Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Identification of Compounds by Chromatographic Analysis
2.2. Total β-Carotene and Lycopene Contents
2.3. Total Polyphenols and Total Flavonoids Contents
2.4. Antioxidant Activity
2.5. Antimicrobial Activity
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Extraction Methods
3.4. UV-Vis Analysis
3.5. UHPLC-ESI-MS Analysis
3.6. Total Polyphenols Content
3.7. Total Flavonoids Content
3.8. Antioxidant Activity by ABTS Method
3.9. Antioxidant Activity by DPPH Method
3.10. Antioxidant Activity by FRAP Method
3.11. Antioxidant Activity by CUPRAC Method
3.12. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demir, N.; Yildiz, O.; Alpaslan, M.; Hayaloglu, A.A. Evaluation of volatiles, phenolic compounds and antioxidant activities of rose hip (Rosa L.) fruits in Turkey. LWT Food Sci. 2014, 57, 126–133. [Google Scholar] [CrossRef]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Chrubasik, C.; Basil, D.; Roufogalis, B.D.; Müller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Turkben, C.; Barut, E.; Copur, O.U.; Durgut, E.; Himelrick, D.G.; Türkben, C.; Barat, E.; Çopur, Ö.U.; Durgut, E.; Himelrick, D.G. Evaluation of rose hips (Rosa spp.) selections. Int. J. Fruit Sci. 2005, 5, 113–121. [Google Scholar] [CrossRef]
- Iancu, P.; Soare, R.; Dinu, M.; Soare, M.; Dorina Bonea, D.; Popescu, M. Analysis of the existing research regarding the use of the species Rosa canina L. Sci. Pap. Ser. B Hortic. 2020, 64, 325–331. [Google Scholar]
- Orhan, D.; Hartevioglu, A.; Kupeli, E.; Yesilada, E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007, 112, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Kubczak, M.; Khassenova, A.B.; Skalski, B.; Michlewska, S.; Wielanek, M.; Aralbayeva, A.N.; Murzakhmetova, M.K.; Zmaraeva, M.; Skłodowska, M.; Bryszewska, M.; et al. Bioactive compounds and antiradical activity of the Rosa canina L. leaf and twig extracts. Agronomy 2020, 10, 1897. [Google Scholar] [CrossRef]
- Andersen, O.M.; Markham, K.R. Flavonoids: Chemistry, Biochemistry and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hambly, A.J.; van Duijneveldt, J.S.; Gates, P.J. Identification of β-carotene oxidation products produced by bleaching clay using UPLC-ESI-MS/MS. Food Chem. 2021, 353, 129455. [Google Scholar] [CrossRef] [PubMed]
- Hvattum, E. Determination of phenolic compounds in rose hip (Rosa canina) using liquid chromatography coupled to electrospray ionization tandem mass spectrometry and diode-array detection. Rapid Commun. Mass Spectrom. 2002, 16, 655–662. [Google Scholar] [CrossRef]
- Yahia, E.M.; García-Solís, P.; Celis, M.E.M. Contribution of Fruits and Vegetables to Human Nutrition and Health. In Postharvest Physiology and Biochemistry of Fruits and Vegetables, 1st ed.; Yahia, E.M., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 19–45. [Google Scholar]
