Identification of Insulin-like Growth Factor (IGF) Family Genes in the Golden Pompano, Trachinotus ovatus: Molecular Cloning, Characterization and Gene Expression
Abstract
:1. Introduction
2. Results
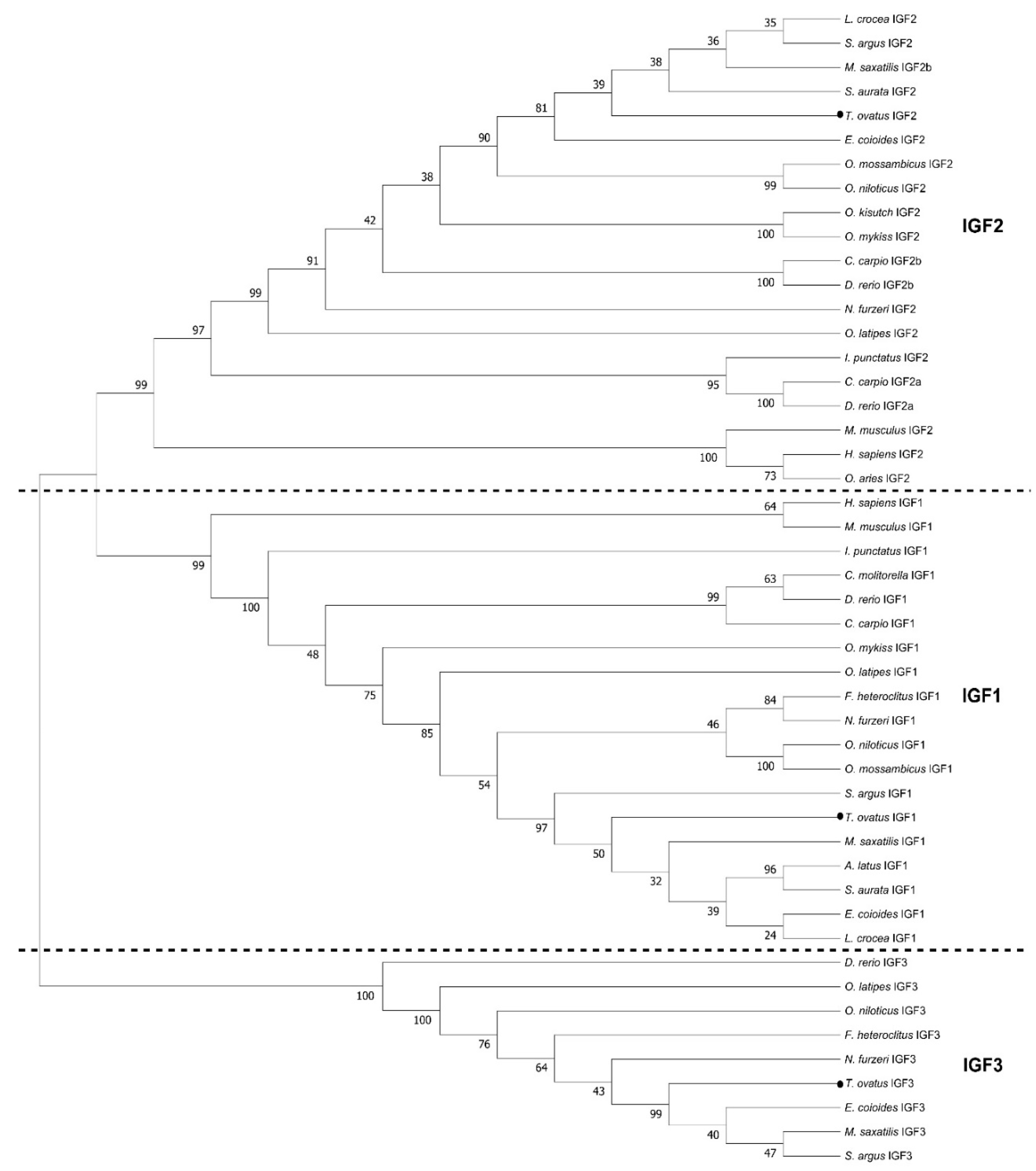
2.1. Molecular Cloning and Sequence Analysis
2.2. Tissue Distribution
2.3. Effects of Fasting on IGF1 and IGF2 Expression
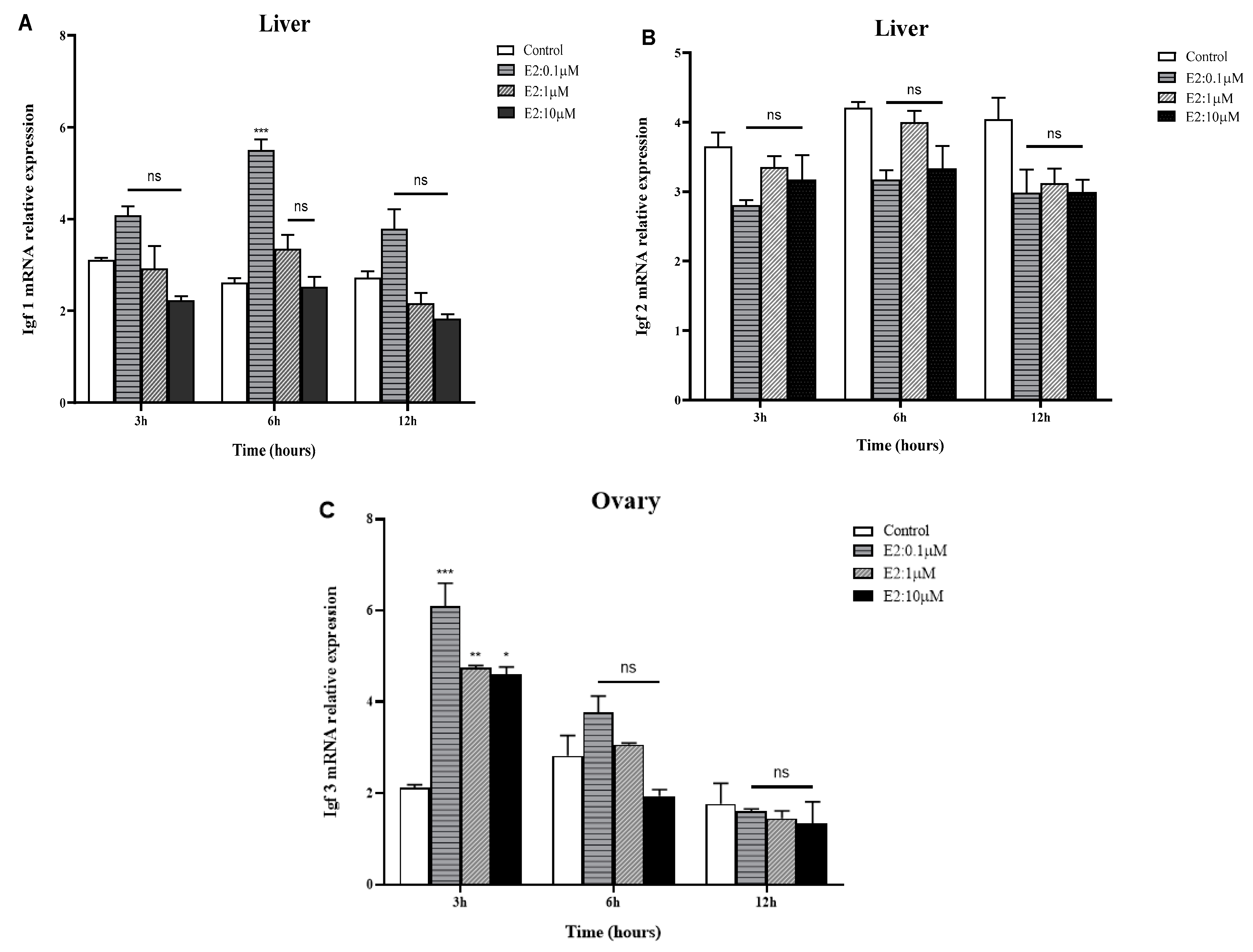
2.4. In Vitro Effects of E2 on IGFs mRNA Expression in the Liver and Gonads of Golden Pompano
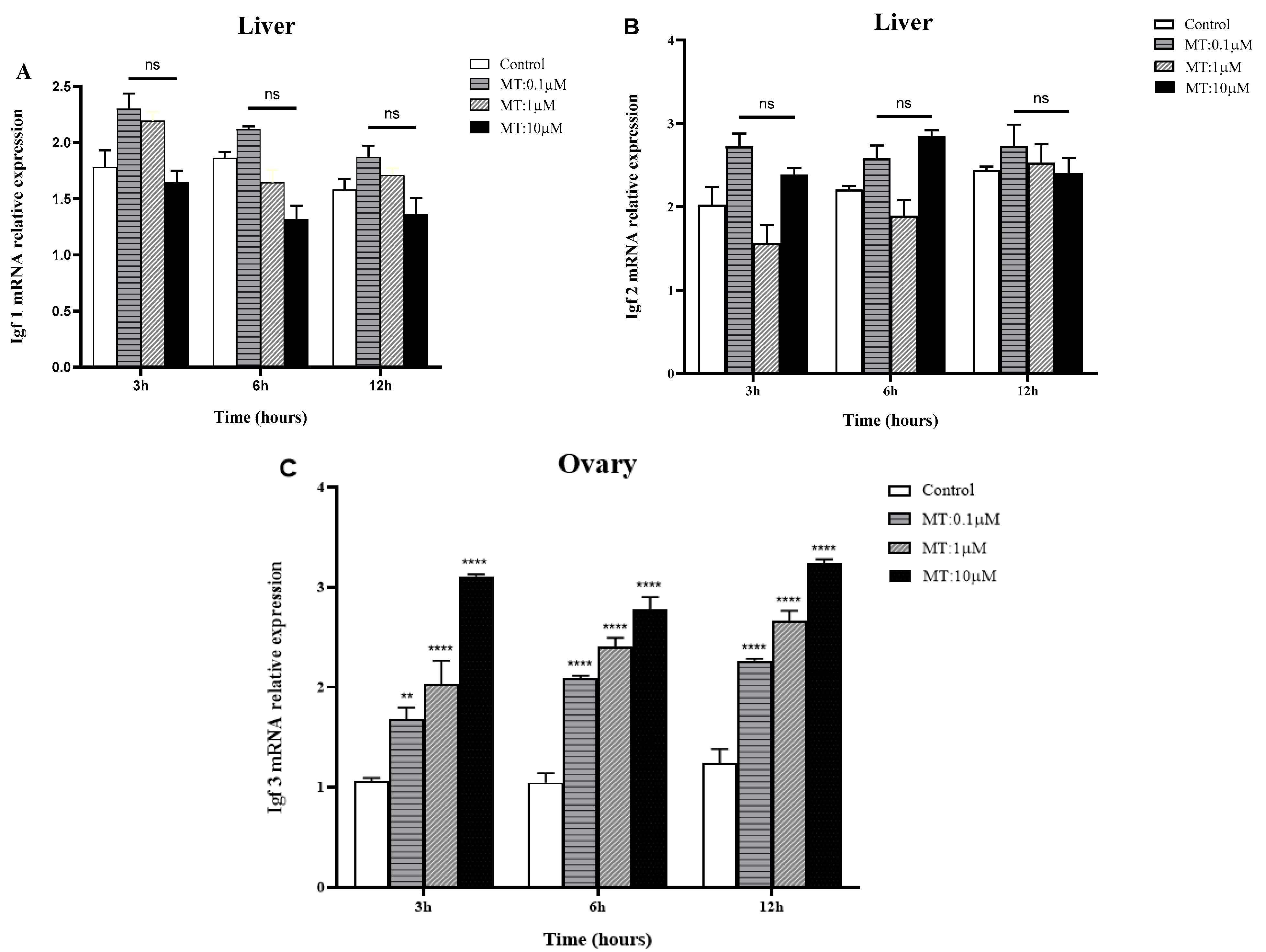
2.5. In Vitro Effects of MT on IGF mRNA Expression in the Liver and Gonads of Golden Pompano
3. Discussion
4. Materials and Methods
4.1. Experimental Fish Collection
4.2. RNA Extraction and cDNA Synthesis
4.3. Molecular Cloning and Sequence Analysis
4.4. Tissue Distribution of IGF Family Genes
4.5. Effects of Fasting on IGF1 and IGF2 Expression in the Liver and Muscles
4.6. In Vitro Incubation of Golden Pompano with E2 and MT on IGF Gene Expression
4.7. Quantitative Real-Time PCR (RT-qPCR) Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, X.; Huang, Y.; Li, X.; Li, Z.; Yang, H.; He, R.; Zhong, H.; Li, G.; Chen, H. Identification and functional characterization of gonadotropin-releasing hormone in pompano (Trachinotus ovatus). Gen. Comp. Endocrinol. 2022, 316, 113958. [Google Scholar] [CrossRef]
- Ren, X.; Liu, J.; Ndandala, C.B.; Li, X.; Guo, Y.; Li, G.; Chen, H. Physiological Effects and Transcriptomic Analysis of sbGnRH on the Liver in Pompano (Trachinotus ovatus). Front. Endocrinol. 2022, 13, 869021. [Google Scholar] [CrossRef]
- Shimizu, M.; Dickhoff, W.W. Circulating insulin-like growth factor binding proteins in fish: Their identities and physiological regulation. Gen. Comp. Endocrinol. 2017, 252, 150–161. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Kang, T.; Li, M.; Wang, D.; Cheng, C.H.K. Igf3: A novel player in fish reproduction. Biol. Reprod. 2021, 104, 1194–1204. [Google Scholar] [CrossRef]
- De la Serrana, D.G.; Macqueen, D.J. Insulin-like growth factor-binding proteins of teleost fishes. Front. Endocrinol. 2018, 9, 80. [Google Scholar] [CrossRef]
- Jia, Y.; Jing, Q.; Gao, Y.; Huang, B. Involvement and expression of growth hormone/insulin-like growth factor member mRNAs in the ovarian development of turbot (Scophthalmus maximus). Fish. Physiol. Biochem. 2019, 45, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Brighton, N.C.; Dai, M.; Farouk, U.; Li, X.; Liu, J.; Huang, H.; Li, G.; Chen, H. Current research and future perspectives of GH and IGFs family genes in somatic growth and reproduction of teleost fish. Aquac. Rep. 2022, 26, 101289. [Google Scholar] [CrossRef]
