The Vitamin C Enantiomers Possess a Comparable Potency in the Induction of Oxidative Stress in Cancer Cells but Differ in Their Toxicity
Abstract
1. Introduction
2. Results
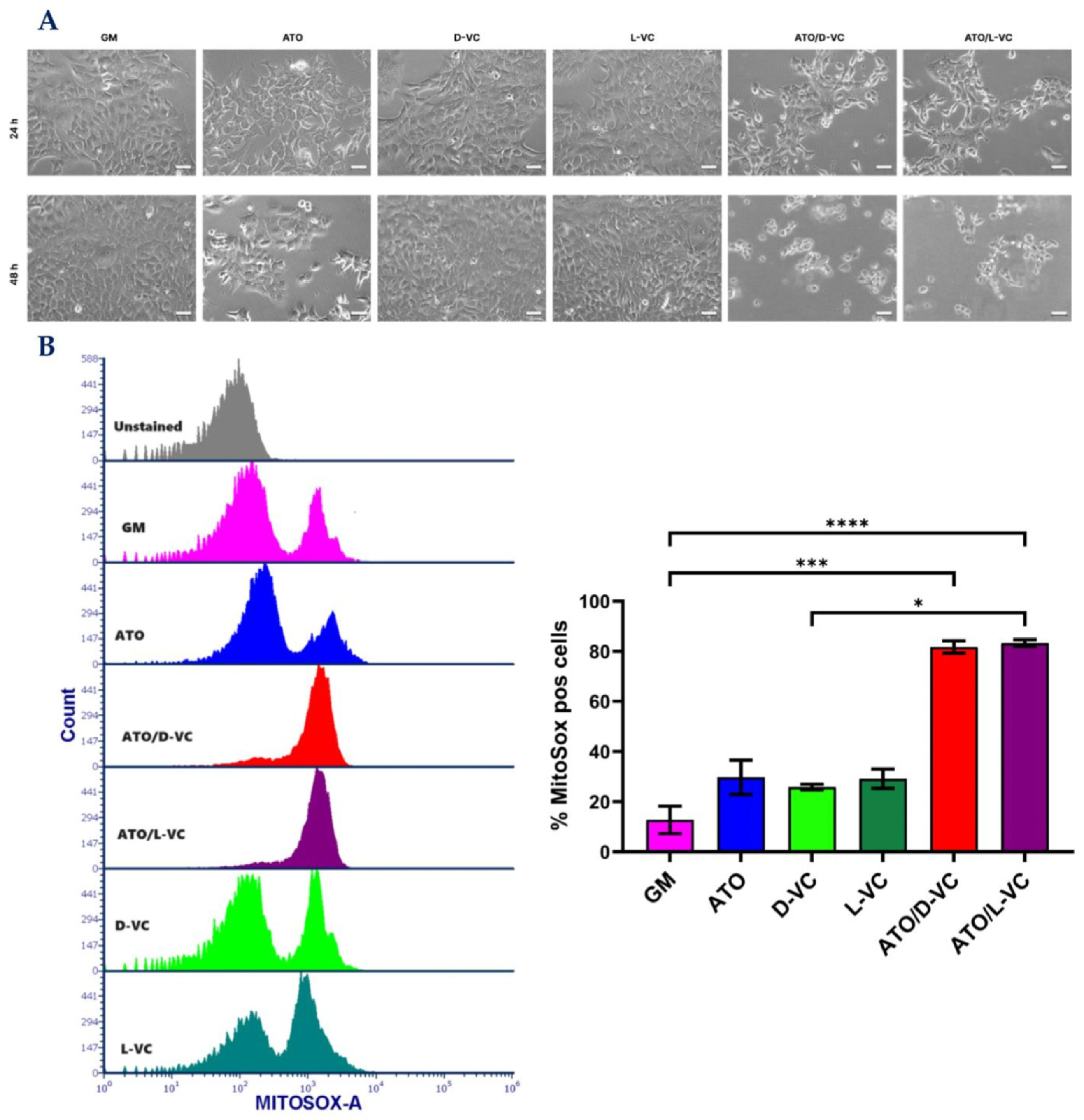
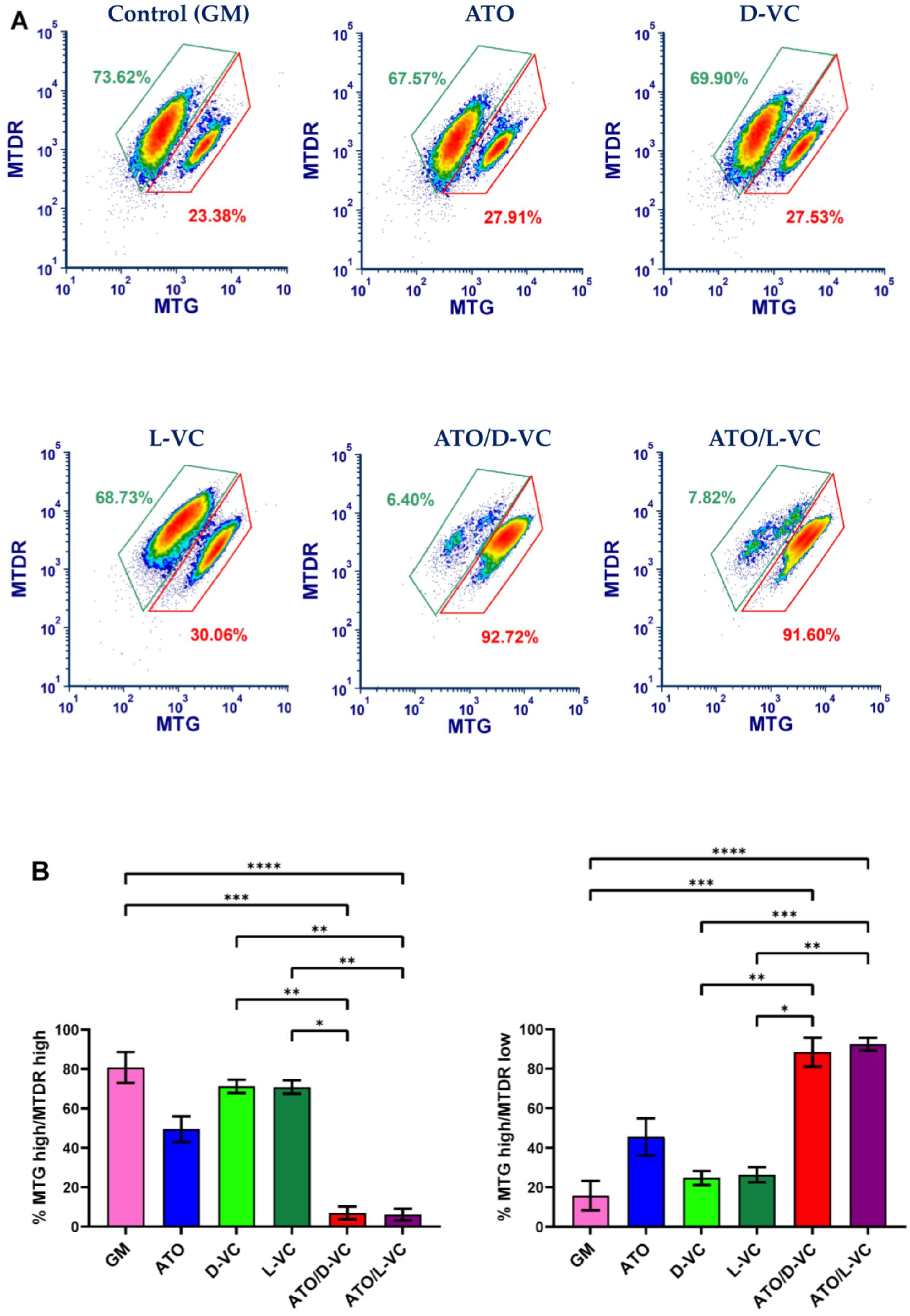
2.1. The Similar Cytotoxic Effects of D-VC and L-VC in KrasG12D-Expressing Cancer Cells
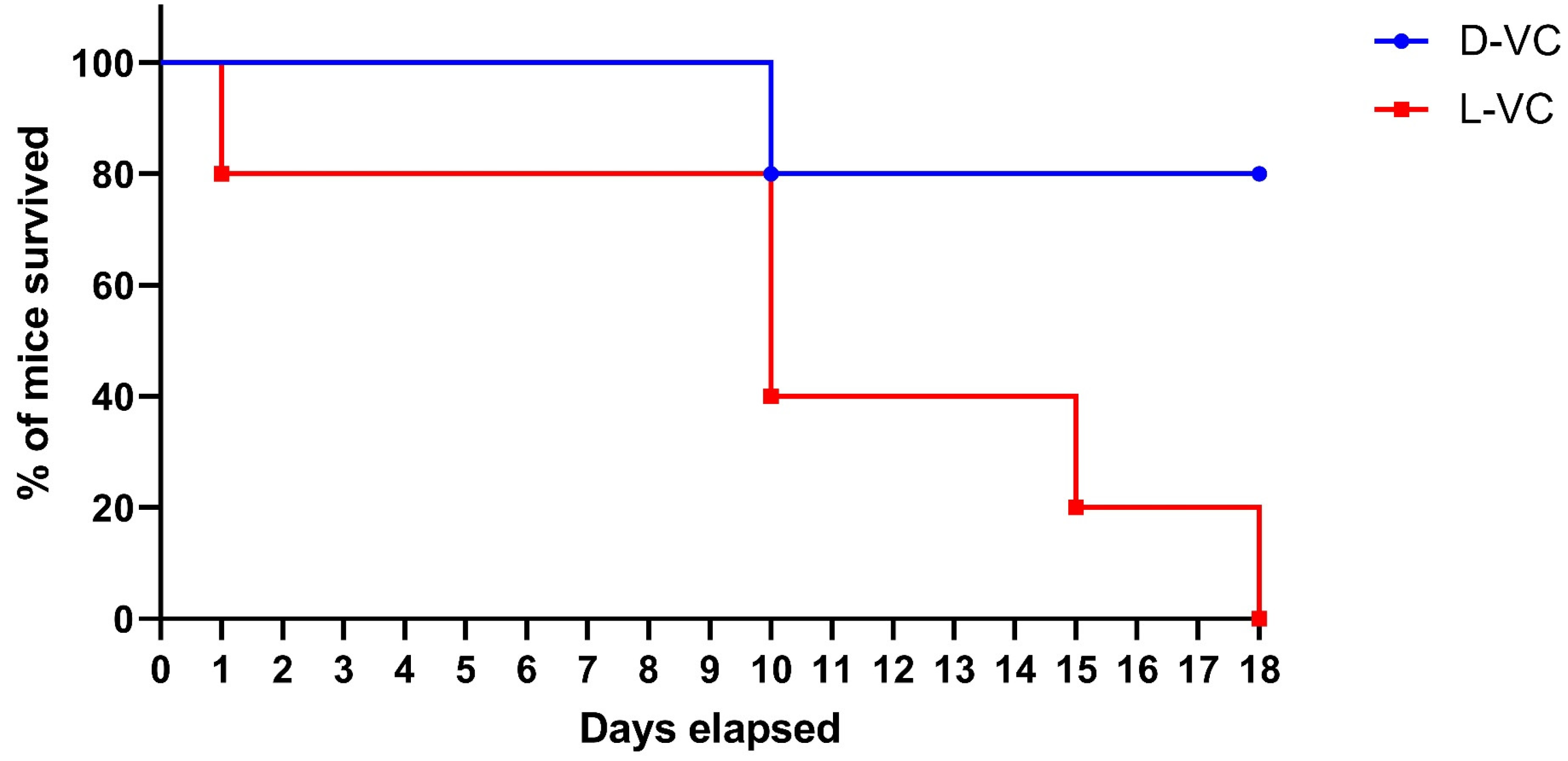
2.2. Evaluations of the Toxic Effects of D-VC and L-VC in Mice
2.3. The Influence of D-VC and L-VC on Leukocyte Count
2.4. Evaluation of the Effects of High Doses of D-VC and L-VC on Internal Organs
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Cell Culture and Treatment
4.3. Flow Cytometry
4.4. Calculation of the Dose of D-VC and L-VC
4.5. Experimental Design
4.6. Hematology (WBC)
4.7. Histopathology Assessment of Tissues
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, Z.; Fan, K.; Yang, C.; Huang, Q.; Gong, Y.; Cheng, H.; Jin, K.; Liu, C.; Ni, Q.; Yu, X.; et al. Critical role of KRAS mutation in pancreatic ductal adenocarcinoma. Transl. Cancer Res. 2018, 7, 1728–1736. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef]
- Tape, C.J.; Ling, S.; Dimitriadi, M.; McMahon, K.M.; Worboys, J.D.; Leong, H.S.; Norrie, I.C.; Miller, C.J.; Poulogiannis, G.; Lauffenburger, D.A.; et al. Oncogenic KRAS Regulates Tumor Cell Signaling via Stromal Reciprocation. Cell 2016, 165, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Burska, A.N.; Ilyassova, B.; Dildabek, A.; Khamijan, M.; Begimbetova, D.; Molnár, F.; Sarbassov, D.D. Enhancing an Oxidative “Trojan Horse” Action of Vitamin C with Arsenic Trioxide for Effective Suppression of KRAS-Mutant Cancers: A Promising Path at the Bedside. Cells 2022, 11, 3454. [Google Scholar] [CrossRef]
