Recombinant Rod Domain of Vimentin Reduces SARS-CoV-2 Viral Replication by Blocking Spike Protein–ACE2 Interactions
Abstract
1. Introduction
2. Results
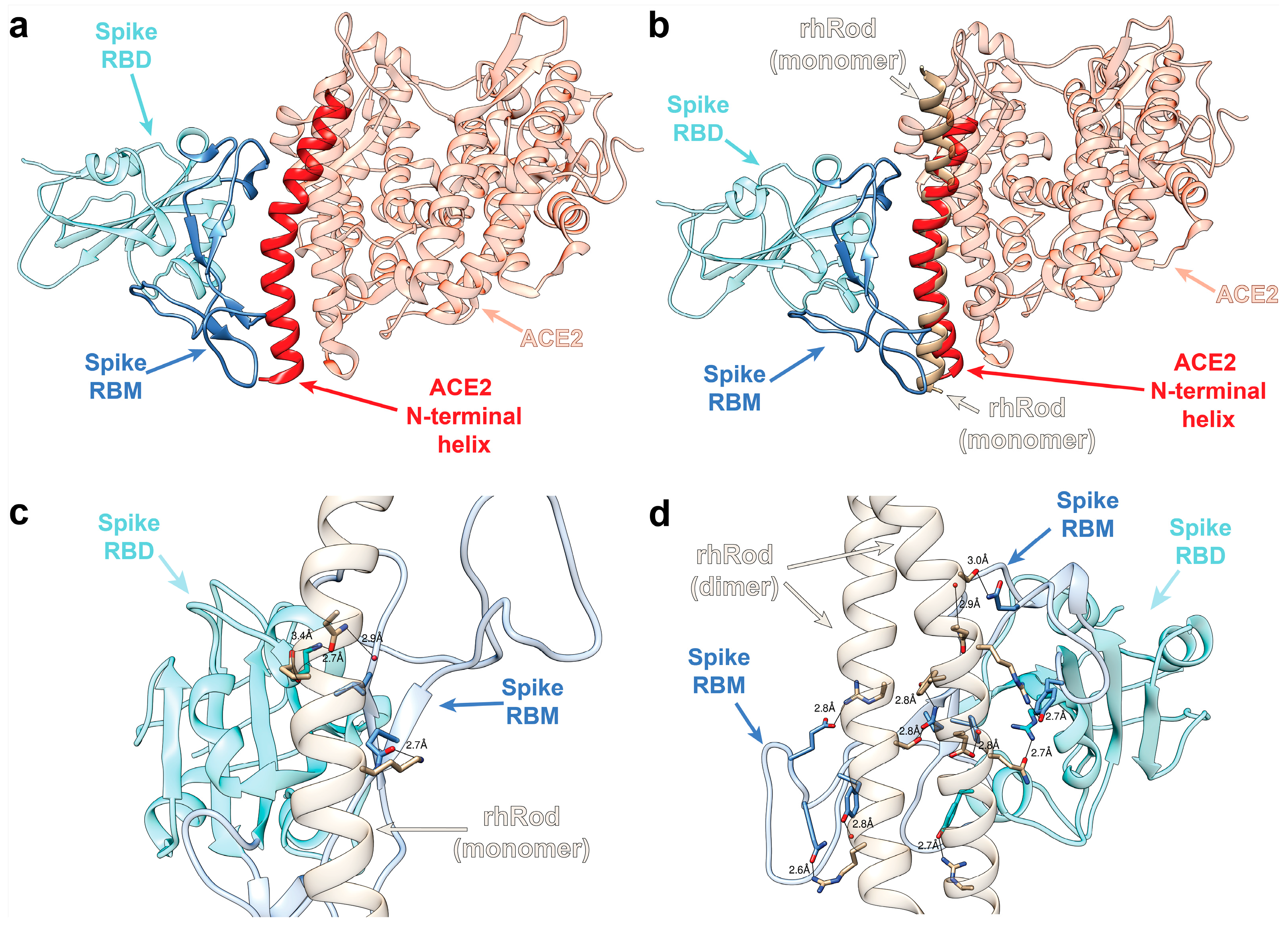
2.1. In Silico Modeling Predicts rhRod Binding to SARS-CoV-2 Spike Protein
2.2. rhRod Binds to SARS-CoV-2 Spike Protein
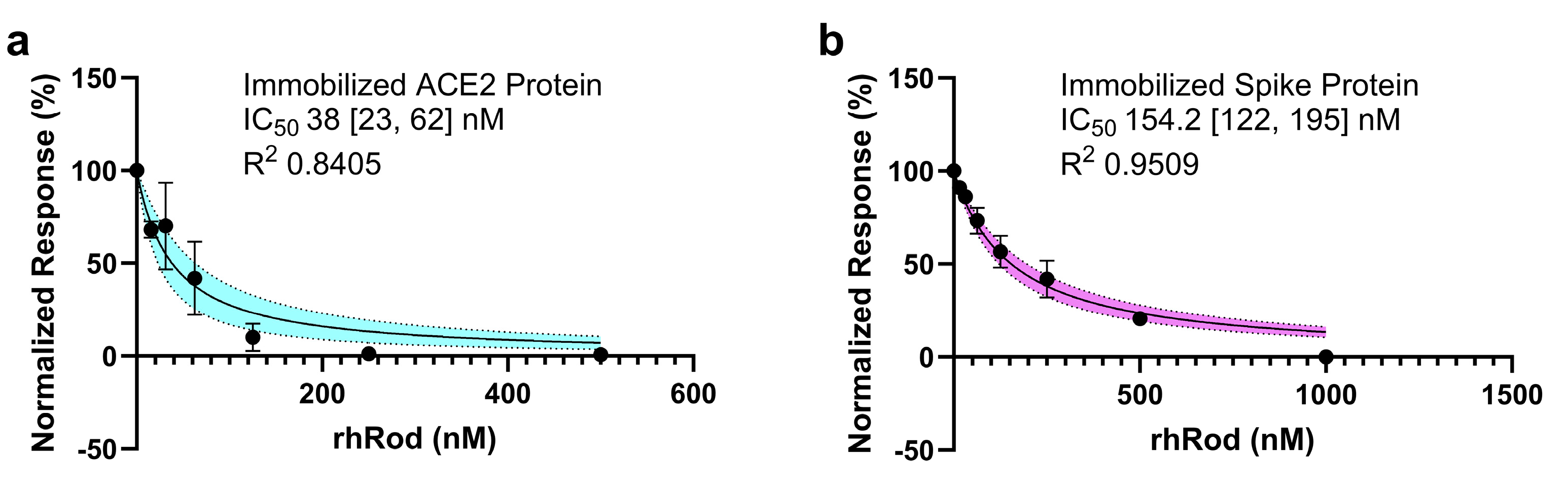
2.3. rhRod Blocks’ SARS-CoV-2 Spike Protein–ACE2 Interactions
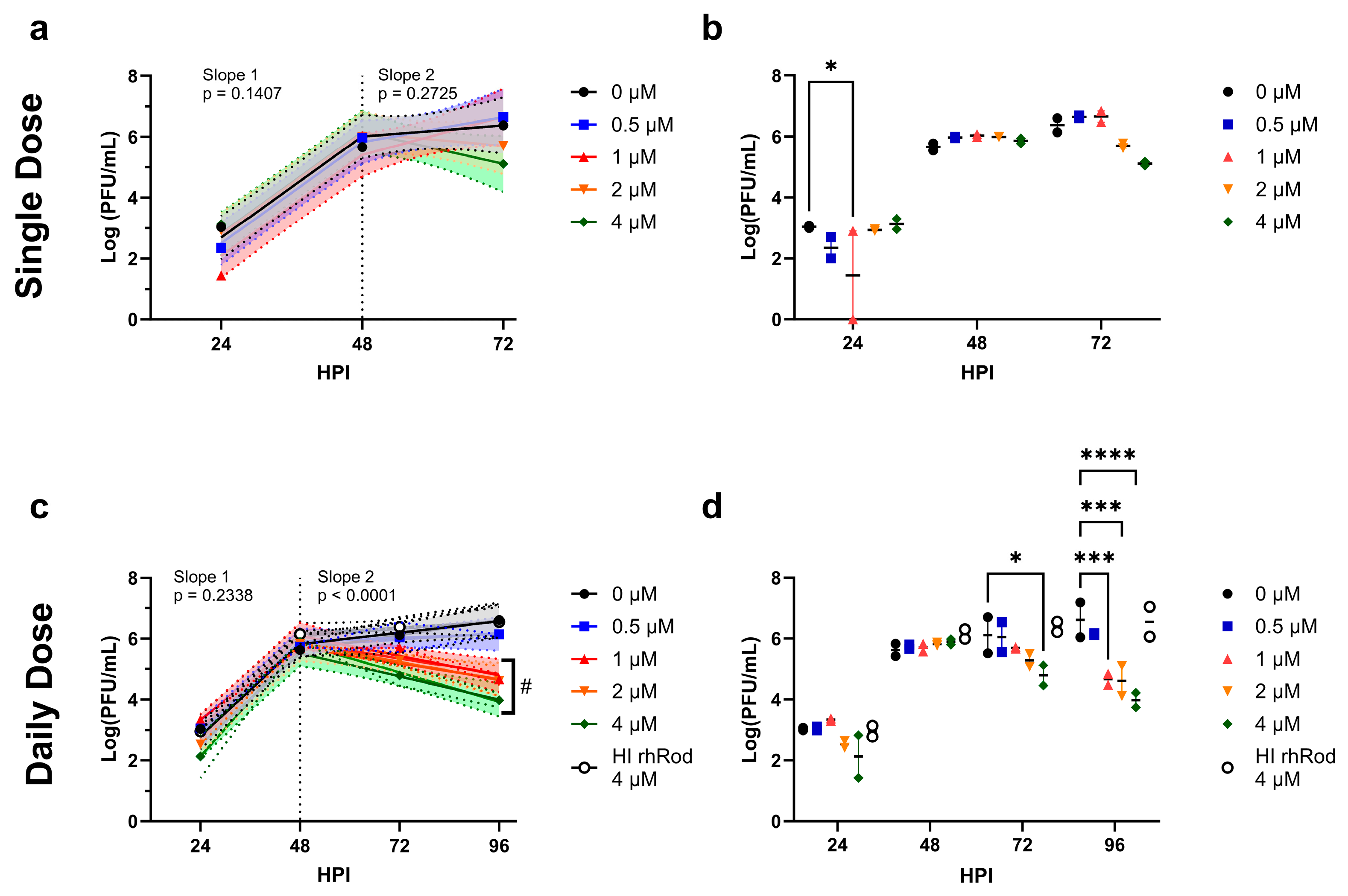
2.4. Daily rhRod Decreases SARS-CoV-2 Replication in Vero E6 Cells
2.5. rhRod Treatment Decreases Lung Inflammation in SARS-CoV-2-Infected K18-hACE2 Mice
3. Discussion
4. Materials and Methods
4.1. Study Approval
4.2. In Silico Modeling of rhRod–Spike Protein Interactions
4.3. Creation of Recombinant Rod Domain of Vimentin (rhRod)
