Nanostructured Coatings Based on Graphene Oxide for the Management of Periprosthetic Infections
Abstract
:1. Introduction
2. Results and Discussions
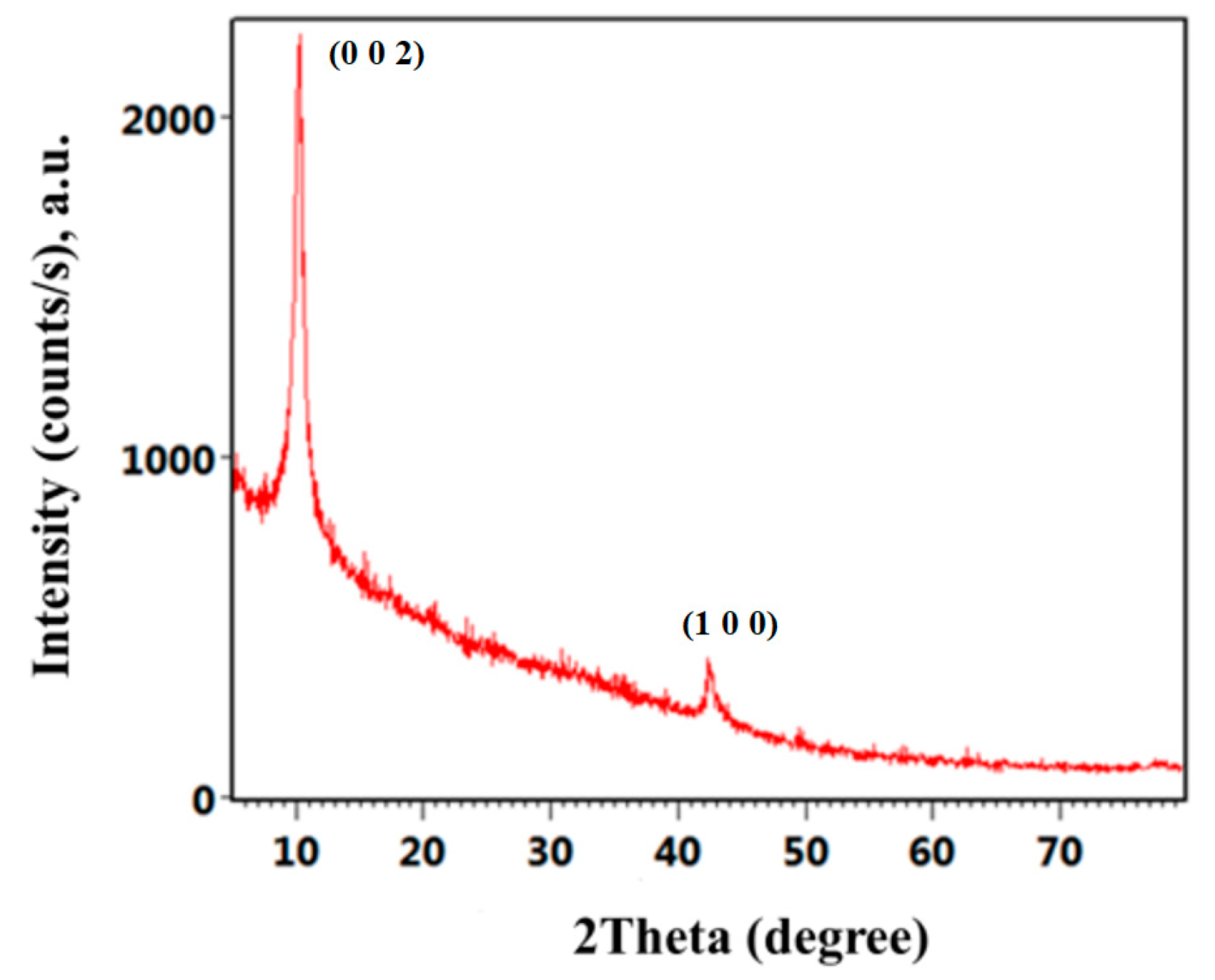
2.1. Physicochemical Characterization of GO Nanomaterial
2.2. Physicochemical Characterization of PLA-nGO-Zin Coatings
2.3. Blood Interaction with PLA-nGO-Zin Coatings
2.3.1. Evaluation of the Hemolytic Potential of PLA-nGO-Zin Coatings
2.3.2. Assessment of the Pro-inflammatory Potential of PLA-nGO-Zin Coatings by Profiling Cytokine Expression
2.4. Anti-Biofilm Efficiency of PLA-nGO-Zin Coatings
3. Materials and Methods
3.1. Materials
3.2. Synthesis Methods
3.2.1. Synthesis of Graphene Oxide (GO) Nanomaterial
3.2.2. Synthesis of PLA-nGO-Zin Coatings
3.3. Physicochemical Investigation Methods
3.3.1. Characterization of GO Nanosheets (nGOs)
3.3.2. Characterization of PLA-nGO-Zin Coatings
3.4. Biological Evaluation of PLA-nGO-Zin Coatings
3.5. Microbiological Evaluation of PLA-nGO-Zin Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abebe, G.M. The role of bacterial biofilm in antibiotic resistance and food contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.M.; Soares, F.A.; Reis, S.; Nunes, C.; Van Dijck, P. Innovative strategies toward the disassembly of the EPS matrix in bacterial biofilms. Front. Microbiol. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.y.; Zare, E.N.; Borzacchiello, A.; Niu, L.n.; Tay, F.R. Metal-based nanomaterials in biomedical applications: Antimicrobial activity and cytotoxicity aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Samrot, A.V.; Abubakar Mohamed, A.; Faradjeva, E.; Si Jie, L.; Hooi Sze, C.; Arif, A.; Chuan Sean, T.; Norbert Michael, E.; Yeok Mun, C.; Xiao Qi, N.; et al. Mechanisms and Impact of Biofilms and Targeting of Biofilms Using Bioactive Compounds—A Review. Medicina 2021, 57, 839. [Google Scholar] [CrossRef]
- Sharahi, J.Y.; Azimi, T.; Shariati, A.; Safari, H.; Tehrani, M.K.; Hashemi, A. Advanced strategies for combating bacterial biofilms. J. Cell. Physiol. 2019, 234, 14689–14708. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Hao, Y.; Liu, Y.; Dong, Z.; Li, K. Biological and Physiochemical Methods of Biofilm Adhesion Resistance Control of Medical-Context Surface. Int. J. Biol. Sci. 2021, 17, 1769–1781. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, P.; Wang, Y.; Hao, Y. Mechanisms and Control Measures of Mature Biofilm Resistance to Antimicrobial Agents in the Clinical Context. ACS Omega 2020, 5, 22684–22690. [Google Scholar] [CrossRef]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob. Resist. Infect. Control 2020, 9, 162. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Niculescu, A.-G.; Slave, Ș.; Bîrcă, A.C.; Dorcioman, G.; Grumezescu, V.; Holban, A.M.; Oprea, O.-C.; Vasile, B.Ș.; Grumezescu, A.M.; et al. Anti-Biofilm Coatings Based on Chitosan and Lysozyme Functionalized Magnetite Nanoparticles. Antibiotics 2021, 10, 1269. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Healthc. Mater. 2020, 9, 2000310. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Negut, I.; Grumezescu, A.M.; Ficai, A.; Dorcioman, G.; Socol, G.; Iordache, F.; Truşcă, R.; Vasile, B.S.; Holban, A.M. MAPLE fabricated coatings based on magnetite nanoparticles embedded into biopolymeric spheres resistant to microbial colonization. Appl. Surf. Sci. 2018, 448, 230–236. [Google Scholar] [CrossRef]
- Prodana, M.; Stoian, A.B.; Burnei, C.; Ionita, D. Innovative Coatings of Metallic Alloys Used as Bioactive Surfaces in Implantology: A Review. Coatings 2021, 11, 649. [Google Scholar] [CrossRef]