- Li, J.; Chu, L.; Sun, X.; Liu, Y.; Cheng, C.H.K. IGFS mediate the action of LH on oocyte maturation in zebrafish. Mol. Endocrinol. 2015, 29, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Eppler, E.; Berishvili, G.; Mazel, P.; Caelers, A.; Hwang, G.; Maclean, N.; Reinecke, M. Distinct organ-specific up- and down-regulation of IGF-I and IGF-II mRNA in various organs of a GH-overexpressing transgenic Nile tilapia. Transgenic Res. 2010, 19, 231–240. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Valdés, J.A.; Molina, A.; Björnsson, B.T. Regulation of skeletal muscle growth in fish by the growth hormone—Insulin-like growth factor system. Gen. Comp. Endocrinol. 2013, 192, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Kwintkiewicz, J.; Giudice, L.C. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin. Reprod. Med. 2009, 27, 43–51. [Google Scholar] [CrossRef]
- Loffing-Cueni, D.; Schmid, A.C.; Reinecke, M. Molecular cloning and tissue expression of the insulin-like growth factor II prohormone in the bony fish Cottus scorpius. Gen. Comp. Endocrinol. 1999, 113, 32–37. [Google Scholar] [CrossRef]
- Perrot, V.; Moiseeva, E.B.; Cozes, Y.; Chan, S.J.; Funkenstein, B. Insulin-like growth factor receptors and their ligands in gonads of a hermaphroditic species, the gilthead seabream (Sparus aurata): Expression and cellular localization. Biol. Reprod. 2000, 63, 229–241. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, K.; Zhu, Y.; Yuan, Y.; Li, M. Medaka igf1 identifies somatic cells and meiotic germ cells of both sexes. Gene 2018, 642, 423–429. [Google Scholar] [CrossRef]
- Duan, C. The insulin-like growth factor system and its biological actions in fish. Am. Zool. 1997, 37, 491–503. [Google Scholar] [CrossRef]
- Delafontaine, P.; Song, Y.H.; Li, Y. Expression, Regulation, and Function of IGF-1, IGF-1R, and IGF-1 Binding Proteins in Blood Vessels. Arter. Thromb. Vasc. Biol. 2004, 24, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.L.; Shimizu, M.; Beckman, B.R.; Baker, D.M.; Dickhoff, W.W. Time course of the GH/IGF axis response to fasting and increased ration in chinook salmon (Oncorhynchus tshawytscha). Gen. Comp. Endocrinol. 2005, 140, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Nelson, S.N.; Van Der Kraak, G. The role of the insulin-like growth factor (IGF) system in zebrafish (Danio rerio) ovarian development. Gen. Comp. Endocrinol. 2010, 168, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Vélez, E.J.; Lutfi, E.; Azizi, S.; Perelló, M.; Salmerón, C.; Riera-Codina, M.; Ibarz, A.; Fernández-Borràs, J.; Blasco, J.; Capilla, E.; et al. Understanding fish muscle growth regulation to optimize aquaculture production. Aquaculture 2017, 467, 28–40. [Google Scholar] [CrossRef]
- Schmid, A.C.; Lutz, I.; Kloas, W.; Reinecke, M. Thyroid hormone stimulates hepatic IGF-I mRNA expression in a bony fish, the tilapia Oreochromis mossambicus, in vitro and in vivo. Gen. Comp. Endocrinol. 2003, 130, 129–134. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Cartas, D.; Miliou, H. Factors influencing GH and IGF-I gene expression on growth in teleost fish: How can aquaculture industry benefit? Rev. Aquac. 2020, 12, 1637–1662. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, S.; Dang, Y.; Wang, M.; Ren, Y. Identification of a New Insulin-like Growth Factor 3 (igf3) in Turbot (Scophthalmus maximus): Comparison and Expression Analysis of IGF System Genes during Gonadal Development. Fishes 2023, 8, 240. [Google Scholar] [CrossRef]