- Peri, F.; Airoldi, C.; Colombo, S.; Martegani, E.; van Neuren, A.S.; Stein, M.; Marinzi, C.; Nicotra, F. Design, synthesis and biological evaluation of sugar-derived Ras inhibitors. Chembiochem 2005, 6, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Gandhi, L.; Mita, M.M.; Damstrup, L.; Campana, F.; Hidalgo, M.; Grande, E.; Hyman, D.M.; Heist, R.S. A phase Ib dose-escalation and expansion study of the oral MEK inhibitor pimasertib and PI3K/MTOR inhibitor voxtalisib in patients with advanced solid tumours. Br. J. Cancer 2018, 119, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Dahlman, J.E.; Tammela, T.; Khan, O.F.; Sood, S.; Dave, A.; Cai, W.; Chirino, L.M.; Yang, G.R.; Bronson, R.; et al. Small RNA combination therapy for lung cancer. Proc. Natl. Acad. Sci. USA 2014, 111, E3553–E3561. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenet. 2018, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Villagran, M.; Ferreira, J.; Martorell, M.; Mardones, L. The Role of Vitamin C in Cancer Prevention and Therapy: A Literature Review. Antioxidants 2021, 10, 1894. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Sinnberg, T.W.; Niessner, H.; Busch, C. Molecular mechanisms of pharmacological doses of ascorbate on cancer cells. Wien Med. Wochenschr. 2015, 165, 251–257. [Google Scholar] [CrossRef]