4.4. Biolayer Interferometry
4.5. SPOT Peptide Array
4.6. Viral and Cell Culture Stocks
4.7. Viral Infection Studies
4.8. In Vivo Animal Studies
4.9. Statistical Analysis
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, P.; Fan, H.; Wu, Q.; Ouyang, J.; Li, K. Compound impact of COVID-19, economy and climate on the spatial distribution of global agriculture and food security. Sci. Total Environ. 2023, 880, 163105. [Google Scholar] [CrossRef]
- Ikram, M.; Sayagh, Y. The Consequences of COVID-19 Disruption on Sustainable Economy in the Top 30 High-Tech Innovative Countries. Glob. J. Flex. Syst. Manag. 2023, 24, 247–269. [Google Scholar] [CrossRef]
- Kosiyaporn, H.; Phaiyarom, M.; Uansri, S.; Kunpeuk, W.; Julchoo, S.; Sinam, P.; Pudpong, N.; Suphanchaimat, R. Characteristics of distance education interventions and related outcomes in primary school children during COVID-19 pandemic: A systematic review. PLoS ONE 2023, 18, e0286674. [Google Scholar] [CrossRef]
- Ciravegna, L.; Michailova, S. Why the world economy needs, but will not get, more globalization in the post-COVID-19 decade. J. Int. Bus. Stud. 2022, 53, 172–186. [Google Scholar] [CrossRef]
- Ahmad, F.B.; Cisewski, J.A.; Anderson, R.N. Provisional Mortality Data—United States, 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.B.; Cisewski, J.A.; Xu, J.; Anderson, R.N. COVID-19 Mortality Update—United States, 2022. Morb. Mortal. Wkly. Rep. 2023, 72, 493–496. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 11 June 2023).