- Pandit, S.; Gaska, K.; Kádár, R.; Mijakovic, I. Graphene-based antimicrobial biomedical surfaces. ChemPhysChem 2021, 22, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Ahmadabadi, H.Y.; Yu, K.; Kizhakkedathu, J.N. Surface modification approaches for prevention of implant associated infections. Colloids Surf. B Biointerfaces 2020, 193, 111116. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, T. Surface treatment strategies to combat implant-related infection from the beginning. J. Orthop. Transl. 2019, 17, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Schulze, A.; Mitterer, F.; Pombo, J.P.; Schild, S. Biofilms by bacterial human pathogens: Clinical relevance—Development, composition and regulation—Therapeutical strategies. Microb. Cell 2021, 8, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, V.; Negut, I.; Gherasim, O.; Birca, A.C.; Grumezescu, A.M.; Hudita, A.; Galateanu, B.; Costache, M.; Andronescu, E.; Holban, A.M. Antimicrobial applications of MAPLE processed coatings based on PLGA and lincomycin functionalized magnetite nanoparticles. Appl. Surf. Sci. 2019, 484, 587–599. [Google Scholar] [CrossRef]
- Norris, G.R.; Checketts, J.X.; Scott, J.T.; Vassar, M.; Norris, B.L.; Giannoudis, P.V. Prevalence of deep surgical site infection after repair of periarticular knee fractures: A systematic review and meta-analysis. JAMA Netw. Open 2019, 2, e199951. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Kihara, S.; Mittwede, P.N.; Maher, P.L.; Rothenberg, A.C.; Falcione, A.D.C.M.; Chen, A.; Urish, K.L.; Tuan, R.S.; Alexander, P.G. Development of a large animal rabbit model for chronic periprosthetic joint infection. Bone Jt. Res. 2021, 10, 156–165. [Google Scholar] [CrossRef]
- Russo, A.; Cavagnaro, L.; Chiarlone, F.; Alessio-Mazzola, M.; Felli, L.; Burastero, G. Predictors of failure of two-stage revision in periprosthetic knee infection: A retrospective cohort study with a minimum two-year follow-up. Arch. Orthop. Trauma Surg. 2022, 142, 481–490. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Akshaya, S.; Rowlo, P.K.; Dukle, A.; Nathanael, A.J. Antibacterial Coatings for Titanium Implants: Recent Trends and Future Perspectives. Antibiotics 2022, 11, 1719. [Google Scholar] [CrossRef]
- Li, J.; Jansen, J.A.; Walboomers, X.F.; van den Beucken, J.J.J.P. Mechanical aspects of dental implants and osseointegration: A narrative review. J. Mech. Behav. Biomed. Mater. 2020, 103, 103574. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Gashi, B.; Hoque, S.; Marian, M.; Rosenkranz, A. Enhancing mechanical and biomedical properties of protheses—Surface and material design. Surf. Interfaces 2021, 27, 101498. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Apaza-Bedoya, K.; Benfatti, C.A.M.; Silva, F.S.; Henriques, B. A Comprehensive Review on the Corrosion Pathways of Titanium Dental Implants and Their Biological Adverse Effects. Metals 2020, 10, 1272. [Google Scholar] [CrossRef]
- Badhe, R.V.; Akinfosile, O.; Bijukumar, D.; Barba, M.; Mathew, M.T. Systemic toxicity eliciting metal ion levels from metallic implants and orthopedic devices—A mini review. Toxicol. Lett. 2021, 350, 213–224. [Google Scholar] [CrossRef]
- Ramos, D.M.; Dhandapani, R.; Subramanian, A.; Sethuraman, S.; Kumbar, S.G. Clinical complications of biodegradable screws for ligament injuries. Mater. Sci. Eng. C 2020, 109, 110423. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Patel, Y.; Jayaswal, A.; Verma, A. Indications for implant removal: A prospective study. Int. J. Res. Orthop. 2019, 5, 253–259. [Google Scholar] [CrossRef]
- Ahn, J.H.; Patel, N.A.; Lin, C.C.; Lee, T.Q. The anterolateral ligament of the knee joint: A review of the anatomy, biomechanics, and anterolateral ligament surgery. Knee Surg. Relat. Res. 2019, 31, 12. [Google Scholar] [CrossRef] [PubMed]
- Chalidis, B.; Pitsilos, C.; Kitridis, D.; Givissis, P. Graft choices for anterolateral ligament knee reconstruction surgery: Current concepts. World J. Clin. Cases 2022, 10, 8463–8473. [Google Scholar] [CrossRef]
- Helito, C.P.; Bonadio, M.B.; Gobbi, R.G.; e Albuquerque, R.F.d.M.; Pécora, J.R.; Camanho, G.L.; Demange, M.K. Combined intra-and extra-articular reconstruction of the anterior cruciate ligament: The reconstruction of the knee anterolateral ligament. Arthrosc. Tech. 2015, 4, e239–e244. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Menge, T.J.; Mitchell, J.J.; Dean, C.S.; LaPrade, R.F. Anterolateral ligament reconstruction technique: An anatomic-based approach. Arthrosc. Tech. 2016, 5, e453–e457. [Google Scholar] [CrossRef] [PubMed]
