zingiberaceae). PLoS ONE 2017, 12, e0180460. [Google Scholar] [CrossRef]
- Flint-Garcia, S.A.; Thornsberry, J.M.; Buckler, E.S.t. Structure of linkage disequilibrium in plants. Annu. Rev. Plant Biol. 2003, 54, 357–374. [Google Scholar] [CrossRef]
- Felsenstein, J. The effect of linkage on directional selection. Genetics 1965, 52, 349–363. [Google Scholar] [CrossRef]
- Nagylaki, T. Quasilinkage equilibrium and the evolution of two-locus systems. Proc. Natl. Acad. Sci. USA 1974, 71, 526–530. [Google Scholar] [CrossRef]
- Kimura, M. Attainment of quasi linkage equilibrium when gene frequencies are changing by natural selection. Genetics 1965, 52, 875–890. [Google Scholar] [CrossRef]
- Charlesworth, B.; Morgan, M.T.; Charlesworth, D. The effect of deleterious mutations on neutral molecular variation. Genetics 1993, 134, 1289–1303. [Google Scholar] [CrossRef]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Stajich, J.E.; Hahn, M.W. Disentangling the effects of demography and selection in human history. Mol. Biol. Evol. 2005, 22, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kosiński, P.; Sękiewicz, K.; Walas, Ł.; Boratynski, A.; Dering, M. Spatial genetic structure of the endemic alpine plant Salix serpillifolia: Genetic swamping on nunataks due to secondary colonization? Alp. Bot. 2019, 129, 107–121. [Google Scholar] [CrossRef]
- Bingham, R.A.; Ranker, T.A. Genetic diversity in alpine and foothill populations of Campanula rotundifolia (Campanulaceae). Int. J. Plant Sci. 2000, 161, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. An Introduction to Conservation Genetics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Catalan, J.; Ninot, J.M.; Aniz, M.M. The high mountain conservation in a changing world. In High Mountain Conservation in a Changing World. Advances in Global Change Research; Catalan, J., Ninot, J.M., Aniz, M.M., Eds.; Springer: Cham, Switzerland, 2017; Volume 62, pp. 3–36. [Google Scholar] [CrossRef][Green Version]
- Rana, S.K.; Price, T.D.; Qian, H. Plant species richness across the Himalaya driven by evolutionary history and current climate. Ecosphere 2019, 10, e02945. [Google Scholar] [CrossRef]
- Chen, Y.X.; Xu, J.; Liu, J.; Li, T.G.; Xiong, Z.F.; Zhang, P.; Yan, H. Climatic and tectonic constraints on the Plio-Pleistocene evolution of the Indonesian Throughflow intermediate water recorded by benthic δ18O from IODP site U1482. Quat. Sci. Rev. 2022, 295, 107666. [Google Scholar] [CrossRef]
- Gossmann, T.I.; Woolfit, M.; Eyre-Walker, A. Quantifying the variation in the effective population size within a genome. Genetics 2011, 189, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Becher, H.; Jackson, B.C.; Charlesworth, B. Patterns of genetic variability in genomic regions with low rates of recombination. Curr. Biol. 2020, 30, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.L.; Halligan, D.L.; Haddrill, P.R.; Charlesworth, B. The relation between recombination rate and patterns of molecular evolution and variation in Drosophila melanogaster. Mol. Biol. Evol. 2014, 31, 1010–1028. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S.C.H.; Harder, L.D.; Worley, A.C. The comparative biology of pollination and mating in flowering plants. Phil. Trans. R. Soc. Lond. B 1996, 351, 1271–1280. [Google Scholar] [CrossRef]
- Burri, R. Evolution: Small populations, low recombination, big trouble? Curr. Biol. 2021, 31, R282–R284. [Google Scholar] [CrossRef]
- Blumthaler, M.; Ambach, W.; Ellinger, R. Increase in solar UV radiation with altitude. J. Photoch. Photobio. B 1997, 39, 130–134. [Google Scholar] [CrossRef]
- Körner, C. Alpine Plant Life; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.J.; Zuo, H.; Zheng, W.K.; Zhang, S.S.; Huang, Y.; Pingcuo, G.S.; Ying, H.; Zhao, F.; Li, Y.R.; et al. Genomic basis of high-altitude adaptation in Tibetan Prunus fruit trees. Curr. Biol. 2021, 31, 3848–3860.e8. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Browning, S.R.; Browning, B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 2007, 81, 1084–1097. [Google Scholar] [CrossRef]
- Chikhi, R.; Medvedev, P. Informed and automated k-mer size selection for genome assembly. Bioinformatics 2014, 30, 31–37. [Google Scholar] [CrossRef]
- Gao, F.; Ming, C.; Hu, W.; Li, H. New software for the fast estimation of population recombination rates (FastEPRR) in the genomic era. G3-Genes Genom. Genet. 2016, 6, 1563–1571. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Vurture, G.W.; Sedlazeck, F.J.; Nattestad, M.; Underwood, C.J.; Fang, H.; Gurtowski, J.; Schatz, M.C. GenomeScope: Fast reference-free genome profiling from short reads. Bioinformatics 2017, 33, 2202–2204. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, W.; Obrycki, J.J.; Meng, L.; Zhou, X.; Chu, D.; Li, B. Optimizing sample size for population genomic study in a global invasive lady beetle, Harmonia Axyridis. Insects 2020, 5, 290. [Google Scholar] [CrossRef]
- Qu, W.M.; Liang, N.; Wu, Z.K.; Zhao, Y.G.; Chu, D. Minimum sample sizes for invasion genomics: Empirical investigation in an invasive whitefly. Ecol. Evol. 2019, 10, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Nadachowska-Brzyska, K.; Burri, R.; Smeds, L.; Ellegren, H. PSMC analysis of effective population sizes in molecular ecology and its application to black-and-white Ficedula flycatchers. Mol. Ecol. 2016, 25, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, L.; Zhang, Z.; Li, M.; Wang, D.; Zhang, X.; Xi, Z.; Keefover-Ring, K.; Smart, L.B.; DiFazio, S.P.; et al. Phylogenomics of the genus Populus reveals extensive interspecific gene flow and balancing selection. New Phytol. 2020, 225, 1370–1382. [Google Scholar] [CrossRef]
- Weissensteiner, M.H.; Bunikis, I.; Catalán, A.; Francoijs, K.J.; Knief, U.; Heim, W.; Peona, V.; Pophaly, S.D.; Sedlazeck, F.J.; Suh, A.; et al. Discovery and population genomics of structural variation in a songbird genus. Nat. Commun. 2020, 11, 3403. [Google Scholar] [CrossRef]
- Li, K.; Zhang, S.; Song, X.; Weyrich, A.; Wang, Y.; Liu, X.; Wan, N.; Liu, J.; Lövy, M.; Cui, H.; et al. Genome evolution of blind subterranean mole rats: Adaptive peripatric versus sympatric speciation. Proc. Natl. Acad. Sci. USA 2020, 117, 32499–32508. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Lenstra, J.A.; Zheng, Z.; Wu, X.; Yang, J.; Li, B.; Yang, Y.; Qiu, Q.; Liu, H.; et al. Evolutionary origin of genomic structural variations in domestic yaks. Nat. Commun. 2023, 14, 5617. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).