- Du, J.; Martin, S.M.; Levine, M.; Wagner, B.A.; Buettner, G.R.; Wang, S.H.; Taghiyev, A.F.; Du, C.; Knudson, C.M.; Cullen, J.J. Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer. Clin. Cancer Res. 2010, 16, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Pires, A.S.; Marques, C.R.; Encarnação, J.C.; Abrantes, A.M.; Marques, I.A.; Laranjo, M.; Oliveira, R.; Casalta-Lopes, J.E.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; et al. Ascorbic Acid Chemosensitizes Colorectal Cancer Cells and Synergistically Inhibits Tumor Growth. Front. Physiol. 2018, 9, 911. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.; Rago, C.; et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Wu, X.; Park, M.; Sarbassova, D.A.; Ying, H.; Lee, M.G.; Bhattacharya, R.; Ellis, L.; Peterson, C.B.; Hung, M.; Lin, H.; et al. A chirality-dependent action of vitamin C in suppressing Kirsten rat sarcoma mutant tumor growth by the oxidative combination: Rationale for cancer therapeutics. Int. J. Cancer 2020, 146, 2822–2828. [Google Scholar] [CrossRef]
- Ying, H.; Kimmelman, A.C.; Lyssiotis, C.A.; Hua, S.; Chu, G.C.; Fletcher-Sananikone, E.; Locasale, J.W.; Son, J.; Zhang, H.; Coloff, J.L.; et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012, 149, 656–670. [Google Scholar] [CrossRef]
- de Brito Monteiro, L.; Davanzo, G.G.; de Aguiar, C.F.; Moraes-Vieira, P.M.M. Using flow cytometry for mitochondrial assays. MethodsX 2020, 7, 100938. [Google Scholar] [CrossRef]