- CDC. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker/?ref=welcometohellworld.com#datatracker-home (accessed on 11 June 2023).
- Bohn, M.K.; Hall, A.; Sepiashvili, L.; Jung, B.; Steele, S.; Adeli, K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression. Physiology 2020, 35, 288–301. [Google Scholar] [CrossRef]
- Bray, M.; Guzel, M.A.; Lam, F.; Yee, A.; Cruz, M.A.; Rumbaut, R.E. High levels of von Willebrand factor with reduced specific activities in hospitalized patients with or without COVID-19. J. Thromb. Thrombolysis 2022, 54, 211–216. [Google Scholar] [CrossRef]
- Brubaker, L.S.; Saini, A.; Nguyen, T.C.; Martinez-Vargas, M.; Lam, F.W.; Yao, Q.; Loor, M.M.; Rosengart, T.K.; Cruz, M.A. Aberrant Fibrin Clot Structure Visualized Ex Vivo in Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Crit. Care Med. 2022, 50, e557–e568. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Mazer, M.B.; Bulut, Y.; Brodsky, N.N.; Lam, F.W.; Sturgill, J.L.; Miles, S.M.; Shein, S.L.; Carroll, C.L.; Remy, K.E.; the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network and BLOODNET Immunology Section; et al. Multisystem Inflammatory Syndrome in Children: Host Immunologic Responses. Pediatr. Crit. Care Med. 2022, 23, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.; Pithon-Curi, T.C.; Curi, R.; Hatanaka, E. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediat. Inflamm. 2020, 2020, 8829674. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.T.; Chien, S.C.; Chen, I.Y.; Lai, C.T.; Tsay, Y.G.; Chang, S.C.; Chang, M.F. Surface vimentin is critical for the cell entry of SARS-CoV. J. Biomed. Sci. 2016, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.W.; Brown, C.A.; Valladolid, C.; Emebo, D.C.; Palzkill, T.G.; Cruz, M.A. The vimentin rod domain blocks P-selectin-P-selectin glycoprotein ligand 1 interactions to attenuate leukocyte adhesion to inflamed endothelium. PLoS ONE 2020, 15, e0240164. [Google Scholar] [CrossRef]
- Lam, F.W.; Da, Q.; Guillory, B.; Cruz, M.A. Recombinant Human Vimentin Binds to P-Selectin and Blocks Neutrophil Capture and Rolling on Platelets and Endothelium. J. Immunol. 2018, 200, 1718–1726. [Google Scholar] [CrossRef]
- Torchala, M.; Moal, I.H.; Chaleil, R.A.; Fernandez-Recio, J.; Bates, P.A. SwarmDock: A server for flexible protein-protein docking. Bioinformatics 2013, 29, 807–809. [Google Scholar] [CrossRef]
- Amraei, R.; Xia, C.; Olejnik, J.; White, M.R.; Napoleon, M.A.; Lotfollahzadeh, S.; Hauser, B.M.; Schmidt, A.G.; Chitalia, V.; Muhlberger, E.; et al. Extracellular vimentin is an attachment factor that facilitates SARS-CoV-2 entry into human endothelial cells. Proc. Natl. Acad. Sci. USA 2022, 119, e2113874119. [Google Scholar] [CrossRef]
- Arrindell, J.; Abou Atmeh, P.; Jayet, L.; Sereme, Y.; Mege, J.L.; Desnues, B. Vimentin is an important ACE2 co-receptor for SARS-CoV-2 in epithelial cells. iScience 2022, 25, 105463. [Google Scholar] [CrossRef] [PubMed]