- Kostrub, O.O.; Kotiuk, V.V.; Liutko, O.B.; Kolov, H.B.; Blonskyi, R.I.; Zasadniuk, I.A. Differential Diagnosis of Reactive and Infectious Arthritis after Anterior Cruciate Ligament Reconstruction. Her. Orthop. Traumatol. Prosthet. 2020, 1, 39–48. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Liu, W.; Zhang, C.; Song, J. Current strategies for enhancement of the bioactivity of artificial ligaments: A mini-review. J. Orthop. Transl. 2022, 36, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Rusu, A.; Lungu, I.-A. The new fifth-generation cephalosporins—A balance between safety and efficacy. Rom. J. Pharm. Pract. 2020, 13, 121–126. [Google Scholar] [CrossRef]
- Bui, T.; Preuss, C.V. Cephalosporins. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mihai, M.M.; Preda, M.; Lungu, I.; Gestal, M.C.; Popa, M.I.; Holban, A.M. Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. Int. J. Mol. Sci. 2018, 19, 1179. [Google Scholar] [CrossRef] [PubMed]
- Anghel, A.G.; Grumezescu, A.M.; Chirea, M.; Grumezescu, V.; Socol, G.; Iordache, F.; Oprea, A.E.; Anghel, I.; Holban, A.M. MAPLE Fabricated Fe3O4@Cinnamomum verum Antimicrobial Surfaces for Improved Gastrostomy Tubes. Molecules 2014, 19, 8981–8994. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Iordache, F.; Vasile, B.S.; Holban, A.M. Bioactive Surfaces of Polylactide and Silver Nanoparticles for the Prevention of Microbial Contamination. Materials 2020, 13, 768. [Google Scholar] [CrossRef]
- Schömig, F.; Perka, C.; Pumberger, M.; Ascherl, R. Implant contamination as a cause of surgical site infection in spinal surgery: Are single-use implants a reasonable solution?—A systematic review. BMC Musculoskelet. Disord. 2020, 21, 634. [Google Scholar] [CrossRef]
- Rădulescu, D.; Voicu, G.; Oprea, A.E.; Andronescu, E.; Grumezescu, V.; Holban, A.M.; Vasile, B.S.; Surdu, A.V.; Grumezescu, A.M.; Socol, G. Mesoporous silica coatings for cephalosporin active release at the bone-implant interface. Appl. Surf. Sci. 2016, 374, 165–171. [Google Scholar] [CrossRef]
- Balaure, P.C.; Grumezescu, A.M. Recent Advances in Surface Nanoengineering for Biofilm Prevention and Control. Part II: Active, Combined Active and Passive, and Smart Bacteria-Responsive Antibiofilm Nanocoatings. Nanomaterials 2020, 10, 1527. [Google Scholar] [CrossRef]
- Marturano, V.; Abate, F.; Ambrogi, V.; Califano, V.; Cerruti, P.; Pepe, G.P.; Vicari, L.R.M.; Ausanio, G. Smart Coatings Prepared via MAPLE Deposition of Polymer Nanocapsules for Light-Induced Release. Molecules 2021, 26, 2736. [Google Scholar] [CrossRef]
- Gherasim, O.; Popescu-Pelin, G.; Florian, P.; Icriverzi, M.; Roseanu, A.; Mitran, V.; Cimpean, A.; Socol, G. Bioactive Ibuprofen-Loaded PLGA Coatings for Multifunctional Surface Modification of Medical Devices. Polymers 2021, 13, 1413. [Google Scholar] [CrossRef]
- Tan, J.; Li, L.; Li, B.; Tian, X.; Song, P.; Wang, X. Titanium Surfaces Modified with Graphene Oxide/Gelatin Composite Coatings for Enhanced Antibacterial Properties and Biological Activities. ACS Omega 2022, 7, 27359–27368. [Google Scholar] [CrossRef]
- Barros, C.H.N.; Casey, E. A Review of Nanomaterials and Technologies for Enhancing the Antibiofilm Activity of Natural Products and Phytochemicals. ACS Appl. Nano Mater. 2020, 3, 8537–8556. [Google Scholar] [CrossRef]
- Pircalabioru, G.G.; Chifiriuc, M.-C. Nanoparticulate drug-delivery systems for fighting microbial biofilms: From bench to bedside. Future Microbiol. 2020, 15, 679–698. [Google Scholar] [CrossRef] [PubMed]
- Ficai, D.; Grumezescu, V.; Fufă, O.M.; Popescu, R.C.; Holban, A.M.; Ficai, A.; Grumezescu, A.M.; Mogoanta, L.; Mogosanu, G.D.; Andronescu, E. Antibiofilm coatings based on PLGA and nanostructured cefepime-functionalized magnetite. Nanomaterials 2018, 8, 633. [Google Scholar] [CrossRef]
- Gherasim, O.; Popescu, R.C.; Grumezescu, V.; Mogoșanu, G.D.; Mogoantă, L.; Iordache, F.; Holban, A.M.; Vasile, B.Ș.; Bîrcă, A.C.; Oprea, O.-C.; et al. MAPLE Coatings Embedded with Essential Oil-Conjugated Magnetite for Anti-Biofilm Applications. Materials 2021, 14, 1612. [Google Scholar] [CrossRef]
- Mercan, D.-A.; Niculescu, A.-G.; Grumezescu, A.M. Nanoparticles for Antimicrobial Agents Delivery—An Up-to-Date Review. Int. J. Mol. Sci. 2022, 23, 13862. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef]
