How Does Radiation Affect Curcumin Raw Material?
Abstract
:1. Introduction
2. Results and Discussion
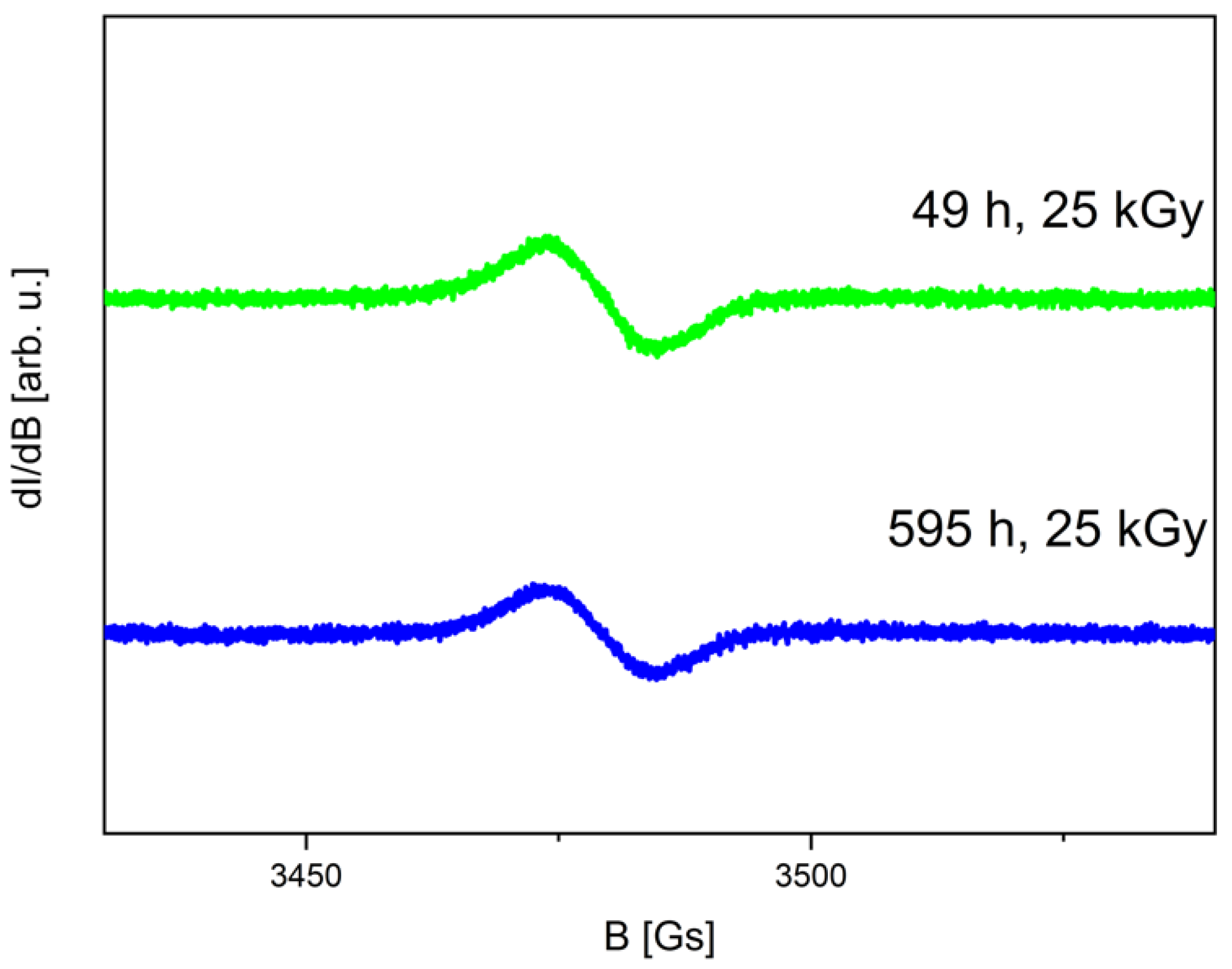
2.1. Electron Paramagnetic Resonance
2.2. Fourier Transform Infrared Spectroscopy
2.3. HPLC-DAD and HPLC-MS Analysis
2.4. Antioxidant Properties
3. Materials and Methods
3.1. Standards and Reagents
3.2. Irradiation
3.3. Electron Paramagnetic Resonance (EPR) Spectroscopy
3.4. Fourier Transform Infrared (FT-IR) Spectroscopy
3.5. High-Performance Liquid Chromatography (HPLC-DAD) Analysis
3.6. HPLC-MS Analysis
3.7. Antioxidant Activity Study
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chatzinasiou, L.; Booker, A.; MacLennan, E.; Mackonochie, M.; Heinrich, M. Turmeric (Curcuma longa L.) products: What quality differences exist? J. Herb. Med. 2019, 17–18, 100281. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Abo Ghanima, M.M.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.R.; Parks, R.J. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Z.Y.; Zheng, X.; Li, D.L.; Zhou, R.P.; Zhang, K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 742–752. [Google Scholar]
- Tang, M.; Taghibiglou, C. The Mechanisms of Action of Curcumin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 58, 1003–1016. [Google Scholar] [CrossRef]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef]
- Available online: https://www.grandviewresearch.com/industry-analysis/turmeric-extract-curcumin-market (accessed on 23 January 2024).
