The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia
Abstract
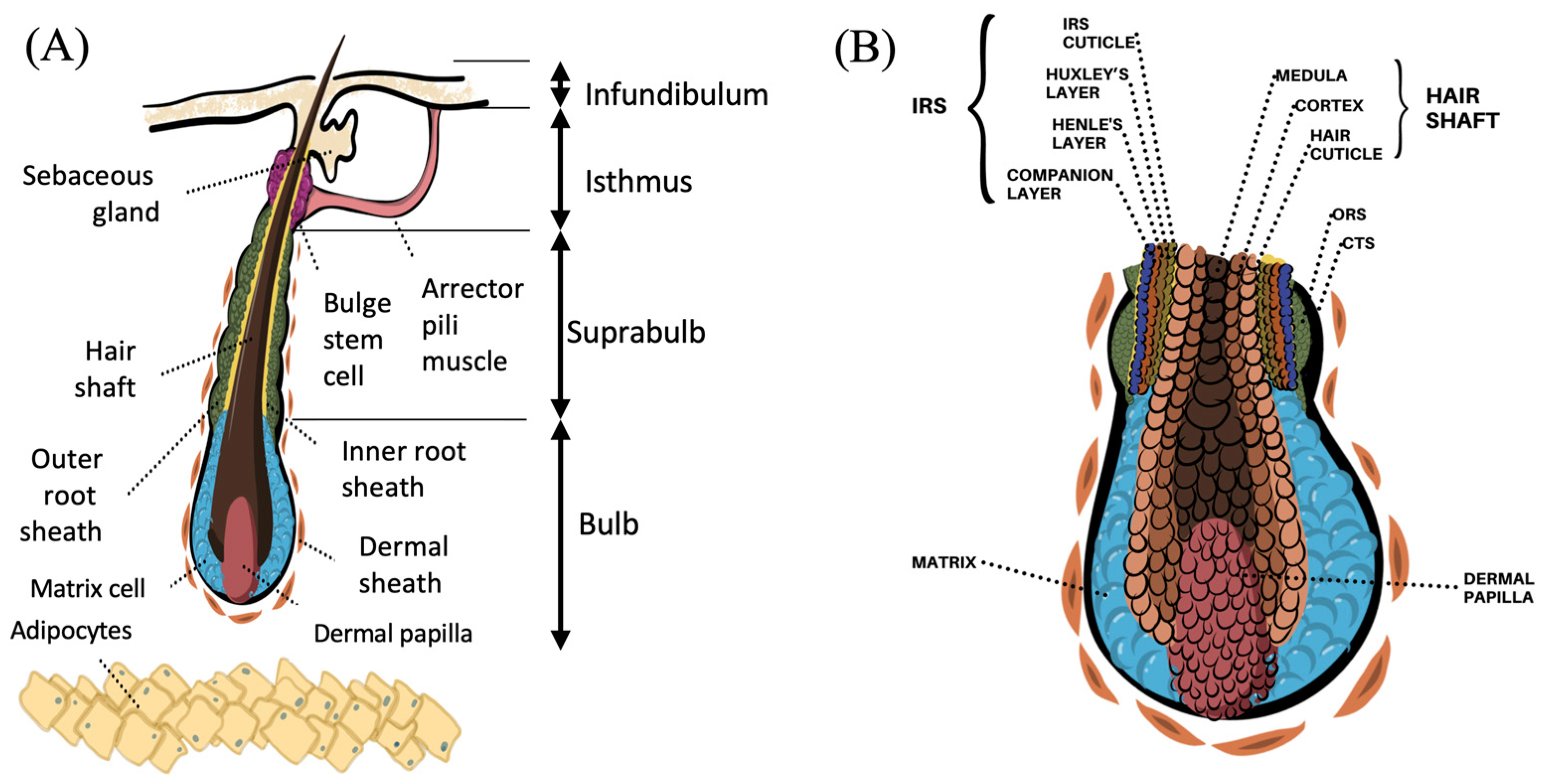
:1. Introduction
2. Functional Hair Follicle Anatomy
3. Hair Follicle Cycling
3.1. Anagen Phase
3.2. Catagen Phase
3.3. Telogen Phase
4. Androgenetic Alopecia (AGA)
5. Transcription Profiling Studies of AGA
6. Genome-Wide Association Studies of AGA
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Norwood, O.T. Male pattern baldness: Classification and incidence. South. Med. J. 1975, 68, 1359–1365. [Google Scholar] [CrossRef]
- Tanaka, Y.; Aso, T.; Ono, J.; Hosoi, R.; Kaneko, T. Androgenetic Alopecia Treatment in Asian Men. J. Clin. Aesthet. Dermatol. 2018, 11, 32–35. [Google Scholar]
- Yildirim, A.M.; Yousaf, A.; Fang, W.; Kolodney, M.S. A cross-sectional study of male balding patterns in people of color. J. Am. Acad. Dermatol. 2022, 87, 1203–1205. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, C.; Shen, Y.; Wang, X.; Ding, X.; Tian, S.; Liu, Y.; Peng, G.; Xue, S.; Zhou, J.; et al. Prevalence of androgenetic alopecia in China: A community-based study in six cities. Br. J. Dermatol. 2010, 162, 843–847. [Google Scholar] [CrossRef]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Lotufo, P.A.; Chae, C.U.; Ajani, U.A.; Hennekens, C.H.; Manson, J.E. Male pattern baldness and coronary heart disease: The Physicians’ Health Study. Arch. Intern. Med. 2000, 160, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Ahouansou, S.; Le Toumelin, P.; Crickx, B.; Descamps, V. Association of androgenetic alopecia and hypertension. Eur. J. Dermatol. 2007, 17, 220–222. [Google Scholar] [PubMed]
- Bakry, O.A.; Shoeib, M.A.M.; El Shafiee, M.K.; Hassan, A. Androgenetic alopecia, metabolic syndrome, and insulin resistance: Is there any association? A case-control study. Indian Dermatol. Online J. 2014, 5, 276–281. [Google Scholar] [CrossRef]
- Zhou, C.K.; Levine, P.H.; Cleary, S.D.; Hoffman, H.J.; Graubard, B.I.; Cook, M.B. Male Pattern Baldness in Relation to Prostate Cancer-Specific Mortality: A Prospective Analysis in the NHANES I Epidemiologic Follow-up Study. Am. J. Epidemiol. 2016, 183, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Hsieh, F.-N.; Lin, L.-Y.; Hsu, C.-K.; Sheu, H.-M.; Chen, W. Higher body mass index is associated with greater severity of alopecia in men with male-pattern androgenetic alopecia in Taiwan: A cross-sectional study. J. Am. Acad. Dermatol. 2014, 70, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Foitzik, K. In search of the ‘hair cycle clock’: A guided tour. Differentiation 2004, 72, 489–511. [Google Scholar] [CrossRef]
- Paus, R.; Cotsarelis, G. The Biology of Hair Follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Knorr, F.; Lademann, J.; Patzelt, A.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Follicular transport route—Research progress and future perspectives. Eur. J. Pharm. Biopharm. 2009, 71, 173–180. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Vogt, A. Human hair follicle: Reservoir function and selective targeting. Br. J. Dermatol. 2011, 165 (Suppl. S2), 13–17. [Google Scholar] [CrossRef]
- Taylor, G.; Lehrer, M.S.; Jensen, P.J.; Sun, T.T.; Lavker, R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000, 102, 451–461. [Google Scholar] [CrossRef]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the epithelial stem cell niche in skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Ohyama, M. Hair follicle bulge: A fascinating reservoir of epithelial stem cells. J. Dermatol. Sci. 2007, 46, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Terunuma, A.; Tock, C.L.; Radonovich, M.F.; Pise-Masison, C.A.; Hopping, S.B.; Brady, J.N.; Udey, M.C.; Vogel, J.C. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J. Clin. Investig. 2006, 116, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648. [Google Scholar] [CrossRef]
- Lyle, S.; Christofidou-Solomidou, M.; Liu, Y.; Elder, D.E.; Albelda, S.; Cotsarelis, G. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell Sci. 1998, 111 Pt 21, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Garza, L.A.; Yang, C.-C.; Zhao, T.; Blatt, H.B.; Lee, M.; He, H.; Stanton, D.C.; Carrasco, L.; Spiegel, J.H.; Tobias, J.W.; et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J. Clin. Investig. 2011, 121, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Bortner, C.D.; Cotsarelis, G.; Reece, J.M.; Trempus, C.S.; Faircloth, R.S.; Tennant, R.W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Investig. Dermatol. 2003, 120, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Aoi, N.; Sato, T.; Yamauchi, Y.; Suga, H.; Eto, H.; Kato, H.; Araki, J.; Yoshimura, K. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab. Investig. 2009, 89, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Poblet, E.; Jiménez, F.; Godínez, J.M.; Pascual-Martín, A.; Izeta, A. The immunohistochemical expression of CD34 in human hair follicles: A comparative study with the bulge marker CK15. Clin. Exp. Dermatol. 2006, 31, 807–812. [Google Scholar] [CrossRef]