- Tadyszak, K.; Wychowaniec, J.K.; Litowczenko, J. Biomedical Applications of Graphene-Based Structures. Nanomaterials 2018, 8, 944. [Google Scholar] [CrossRef]
- Lazăr, A.-I.; Aghasoleimani, K.; Semertsidou, A.; Vyas, J.; Roșca, A.-L.; Ficai, D.; Ficai, A. Graphene-Related Nanomaterials for Biomedical Applications. Nanomaterials 2023, 13, 1092. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Kunduru, K.R.; Basu, A.; Mizrahi, B.; Domb, A.J.; Khan, W. Injectable formulations of poly(lactic acid) and its copolymers in clinical use. Adv. Drug Deliv. Rev. 2016, 107, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Nofar, M.; Sacligil, D.; Carreau, P.J.; Kamal, M.R.; Heuzey, M.-C. Poly (lactic acid) blends: Processing, properties and applications. Int. J. Biol. Macromol. 2019, 125, 307–360. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, T.; Zhao, X.; Wang, X.; Zhou, L.; Yang, Y.; Liao, F.; Ju, Y. Poly(lactic acid)/graphene oxide–ZnO nanocomposite films with good mechanical, dynamic mechanical, anti-UV and antibacterial properties. J. Chem. Technol. Biotechnol. 2015, 90, 1677–1684. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Marino, A.; Nostro, A. Antimicrobial additives for poly(lactic acid) materials and their applications: Current state and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 7739–7756. [Google Scholar] [CrossRef]
- Arriagada, P.; Palza, H.; Palma, P.; Flores, M.; Caviedes, P. Poly(lactic acid) composites based on graphene oxide particles with antibacterial behavior enhanced by electrical stimulus and biocompatibility. J. Biomed. Mater. Res. Part A 2018, 106, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Ikram, R.; Jan, B.M.; Ahmad, W. An overview of industrial scalable production of graphene oxide and analytical approaches for synthesis and characterization. J. Mater. Res. Technol. 2020, 9, 11587–11610. [Google Scholar] [CrossRef]
- Singh, J.; Jindal, N.; Kumar, V.; Singh, K. Role of green chemistry in synthesis and modification of graphene oxide and its application: A review study. Chem. Phys. Impact 2023, 6, 100185. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Liu, Y.; Zhao, B. Progress in preparation, characterization, surface functional modification of graphene oxide: A review. J. Saudi Chem. Soc. 2022, 26, 101560. [Google Scholar] [CrossRef]
- Kashif, M.; Alsaiari, N.S.; Jaafar, E.; Low, F.W.; Oon, C.S.; Sahari, S.K.; Almuaikel, N.S. Reaction-Time-Dependent Opto-Electrical Properties of Graphene Oxide. Crystals 2022, 12, 1303. [Google Scholar] [CrossRef]
- Hou, Y.; Lv, S.; Liu, L.; Liu, X. High-quality preparation of graphene oxide via the Hummers’ method: Understanding the roles of the intercalator, oxidant, and graphite particle size. Ceram. Int. 2020, 46, 2392–2402. [Google Scholar] [CrossRef]
- Siburian, R.; Simanjuntak, C.; Supeno, M.; Lumbanraja, S.; Sihotang, H. New route to synthesize of graphene nano sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef]
- Kesavan, S.; Meena, K.S.; Dhakshinamoorthy, R. Bioactive polysaccharides based graphene oxide nanoparticle as a promising carrier for anticancer drug delivery. Biointerface Res. Appl. Chem. 2022, 12, 3429–3445. [Google Scholar]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Sohail, M.; Saleem, M.; Ullah, S.; Saeed, N.; Afridi, A.; Khan, M.; Arif, M. Modified and improved Hummer’s synthesis of graphene oxide for capacitors applications. Mod. Electron. Mater. 2017, 3, 110–116. [Google Scholar] [CrossRef]
- Sengupta, I.; Kumar, S.S.S.S.; Pal, S.K.; Chakraborty, S. Investigating the effect of graphite pretreatment and contribution of the oxidizer in the synthesis of graphite oxide by hummers approach. Fuller. Nanotub. Carbon. Nanostruct. 2022, 30, 626–637. [Google Scholar] [CrossRef]
- Zhou, A.; Yu, T.; Liang, X.; Yin, S. H2O2-free strategy derived from Hummers method for preparing graphene oxide with high oxidation degree. FlatChem 2023, 38, 100487. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Andronescu, E.; Negut, I.; Bîrcă, A.C.; Gălățeanu, B.; Hudiță, A. Bioactive Coatings Loaded with Osteogenic Protein for Metallic Implants. Polymers 2021, 13, 4303. [Google Scholar] [CrossRef]