- Rebane-Klemm, E.; Truu, L.; Reinsalu, L.; Puurand, M.; Shevchuk, I.; Chekulayev, V.; Timohhina, N.; Tepp, K.; Bogovskaja, J.; Afanasjev, V.; et al. Mitochondrial Respiration in KRAS and BRAF Mutated Colorectal Tumors and Polyps. Cancers 2020, 12, 815. [Google Scholar] [CrossRef]
- Presley, A.D.; Fuller, K.M.; Arriaga, E.A. MitoTracker Green labeling of mitochondrial proteins and their subsequent analysis by capillary electrophoresis with laser-induced fluorescence detection. J. Chromatogr. B 2003, 793, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D. Detection of Mitochondrial Mass, Damage, and Reactive Oxygen Species by Flow Cytometry. Cold Spring Harb. Protoc. 2015, 2015, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef]
- Campbell, E.J.; Vissers, M.C.; Wohlrab, C.; Hicks, K.O.; Strother, R.M.; Bozonet, S.M.; Robinson, B.A.; Dachs, G.U. Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic. Biol. Med. 2016, 99, 451–462. [Google Scholar] [CrossRef]
- Goldman, H.M.; Gould, B.S.; Munro, H.N. The antiscorbutic action of l-ascorbic acid and d-isoascorbic acid (erythorbic acid) in the guinea pig. Am. J. Clin. Nutr. 1981, 34, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Rivers, J.M.; Huang, E.D.; Dodds, M.L. Human Metabolism of L-Ascorbic Acid and Erythorbic Acid. J. Nutr. 1963, 81, 163–168. [Google Scholar] [CrossRef]
- Dayton, P.G.; Burns, J.J. Metabolism of D-ascorbic acid-1-C14 in guinea pigs and rats. J. Biol. Chem. 1958, 231, 85–91. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef]