- Qin, Q.; Chen, X.; Zhu, X.; Li, X.; Zhao, Y.; Xu, Z.; Liu, W. Insulin-like growth factor I of Yellow catfish (Pelteobagrus fulvidraco): cDNA characterization, tissue distribution, and expressions in response to starvation and refeeding. Fish. Physiol. Biochem. 2020, 46, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Canosa, L.F.; Bertucci, J.I. The effect of environmental stressors on growth in fish and its endocrine control. Front. Endocrinol. 2023, 14, 1109461. [Google Scholar] [CrossRef] [PubMed]
- Fukada, H.; Murashita, K.; Furutani, T.; Masumoto, T. Yellowtail insulin-like growth factor 1: Molecular cloning and response to various nutritional conditions. Domest. Anim. Endocrinol. 2012, 42, 220–229. [Google Scholar] [CrossRef]
- Canosa, L.F.; Bertucci, J.I. Nutrient regulation of somatic growth in teleost fish. The interaction between somatic growth, feeding and metabolism. Mol. Cell Endocrinol. 2020, 518, 111029. [Google Scholar] [CrossRef]
- Caruso, M.A.; Sheridan, M.A. New insights into the signaling system and function of insulin in fish. Gen. Comp. Endocrinol. 2011, 173, 227–247. [Google Scholar] [CrossRef]
- Chen, H.; Huang, H.; Chen, X.; Deng, S.; Zhu, C.; Huang, H.; Li, G. Structural and functional characterization of neuromedin S in the teleost fish, zebrafish (Danio rerio). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 191, 76–83. [Google Scholar] [CrossRef]
- Assan, D.; Huang, Y.; Mustapha, U.F.; Addah, M.N.; Li, G.; Chen, H. Fish Feed Intake, Feeding Behavior, and the Physiological Response of Apelin to Fasting and Refeeding. Front. Endocrinol. 2021, 12, 798903. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Kaneko, N.; Fukuda, M.; Nakano, Y.; Kimura, S.; Hara, A.; Shimizu, M. Responses of insulin-like growth factor (IGF)-I and two IGF-binding protein-1 subtypes to fasting and re-feeding, and their relationships with individual growth rates in yearling masu salmon (Oncorhynchus masou). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 191–198. [Google Scholar] [CrossRef]
- Codina, M.; Montserrat, N.; De La Serrana, D.G.; Rallière, C.; Gabillard, J.C.; Navarro, I.; Rescan, P.Y.; Gutierrez, J. Role of insulin and IGFs in fish muscle development and quality. Arch. Tierzucht. 2006, 49, 33. [Google Scholar]
- Reinecke, M. Insulin-like growth factors and fish reproduction. Biol. Reprod. 2010, 82, 656–661. [Google Scholar] [CrossRef]
- Chandhini, S.; Trumboo, B.; Jose, S.; Varghese, T.; Rajesh, M.; Kumar, V.J.R. Insulin-like growth factor signalling and its significance as a biomarker in fish and shellfish research. Fish. Physiol. Biochem. 2021, 47, 1011–1031. [Google Scholar] [CrossRef]