- Živković, J.; Stojković, D.; Petrović, J.; Zdunić, G.; Glamočlija, J.; Soković, M. Rosa canina L.—new possibilities for an old medicinal herb. Food Funct. 2015, 6, 3687–3692. [Google Scholar] [CrossRef]
- Soltan, O.I.A.; Gazwi, H.S.S.; Ragab, A.E.; Aljohani, A.S.M.; El-Ashmawy, I.M.; Batiha, G.E.-S.; Hafiz, A.A.; Abdel-Hameed, S.M. Assessment of bioactive phytochemicals and utilization of Rosa canina fruit extract as a novel natural antioxidant for mayonnaise. Molecules 2023, 28, 3350. [Google Scholar] [CrossRef] [PubMed]
- Andreea Stanila, A.; Diaconeasa, Z.; Roman, I.; Sima, N.; Maniutiu, D.; Roman, A.; Sima, R. Extraction and characterization of phenolic compounds from rose hip (Rosa canina L.) using liquid chromatography coupled with electrospray ionization—Mass spectrometry. Not. Bot. Horti Agrobot. 2015, 43, 349–354. [Google Scholar] [CrossRef]
- Fetni, S.; Bertella, N.; Ouahab, A.; Miguel Martinez Zapater, J.; De Pascual-Teresa Fernandez, S. Composition and biological activity of the Algerian plant Rosa canina L. by HPLC-UV-MS. Arab. J. Chem. 2020, 13, 1105–1119. [Google Scholar] [CrossRef]
- Girennavar, B.; Jayaprakasha, G.K.; Jifon, J.L.; Patil, B.S. Variation of bioactive furocoumarins and flavonoids in different varieties of grapefruits and pummel. Eur. Food Res. Technol. 2008, 226, 1269–1275. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 841–1856. [Google Scholar] [CrossRef] [PubMed]
- Vallverdu-Queralt, A.; Jauregui, O.; Medina-Remon, A.; Andres-Lacueva, C.; Lamuela-Raventos, R.M. Improved characterization of tomato polyphenols using liquid chromatography/electrospray ionization linear ion trap quadrupole orbitrap mass spectrometry and liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2986–2992. [Google Scholar] [CrossRef]
- De Souza, R.F.V.; De Giovani, W.F. Synthesis, spectral and electrochemical properties of Al(III) and Zn(II) complexes with flavonoids. Spectrochim. Acta A 2005, 61, 1985–1990. [Google Scholar] [CrossRef]
- Re, R.; Bramley, P.M.; Rice-Evans, C. Effects of food processing on flavonoids and lycopene status in a Mediterranean tomato variety. Free Radic. Res. 2002, 36, 803–810. [Google Scholar] [CrossRef]
- Patil, B.S.; Jayaprakasha, G.K.; Chidambara Murthy, K.N.; Vikram, A. Bioactive compounds: Historical perspectives, opportunities, and challenges. J. Agric. Food Chem. 2009, 57, 8142–8160. [Google Scholar] [CrossRef]
- Ng, L.-K.; Lafontaine, P.; Vanier, M. Characterization of migarette tobacco by direct electrospray ionization-ion trap mass spectrometry (ESI-ITMS) analysis of the aqueous extracts—A novel and simple approach. J. Agric. Food Chem. 2004, 52, 7251–7257. [Google Scholar] [CrossRef] [PubMed]