- Ito, M.; Kizawa, K.; Hamada, K.; Cotsarelis, G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 2004, 72, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Cotsarelis, G.; Wei, Z.-G.; Fryer, E.; Margolis-Fryer, J.; Ostead, M.; Tokarek, R.; Sun, T.-T.; Lavker, R.M. Cells within the bulge region of mouse hair follicle transiently proliferate during early anagen: Heterogeneity and functional differences of various hair cycles. Differentiation 1994, 55, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Aiba, S.; Kurosawa, M.; Tagami, H. Ber-EP4 antigen is a marker for a cell population related to the secondary hair germ. Exp. Dermatol. 2004, 13, 401–405. [Google Scholar] [CrossRef]
- Mardaryev, A.N.; Meier, N.; Poterlowicz, K.; Sharov, A.A.; Sharova, T.Y.; Ahmed, M.I.; Rapisarda, V.; Lewis, C.; Fessing, M.Y.; Ruenger, T.M.; et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development 2011, 138, 4843–4852. [Google Scholar] [CrossRef]
- Veniaminova, N.A.; Vagnozzi, A.N.; Kopinke, D.; Do, T.T.; Murtaugh, L.C.; Maillard, I.; Dlugosz, A.A.; Reiter, J.F.; Wong, S.Y. Keratin 79 identifies a novel population of migratory epithelial cells that initiates hair canal morphogenesis and regeneration. Development 2013, 140, 4870–4880. [Google Scholar] [CrossRef]
- Shirokova, V.; Biggs, L.C.; Jussila, M.; Ohyama, T.; Groves, A.K.; Mikkola, M.L. Foxi3 Deficiency Compromises Hair Follicle Stem Cell Specification and Activation. Stem Cells 2016, 34, 1896–1908. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef]
- Panteleyev, A.A.; Jahoda, C.A.; Christiano, A.M. Hair follicle predetermination. J. Cell Sci. 2001, 114, 3419–3431. [Google Scholar] [CrossRef]
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.-C.; Rose, C.; Yoon, G.S.; Lee, S.-J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef]
- Panteleyev, A.A. Functional anatomy of the hair follicle: The Secondary Hair Germ. Exp. Dermatol. 2018, 27, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Detmar, M.; Schaart, F.M.; Blume, U.; Orfanos, C.E. Culture of hair matrix and follicular keratinocytes. J. Investig. Dermatol. 1993, 101, 130S–134S. [Google Scholar] [CrossRef] [PubMed]
- Woo, W.-M.; Zhen, H.H.; Oro, A.E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012, 26, 1235–1246. [Google Scholar] [CrossRef]
- Rompolas, P.; Mesa, K.R.; Greco, V. Spatial organization within a niche as a determinant of stem-cell fate. Nature 2013, 502, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Enshell-Seijffers, D.; Lindon, C.; Kashiwagi, M.; Morgan, B.A. beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 2010, 18, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Langbein, L.; Schweizer, J. Keratins of the human hair follicle. Int. Rev. Cytol. 2005, 243, 1–79. [Google Scholar]
- Ebling, F.J. The hair cycle and its regulation. Clin. Dermatol. 1988, 6, 67–73. [Google Scholar] [CrossRef]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; McKay, I.A.; Stenn, K.S. A Comprehensive Guide for the Accurate Classification of Murine Hair Follicles in Distinct Hair Cycle Stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef]
- Collins, I.S. The role of the clinical pharmacologist in the teaching hospital. Med. J. Aust. 1975, 1, 787–789. [Google Scholar] [CrossRef]
- Paus, R.; Christoph, T.; Müller-Röver, S. Immunology of the hair follicle: A short journey into terra incognita. J. Investig. Dermatol. Symp. Proc. 1999, 4, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Mayer, J.A.; de la Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.-M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef]
- Danilenko, D.M.; Ring, B.D.; Pierce, G.F. Growth factors and cytokines in hair follicle development and cycling: Recent insights from animal models and the potentials for clinical therapy. Mol. Med. Today 1996, 2, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Stenn, K.S.; Combates, N.J.; Eilertsen, K.J.; Gordon, J.S.; Pardinas, J.R.; Parimoo, S.; Prouty, S.M. Hair follicle growth controls. Dermatol. Clin. 1996, 14, 543–558. [Google Scholar] [CrossRef] [PubMed]