- Wang, D.S.; Jiao, B.; Hu, C.; Huang, X.; Liu, Z.; Cheng, C.H.K. Discovery of a gonad-specific IGF subtype in teleost. Biochem. Biophys. Res. Commun. 2008, 367, 336–341. [Google Scholar] [CrossRef]
- Mahardini, A.; Yamauchi, C.; Takeuchi, Y.; Rizky, D.; Takekata, H.; Takemura, A. Changes in mRNA abundance of insulin-like growth factors in the brain and liver of a tropical damselfish, Chrysiptera cyanea, in relation to seasonal and food-manipulated reproduction. Gen. Comp. Endocrinol. 2018, 269, 112–121. [Google Scholar] [CrossRef]
- Higuchi, K.; Kazeto, Y.; Ozaki, Y.; Izumida, D.; Hotta, T.; Soyano, K.; Gen, K. Insulin-like growth factors 1 and 2 regulate gene expression and enzymatic activity of cyp17a1 in ovarian follicles of the yellowtail, Seriola quinqueradiata. Heliyon 2020, 6, e04181. [Google Scholar] [CrossRef]
- Pierce, A.L.; Breves, J.P.; Moriyama, S.; Hirano, T.; Grau, E.G. Differential regulation of Igf1 and Igf2 mRNA levels in tilapia hepatocytes: Effects of insulin and cortisol on GH sensitivity. J. Endocrinol. 2011, 211, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Baroiller, J.F.; D’Cotta, H.; Shved, N.; Berishvili, G.; Toguyeni, A.; Fostier, A.; Eppler, E.; Reinecke, M. Oestrogen and insulin-like growth factors during the reproduction and growth of the tilapia Oreochromis niloticus and their interactions. Gen. Comp. Endocrinol. 2014, 205, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Terova, G.; Rimoldi, S.; Chini, V.; Gornati, R.; Bernardini, G.; Saroglia, M. Cloning and expression analysis of insulin-like growth factor I and II in liver and muscle of sea bass (Dicentrarchus labrax, L.) during long-term fasting and refeeding. J. Fish. Biol. 2007, 70, 219–233. [Google Scholar] [CrossRef]
- Zou, S.; Kamei, H.; Modi, Z.; Duan, C. Zebrafish IGF genes: Gene duplication, conservation and divergence, and novel roles in midline and notochord development. PLoS ONE 2009, 4, e7026. [Google Scholar] [CrossRef] [PubMed]
- Tse, M.C.L.; Vong, Q.P.; Cheng, C.H.K.; Chan, K.M. PCR-cloning and gene expression studies in common carp (Cyprinus carpio) insulin-like growth factor-II. Biochim. Biophys. Acta Gene Struct. Expr. 2002, 1575, 63–74. [Google Scholar] [CrossRef]
- Patruno, M.; Maccatrozzo, L.; Funkenstein, B.; Radaelli, G. Cloning and expression of insulin-like growth factors I and II in the shi drum (Umbrina cirrosa). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 137–151. [Google Scholar] [CrossRef]
- Peterson, B.C.; Bosworth, B.G.; Bilodeau, A.L. Differential gene expression of IGF-I, IGF-II, and toll-like receptors 3 and 5 during embryogenesis in hybrid (channelxblue) and channel catfish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2005, 141, 42–47. [Google Scholar] [CrossRef]