- Di Lecce, G.; Boselli, E.; D’Ignazi, G.; Frega, N.G. Evolution of phenolics and glutathione in Verdicchio wine obtained with maceration under reductive conditions. LWT Food Sci. Technol. 2013, 53, 54–60. [Google Scholar] [CrossRef]
- Mihaylova, D.; Georgieva, L.; Pavlov, A. Antioxidant activity and bioactive compounds of Rosa canina L. herbal preparations. Sci. Bull. F Biotechnol. 2015, XIX, 160–165. [Google Scholar]
- Aresenescu, A. Pharmacognostical Research on the Species Rosa canina L.; UMF Cluj-Napoca: Cluj-Napoca, Romania, 2008. (In Romanian) [Google Scholar]
- Steenson, D.F.; Min, D.B. Effects of β-carotene and lycopene thermal degradation products on the oxidative stability of soybean oil. J. Am. Oil Chem. Soc. 2000, 77, 1153–1160. [Google Scholar] [CrossRef]
- Andersson, S.C.; Rumpunen, K.; Johansson, E.; Olsson, M.E. Carotenoid content and composition in rose hips (Rosa spp.) during ripening, determination of suitable maturity marker and implications for health promoting food products. Food Chem. 2011, 128, 689–696. [Google Scholar] [CrossRef]
- Novotny, J.A.; Harrison, D.J.; Pawlosky, R.; Flanagan, V.P.; Harrison, E.H.; Kurilich, A.C. β-carotene conversion to vitamin A decreases as the dietary dose increases in humans. J. Nutr. 2010, 140, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, O.; Alpaslan, M. Properties of Rose hip marmelades. Food Technol. Biotechnol. 2012, 50, 98–106. [Google Scholar]
- Turkben, C.; Uylaser, V.; Incedayi, B. Influence of traditional processing on some compounds of rose hip (Rosa canina L.) fruits collected from habitat in Bursa, Turkey. Asian J. Chem. 2010, 22, 2309–2318. [Google Scholar]
- Razungles, A.; Oszmianski, J.; Sapis, J.-C. Determination of carotenoids in fruits of Rosa sp. (Rosa Canina and Rosa Rugosa) and of chokeberry (Aronia Melanocarpa). J. Food Sci. 1989, 54, 774–775. [Google Scholar] [CrossRef]
- Martinez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Kotikova, Z.; Lachman, J.; Hejtmankova, A.; Hejtmankova, K. Determination of antioxidant activity and antioxidant content in tomato varieties and evaluation of mutual interactions between antioxidants. LWT Food Sci. Technol. 2011, 44, 1703–1710. [Google Scholar] [CrossRef]
- Available online: https://www.mayoclinic.org/drugs-supplements/beta-carotene-oral-route/proper-use/drg-20066795#:~:text=Adults%20and%20teenagers%3A%206%20to,vitamin%20A%20activity)%20per%20day (accessed on 22 December 2023).
- Kapała, A.; Szlendak, M.; Motacka, E. The anti-cancer activity of lycopene: A systematic review of human and animal studies. Nutrients 2022, 14, 5152. [Google Scholar] [CrossRef]