- Oshimori, N.; Fuchs, E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 2012, 10, 63–75. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.; Paus, R. Molecular principles of hair follicle induction and morphogenesis. Bioessays 2005, 27, 247–261. [Google Scholar] [CrossRef]
- Ghadially, F.N. Effect of trauma on growth of hair. Nature 1958, 181, 993. [Google Scholar] [CrossRef] [PubMed]
- Li, L.F.; Fiedler, V.C.; Kumar, R. Induction of hair growth by skin irritants and its relation to skin protein kinase C isoforms. Br. J. Dermatol. 1999, 140, 616–623. [Google Scholar] [CrossRef]
- Chase, H.B.; Eaton, G.J. The growth of hair follicles in waves. Ann. N. Y. Acad. Sci. 1959, 83, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Foitzik, K. Biology of the Hair Follicle: The Basics. Semin. Cutan. Med. Surg. 2006, 25, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Paus, R. Melanogenesis is coupled to murine anagen: Toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J. Investig. Dermatol. 1993, 101, 90S–97S. [Google Scholar] [CrossRef]
- Van Neste, D.; Tobin, D.J. Hair cycle and hair pigmentation: Dynamic interactions and changes associated with aging. Micron 2004, 35, 193–200. [Google Scholar] [CrossRef]
- Mecklenburg, L.; Tobin, D.J.; Müller-Röver, S.; Handjiski, B.; Wendt, G.; Peters, E.M.; Pohl, S.; Moll, I.; Paus, R. Active hair growth (anagen) is associated with angiogenesis. J. Investig. Dermatol. 2000, 114, 909–916. [Google Scholar] [CrossRef]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef]
- Hansen, L.A.; Alexander, N.; Hogan, M.E.; Sundberg, J.P.; Dlugosz, A.; Threadgill, D.W.; Magnuson, T.; Yuspa, S.H. Genetically null mice reveal a central role for epidermal growth factor receptor in the differentiation of the hair follicle and normal hair development. Am. J. Pathol. 1997, 150, 1959–1975. [Google Scholar] [PubMed]
- Murillas, R.; Larcher, F.; Conti, C.J.; Santos, M.; Ullrich, A.; Jorcano, J.L. Expression of a dominant negative mutant of epidermal growth factor receptor in the epidermis of transgenic mice elicits striking alterations in hair follicle development and skin structure. EMBO J. 1995, 14, 5216–5223. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Paus, R.; Plonka, P.; Chakraborty, A.; Maurer, M.; Pruski, D.; Lukiewicz, S. Melanogenesis During the Anagen-Catagen-Telogen Transformation of the Murine Hair Cycle. J. Investig. Dermatol. 1994, 102, 862–869. [Google Scholar] [CrossRef]
- Ahmad, W.; Haque, M.F.U.; Brancolini, V.; Tsou, H.C.; Haque, S.U.; Lam, H.; Aita, V.M.; Owen, J.; Deblaquiere, M.; Frank, J.; et al. Alopecia Universalis Associated with a Mutation in the Human hairless Gene. Science 1998, 279, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Panteleyev, A.A.; van der Veen, C.; Rosenbach, T.; Müller-Röver, S.; Sokolov, V.E.; Paus, R. Towards Defining the Pathogenesis of the Hairless Phenotype. J. Investig. Dermatol. 1998, 110, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.B.; Beaudoin, G.M.; DeRenzo, C.L.; Zarach, J.M.; Chen, S.H.; Thompson, C.C. The hairless gene mutated in congenital hair loss disorders encodes a novel nuclear receptor corepressor. Genes. Dev. 2001, 15, 2687–2701. [Google Scholar] [CrossRef] [PubMed]
- Sprecher, E.; Bergman, R.; Szargel, R.; Friedman-Birnbaum, R.; Cohen, N. Identification of a Genetic Defect in the Hairless Gene in Atrichia with Papular Lesions: Evidence for Phenotypic Heterogeneity among Inherited Atrichias. Am. J. Hum. Genet. 1999, 64, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Cichon, S.; Anker, M.; Vogt, I.R.; Rohleder, H.; Pützstück, M.; Hillmer, A.; Farooq, S.A.; Al-Dhafri, K.S.; Ahmad, M.; Haque, S.; et al. Cloning, Genomic Organization, Alternative Transcripts and Mutational Analysis of the Gene Responsible for Autosomal Recessive Universal Congenital Alopecia. Hum. Mol. Genet. 1998, 7, 1671–1679. [Google Scholar] [CrossRef]
- Kealey, T.; Philpott, M.; Guy, R. 1 The regulatory biology of the human pilosebaceous unit. Baillière’s Clin. Obs. Gynaecol. 1997, 11, 205–227. [Google Scholar] [CrossRef]
- Lindner, G.; Menrad, A.; Gherardi, E.; Merlino, G.; Welker, P.; Handjiski, B.; Roloff, B.; Paus, R. Involvement of hepatocyte growth factor/scatter factor and Met receptor signaling in hair follicle morphogenesis and cycling. FASEB J. 2000, 14, 319–332. [Google Scholar] [CrossRef]