- Wood, A.W.; Duan, C.; Bern, H.A. Insulin-like growth factor signaling in fish. Int. Rev. Cytol. 2005, 243, 215–285. [Google Scholar]
- Song, F.; Wang, L.; Zhu, W.; Fu, J.; Dong, J.; Dong, Z. A novel igf3 gene in common carp (Cyprinus carpio): Evidence for its role in regulating gonadal development. PLoS ONE 2016, 11, e0168874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Guo, Y.; Nong, C.; Ndandala, C.B.; Yang, H.; Huang, H.; Li, G.; Chen, H. Analysis on lncRNA and mRNA expression profiles of IGF3-induced ovarian maturation in spotted scat (Scatophagus argus). Aquac. Rep. 2022, 27, 101367. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, D.; Chu, L.; Liu, Y.; Sun, X.; Li, J.; Cheng, C.H.K. Igf3 is essential for ovary differentiation in zebrafish†. Biol. Reprod. 2021, 104, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Niu, C.; Cheng, C.H. Igf3 serves as a mediator of LH in zebrafish ovulation. Biol. Reprod. 2018, 99, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Swanson, P.; Dickhoff, W.W. Free and protein-bound insulin-like growth factor-I (IGF-I) and IGF- binding proteins in plasma of coho salmon, Oncorhynchus kisutch. Gen. Comp. Endocrinol. 1999, 115, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Picha, M.E.; Turano, M.J.; Tipsmark, C.K.; Borski, R.J. Regulation of endocrine and paracrine sources of Igfs and Gh receptor during compensatory growth in hybrid striped bass (Morone chrysops X Morone saxatilis). J. Endocrinol. 2008, 199, 81–94. [Google Scholar] [CrossRef]
- Nebo, C.; Portella, M.C.; Carani, F.R.; de Almeida, F.L.A.; Padovani, C.R.; Carvalho, R.F.; Dal-Pai-Silva, M. Short periods of fasting followed by refeeding change the expression of muscle growth-related genes in juvenile Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 164, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Hasanpoor, S.; Ghomi Marzdashti, M.R.; Vahabzadeh Roodsari, H.; Kazemi, R.; Moosavi Sabet, S.H. IGF-I gene expression in liver and white muscles confirming promotion effect of dietary NaCl on Growth indices of Giant sturgeon (Huso huso) juveniles. Iran. J. Fish. Sci. 2020, 19, 2634–2648. [Google Scholar]
- Peterson, B.C.; Waldbieser, G.C. Effects of fasting on IGF-I, IGF-II, and IGF-binding protein mRNA concentrations in channel catfish (Ictalurus punctatus). Domest. Anim. Endocrinol. 2009, 37, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.C.; Small, B.C. Effects of fasting on circulating IGF-binding proteins, glucose, and cortisol in channel catfish (Ictalurus punctatus). Domest. Anim. Endocrinol. 2004, 26, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Terova, G.; Rimoldi, S.; Gornati, R.; Bernardini, G.; Saroglia, M. Cloning and expression analysis of myostatin, fibroblast growth factor 6, insulin-like growth factor I and II in liver and muscle of sea bass (Dicentrarchus labrax, L.) during long-term fasting and refeeding. Ital. J. Anim. Sci. 2007, 6 (Suppl. S1), 826–828. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yang, X.; Cao, Z.; Chen, X.; Qin, Q.; Liu, C.; Sun, Y. Insulin-like growth factor binding protein 3 gene of golden pompano (TroIGFBP3) promotes antimicrobial immune defense. Fish Shellfish. Immunol. 2020, 103, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Bouraoui, L.; Capilla, E.; Gutiérrez, J.; Navarro, I. Insulin and insulin-like growth factor I signaling pathways in rainbow trout (Oncorhynchus mykiss) during adipogenesis and their implication in glucose uptake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R33–R41. [Google Scholar] [CrossRef] [PubMed]
- Breves, J.P.; Phipps-Costin, S.K.; Fujimoto, C.K.; Einarsdottir, I.E.; Regish, A.M.; Björnsson, B.T.; McCormick, S.D. Hepatic insulin-like growth-factor binding protein (igfbp) responses to food restriction in Atlantic salmon smolts. Gen. Comp. Endocrinol. 2016, 233, 79–87. [Google Scholar] [CrossRef]
- Bertucci, J.I.; Blanco, A.M.; Sundarrajan, L.; Rajeswari, J.J.; Velasco, C.; Unniappan, S. Nutrient regulation of endocrine factors influencing feeding and growth in fish. Front. Endocrinol. 2019, 10, 83. [Google Scholar] [CrossRef]
- Peterson, B.C.; Waldbieser, G.C.; Bilodeau, L. IGF-I and IGF-II mRNA expression in slow and fast growing families of USDA103 channel catfish (Ictalurus punctatus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2004, 139, 317–323. [Google Scholar] [CrossRef]
- Eppler, E.; Caelers, A.; Shved, N.; Hwang, G.; Rahman, A.M.; MacLean, N.; Zapf, J.; Reinecke, M. Insulin-like growth factor I (IGF-I) in a growth-enhanced transgenic (GH-overexpressing) bony fish, the tilapia (Oreochromis niloticus): Indication for a higher impact of autocrine/paracrine than of endocrine IGF-I. Transgenic Res. 2007, 16, 479–489. [Google Scholar] [CrossRef]
- Krogdahl, Å.; Bakke-McKellep, A.M. Fasting and refeeding cause rapid changes in intestinal tissue mass and digestive enzyme capacities of Atlantic salmon (Salmo salar L.). Comp Biochem Physiol. A Mol Integr Physiol. 2005, 141, 450–460. [Google Scholar] [CrossRef]
- Carnevali, O.; Cardinali, M.; Maradonna, F.; Parisi, M.; Olivotto, I.; Polzonetti-Magni, A.M.; Mosconi, G.; Funkenstein, B. Hormonal regulation of hepatic IGF-I and IGF-II gene expression in the marine teleost Sparus aurata. Mol. Reprod. Dev. 2005, 71, 12–18. [Google Scholar] [CrossRef]
- Norbeck, L.A.; Sheridan, M.A. An in vitro model for evaluating peripheral regulation of growth in fish: Effects of 17β-estradiol and testosterone on the expression of growth hormone receptors, insulin-like growth factors, and insulin-like growth factor type 1 receptors in rainbow trou. Gen. Comp. Endocrinol. 2011, 173, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Dai, S.; Xiao, H.; Qi, S.; Li, Y.; Zheng, Q.; Jie, M.; Cheng, C.H.K.; Wang, D. Regulation of spermatogenesis and reproductive capacity by Igf3 in tilapia. Cell Mol. Life Sci. 2020, 77, 4921–4938. [Google Scholar] [CrossRef] [PubMed]