- Tomita, T.; Kato, M.; Hiratsuka, S. Regulation of vascular permeability in cancer metastasis. Cancer Sci. 2021, 112, 2966–2974. [Google Scholar] [CrossRef] [PubMed]
- Baguley, B.C.; Ding, Q.; Richardson, E. Preliminary Evidence That High-Dose Vitamin C has a Vascular Disrupting Action in Mice. Front. Oncol. 2014, 4, 310. [Google Scholar] [CrossRef]
- Karasuyama, H.; Tsujimura, Y.; Obata, K.; Mukai, K. Role for basophils in systemic anaphylaxis. Chem. Immunol. Allergy 2010, 95, 85–97. [Google Scholar] [CrossRef]
- Olingy, C.E.; Dinh, H.Q.; Hedrick, C.C. Monocyte heterogeneity and functions in cancer. J. Leukoc. Biol. 2019, 106, 309–322. [Google Scholar] [CrossRef]
- Patysheva, M.; Frolova, A.; Larionova, I.; Afanas’ev, S.; Tarasova, A.; Cherdyntseva, N.; Kzhyshkowska, J. Monocyte programming by cancer therapy. Front. Immunol. 2022, 13, 994319. [Google Scholar] [CrossRef]
- Van Gorkom, G.N.Y.; Klein Wolterink, R.G.J.; Van Elssen, C.H.M.J.; Wieten, L.; Germeraad, W.T.V.; Bos, G.M.J. Influence of Vitamin C on Lymphocytes: An Overview. Antioxidants 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed]
- Vacca, P.; Montaldo, E.; Croxatto, D.; Moretta, F.; Bertaina, A.; Vitale, C.; Locatelli, F.; Mingari, M.C.; Moretta, L. NK Cells and Other Innate Lymphoid Cells in Hematopoietic Stem Cell Transplantation. Front. Immunol. 2016, 7, 188. [Google Scholar] [CrossRef] [PubMed]
- AAT Bioquest, Inc. Available online: https://www.aatbio.com/resources/toxicity-lethality-median-dose-td50-ld50/vitamin-c (accessed on 18 October 2023).