- Foitzik, K.; Lindner, G.; Mueller-Roever, S.; Maurer, M.; Botchkareva, N.; Botchkarev, V.; Handjiski, B.; Metz, M.; Hibino, T.; Soma, T.; et al. Control of murine hair follicle regression (catagen) by TGF-β1 in vivo. FASEB J. 2000, 14, 752–760. [Google Scholar]
- Botchkarev, V.A.; Botchkareva, N.V.; Albers, K.M.; van der Veen, C.; Lewin, G.R.; Paus, R. Neurotrophin-3 Involvement in the Regulation of Hair Follicle Morphogenesis. J. Investig. Dermatol. 1998, 111, 279–285. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Botchkareva, N.V.; Lommatzsch, M.; Peters, E.M.J.; Lewin, G.R.; Subramaniam, A.; Braun, A.; Renz, H.; Paus, R. BDNF overexpression induces differential increases among subsets of sympathetic innervation in murine back skin. Eur. J. Neurosci. 1998, 10, 3276–3283. [Google Scholar] [CrossRef]
- Botchkarev, V.A.; Peters, E.M.J.; Botchkareva, N.V.; Maurer, M.; Paus, R. Hair Cycle-Dependent Changes in Adrenergic Skin Innervation, and Hair Growth Modulation by Adrenergic Drugs. J. Investig. Dermatol. 1999, 113, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Handjiski, B.; Czarnetzki, B.M.; Eichmüller, S. A Murine Model for Inducing and Manipulating Hair Follicle Regression (Catagen): Effects of Dexamethasone and Cyclosporin A. J. Investig. Dermatol. 1994, 103, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Ohnemus, U.; Paus, R.; Ünalan, M.; Handjiski, B. Topical Estrogen Accelerates Hair Regrowth in Mice After Chemotherapy-Induced Alopecia by Favoring the Dystrophic Catagen Response Pathway to Damage. J. Investig. Dermatol. 2004, 122, 7–13. [Google Scholar] [CrossRef]
- Kwack, M.H.; Ben Hamida, O.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Dexamethasone, a Synthetic Glucocorticoid, Induces the Activity of Androgen Receptor in Human Dermal Papilla Cells. Ski. Pharmacol. Physiol. 2022, 35, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Arck, P.C.; Handjiski, B.; Peters, E.M.J.; Peter, A.S.; Hagen, E.; Fischer, A.; Klapp, B.F.; Paus, R. Stress Inhibits Hair Growth in Mice by Induction of Premature Catagen Development and Deleterious Perifollicular Inflammatory Events via Neuropeptide Substance P-Dependent Pathways. Am. J. Pathol. 2003, 162, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Bell, F.P.; Vidmar, T.J.; Raymond, T.L. L-carnitine administration and withdrawal affect plasma and hepatic carnitine concentrations, plasma lipid and lipoprotein composition, and in vitro hepatic lipogenesis from labeled mevalonate and oleate in normal rabbits. J. Nutr. 1992, 122, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Chanda, S.; Robinette, C.L.; Couse, J.F.; Smart, R.C. 17beta-estradiol and ICI-182780 regulate the hair follicle cycle in mice through an estrogen receptor-alpha pathway. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E202–E210. [Google Scholar] [CrossRef] [PubMed]
- Ohnemus, U.; Uenalan, M.; Inzunza, J.; Gustafsson, J.-A.; Paus, R. The Hair Follicle as an Estrogen Target and Source. Endocr. Rev. 2006, 27, 677–706. [Google Scholar] [CrossRef]
- Norwood, O.T. Incidence of female androgenetic alopecia (female pattern alopecia). Dermatol. Surg. 2001, 27, 53–54. [Google Scholar]
- Jahoda, C.A.; Reynolds, A.J. Dermal-epidermal interactions. Adult follicle-derived cell populations and hair growth. Dermatol. Clin. 1996, 14, 573–583. [Google Scholar] [CrossRef]
- Kaufman, K.D. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol. Clin. 1996, 14, 697–711. [Google Scholar] [CrossRef]
- Ludwig, E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br. J. Dermatol. 1977, 97, 247–254. [Google Scholar] [CrossRef]
- Hamilton, J.B. Patterned loss of hair in man; types and incidence. Ann. N. Y. Acad. Sci. 1951, 53, 708–728. [Google Scholar] [CrossRef]
- Hibberts, N.A.; Howell, A.E.; Randall, V.A. Balding hair follicle dermal papilla cells contain higher levels of androgen receptors than those from non-balding scalp. J. Endocrinol. 1998, 156, 59–65. [Google Scholar] [CrossRef]