- Deptula, P.; Fiedoruk, K.; Wasilewska, M.; Suprewicz, L.; Ciesluk, M.; Zeliszewska, P.; Ocwieja, M.; Adamczyk, Z.; Pogoda, K.; Bucki, R. Physicochemical Nature of SARS-CoV-2 Spike Protein Binding to Human Vimentin. ACS Appl. Mater. Interfaces 2023, 15, 34172–34180. [Google Scholar] [CrossRef] [PubMed]
- Vermeire, P.J.; Lilina, A.V.; Hashim, H.M.; Dlabolova, L.; Fiala, J.; Beelen, S.; Kukacka, Z.; Harvey, J.N.; Novak, P.; Strelkov, S.V. Molecular structure of soluble vimentin tetramers. Sci. Rep. 2023, 13, 8841. [Google Scholar] [CrossRef]
- Puhm, F.; Allaeys, I.; Lacasse, E.; Dubuc, I.; Galipeau, Y.; Zaid, Y.; Khalki, L.; Belleannee, C.; Durocher, Y.; Brisson, A.R.; et al. Platelet activation by SARS-CoV-2 implicates the release of active tissue factor by infected cells. Blood Adv. 2022, 6, 3593–3605. [Google Scholar] [CrossRef] [PubMed]
- Bhargavan, B.; Kanmogne, G.D. SARS-CoV-2 Spike Proteins and Cell-Cell Communication Induce P-Selectin and Markers of Endothelial Injury, NETosis, and Inflammation in Human Lung Microvascular Endothelial Cells and Neutrophils: Implications for the Pathogenesis of COVID-19 Coagulopathy. Int. J. Mol. Sci. 2023, 24, 12585. [Google Scholar] [CrossRef]
- Karahasan Yagci, A.; Sarinoglu, R.C.; Bilgin, H.; Yanilmaz, O.; Sayin, E.; Deniz, G.; Guncu, M.M.; Doyuk, Z.; Baris, C.; Kuzan, B.N.; et al. Relationship of the cycle threshold values of SARS-CoV-2 polymerase chain reaction and total severity score of computerized tomography in patients with COVID-19. Int. J. Infect. Dis. 2020, 101, 160–166. [Google Scholar] [CrossRef]
- Kociolek, L.K.; Muller, W.J.; Yee, R.; Dien Bard, J.; Brown, C.A.; Revell, P.A.; Wardell, H.; Savage, T.J.; Jung, S.; Dominguez, S.; et al. Comparison of Upper Respiratory Viral Load Distributions in Asymptomatic and Symptomatic Children Diagnosed with SARS-CoV-2 Infection in Pediatric Hospital Testing Programs. J. Clin. Microbiol. 2020, 59, e02593-20. [Google Scholar] [CrossRef]
- Salto-Alejandre, S.; Berastegui-Cabrera, J.; Camacho-Martinez, P.; Infante-Dominguez, C.; Carretero-Ledesma, M.; Crespo-Rivas, J.C.; Marquez, E.; Lomas, J.M.; Bueno, C.; Amaya, R.; et al. SARS-CoV-2 viral load in nasopharyngeal swabs is not an independent predictor of unfavorable outcome. Sci. Rep. 2021, 11, 12931. [Google Scholar] [CrossRef]
- Dadras, O.; Afsahi, A.M.; Pashaei, Z.; Mojdeganlou, H.; Karimi, A.; Habibi, P.; Barzegary, A.; Fakhfouri, A.; Mirzapour, P.; Janfaza, N.; et al. The relationship between COVID-19 viral load and disease severity: A systematic review. Immun. Inflamm. Dis. 2022, 10, e580. [Google Scholar] [CrossRef]
- Winkler, E.S.; Bailey, A.L.; Kafai, N.M.; Nair, S.; McCune, B.T.; Yu, J.; Fox, J.M.; Chen, R.E.; Earnest, J.T.; Keeler, S.P.; et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat. Immunol. 2020, 21, 1327–1335. [Google Scholar] [CrossRef]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Barcena, M.; et al. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Pires De Souza, G.A.; Le Bideau, M.; Boschi, C.; Wurtz, N.; Colson, P.; Aherfi, S.; Devaux, C.; La Scola, B. Choosing a cellular model to study SARS-CoV-2. Front. Cell Infect. Microbiol. 2022, 12, 1003608. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Frank, R. The SPOT-synthesis technique. Synthetic peptide arrays on membrane supports–principles and applications. J. Immunol. Methods 2002, 267, 13–26. [Google Scholar] [CrossRef] [PubMed]