- Van Hung, P. Phenolic compounds of cereals and their antioxidant capacity. Crit. Rev. Food Sci. Nutr. 2014, 56, 25–35. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Keen, C.; Holt, R.; Oteiza, P.; Fraga, C.; Schmitz, H. Cocoa antioxidants and cardiovascular health. Am. J. Clin. Nutr. 2005, 81, 298S–303S. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Crowe-White, K.M.; Evans, L.W.; Kuhnle, G.G.C.; Milenkovic, D.; Stote, K.; Wallace, T.; Handu, D.; Senkus, K.E. Flavan-3-ols and cardiometabolic health: First ever dietary bioactive guideline. Adv. Nutr. 2022, 13, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Nadpal, J.D.; Lesjak, M.M.; Mrkonjić, Z.O.; Majkić, T.M.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Beara, I.N. Phytochemical composition and in vitro functional properties of three wild rose hips and their traditional preserves. Food Chem. 2018, 241, 290–300. [Google Scholar] [CrossRef]
- Ozkan, G.; Stübler, A.-S.; Aganovic, K.; Draeger, G.; Esatbeyoglu, T.; Capanoglu, E. A comparative study on physicochemical properties and in vitro bioaccessibility of bioactive compounds in rosehip (Rosa canina L.) infusions treated by non-thermal and thermal treatments. J. Food Process. Preserv. 2022, 46, e16096. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, I.; Hafsa, I.; Hamdi, S.; Charbonnel, C.; Ghoul, M. Review of the effects of food processing and formulation on flavonol and anthocyanin behaviour. J. Food Eng. 2012, 111, 208–217. [Google Scholar] [CrossRef]
- Wołosiak, R.; Drużyńska, B.; Derewiaka, D.; Piecyk, M.; Majewska, E.; Ciecierska, M.; Worobiej, E.; Pakosz, P. Verification of the conditions for determination of antioxidant activity by ABTS and DPPH Assays—A practical approach. Molecules 2022, 27, 50. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Özyürek, M.; Çelik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Özyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Exotic fruits as a source of important phytochemicals: Improving the traditional use of Rosa canina fruits in Portugal. Food Res. Int. 2011, 44, 2233–2236. [Google Scholar] [CrossRef]
- Dimitrijevic, D.; Kostic, D.; Stojanovic, G.; Mitic, S.S.; Mitic, M.; Djordjevic, A. Phenolic composition, antioxidant activity, mineral content and antimicrobial activity of fresh fruit extracts of Morus alba L. J. Food Nutr. Res. 2014, 53, 22–30. [Google Scholar]
- Korotkova, E.I.; Karbainov, Y.A.; Shevchuk, A.V. Study of antioxidant properties by voltammetry. J. Electroanal. Chem. 2002, 518, 56–60. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Gupta, V.; Kohli, K.; Ghaiye, P.; Bansal, P.; Lather, A. Pharmacological potentials of Citrus paradisi—An overview. Int. J. Phytother. Res. 2011, 1, 8–17. [Google Scholar]
- Hung, T.M.; Na, M.K.; Thuong, P.T.; Su, N.D.; Sok, D.E.; Song, K.S.; Seong, Y.H.; Bae, K.H. Antioxidant activity of caffeoyl quinic acid derivatives from the roots of Dipsacus asper wall. J. Ethnopharmacol. 2006, 108, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Anese, M.; Manzocco, L.; Nicoli, M.C.; Lerici, C.R. Antioxidant properties of tomato juice as affected by heating. J. Sci. Food Agric. 1999, 79, 750–754. [Google Scholar] [CrossRef]