- Chang, C.; Saltzman, A.; Yeh, S.; Young, W.; Keller, E.T.; Lee, H.-J.; Wang, C.; Mizokami, A. Androgen receptor: An overview. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Yamaguchi, Y.; Hamada, K.; Yoshikawa, K.; Itami, S. Expression of mRNA for androgen receptor, 5alpha-reductase and 17beta-hydroxysteroid dehydrogenase in human dermal papilla cells. Br. J. Dermatol. 1999, 141, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Premature senescence of balding dermal papilla cells in vitro is associated with p16(INK4a) expression. J. Investig. Dermatol. 2008, 128, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Randall, V.A.; Hibberts, N.A.; Hamada, K. A comparison of the culture and growth of dermal papilla cells from hair follicles from non-balding and balding (androgenetic alopecia) scalp. Br. J. Dermatol. 1996, 134, 437–444. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Huang, Y.-C.; Huang, K.-S.; Chan, C.-C.; Chiu, H.-Y.; Tsai, R.-Y.; Chan, J.-Y.; Lin, S.-J. Stress-induced premature senescence of dermal papilla cells compromises hair follicle epithelial-mesenchymal interaction. J. Dermatol. Sci. 2017, 86, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Lee, S.A.; Li, K.N.; Tumbar, T. Stem cell-intrinsic mechanisms regulating adult hair follicle homeostasis. Exp. Dermatol. 2021, 30, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Jaworsky, C.; Kligman, A.M.; Murphy, G.F. Characterization of inflammatory infiltrates in male pattern alopecia: Implications for pathogenesis. Br. J. Dermatol. 1992, 127, 239–246. [Google Scholar] [CrossRef]
- Peyravian, N.; Deo, S.; Daunert, S.; Jimenez, J.J. The Inflammatory Aspect of Male and Female Pattern Hair Loss. J. Inflamm. Res. 2020, 13, 879–881. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.A. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J. Am. Acad. Dermatol. 1993, 28, 755–763. [Google Scholar] [CrossRef]
- Lattanand, A.; Johnson, W.C. Male pattern alopecia a histopathologic and histochemical study. J. Cutan. Pathol. 1975, 2, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Sueki, H.; Stoudemayer, T.; Kligman, A.M.; Murphy, G.F. Quantitative and ultrastructural analysis of inflammatory infiltrates in male pattern alopecia. Acta Derm. Venereol. 1999, 79, 347–350. [Google Scholar] [PubMed]
- Magro, C.M.; Rossi, A.; Poe, J.; Manhas-Bhutani, S.; Sadick, N. The role of inflammation and immunity in the pathogenesis of androgenetic alopecia. J. Drugs Dermatol. 2011, 10, 1404–1411. [Google Scholar]
- Martinez-Jacobo, L.; Ancer-Arellano, C.I.; Ortiz-Lopez, R.; Salinas-Santander, M.; Villarreal-Villarreal, C.D.; Ancer-Rodriguez, J.; Camacho-Zamora, B.; Zomosa-Signoret, V.; la Garza, C.E.M.-D.; Ocampo-Candiani, J.; et al. Evaluation of the Expression of Genes Associated with Inflammation and Apoptosis in Androgenetic Alopecia by Targeted RNA-Seq. Ski. Appendage Disord. 2018, 4, 268–273. [Google Scholar] [CrossRef]
- Mahe, Y.F.; Michelet, J.-F.; Billoni, N.; Jarrousse, F.; Buan, B.; Commo, S.; Saint-Leger, D.; Bernard, B.A. Androgenetic alopecia and microinflammation. Int. J. Dermatol. 2000, 39, 576–584. [Google Scholar] [CrossRef]
- Ho, B.S.-Y.; Ho, E.X.P.; Chu, C.W.; Ramasamy, S.; Bigliardi-Qi, M.; de Sessions, P.F.; Bigliardi, P.L. Microbiome in the hair follicle of androgenetic alopecia patients. PLoS ONE 2019, 14, e0216330. [Google Scholar] [CrossRef]
- Rushton, D.H. Nutritional factors and hair loss. Clin. Exp. Dermatol. 2002, 27, 396–404. [Google Scholar] [CrossRef]
- Trüeb, R.M. Is androgenetic alopecia a photoaggravated dermatosis? Dermatology 2003, 207, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Gibney, E.R.; Nolan, C.M. Epigenetics and gene expression. Heredity 2010, 105, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Naue, J.; Winkelmann, J.; Schmidt, U.; Lutz-Bonengel, S. Analysis of age-dependent DNA methylation changes in plucked hair samples using massive parallel sequencing. Rechtsmedizin 2021, 31, 226–233. [Google Scholar] [CrossRef]
- Dai, Z.; Ramesh, V.; Locasale, J.W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 2020, 21, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Rückert, R.; Lindner, G.; Bulfone-Paus, S.; Paus, R. High-dose proinflammatory cytokines induce apoptosis of hair bulb keratinocytes in vivo. Br. J. Dermatol. 2000, 143, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Chovarda, E.; Sotiriou, E.; Lazaridou, E.; Vakirlis, E.; Ioannides, D. The role of prostaglandins in androgenetic alopecia. Int. J. Dermatol. 2021, 60, 730–735. [Google Scholar] [CrossRef]
- Kaufman, K.D. Androgens and alopecia. Mol. Cell. Endocrinol. 2002, 198, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Won, C.H.; Kwon, O.S.; Kim, Y.K.; Kang, Y.J.; Kim, B.J.; Choi, C.W.; Eun, H.C.; Cho, K.H. Dermal fibrosis in male pattern hair loss: A suggestive implication of mast cells. Arch. Dermatol. Res. 2008, 300, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Plotczyk, M.; Jiménez, F.; Limbu, S.; Boyle, C.J.; Ovia, J.; Almquist, B.D.; Higgins, C.A. Anagen hair follicles transplanted into mature human scars remodel fibrotic tissue. NPJ Regen. Med. 2023, 8, 1. [Google Scholar] [CrossRef]