- Jeswal, P.; Kumar, D. Mycobiota and Natural Incidence of Aflatoxins, Ochratoxin A, and Citrinin in Indian Spices Confirmed by LC-MS/MS. Int. J. Microbiol. 2015, 2015, 242486. [Google Scholar] [CrossRef] [PubMed]
- Khazaeli, P.; Mehrabani, M.; Heidari, M.R.; Asadikaram, G.; Lari Najafi, M. Prevalence of Aflatoxin Contamination in Herbs and Spices in Different Regions of Iran. Iran. J. Public. Health 2017, 46, 1540–1545. [Google Scholar]
- Alameri, M.M.; Kong, A.S.; Aljaafari, M.N.; Ali, H.A.; Eid, K.; Sallagi, M.A.; Cheng, W.H.; Abushelaibi, A.; Lim, S.E.; Loh, J.Y.; et al. Aflatoxin Contamination: An Overview on Health Issues, Detection and Management Strategies. Toxins 2023, 15, 246. [Google Scholar] [CrossRef] [PubMed]
- Ghisleni, D.D.; Braga Mde, S.; Kikuchi, I.S.; Braşoveanu, M.; Nemţanu, M.R.; Dua, K.; Pinto Tde, J. The Microbial Quality Aspects and Decontamination Approaches for the Herbal Medicinal Plants and Products: An in-Depth Review. Curr. Pharm. Des. 2016, 22, 4264–4287. [Google Scholar] [CrossRef]
- Dhanya, R.; Mishra, B.B.; Khaleel, K.M. Effect of gamma irradiation on curcuminoids and volatile oils of fresh turmeric (Curcuma longa). Radiat. Phys. Chem. 2011, 80, 1247–1249. [Google Scholar] [CrossRef]
- Chosdu, R.; Erizal; Iriawan, T.; Hilmy, N. The effect of gamma irradiation on curcumin component of Curcuma domestica. Radiat. Phys. Chem. 1995, 46, 663–667. [Google Scholar] [CrossRef]
- Esmaeili, S.; Barzegar, M.; Sahari, M.A.; Berengi-Ardestani, S. Effect of gamma irradiation under various atmospheres of packaging on the microbial and physicochemical properties of turmeric powder. Radiat. Phys. Chem. 2018, 148, 60–67. [Google Scholar] [CrossRef]
- Almeida, M.C.; Sampaio, G.R.; Bastos, D.H.M.; Villavicencio, A.L.C.H. Effect of gamma radiation processing on turmeric: Antioxidant activity and curcumin content. Radiat. Phys. Chem. 2018, 152, 12–16. [Google Scholar] [CrossRef]
- Chatterjee, S.; Desai, S.R.P.; Thomas, P. Effect of gamma-irradiation on the antioxidant activity of turmeric (Curcuma longa L.) extracts. Food Res. Int. 1999, 32, 487–490. [Google Scholar] [CrossRef]
- Fifield, L.S.; Pharr, M.; Staack, D.; Pillai, S.D.; Nichols, L.; McCoy, J.; Faucette, T.; Bisel, T.T.; Huang, M.; Hasan, M.K.; et al. Direct comparison of gamma, electron beam and X-ray irradiation doses on characteristics of low-density polyethylene, polypropylene homopolymer, polyolefin elastomer and chlorobutyl rubber medical device polymers. Radiat. Phys. Chem. 2021, 186, 109505. [Google Scholar] [CrossRef]
- Lanza, C.M.; Mazzaglia, A.; Paladino, R.; Auditore, L.; Barnà, R.C.; Loria, D.; Trifirò, A.; Trimarchi, M.; Bellia, G. Characterization of peeled and unpeeled almond (Prunus amygdalus) flour after electron beam processing. Radiat. Phys. Chem. 2013, 86, 140–144. [Google Scholar] [CrossRef]
- Cuervo, M.P.; Lucia, L.M.; Castillo, A. Efficacy of Traditional Almond Decontamination Treatments and Electron Beam Irradiation against Heat-Resistant Salmonella Strains. J. Food Prot. 2016, 79, 369–375. [Google Scholar] [CrossRef]