- Croitoru, A.; Oprea, O.; Nicoara, A.; Trusca, R.; Radu, M.; Neacsu, I.; Ficai, D.; Ficai, A.; Andronescu, E. Multifunctional Platforms Based on Graphene Oxide and Natural Products. Medicina 2019, 55, 230. [Google Scholar] [CrossRef]
- Zhu, Y.; Kong, G.; Pan, Y.; Liu, L.; Yang, B.; Zhang, S.; Lai, D.; Che, C. An improved Hummers method to synthesize graphene oxide using much less concentrated sulfuric acid. Chin. Chem. Lett. 2022, 33, 4541–4544. [Google Scholar] [CrossRef]
- Croitoru, A.-M.; Karaçelebi, Y.; Saatcioglu, E.; Altan, E.; Ulag, S.; Aydoğan, H.K.; Sahin, A.; Motelica, L.; Oprea, O.; Tihauan, B.-M.; et al. Electrically Triggered Drug Delivery from Novel Electrospun Poly(Lactic Acid)/Graphene Oxide/Quercetin Fibrous Scaffolds for Wound Dressing Applications. Pharmaceutics 2021, 13, 957. [Google Scholar] [CrossRef]
- Bîrcă, A.C.; Chircov, C.; Niculescu, A.G.; Hildegard, H.; Baltă, C.; Roșu, M.; Mladin, B.; Gherasim, O.; Mihaiescu, D.E.; Vasile, B.Ș.; et al. H2O2-PLA-(Alg)2Ca Hydrogel Enriched in Matrigel® Promotes Diabetic Wound Healing. Pharmaceutics 2023, 15, 857. [Google Scholar] [CrossRef] [PubMed]
- Wieser, J.; Sturm, H.; Pichler, A.; Hotter, A.; Martin, N.; Langes, C.; Griesser, U. Crystalline Forms of Ceftaroline Fosamil. U.S. Patent US9534004B2, 3 January 2017. Available online: https://patents.google.com/patent/US9534004/en20 (accessed on 5 January 2024).
- Xu, W.; Zhang, A.; Zhang, X.; Gan, Z. Ceftaroline Fosamil Amino Acid Salt and Crystal Thereof. Patent WO2016008393A1, 21 January 2016. Available online: https://patents.google.com/patent/WO2016008393A1/en (accessed on 5 January 2024).
- Fulmali, A.; Bharate, S.S. Phosphate moiety in FDA-approved pharmaceutical salts and prodrugs. Drug Dev. Res. 2022, 83, 1059–1074. [Google Scholar] [CrossRef]
- del Pino, A.P.; György, E.; Logofatu, C.; Duta, A. Study of the deposition of graphene oxide by matrix-assisted pulsed laser evaporation. J. Phys. D Appl. Phys. 2013, 46, 505309. [Google Scholar] [CrossRef]
- Sima, L.E.; Chiritoiu, G.; Negut, I.; Grumezescu, V.; Orobeti, S.; Munteanu, C.V.A.; Sima, F.; Axente, E. Functionalized graphene oxide thin films for anti-tumor drug delivery to melanoma cells. Front. Chem. 2020, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- György, E.; Logofatu, C.; Pérez del Pino, Á.; Datcu, A.; Pascu, O.; Ivan, R. Enhanced UV- and visible-light driven photocatalytic performances and recycling properties of graphene oxide/ZnO hybrid layers. Ceram. Int. 2018, 44, 1826–1835. [Google Scholar] [CrossRef]
- Pérez del Pino, Á.; Ramadan, M.A.; Garcia Lebière, P.; Ivan, R.; Logofatu, C.; Yousef, I.; György, E. Fabrication of graphene-based electrochemical capacitors through reactive inverse matrix assisted pulsed laser evaporation. Appl. Surf. Sci. 2019, 484, 245–256. [Google Scholar] [CrossRef]
- Dinca, V.; Liu, Q.; Brajnicov, S.; Bonciu, A.; Vlad, A.; Dinu, C.Z. Composites formed from tungsten trioxide and graphene oxide for the next generation of electrochromic interfaces. Compos. Commun. 2020, 17, 115–122. [Google Scholar] [CrossRef]
- Queraltó, A.; Pérez del Pino, Á.; Logofatu, C.; Calota, A.; Amade, R.; Alshaikh, I.; Bertran, E.; Urzica, I.; György, E. MAPLE synthesis of reduced graphene oxide/silver nanocomposite electrodes: Influence of target composition and gas ambience. J. Alloys Compd. 2017, 726, 1003–1013. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Li, D.; Tang, Z.; Wang, Y.; Chen, G.; Chen, H.; Brash, J.L. Blood compatible materials: State of the art. J. Mater. Chem. B 2014, 2, 5718–5738. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Steinle, H.; Golombek, S.; Hann, L.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Blood-contacting biomaterials: In vitro evaluation of the hemocompatibility. Front. Bioeng. Biotechnol. 2018, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- ASTM F756–00; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM International: West Conshohocken, PA, USA, 2004.