- English, R.S. A hypothetical pathogenesis model for androgenic alopecia: Clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Med. Hypotheses 2018, 111, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.V.; Hermann, D.J.; Cunningham, G.R.; Wilson, T.H.; Morrill, B.B.; Hobbs, S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor. J. Clin. Endocrinol. Metab. 2004, 89, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Arif, T.; Dorjay, K.; Adil, M.; Sami, M. Dutasteride in Androgenetic Alopecia: An Update. Curr. Clin. Pharmacol. 2017, 12, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, J.; Kaufman, K.D. Use of finasteride in the treatment of men with androgenetic alopecia (male pattern hair loss). J. Investig. Dermatol. Symp. Proc. 2003, 8, 20–23. [Google Scholar] [CrossRef]
- Zappacosta, A.R. Reversal of baldness in patient receiving minoxidil for hypertension. N. Engl. J. Med. 1980, 303, 1480–1481. [Google Scholar]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Buhl, A.E.; Waldon, D.J.; Baker, C.A.; Johnson, G.A. Minoxidil sulfate is the active metabolite that stimulates hair follicles. J. Investig. Dermatol. 1990, 95, 553–557. [Google Scholar] [CrossRef]
- Dooley, T.P.; Walker, C.J.; Hirshey, S.J.; Falany, C.N.; Diani, A.R. Localization of minoxidil sulfotransferase in rat liver and the outer root sheath of anagen pelage and vibrissa follicles. J. Investig. Dermatol. 1991, 96, 65–70. [Google Scholar] [CrossRef]
- Roberts, J.; Desai, N.; McCoy, J.; Goren, A. Sulfotransferase activity in plucked hair follicles predicts response to topical minoxidil in the treatment of female androgenetic alopecia. Dermatol. Ther. 2014, 27, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Whiting, D.; Bergfeld, W.; Miller, J.; Hordinsky, M.; Wanser, R.; Zhang, P.; Kohut, B. A multicenter, randomized, placebo-controlled, double-blind clinical trial of a novel formulation of 5% minoxidil topical foam versus placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2007, 57, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Lucky, A.W.; Piacquadio, D.J.; Ditre, C.M.; Dunlap, F.; Kantor, I.; Pandya, A.G.; Savin, R.C.; Tharp, M.D. A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J. Am. Acad. Dermatol. 2004, 50, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Dunlap, F.E.; Funicella, T.; Koperski, J.A.; Swinehart, J.M.; Tschen, E.H.; Trancik, R.J. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J. Am. Acad. Dermatol. 2002, 47, 377–385. [Google Scholar] [CrossRef]
- Ghonemy, S.; Alarawi, A.; Bessar, H. Efficacy and safety of a new 10% topical minoxidil versus 5% topical minoxidil and placebo in the treatment of male androgenetic alopecia: A trichoscopic evaluation. J. Dermatol. Treat. 2021, 32, 236–241. [Google Scholar] [CrossRef]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J.L. Minoxidil upregulates the expression of vascular endothelial growth factor in human hair dermal papilla cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Kwack, M.H.; Kang, B.M.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Minoxidil activates β-catenin pathway in human dermal papilla cells: A possible explanation for its anagen prolongation effect. J. Dermatol. Sci. 2011, 62, 154–159. [Google Scholar] [CrossRef]
- Michelet, J.F.; Commo, S.; Billoni, N.; Mahé, Y.F.; Bernard, B.A. Activation of cytoprotective prostaglandin synthase-1 by minoxidil as a possible explanation for its hair growth-stimulating effect. J. Investig. Dermatol. 1997, 108, 205–209. [Google Scholar] [CrossRef]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci. Transl. Med. 2012, 4, 126ra34. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The effectiveness of treatments for androgenetic alopecia: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes. Dev. 2000, 14, 1181–1185. [Google Scholar] [CrossRef] [PubMed]
- Myung, P.S.; Takeo, M.; Ito, M.; Atit, R.P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 2013, 133, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Yoon, J.; Shin, S.H.; Zahoor, M.; Kim, H.J.; Park, P.J.; Park, W.-S.; Min, D.S.; Kim, H.-Y.; Choi, K.-Y. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS ONE 2012, 7, e34152. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.C.; Park, J.; Kim, Y.-R.; Choi, S.; Kim, G.-U.; Kim, E.; Hwang, Y.; Kim, H.; Han, G.; Lee, S.-H.; et al. CXXC5 Mediates DHT-Induced Androgenetic Alopecia via PGD2. Cells 2023, 12, 555. [Google Scholar] [CrossRef] [PubMed]