- Kong, Q.; Wu, A.; Qi, W.; Qi, R.; Carter, J.M.; Rasooly, R.; He, X. Effects of electron-beam irradiation on blueberries inoculated with Escherichia coli and their nutritional quality and shelf life. Postharvest Biol. Technol. 2014, 95, 28–35. [Google Scholar] [CrossRef]
- Gomes, C.; Da Silva, P.; Chimbombi, E.; Kim, J.; Castell-Perez, E.; Moreira, R.G. Electron-beam irradiation of fresh broccoli heads (Brassica oleracea L. italica). LWT Food Sci. Technol. 2008, 41, 1828–1833. [Google Scholar] [CrossRef]
- Ramakrishnan, S.R.; Jo, Y.; Nam, H.-A.; Gu, S.-Y.; Baek, M.-E.; Kwon, J.-H. Implications of low-dose e-beam irradiation as a phytosanitary treatment on physicochemical and sensory qualities of grapefruit and lemons during postharvest cold storage. Sci. Hortic. 2019, 245, 1–6. [Google Scholar] [CrossRef]
- Jin, Y.; Shin, H.; Song, K.B. Electron beam irradiation improves shelf lives of Korean ginseng (Panax ginseng C.A. Meyer) and red ginseng. J. Food Sci. 2007, 72, C217–C222. [Google Scholar] [CrossRef]
- Ebrahimi-Mahmoudabad, S.R.; Taghinejad-Roudbaneh, M. Investigation of electron beam irradiation effects on anti-nutritional factors, chemical composition and digestion kinetics of whole cottonseed, soybean and canola seeds. Radiat. Phys. Chem. 2011, 80, 1441–1447. [Google Scholar] [CrossRef]
- Yamaoki, R.; Kimura, S. Effectiveness of electron beam irradiation for microbial decontamination of turmeric powder (Curcuma longa Linne) and analysis of curcuminoid degradation. J. Food Process. Preserv. 2018, 42, e13334. [Google Scholar] [CrossRef]
- Zhuan, R.; Wang, J. Degradation of sulfamethoxazole by ionizing radiation: Kinetics and implications of additives. Sci. Total Env. 2019, 668, 67–73. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Cielecka-Piontek, J.; Garbacki, P.; Lewandowska, K.; Bednarski, W.; Barszcz, B.; Zalewski, P.; Kycler, W.; Oszczapowicz, I.; Jelińska, A. Radiation sterilization of anthracycline antibiotics in solid state. Sci. World J. 2013, 2013, 258758. [Google Scholar] [CrossRef] [PubMed]
- Kilińska, K.; Cielecka-Piontek, J.; Skibiński, R.; Szymanowska, D.; Miklaszewski, A.; Bednarski, W.; Tykarska, E.; Stasiłowicz, A.; Zalewski, P. The Radiostability of Meropenem Trihydrate in Solid State. Molecules 2018, 23, 2738. [Google Scholar] [CrossRef]
- Marciniec, B.; Stawny, M.; Kozak, M.; Naskrent, M. The influence of radiation sterilization on thiamphenicol. Spectrochim. Acta Part 2008, 69, 865–870. [Google Scholar] [CrossRef]
- Zalewski, P.; Skibiński, R.; Szymanowska-Powałowska, D.; Piotrowska, H.; Kozak, M.; Pietralik, Z.; Bednarski, W.; Cielecka-Piontek, J. The radiolytic studies of cefpirome sulfate in the solid state. J. Pharm. Biomed. Anal. 2016, 118, 410–416. [Google Scholar] [CrossRef]
- Garbacki, P.; Zalewski, P.; Skibiński, R.; Kozak, M.; Ratajczak, M.; Lewandowska, K.; Bednarski, W.; Podborska, A.; Mizera, M.; Jelińska, A.; et al. Radiostability of cefoselis sulfate in the solid state. X-ray Spectrom. 2015, 44, 344–350. [Google Scholar] [CrossRef]