- Fu, X.; Shen, Y.; Shen, X.; Jiang, X.; Huang, D.; Yan, Y. Chitosan derivatives with dual-antibacterial functional groups for antimicrobial finishing of cotton fabrics. Carbohydr. Polym. 2011, 85, 221–227. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. Rev. 2022, 19, 1–30. [Google Scholar] [CrossRef]
- Pero, R.W.; Lund, H.; Leanderson, T. Antioxidant metabolism induced by quinic acid. Increased urinary excretion of tryptophan and nicotinamide. Phytother. Res. 2009, 23, 335–346. [Google Scholar] [CrossRef]
- Montazeri, N.; Baher, E.; Mirzajani, F.; Barami, Z.; Yousefian, S. Phytochemical contents and biological activities of Rosa canina fruit from Iran. J. Med. Plant Res. 2011, 5, 4584–4589. [Google Scholar]
- Ozturk Yilmaz, S.; Ercisli, S. Antibacterial and antioxidant activity of fruits of some rose species from Turkey. Rom. Biotechnol. Lett. 2011, 16, 6407–6411. [Google Scholar]
- Oyedemi, S.O.; Oyedemi, B.O.; Prieto, J.M.; Coopoosamy, R.M.; Stapleton, P.; Gibbons, S. In vitro assessment of antibiotic-resistance reversal of a methanol extract from Rosa canina L. S. Afr. J. Bot. 2016, 105, 337–342. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruits and vegetables are from additive and synergistic combination of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wu, Y.; Zhong, K.; Xiao, K.; Liu, L.; Huang, Y.; Wang, Z.; Gao, H. A comparative study on the effects of quinic acid and shikimic acid on cellular functions of Staphylococcus aureus. J. Food Prot. 2018, 81, 1187–1192. [Google Scholar] [CrossRef]
- Burel, C.; Kala, A.; Purevdorj-Gage, L. Impact of pH on citric acid antimicrobial activity against Gram-negative bacteria. Lett. Appl. Microbiol. 2021, 72, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Eliuz, E. Antimicrobial activity of citric acid against Escherichia coli, Staphylococcus aureus and Candida albicans as a sanitizer agent. Eurasian J. Forest Sci. 2020, 8, 295–301. [Google Scholar] [CrossRef]
- Kepa, M.; Miklasinska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smolen-Dzirba, J.; Wasik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Pinho, E.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. BioMed. Res. Int. 2014, 2014, 814590. [Google Scholar] [CrossRef]
- Wang, L.; Bi, C.; Cai, H.; Liu, B.; Zhong, X.; Deng, X.; Wang, T.; Xiang, H.; Niu, X.; Wang, D. The therapeutic effect of chlorogenic acid against Staphylococcus aureus infection through sortase A inhibition. Front. Microbiol. 2015, 6, 1031. [Google Scholar] [CrossRef]
- Orhan, D.D.; Ozçelik, B.; Ozgen, S.; Ergun, F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. [Google Scholar] [CrossRef]