- Hebert, A.; Thiboutot, D.; Gold, L.S.; Cartwright, M.; Gerloni, M.; Fragasso, E.; Mazzetti, A. Efficacy and Safety of Topical Clascoterone Cream, 1%, for Treatment in Patients with Facial Acne: Two Phase 3 Randomized Clinical Trials. JAMA Dermatol. 2020, 156, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gao, Y.; Yuan, Y.; Yang, K.; Shen, C.; Wang, J.; Tian, J. Janus Kinase Inhibitors for Alopecia Areata: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2320351. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Driscoll, M.S. Review of Baricitinib in the Treatment of Alopecia Areata. J. Drugs Dermatol. 2023, 22, 935–940. [Google Scholar]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Johnstone, M.A. Hypertrichosis and increased pigmentation of eyelashes and adjacent hair in the region of the ipsilateral eyelids of patients treated with unilateral topical latanoprost. Am. J. Ophthalmol. 1997, 124, 544–547. [Google Scholar] [CrossRef]
- Johnstone, M.A.; Albert, D.M. Prostaglandin-induced hair growth. Surv. Ophthalmol. 2002, 47 (Suppl. S1), S185–S202. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Lönnfors, S.; Hillmann, K.; Garcia Bartels, N. A randomized double-blind placebo-controlled pilot study to assess the efficacy of a 24-week topical treatment by latanoprost 0.1% on hair growth and pigmentation in healthy volunteers with androgenetic alopecia. J. Am. Acad. Dermatol. 2012, 66, 794–800. [Google Scholar] [CrossRef]
- Chew, E.G.Y.; Ho, B.S.-Y.; Ramasamy, S.; Dawson, T.; Tennakoon, C.; Liu, X.; Leong, W.M.S.; Yang, S.Y.S.; Lim, S.Y.D.; Jaffar, H.; et al. Comparative transcriptome profiling provides new insights into mechanisms of androgenetic alopecia progression. Br. J. Dermatol. 2017, 176, 265–269. [Google Scholar] [CrossRef]
- Michel, L.; Reygagne, P.; Benech, P.; Jean-Louis, F.; Scalvino, S.; So, S.L.K.; Hamidou, Z.; Bianovici, S.; Pouch, J.; Ducos, B.; et al. Study of gene expression alteration in male androgenetic alopecia: Evidence of predominant molecular signalling pathways. Br. J. Dermatol. 2017, 177, 1322–1336. [Google Scholar] [CrossRef]
- Dey-Rao, R.; Sinha, A.A. A genomic approach to susceptibility and pathogenesis leads to identifying potential novel therapeutic targets in androgenetic alopecia. Genomics 2017, 109, 165–176. [Google Scholar] [CrossRef]
- Vogt, A.; Pfannes, E.K.B.; Fimmel, S.; Hadam, S.; Andruck, A.; Kottner, J.; Blume-Peytavi, U. Infundibular protein and RNA microarray analyses from affected and clinically non-affected scalp in male androgenetic alopecia patients. Exp. Dermatol. 2017, 26, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Yu, A.; Luo, Y.; Tian, T.; Dong, Y.; Zong, H.; Chen, H.; Gao, X.; Xu, X.; Li, Y. Genomewide differential expression profiling of long non-coding RNAs in androgenetic alopecia in a Chinese male population. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Hochfeld, L.M.; Keller, A.; Anhalt, T.; Fricker, N.; Nöthen, M.M.; Heilmann-Heimbach, S. Insights into Male Androgenetic Alopecia: Differential Gene Expression Profiling of Plucked Hair Follicles and Integration with Genetic Data. J. Investig. Dermatol. 2019, 139, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Qu, Q.; Jiang, W.; Shi, P.-L.; Fan, Z.-X.; Du, L.-J.; Wang, G.-F.; Liu, X.-N.; Guo, Z.-H.; Liu, Y.; et al. Identification of Functional Patterns of Androgenetic Alopecia Using Transcriptome Profiling in Distinct Locations of Hair Follicles. J. Investig. Dermatol. 2018, 138, 972–975. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, Y.; Huang, Y.; Wang, J.; Yang, K.; Zhang, Y.; Pu, W.; Liu, J.; Shi, X.; Ma, Y.; et al. Insights into male androgenetic alopecia using comparative transcriptome profiling: Hypoxia-inducible factor-1 and Wnt/β-catenin signalling pathways. Br. J. Dermatol. 2022, 187, 936–947. [Google Scholar] [CrossRef]
- Lin, K.K.; Chudova, D.; Hatfield, G.W.; Smyth, P.; Andersen, B. Identification of hair cycle-associated genes from time-course gene expression profile data by using replicate variance. Proc. Natl. Acad. Sci. USA 2004, 101, 15955–15960. [Google Scholar] [CrossRef]