- McCray, P.B., Jr.; Pewe, L.; Wohlford-Lenane, C.; Hickey, M.; Manzel, L.; Shi, L.; Netland, J.; Jia, H.P.; Halabi, C.; Sigmund, C.D.; et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J. Virol. 2007, 81, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Harcourt, J.; Tamin, A.; Lu, X.; Kamili, S.; Sakthivel, S.K.; Murray, J.; Queen, K.; Tao, Y.; Paden, C.R.; Zhang, J.; et al. Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease, United States. Emerg. Infect. Dis. 2020, 26, 1266–1273. [Google Scholar] [CrossRef]
- Zheng, J.; Wong, L.R.; Li, K.; Verma, A.K.; Ortiz, M.E.; Wohlford-Lenane, C.; Leidinger, M.R.; Knudson, C.M.; Meyerholz, D.K.; McCray, P.B., Jr.; et al. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 2021, 589, 603–607. [Google Scholar] [CrossRef]
- Oladunni, F.S.; Park, J.G.; Pino, P.A.; Gonzalez, O.; Akhter, A.; Allue-Guardia, A.; Olmo-Fontanez, A.; Gautam, S.; Garcia-Vilanova, A.; Ye, C.; et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin-converting enzyme 2 transgenic mice. Nat. Commun. 2020, 11, 6122. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M.; On behalf of the Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef]



| Lot | KD [95% CI] (nM) | R2 |
|---|---|---|
| rhRod-1 | 156 [115, 212] | 0.9722 |
| rhRod-2 | 384 [168, 1000] | 0.9584 |
| rhRod-3 | 191 [98, 381] | 0.8976 |
| rhRod-4 | 175 [90, 350] | 0.7787 |
| rhRod-5 | 135 [97, 188] | 0.9662 |
| rhRod-6 | 227 [126, 425] | 0.977 |
| rhRod-7 | 104 [57, 188] | 0.9636 |
| *HI rhRod-2 | Indeterminate | 0.8819 |
| *HI-rhRod-3 | 146 [−2657, infinity] | 0.3646 |
| *HI rhRod-4 | 525 [181, 2439] | 0.9412 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, F.W.; Brown, C.A.; Ronca, S.E. Recombinant Rod Domain of Vimentin Reduces SARS-CoV-2 Viral Replication by Blocking Spike Protein–ACE2 Interactions. Int. J. Mol. Sci. 2024, 25, 2477. https://doi.org/10.3390/ijms25052477
Lam FW, Brown CA, Ronca SE. Recombinant Rod Domain of Vimentin Reduces SARS-CoV-2 Viral Replication by Blocking Spike Protein–ACE2 Interactions. International Journal of Molecular Sciences. 2024; 25(5):2477. https://doi.org/10.3390/ijms25052477
Chicago/Turabian StyleLam, Fong Wilson, Cameron August Brown, and Shannon Elizabeth Ronca. 2024. "Recombinant Rod Domain of Vimentin Reduces SARS-CoV-2 Viral Replication by Blocking Spike Protein–ACE2 Interactions" International Journal of Molecular Sciences 25, no. 5: 2477. https://doi.org/10.3390/ijms25052477
APA StyleLam, F. W., Brown, C. A., & Ronca, S. E. (2024). Recombinant Rod Domain of Vimentin Reduces SARS-CoV-2 Viral Replication by Blocking Spike Protein–ACE2 Interactions. International Journal of Molecular Sciences, 25(5), 2477. https://doi.org/10.3390/ijms25052477