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.; Kirkham, P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signalling. J. Inflamm. 2012, 9, 1. [Google Scholar] [CrossRef]
- Chaiwut, R.; Kasinrerk, W. Very low concentration of lipopolysaccharide can induce the production of various cytokines and chemokines in human primary monocytes. BMC Res. Notes 2022, 15, 42. [Google Scholar] [CrossRef]
- Hoyle, C.; Rivers-Auty, J.; Lemarchand, E.; Vranic, S.; Wang, E.; Buggio, M.; Rothwell, N.J.; Allan, S.M.; Kostarelos, K.; Brough, D. Small, Thin Graphene Oxide Is Anti-inflammatory Activating Nuclear Factor Erythroid 2-Related Factor 2 via Metabolic Reprogramming. ACS Nano 2018, 12, 11949–11962. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-S.; Kung, M.-L.; Chen, F.-C.; Ke, Y.-C.; Shen, C.-C.; Yang, Y.-C.; Tang, C.-M.; Yeh, C.-A.; Hsieh, H.-H.; Hsu, S.-h. Nanogold-Carried Graphene Oxide: Anti-Inflammation and Increased Differentiation Capacity of Mesenchymal Stem Cells. Nanomaterials 2021, 11, 2046. [Google Scholar] [CrossRef]
- Zupančič, U.; Jolly, P.; Estrela, P.; Moschou, D.; Ingber, D.E. Graphene Enabled Low-Noise Surface Chemistry for Multiplexed Sepsis Biomarker Detection in Whole Blood. Adv. Funct. Mater. 2021, 31, 2010638. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Shao, Y.; Sun, Y.; Si, H.; Miao, J.; Xu, Y. Chitosan functionalized graphene oxide nanocomposites for fluorescence imaging of apoptotic processes and targeted anti-inflammation study. Carbohydr. Polym. 2021, 269, 118345. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.H.; Singh, G.; More, N.; Keerthana, M.; Sharma, A.; Jawade, D.; Balu, A.; Kapusetti, G. Ultrahigh sensitive graphene oxide/conducting polymer composite based biosensor for cholesterol and bilirubin detection. Biosens. Bioelectron. X 2023, 13, 100290. [Google Scholar] [CrossRef]
- Borges-Vilches, J.; Figueroa, T.; Guajardo, S.; Carmona, S.; Mellado, C.; Meléndrez, M.; Aguayo, C.; Fernández, K. Novel and effective hemostats based on graphene oxide-polymer aerogels: In vitro and in vivo evaluation. Biomater. Adv. 2022, 139, 213007. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Xiao, M.; Dong, J.; Huang, Y.; Sun, H.; Wang, D. Synergistic anti-inflammatory effects of graphene oxide quantum dots and trans-10-hydroxy-2-decenoic acid on LPS-stimulated RAW 264.7 macrophage cells. Biomater. Adv. 2022, 136, 212774. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Kharaziha, M.; Raeissi, K. Electrophoretic deposition of chitosan reinforced graphene oxide-hydroxyapatite on the anodized titanium to improve biological and electrochemical characteristics. Mater. Sci. Eng. C 2019, 98, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, I.A.U. Biocompatibility of Alginate-Graphene Oxide Film for Tissue Engineering Applications. Key Eng. Mater. 2021, 900, 26–33. [Google Scholar] [CrossRef]
- Yılmaz, E.; Çakıroğlu, B.; Gökçe, A.; Findik, F.; Gulsoy, H.O.; Gulsoy, N.; Mutlu, Ö.; Özacar, M. Novel hydroxyapatite/graphene oxide/collagen bioactive composite coating on Ti16Nb alloys by electrodeposition. Mater. Sci. Eng. C 2019, 101, 292–305. [Google Scholar] [CrossRef]
- Murugan, N.; Murugan, C.; Sundramoorthy, A.K. In vitro and in vivo characterization of mineralized hydroxyapatite/polycaprolactone-graphene oxide based bioactive multifunctional coating on Ti alloy for bone implant applications. Arab. J. Chem. 2018, 11, 959–969. [Google Scholar] [CrossRef]
- Fei, F.; Yao, H.; Wang, Y.; Wei, J. Graphene Oxide/RhPTH(1-34)/Polylactide Composite Nanofibrous Scaffold for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 5799. [Google Scholar] [CrossRef]
- Zhang, D.; Yao, Y.; Duan, Y.; Yu, X.; Shi, H.; Nakkala, J.R.; Zuo, X.; Hong, L.; Mao, Z.; Gao, C. Surface-Anchored Graphene Oxide Nanosheets on Cell-Scale Micropatterned Poly(d,l-lactide-co-caprolactone) Conduits Promote Peripheral Nerve Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 7915–7930. [Google Scholar] [CrossRef]
- Ciftci, F.; Ayan, S.; Duygulu, N.; Yilmazer, Y.; Karavelioglu, Z.; Vehapi, M.; Cakır Koc, R.; Sengor, M.; Yılmazer, H.; Ozcimen, D.; et al. Selenium and clarithromycin loaded PLA-GO composite wound dressings by electrospinning method. Int. J. Polym. Mater. Polym. Biomater. 2022, 71, 898–909. [Google Scholar] [CrossRef]
- Fraczek-Szczypta, A.; Jantas, D.; Ciepiela, F.; Grzonka, J. Graphene oxide-conductive polymer nanocomposite coatings obtained by the EPD method as substrates for neurite outgrowth. Diam. Relat. Mater. 2020, 102, 107663. [Google Scholar] [CrossRef]