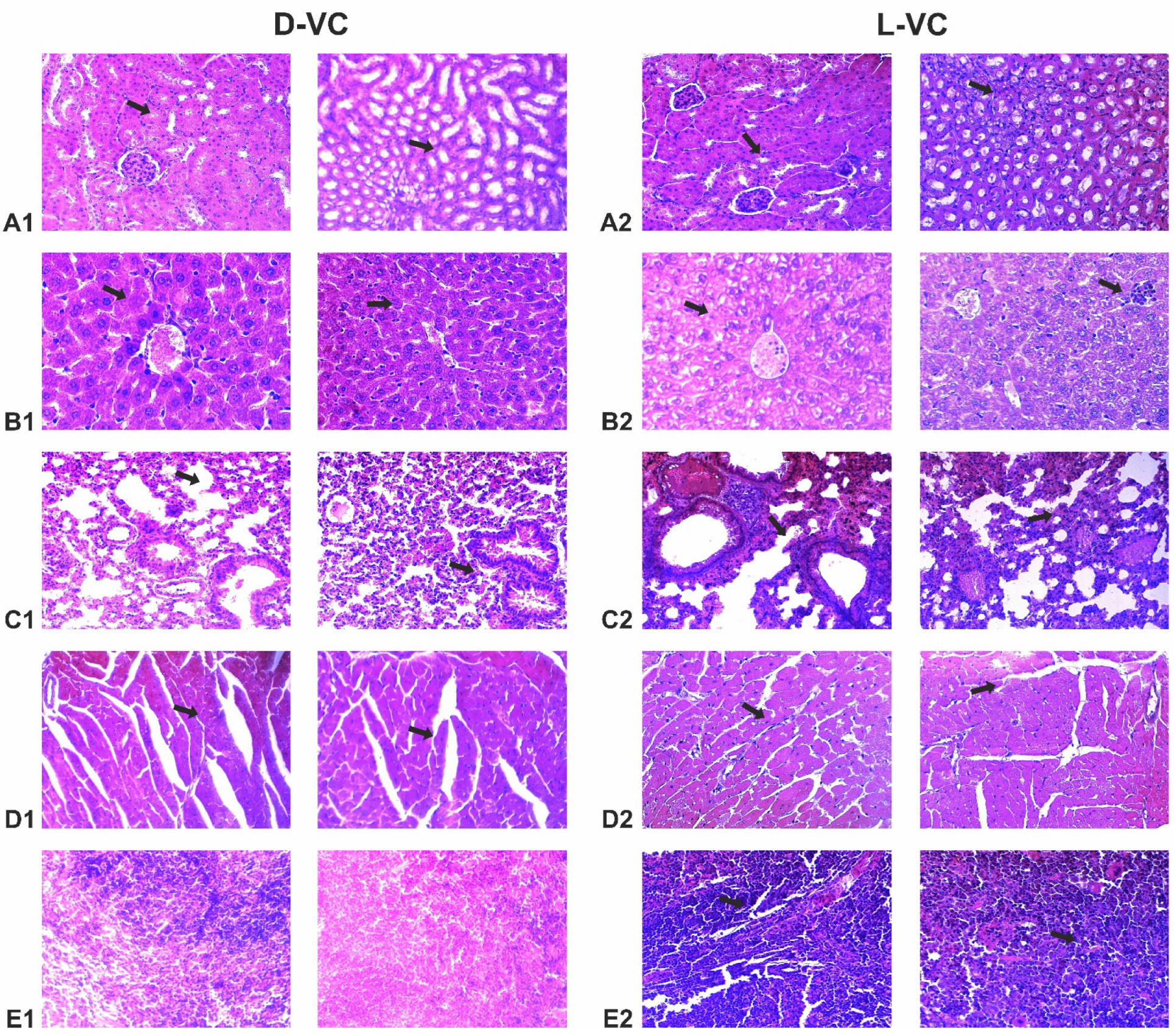
| Organs | D-VC | L-VC |
|---|---|---|
| Kidneys | Swelling of the stroma, sludged erythrocytes in the lumen of blood vessels. The epithelium of the renal tubules is swollen, with pronounced dystrophic changes, and the lumens of the tubules are narrowed. | Dystrophy, swelling of the epithelium of the renal tubules, in the center of the capillary glomerulus with symptoms of erythrodiapedesis, uneven hyperplasia of podocytes. Perevascular lymphocytic infiltration. |
| Liver | Protein edema of the stroma, pronounced congestion of blood vessels, single perivascular lymphocytic infiltrates. | Congestion of blood vessels, protein degeneration of hepatocytes, lysed erythrocytes in the lumen of arterioles. |
| Lung | Erythrostasis in the microcirculatory bed, acute vascular congestion, sludge of erythrocytes in the lumen of the areolas. Rupture of interalveolar septa, foci of distelectasis. Perivascular lymphocytic infiltrates. | Acute vascular congestion with multiple confluent hemorrhages, thrombosis of microvasculature vessels, sludged erythrocytes. Multiple foci of thrombosis and perivascular hemorrhages are signs of severe intoxication. Fibrinoid necrosis of the vascular wall is a sign of long-term destruction of the vascular walls. Signs of acute inflammation (interstitial pneumonia)—lymphocytic—plasmacytic infiltration of peribronchial tissue and interalveolar septa (interstitial pneumonia—a reaction to intoxication in the organism). Collapse of lung tissue. |
| Myocardium | Protein dystrophy of cardiomyocytes, small foci of wave-like contracture of muscle fibers, sludge of erythrocytes in the lumen of some vessels. | Protein dystrophy of cardiomyocytes, foci of wavy contracture of fibers, sludge of erythrocytes, foci of destruction of cardiomyocytes, proteinaceous edema of the stroma. Perivascular fibrosis with lymphocytic infiltration. |