- Komen, J.; Lodder, P.A.J.; Huskens, F.; Richter, C.J.J.; Huisman, E.A. Effects of oral administration of 17α-methyltestosterone and 17β-estradiol on gonadal development in common carp, Cyprinus carpio L. Aquaculture 1989, 78, 349–363. [Google Scholar] [CrossRef]
- Davis, L.K.; Hiramatsu, N.; Hiramatsu, K.; Reading, B.J.; Matsubara, T.; Hara, A.; Sullivan, C.V.; Pierce, A.L.; Hirano, T.; Grau, E.G. Induction of three vitellogenins by 17beta-estradiol with concurrent inhibition of the growth hormone-insulin-like growth factor 1 axis in a euryhaline teleost, the tilapia (Oreochromis mossambicus). Biol. Reprod. 2007, 77, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Karsli, Z. Effects of synthetic androgen (17α-methyltestosterone) and estrogen (17β-estradiol) on growth and skin coloration in emperor red cichlid, aulonocara nyassae (actinopterygii: Cichliformes: Cichlidae). Acta Ichthyol. Piscat. 2021, 51, 357–363. [Google Scholar] [CrossRef]
- Lee, S.L.J.; Horsfield, J.A.; Black, M.A.; Rutherford, K.; Fisher, A.; Gemmell, N.J. Histological and transcriptomic effects of 17α-methyltestosterone on zebrafish gonad development. BMC Genom. 2017, 18, 557. [Google Scholar] [CrossRef] [PubMed]





| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1: Trachinotus ovatus | 100 | |||||||||
| 2: Scatophagus argus | 96.76 | 100 | ||||||||
| 3: Epinephelus coioides | 98.92 | 97.85 | 100 | |||||||
| 4: Fundulus heteroclitus | 80.87 | 80.98 | 80.98 | 100 | ||||||
| 5: Larimichthys crocea | 98.92 | 97.85 | 100 | 80.98 | 100 | |||||
| 6: Nothobranchius furzeri | 88.59 | 87.57 | 88.65 | 82.89 | 88.65 | 100 | ||||
| 7: Oncorhynchus mykiss | 89.02 | 87.36 | 89.08 | 73.99 | 89.08 | 82.66 | 100 | |||
| 8: Oreaochromis niloticus | 88.95 | 89.56 | 90.11 | 79.44 | 90.11 | 87.29 | 85.38 | 100 | ||
| 9: Oryzias latipes | 86.89 | 84.78 | 86.96 | 76.50 | 86.96 | 84.25 | 80.81 | 82.22 | 100 | |
| 10: Danio rerio | 80.38 | 77.99 | 79.87 | 68.79 | 79.87 | 77.22 | 86.67 | 78.48 | 78.34 | 100 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1: Trachinotus ovatus | 100 | |||||||||||
| 2: Scatophagus argus | 96.28 | 100 | ||||||||||
| 3: Epinephelus coioides | 94.88 | 95.81 | 100 | |||||||||
| 4: Larimichthys crocea | 95.35 | 97.21 | 95.35 | 100 | ||||||||
| 5: Nothobranchius furzeri | 84.04 | 85.45 | 84.98 | 84.51 | 100 | |||||||
| 6: Oncorhynchus mykiss | 84.11 | 84.58 | 83.64 | 85.05 | 78.30 | 100 | ||||||
| 7: Oreaochromis niloticus | 89.77 | 90.70 | 89.30 | 90.23 | 82.16 | 80.84 | 100 | |||||
| 8: Oryzias latipes | 75 | 74.53 | 75 | 74.06 | 68.10 | 68.72 | 69.81 | 100 | ||||
| 9: Cyprinus carpio (a) | 52.71 | 53.20 | 53.69 | 53.69 | 52.22 | 54.68 | 53.69 | 46 | 100 | |||
| 10: Cyprinus carpio (b) | 77.36 | 78.77 | 78.77 | 79.72 | 75.47 | 75.94 | 74.06 | 63.16 | 54.68 | 100 | ||