- Talebi, A.; Labbaf, S.; Karimzadeh, F.; Masaeli, E.; Nasr Esfahani, M.-H. Electroconductive Graphene-Containing Polymeric Patch: A Promising Platform for Future Cardiac Repair. ACS Biomater. Sci. Eng. 2020, 6, 4214–4224. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, L.; Hou, Y.; Zhang, Z.; Chen, M.; Wang, M.; Liu, J.; Wang, J.; Zhao, Z.; Xie, C.; et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact. Mater. 2022, 18, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Y.; Li, H.; Liu, Y.; Bai, X.; Chau, W.; Zheng, Y.; Qin, L. Magnesium alloy based interference screw developed for ACL reconstruction attenuates peri-tunnel bone loss in rabbits. Biomaterials 2018, 157, 86–97. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Miao, H.; Xu, J.; Ong, M.T.-y.; Wang, C.; Zheng, L.; Wang, J.; Huang, L.; Zu, H.; et al. High formability Mg-Zn-Gd wire facilitates ACL reconstruction via its swift degradation to accelerate intra-tunnel endochondral ossification. J. Magnes. Alloys 2022, in press. [Google Scholar] [CrossRef]
- Veizi, E.; Alkan, H.; Çay, N.; Şahin, A.; Çepni, Ş.; Tecimel, O.; Fırat, A. Clinical and radiological comparison of bioactive glass and poly-L-lactic acid/hydroxyapatite bioabsorbable interference screws for tibial graft fixation in anterior cruciate ligament reconstruction. Orthop. Traumatol. Surg. Res. OTSR 2022, 108, 103247. [Google Scholar] [CrossRef]
- Ficek, K.; Rajca, J.; Stolarz, M.; Stodolak-Zych, E.; Wieczorek, J.; Muzalewska, M.; Wyleżoł, M.; Wróbel, Z.; Binkowski, M.; Błażewicz, S. Bioresorbable Stent in Anterior Cruciate Ligament Reconstruction. Polymers 2019, 11, 1961. [Google Scholar] [CrossRef]
- Stodolak-Zych, E.; Ficek, K.; Wieczorek, J.; Kajor, M.; Gryń, K.; Rapacz-Kmita, A.; Rajca, J.; Kosenyuk, Y.; Stolarz, M.; Błażewicz, S. Assessment of sheep knee joint after ACL replacement with Achilles tendon autograft and PLA-based implant. J. Mech. Behav. Biomed. Mater. 2022, 125, 104923. [Google Scholar] [CrossRef]
- Borjali, A.; Farrahi, G.; Chizari, M. Sheathed fixation improves BASHTI technique in an anterior cruciate ligament reconstruction. Proc. Inst. Mech. Engineers. Part H J. Eng. Med. 2023, 237, 375–384. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Jiang, J.; Lv, F.; Chang, J.; Chen, S.; Wu, C. An osteogenesis/angiogenesis-stimulation artificial ligament for anterior cruciate ligament reconstruction. Acta Biomater. 2017, 54, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, Y.; Ai, C.; Jiang, J.; Cai, J.; Sheng, D.; Wan, F.; Liu, X.; Hao, Y.; Chen, J. Enhance the biocompatibility and osseointegration of polyethylene terephthalate ligament by plasma spraying with hydroxyapatite in vitro and in vivo. Int. J. Nanomed. 2018, 13, 3609–3623. [Google Scholar] [CrossRef] [PubMed]
- Kanniyappan, H.; Venkatesan, M.; Panji, J.; Ramasamy, M.; Muthuvijayan, V. Evaluating the inherent osteogenic and angiogenic potential of mesoporous silica nanoparticles to augment vascularized bone tissue formation. Microporous Mesoporous Mater. 2021, 311, 110687. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Shkarina, S.; Syromotina, D.S.; Melnik, E.V.; Shkarin, R.; Selezneva, I.I.; Ermakov, A.M.; Ivlev, S.I.; Cecilia, A.; Weinhardt, V.; et al. Characterization of biomimetic silicate- and strontium-containing hydroxyapatite microparticles embedded in biodegradable electrospun polycaprolactone scaffolds for bone regeneration. Eur. Polym. J. 2019, 113, 67–77. [Google Scholar] [CrossRef]
- Egawa, T.; Inagaki, Y.; Akahane, M.; Furukawa, A.; Inoue, K.; Ogawa, M.; Tanaka, Y. Silicate-substituted strontium apatite nano coating improves osteogenesis around artificial ligament. BMC Musculoskelet. Disord. 2019, 20, 396. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Carrothers, T.J.; Riccobene, T.; Stone, G.G.; Kantecki, M. Ceftaroline Fosamil for Treatment of Pediatric Complicated Skin and Soft Tissue Infections and Community-Acquired Pneumonia. Pediatr. Drugs 2021, 23, 549–563. [Google Scholar] [CrossRef]
- Lázaro-Díez, M.; Remuzgo-Martínez, S.; Rodríguez-Mirones, C.; Acosta, F.; Icardo, J.M.; Martínez-Martínez, L.; Ramos-Vivas, J. Effects of Subinhibitory Concentrations of Ceftaroline on Methicillin-Resistant Staphylococcus aureus (MRSA) Biofilms. PLoS ONE 2016, 11, e0147569. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Antibacterial Action of Nanoparticle Loaded Nanocomposites Based on Graphene and Its Derivatives: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 3563. [Google Scholar] [CrossRef]
- Liauw, C.M.; Vaidya, M.; Slate, A.J.; Hickey, N.A.; Ryder, S.; Martínez-Periñán, E.; McBain, A.J.; Banks, C.E.; Whitehead, K.A. Analysis of Cellular Damage Resulting from Exposure of Bacteria to Graphene Oxide and Hybrids Using Fourier Transform Infrared Spectroscopy. Antibiotics 2023, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Ma, H.; Liu, B.; Wang, J. Graphene Oxide Reinforced Polylactic Acid/Polyurethane Antibacterial Composites. J. Nanomater. 2013, 2013, 373414. [Google Scholar] [CrossRef]