- Hraishawi, R.M.O.; Abdul-Razak, A.S.; Al-Hayder, M.N.; Al-wafi, H. Investigation the antimicrobial and antioxidant activity of lycopene extraction from Solanum Lycopersicum. Eur. Asian J. BioSci. 2020, 14, 5305–5310. [Google Scholar]
- Tomar, A.; Broor, S.; Kaushik, S.; Bharara, T.; Arya, D.S. Antibacterial potential of citrus flavonoid, naringenin with β-lactam antibiotics against methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Curr. Microbiol. App. Sci. 2021, 10, 1799–1809. [Google Scholar]
- K Perkins-Veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene content differs among red fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 81, 983–987. [Google Scholar] [CrossRef]
- Abdul-Hammed, M.; Bello, I.A.; Oladoye, S.O. Simultaneous spectrophotometric determination of lycopene and beta-carotene concentrations in carotenoid mixtures of the extracts from tomatoes, papaya and orange juice. Pak. J. Sci. Ind. Res. B Biol. Sci. 2013, 56, 90–97. [Google Scholar] [CrossRef]
- Swain, T.; Hillis, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Arts, M.; Haenen, G.; Voss, H.P.; Bast, A. Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food Chem. Toxicol. 2004, 42, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Karori, S.M.; Wachira, F.N.; Wanyoko, J.K.; Ngure, R.M. Antioxidant capacity of different types of tea products. Afr. J. Biotechnol. 2007, 6, 2287–2296. [Google Scholar] [CrossRef]
- Vijayalakshmi, M.; Ruckmani, K. Ferric reducing anti-oxidant power assay in plant extract. Bangladesh J. Pharmacol. 2016, 11, 570–572. [Google Scholar] [CrossRef]
- Apak, R.; Guclu, K.; Ozyurek, M.; Karademir, S.E.; Erca, E. The cupric ion reducing antioxidant capacity (CUPRAC) and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 32nd ed.; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2022; ISBN 978-1-68440-135-2. [Google Scholar]

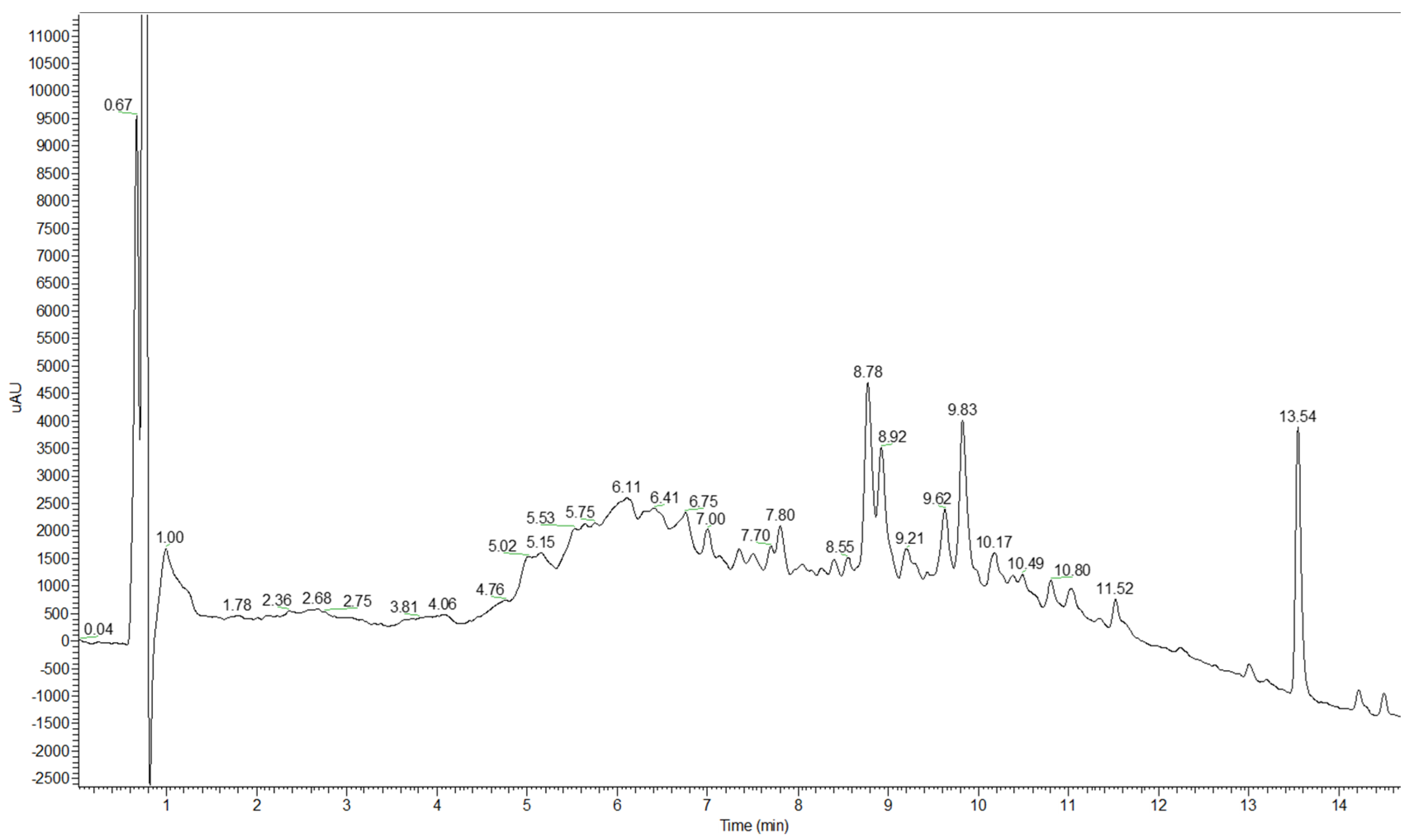

| Peak | tR [min] | λmax [nm] | Molecular Ion [M − H]− m/z | MS/MS Fragment Ions m/z | Assignment | Presence in Sample |
|---|---|---|---|---|---|---|
| 1. | 0.75 | − | 191 | 173, 127, 111, 85 (100%) | Quinic acid [16] | + |
| 2. | 0.92 | − | 191 | 173, 111 (100%) | Citric acid (standard) | + |
| 3. | 1.50 | − | 179 | 161, 143, 131, 119, 113, 101, 89 (100%), 71 | Caffeic acid [17] | + |
| 4. | 5.30 | 325 303sh | 353 | 191 (100%), 179, 173 | Chlorogenic acid (standard) | + |
| 5. | 5.40 | 318 298sh | 343 | 181 (100%), 137 | Homovanilic acid hexoside [18] | + |
| 6. | 5.50 | − | 477 | 431 (100%) | not identified | + |
| 7. | 5.76 | 325 300sh | 353 | 191, 179, 173 (100%), 135 | 4-O-caffeoyl-quinic acid | + |
| 8. | 6.23 | − | 457 | 411 (100%) | not identified | + |
| 9. | 6.63 | − | 457 | 411 (100%), 341 | not identified | + |
| 10. | 8.18 | 354 262 | 471 | 425 (100%), 263 | not identified flavonoid | + |