- Zalewski, P.; Skibiński, R.; Szymanowska-Powałowska, D.; Piotrowska, H.; Bednarski, W.; Cielecka-Piontek, J. Radiolytic studies of cefozopran hydrochloride in the solid state. Electron. J. Biotechnol. 2017, 25, 28–32. [Google Scholar] [CrossRef]
- Janiaczyk, M.; Jelińska, A.; Woźniak-Braszak, A.; Bilski, P.; Popielarz-Brzezińska, M.; Wachowiak, M.; Baranowski, M.; Tomczak, S.; Ogrodowczyk, M. Electron Beam Radiation as a Safe Method for the Sterilization of Aceclofenac and Diclofenac—The Usefulness of EPR and 1H-NMR Methods in Determination of Molecular Structure and Dynamics. Pharmaceutics 2022, 14, 1331. [Google Scholar] [CrossRef] [PubMed]
- Marciniec, B.; Stawny, M.; Olszewski, K.; Kozak, M.; Naskrent, M. Analytical study on irradiated methylxanthine derivatives. J. Therm. Anal. Calorim. 2013, 111, 2165–2170. [Google Scholar] [CrossRef]
- Supriya, P.; Sridhar, K.R.; Ganesh, S. Fungal decontamination and enhancement of shelf life of edible split beans of wild legume Canavalia maritima by the electron beam irradiation. Radiat. Phys. Chem. 2014, 96, 5–11. [Google Scholar] [CrossRef]
- Mohamed, A.B.; Chavez, R.A.; Wagacha, M.J.; Mutegi, C.K.; Muthomi, J.W.; Pillai, S.D.; Stasiewicz, M.J. Efficacy of electron beam irradiation in reduction of mycotoxin-producing fungi, aflatoxin, and fumonisin, in naturally contaminated maize slurry. Toxicon X 2022, 16, 100141. [Google Scholar] [CrossRef] [PubMed]
- Özer, A.Y.; Turker, S.; Çolak, S.; Korkmaz, M.; Kiliç, E.; Özalp, M. The eff ects of gamma irradiation on diclofenac sodium, liposome and noisome ingredients for rheumatoid arthritis. Int. Med. Appl. Sci. 2013, 5, 122–130. [Google Scholar]
- Marciniec, B.; Kozak, M.; Naskrent, M.; Dettlaff, K.; Ogrodowczyk, M.; Stawny, M.; Wachowski, L. Thermal study of four irradiated imidazoline derivatives in solid state. J. Therm. Anal. Calorim 2007, 88, 337–342. [Google Scholar] [CrossRef]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Boone, C.W.; Simic, M.G. H-Atom Transfer Is A Preferred Antioxidant Mechanism of Curcumin. J. Am. Chem. Soc. 1999, 121, 9677–9681. [Google Scholar] [CrossRef]
- Barclay, L.R.C.; Vinqvist, M.R.; Mukai, K.; Goto, H.; Hashimoto, Y.; Tokunaga, A.; Uno, H. On the Antioxidant Mechanism of Curcumin: Classical Methods Are Needed To Determine Antioxidant Mechanism and Activity. Org. Lett. 2000, 2, 2841–2843. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Álvarez-Diduk, R.; Ramírez-Silva, M.T.; Alarcón-Ángeles, G.; Rojas-Hernández, A. Role of the reacting free radicals on the antioxidant mechanism of curcumin. Chem. Phys. 2009, 363, 13–23. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Abnormal solvent effects on hydrogen atom abstraction. 2. Resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J. Org. Chem. 2004, 69, 5888–5896. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-S.; Chen, T.-H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.-F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef] [PubMed]
- Stasiłowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-cyclodextrin as an effective carrier of curcumin—Piperine nutraceutical system with improved enzyme inhibition properties. J. Enzym. Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef]
- Sip, S.; Sip, A.; Miklaszewski, A.; Żarowski, M.; Cielecka-Piontek, J. Zein as an Effective Carrier for Hesperidin Delivery Systems with Improved Prebiotic Potential. Molecules 2023, 28, 5209. [Google Scholar] [CrossRef] [PubMed]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Zalewski, P.; Szwajgier, D.; Baranowska-Wójcik, E.; Żarowski, M.; Plech, T.; Cielecka-Piontek, J. Permeability of Hypogymnia physodes Extract Component-Physodic Acid through the Blood-Brain Barrier as an Important Argument for Its Anticancer and Neuroprotective Activity within the Central Nervous System. Cancers 2021, 13, 1717. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Włodarczyk, A.; Rewers, M.; Sliwinska, E.; Studzińska-Sroka, E.; Witkowska-Banaszczak, E.; Stochmal, A.; Żuchowski, J.; Dlugaszewska, J.; Thiem, B. Micropropagation of Chaenomeles japonica: A Step towards Production of Polyphenol-rich Extracts Showing Antioxidant and Antimicrobial Activities. Molecules 2019, 24, 1314. [Google Scholar] [CrossRef] [PubMed]




| Name | Retention Time (min) | Measured Mass (m/z) | Theoretical Mass (m/z) | Mass Error (ppm) | Molecular Ion Formula [M-H−] | MS/MS Fragmentation Ions (m/z) | MS/MS Fragment Formula |
|---|---|---|---|---|---|---|---|
Curcumin | 6.9 | 367.1167 | 367.1187 | 5.38 | C21H19O6 | 217.0505 173.0603 158.0371 149.0604 132.0237 | C12H9O4 C11H8O2 C10H6O2 C9H9O2 C8H6O2 |
Demethoxycurcumin | 6.75 | 337.1084 | 337.1081 | 0.3 | C20H17O5 | 217.0508 173.0609 158.0356 134.0219 119.0501 | C12H9O4 C11H8O2 C10H6O2 C8H4O2 C8H7O |
Bisdemethoxycurcumin | 6.6 | 307.0967 | 307.0976 | 2.77 | C19H15O4 | 187.0379 143.0476 158.0356 119.0491 | C11H9O3 C10H7O C10H6O2 C8H7O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosiak, N.; Garbiec, E.; Bednarski, W.; Skibiński, R.; Lewandowska, K.; Bazan-Woźniak, A.; Pietrzak, R.; Cielecka-Piontek, J.; Zalewski, P. How Does Radiation Affect Curcumin Raw Material? Int. J. Mol. Sci. 2024, 25, 2524. https://doi.org/10.3390/ijms25052524
Rosiak N, Garbiec E, Bednarski W, Skibiński R, Lewandowska K, Bazan-Woźniak A, Pietrzak R, Cielecka-Piontek J, Zalewski P. How Does Radiation Affect Curcumin Raw Material? International Journal of Molecular Sciences. 2024; 25(5):2524. https://doi.org/10.3390/ijms25052524
Chicago/Turabian StyleRosiak, Natalia, Ewa Garbiec, Waldemar Bednarski, Robert Skibiński, Kornelia Lewandowska, Aleksandra Bazan-Woźniak, Robert Pietrzak, Judyta Cielecka-Piontek, and Przemysław Zalewski. 2024. "How Does Radiation Affect Curcumin Raw Material?" International Journal of Molecular Sciences 25, no. 5: 2524. https://doi.org/10.3390/ijms25052524
APA StyleRosiak, N., Garbiec, E., Bednarski, W., Skibiński, R., Lewandowska, K., Bazan-Woźniak, A., Pietrzak, R., Cielecka-Piontek, J., & Zalewski, P. (2024). How Does Radiation Affect Curcumin Raw Material? International Journal of Molecular Sciences, 25(5), 2524. https://doi.org/10.3390/ijms25052524