- Chen, Q.; Mangadlao, J.D.; Wallat, J.; De Leon, A.; Pokorski, J.K.; Advincula, R.C. 3D Printing Biocompatible Polyurethane/Poly(lactic acid)/Graphene Oxide Nanocomposites: Anisotropic Properties. ACS Appl. Mater. Interfaces 2017, 9, 4015–4023. [Google Scholar] [CrossRef]
- Mohamad, S.N.; Ramli, I.; Abdullah, L.C.; Mohamed, N.H.; Islam, M.S.; Ibrahim, N.A.; Ishak, N.S. Evaluation on Structural Properties and Performances of Graphene Oxide Incorporated into Chitosan/Poly-Lactic Acid Composites: CS/PLA versus CS/PLA-GO. Polymers 2021, 13, 1839. [Google Scholar] [CrossRef]
- Khan, M.U.; Yaqoob, Z.; Ansari, M.N.; Razak, S.I.; Raza, M.A.; Sajjad, A.; Haider, S.; Busra, F.M. Chitosan/Poly Vinyl Alcohol/Graphene Oxide Based pH-Responsive Composite Hydrogel Films: Drug Release, Anti-Microbial and Cell Viability Studies. Polymers 2021, 13, 3124. [Google Scholar] [CrossRef] [PubMed]
- Ericson Morgan, G.; Martin, R.; Welch, H.; Morris, K. Quantitative Weight Bearing and non-weight Bearing Measures of Stiffness in the Achilles Tendon and Gastrocnemius Muscle. Muscles Ligaments Tendons J. 2020, 10, 100. [Google Scholar] [CrossRef]
- Silva, M.; Gomes, C.; Pinho, I.; Gonçalves, H.; Vale, A.C.; Covas, J.A.; Alves, N.M.; Paiva, M.C. Poly(Lactic Acid)/Graphite Nanoplatelet Nanocomposite Filaments for Ligament Scaffolds. Nanomaterials 2021, 11, 2796. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qu, Z.; Liu, Z.; Ren, G. Mechanism of Oxidization of Graphite to Graphene Oxide by the Hummers Method. ACS Omega 2022, 7, 23503–23510. [Google Scholar] [CrossRef] [PubMed]
- Cruz, R.; Nisar, M.; Palza, H.; Yazdani-Pedram, M.; Aguilar-Bolados, H.; Quijada, R. Development of bio degradable nanocomposites based on PLA and functionalized graphene oxide. Polym. Test. 2023, 124, 108066. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Șuhan, R.; Niculescu, A.-G.; Grumezescu, V.; Negut, I.; Holban, A.M.; Oprea, O.-C.; Bîrcă, A.C.; Vasile, B.Ș.; Grumezescu, A.M.; et al. Biofilm-Resistant Nanocoatings Based on ZnO Nanoparticles and Linalool. Nanomaterials 2021, 11, 2564. [Google Scholar] [CrossRef]
- Hudiță, A.; Radu, I.C.; Zaharia, C.; Ion, A.C.; Ginghină, O.; Gălățeanu, B.; Măruțescu, L.; Grama, F.; Tsatsakis, A.; Gurevich, L.; et al. Bio- and Hemo-Compatible Silk Fibroin PEGylated Nanocarriers for 5-Fluorouracil Chemotherapy in Colorectal Cancer: In Vitro Studies. Pharmaceutics 2021, 13, 755. [Google Scholar] [CrossRef]
- Gheran, C.V.; Voicu, S.N.; Galateanu, B.; Callewaert, M.; Moreau, J.; Cadiou, C.; Chuburu, F.; Dinischiotu, A. In Vitro Studies Regarding the Safety of Chitosan and Hyaluronic Acid-Based Nanohydrogels Containing Contrast Agents for Magnetic Resonance Imaging. Int. J. Mol. Sci. 2022, 23, 3258. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Ficai, A.; Grumezescu, V.; Holban, A.M.; Gălățeanu, B.; Hudiță, A. Composite P(3HB-3HV)-CS Spheres for Enhanced Antibiotic Efficiency. Polymers 2021, 13, 989. [Google Scholar] [CrossRef]
- Hudiță, A.; Grumezescu, V.; Gherasim, O.; Grumezescu, A.M.; Dorcioman, G.; Negut, I.; Oprea, O.-C.; Vasile, B.Ș.; Gălățeanu, B.; Curuțiu, C.; et al. MAPLE Processed Nanostructures for Antimicrobial Coatings. Int. J. Mol. Sci. 2022, 23, 15355. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinescu, S.; Niculescu, A.-G.; Hudiță, A.; Grumezescu, V.; Rădulescu, D.; Bîrcă, A.C.; Dorcioman, G.; Gherasim, O.; Holban, A.M.; Gălățeanu, B.; et al. Nanostructured Coatings Based on Graphene Oxide for the Management of Periprosthetic Infections. Int. J. Mol. Sci. 2024, 25, 2389. https://doi.org/10.3390/ijms25042389
Constantinescu S, Niculescu A-G, Hudiță A, Grumezescu V, Rădulescu D, Bîrcă AC, Dorcioman G, Gherasim O, Holban AM, Gălățeanu B, et al. Nanostructured Coatings Based on Graphene Oxide for the Management of Periprosthetic Infections. International Journal of Molecular Sciences. 2024; 25(4):2389. https://doi.org/10.3390/ijms25042389
Chicago/Turabian StyleConstantinescu, Sorin, Adelina-Gabriela Niculescu, Ariana Hudiță, Valentina Grumezescu, Dragoș Rădulescu, Alexandra Cătălina Bîrcă, Gabriela Dorcioman, Oana Gherasim, Alina Maria Holban, Bianca Gălățeanu, and et al. 2024. "Nanostructured Coatings Based on Graphene Oxide for the Management of Periprosthetic Infections" International Journal of Molecular Sciences 25, no. 4: 2389. https://doi.org/10.3390/ijms25042389
APA StyleConstantinescu, S., Niculescu, A. -G., Hudiță, A., Grumezescu, V., Rădulescu, D., Bîrcă, A. C., Dorcioman, G., Gherasim, O., Holban, A. M., Gălățeanu, B., Vasile, B. Ș., Grumezescu, A. M., Bolocan, A., & Rădulescu, R. (2024). Nanostructured Coatings Based on Graphene Oxide for the Management of Periprosthetic Infections. International Journal of Molecular Sciences, 25(4), 2389. https://doi.org/10.3390/ijms25042389