| Spleen | The structure is not broken. | Splenomegaly, vascular congestion, areas of thrombosis and hemorrhage. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Begimbetova, D.; Burska, A.N.; Baltabekova, A.; Kussainova, A.; Kukanova, A.; Fazyl, F.; Ibragimova, M.; Manekenova, K.; Makishev, A.; Bersimbaev, R.I.; et al. The Vitamin C Enantiomers Possess a Comparable Potency in the Induction of Oxidative Stress in Cancer Cells but Differ in Their Toxicity. Int. J. Mol. Sci. 2024, 25, 2531. https://doi.org/10.3390/ijms25052531
Begimbetova D, Burska AN, Baltabekova A, Kussainova A, Kukanova A, Fazyl F, Ibragimova M, Manekenova K, Makishev A, Bersimbaev RI, et al. The Vitamin C Enantiomers Possess a Comparable Potency in the Induction of Oxidative Stress in Cancer Cells but Differ in Their Toxicity. International Journal of Molecular Sciences. 2024; 25(5):2531. https://doi.org/10.3390/ijms25052531
Chicago/Turabian StyleBegimbetova, Dinara, Agata N. Burska, Aidana Baltabekova, Assiya Kussainova, Assiya Kukanova, Fatima Fazyl, Milana Ibragimova, Kenzhekyz Manekenova, Abay Makishev, Rakhmetkazhi I. Bersimbaev, and et al. 2024. "The Vitamin C Enantiomers Possess a Comparable Potency in the Induction of Oxidative Stress in Cancer Cells but Differ in Their Toxicity" International Journal of Molecular Sciences 25, no. 5: 2531. https://doi.org/10.3390/ijms25052531
APA StyleBegimbetova, D., Burska, A. N., Baltabekova, A., Kussainova, A., Kukanova, A., Fazyl, F., Ibragimova, M., Manekenova, K., Makishev, A., Bersimbaev, R. I., & Sarbassov, D. D. (2024). The Vitamin C Enantiomers Possess a Comparable Potency in the Induction of Oxidative Stress in Cancer Cells but Differ in Their Toxicity. International Journal of Molecular Sciences, 25(5), 2531. https://doi.org/10.3390/ijms25052531