| 11. | 8.99 | 351 289 | 597 | 487, 387, 357 (100%) | Phloretin-C-diglycoside [19] | + |
| 12. | 8.99 | 355 258 | 609 | 301 (100%), 299, 271 | Rutin (quercetin-3-O-rutinoside) (standard) | + |
| 13. | 9.26 | 321 293 | 579 | 533, 459 (100%), 357, 313, 271, 235 | not identified | + |
| 14. | 9.48 | 324 295 | 503 | 324 295 | not identified | + |
| 15. | 9.70 | − | 609 | 489, 301 (100%) | Quercetin-hexoside-rhamnoside (tent.) | + |
| 16. | 10.90 | − | 537 # | 457 (100%) | Lycopene or β-carotene [20] | + |
| 17. | 11.40 | 291 | 271 | 227, 177, 151 (100%), 107, 93 | Naringenin [16,21] | + |
| 18. | 13.47 | 343 | 386 # | 201 (100%) | not identified | + |
| 19. | 14.25 | 358 | 274 # | 256 (100%), 230, 106, 102, 88 | not identified | + |
| Total β-Carotene and Lycopene Contents [mg/100 g FW] | TPC and TFC [mg·kg−1] | ABTS | DPPH | FRAP | CUPRAC | ||
|---|---|---|---|---|---|---|---|
| Csr ± SD [μmol/100 g FW] | |||||||
| 7.25 ± 0.09 (RSD = 7.04%) | 2.34 ± 0.06 (RSD = 2.35%) | 2980.94 ± 7 (RSD = 2.3%) | 1454.73 ± 20 (RSD = 1.35%) | 12.30 ± 0.78 | 6.84 ± 0.03 | 52.04 ± 1.02 | 15,425.38 ± 35.86 |
| Total Polyphenols | Total Flavonoids | ABTS | DPPH | FRAP | CUPRAC | |
|---|---|---|---|---|---|---|
| Total polyphenols | 1.0000 | |||||
| Total flavonoids | 0.9935 | 1.0000 | ||||
| ABTS | 0.9935 | 0.9740 | 1.0000 | |||
| DPPH | 0.9899 | 0.9996 | 0.9672 | 1.0000 | ||
| FRAP | 0.9861 | 0.9986 | 0.9607 | 0.9997 | 1.0000 | |
| CUPRAC | 0.9935 | 0.9982 | 0.9740 | 0.9996 | 0.9986 | 1.0000 |
| Microbial Strain | Minimum Inhibitory (MIC)/ Minimum Microbicidal Concentration (MMC) [mg∙mL−1] |
|---|---|
| Gram (+) bacteria | |
| Staphylococcus aureus | 4.0/0 |
| Enterococcus faecalis | 4.0/0 |
| Bacillus cereus | 4.0/0 |
| Gram (−) bacteria | |
| Escherichia coli | 4.0/0 |
| Salmonella enteritidis | 4.0/4.0 |
| Enteroabacter aerogenes | 4.0/0 |
| Pseudomonas aeruginosa | 2.0/0 |
| Yeast | |
| Candida albicans | 2.0/0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljković, V.M.; Nikolić, L.; Mrmošanin, J.; Gajić, I.; Mihajilov-Krstev, T.; Zvezdanović, J.; Miljković, M. Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia. Int. J. Mol. Sci. 2024, 25, 2518. https://doi.org/10.3390/ijms25052518
Miljković VM, Nikolić L, Mrmošanin J, Gajić I, Mihajilov-Krstev T, Zvezdanović J, Miljković M. Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia. International Journal of Molecular Sciences. 2024; 25(5):2518. https://doi.org/10.3390/ijms25052518
Chicago/Turabian StyleMiljković, Vojkan M., Ljubiša Nikolić, Jelena Mrmošanin, Ivana Gajić, Tatjana Mihajilov-Krstev, Jelena Zvezdanović, and Milena Miljković. 2024. "Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia" International Journal of Molecular Sciences 25, no. 5: 2518. https://doi.org/10.3390/ijms25052518