| 11: Danio rerio (a) | 55.84 | 56.35 | 56.85 | 57.36 | 55.84 | 58.88 | 56.35 | 47.42 | 83.25 | 55.84 | 100 | |
| 12: Danio rerio (b) | 77.36 | 78.77 | 78.77 | 79.72 | 75.47 | 76.89 | 74.06 | 63.16 | 54.19 | 95.28 | 55.33 | 100 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1: Trachinotus ovatus | 100 | ||||||||
| 2: Scatophagus argus | 77.60 | 100 | |||||||
| 3: Epinephelus coioides | 72.02 | 74.62 | 100 | ||||||
| 4: Fundulus heteroclitus | 59.78 | 62.77 | 59.47 | 100 | |||||
| 5: Morone saxatilis | 77.20 | 80.20 | 73.87 | 64.21 | 100 | ||||
| 6: Nothobranchius furzeri | 64.77 | 67.78 | 63.74 | 69.78 | 66.48 | 100 | |||
| 7: Oreaochromis niloticus | 59.09 | 60.00 | 59.89 | 54.24 | 61.54 | 57.31 | 100 | ||
| 8: Oryzias latipes | 56.88 | 52.96 | 51.52 | 51.22 | 52.73 | 53.75 | 47.83 | 100 | |
| 9: Danio rerio | 38.37 | 38.07 | 39.89 | 36.52 | 37.64 | 37.64 | 33.73 | 38.61 | 100 |
| Primer Name | Sequence (5′-3′) | Application | Reference |
|---|---|---|---|
| IGF1 F | GACCCGTGGGGATGTCTAGC | Cloning of ORF | Designed by author |
| IGF1 R | ACAGAATGTAGGGAAGGAGC | Cloning of ORF | Designed by author |
| IGF2 F | GACTACTGCCATCTGACATG | Cloning of ORF | Designed by author |
| IGF2 R | GCACAGACAAGAGTTTGGAG | Cloning of ORF | Designed by author |
| IGF3 F | GATGCACTCCTCCTGCCGCTC | Cloning of ORF | Designed by author |
| IGF3 R | CGTGACCTCTGACCTCATGGCC | Cloning of ORF | Designed by author |
| IGF1 F | TACTGCTGTGTGTCCTCAC | qPCR | Designed by author |
| IGF1 R | CGCCTGGAGATGTACTGTGCA | qPCR | Designed by author |
| IGF2 F | TCGGCGGAGACGCTGTGTG | qPCR | Designed by author |
| IGF2 R | TTCCCGTGATGCCCGCACTA | qPCR | Designed by author |
| IGF3 F | GGGGATCGAGGAATCTATT | qPCR | Designed by author |
| IGF3 R | AGTTCCAAACAGTATTTCACAA | qPCR | Designed by author |
| β-actin F | GAGAGGTTCCGTTGCCCAGAG | qPCR | [2] |
| β-actin R | CAGACAGCACAGTGTTGGCGT | qPCR | [2] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndandala, C.B.; Zhou, Q.; Li, Z.; Guo, Y.; Li, G.; Chen, H. Identification of Insulin-like Growth Factor (IGF) Family Genes in the Golden Pompano, Trachinotus ovatus: Molecular Cloning, Characterization and Gene Expression. Int. J. Mol. Sci. 2024, 25, 2499. https://doi.org/10.3390/ijms25052499
Ndandala CB, Zhou Q, Li Z, Guo Y, Li G, Chen H. Identification of Insulin-like Growth Factor (IGF) Family Genes in the Golden Pompano, Trachinotus ovatus: Molecular Cloning, Characterization and Gene Expression. International Journal of Molecular Sciences. 2024; 25(5):2499. https://doi.org/10.3390/ijms25052499
Chicago/Turabian StyleNdandala, Charles Brighton, Qi Zhou, Zhiyuan Li, Yuwen Guo, Guangli Li, and Huapu Chen. 2024. "Identification of Insulin-like Growth Factor (IGF) Family Genes in the Golden Pompano, Trachinotus ovatus: Molecular Cloning, Characterization and Gene Expression" International Journal of Molecular Sciences 25, no. 5: 2499. https://doi.org/10.3390/ijms25052499