- Joost, S.; Zeisel, A.; Jacob, T.; Sun, X.; La Manno, G.; Lönnerberg, P.; Linnarsson, S.; Kasper, M. Single-Cell Transcriptomics Reveals that Differentiation and Spatial Signatures Shape Epidermal and Hair Follicle Heterogeneity. Cell Syst. 2016, 3, 221–237.e9. [Google Scholar] [CrossRef]
- Joost, S.; Annusver, K.; Jacob, T.; Sun, X.; Dalessandri, T.; Sivan, U.; Sequeira, I.; Sandberg, R.; Kasper, M. The Molecular Anatomy of Mouse Skin during Hair Growth and Rest. Cell Stem Cell 2020, 26, 441–457.e7. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, R.; Grzenda, A.; Allison, T.F.; Rawnsley, J.; Balin, S.J.; Sabri, S.; Plath, K.; Lowry, W.E. Defining Transcriptional Signatures of Human Hair Follicle Cell States. J. Investig. Dermatol. 2020, 140, 764–773.e4. [Google Scholar] [CrossRef] [PubMed]
- Nyholt, D.R.; Gillespie, N.A.; Heath, A.C.; Martin, N.G. Genetic basis of male pattern baldness. J. Investig. Dermatol. 2003, 121, 1561–1564. [Google Scholar] [PubMed]
- Ellis, J.A.; Stebbing, M.; Harrap, S.B. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J. Investig. Dermatol. 2001, 116, 452–455. [Google Scholar] [CrossRef]
- Hillmer, A.M.; Hanneken, S.; Ritzmann, S.; Becker, T.; Freudenberg, J.; Brockschmidt, F.F.; Flaquer, A.; Freudenberg-Hua, Y.; Jamra, R.A.; Metzen, C.; et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am. J. Hum. Genet. 2005, 77, 140–148. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Carithers, L.J.; Moore, H.M. The Genotype-Tissue Expression (GTEx) Project. Biopreserv. Biobank. 2015, 13, 307–308. [Google Scholar] [CrossRef]
- Hillmer, A.M.; Brockschmidt, F.F.; Hanneken, S.; Eigelshoven, S.; Steffens, M.; Flaquer, A.; Herms, S.; Becker, T.; Kortüm, A.-K.; Nyholt, D.R.; et al. Susceptibility variants for male-pattern baldness on chromosome 20p11. Nat. Genet. 2008, 40, 1279–1281. [Google Scholar] [CrossRef] [PubMed]



| Sample Size | Comparisons | Extraction Method | Number of DEGs in AGA | Upregulated Gene Sets in AGA | Down Regulated Gene Sets in AGA | Technology/ Platform | PMID/ Repository |
|---|---|---|---|---|---|---|---|
| 5 male patients | HFs from bald vs. haired regions | FUE | 250 (169 up, 81 down) | Immune response | Keratins | Affymetrix HG-U133A | 22440736/GSE36169 |
| 20 patients, 10 healthy controls | Hair bulbs were used. Patient vertex vs. control vertex, patient vertex vs. patient occipital, patient occipital vs. control occipital, control occipital vs. control vertex | FUE | 1339 (692 up, 647 down) (Patient vertex vs. Control vertex + occipital) | Lipid synthesis, electron carrier activity, metabolites, and energy | Keratin, epidermis development, cell cycle, hair follicle morphogenesis | Illumina HiSeq | 27239811/NA |
| 14 young (<35) patients (premature AGA) and 14 healthy controls | Scalp vertex, patients vs. controls | Biopsy | 333 (184 up, 149 down) | Immune and inflammatory responses | WNT/β-catenin, TGF-β, BMP, and vitamin D biosynthesis | Agilent Whole Human Genome Oligo Microarrays | 28403520/GSE90594 |
| 3 male patients | Scalp biopsies from bald and haired portions | Biopsy | 431 (258 up, 173 down) | Cell proliferation, apoptosis, MAPK signaling, WNT signaling, EMT, TGF-β, GDF, and Activin * | Affymetrix Human Genome U952A2 microarray chips | 28263792/NA | |
| 6 male patients | HFs from frontotemporal, vertex, and occipital regions | plucking | 744 (336 up, 308 down) | Apoptosis, RNA methylation, ion channels | Cholinergic receptors, keratin production | Agilent Whole Human Genome Oligo Microarrays | 28266729/GSE78722 |
| 10 male patients | Paired HFs from the balding and haired regions | Biopsy | 2143 lncRNAs (770 up, 1373 down) | Metabolic process, T-cell receptor signaling | Developmental process, nervous system development, Hedgehog signaling | Human lncRNA Expression Microarray | 28419572/GSE84839 |
| 24 male patients | Paired HFs from frontal vs. occipital scalp | plucking | 143 miRNAs and 2836 mRNAs | Ceramide biosynthesis, GADD45 signaling * | Illumina HT12 Gene expression BeadChip, Affymetrix miRNA 4.0 Array | 30009830/EGAS00001002832 | |
| 12 male patients, 12 healthy controls | Bulge HFSCs vs. DP, AGA vs. controls, balding vs. non-balding areas | FUE | NA | Inflammation, stress, fibrosis | NA | Illumina HiSeq 2500 | 29122575/GSE101451 |
| 10 male patients | Paired HFs from the vertex and occipital scalp of 10 male patients with AGA | FUE | mRNAs: 308 up, 198 down; miRNAs: 35 up, 20 down; lncRNAs: 53 up, 74 down | HIF-1 signaling pathway | WNT and Hippo signaling pathways | Illumina HiSeqX Ten | 35862273/NA |
| 10 male patients | Paired HFs from the frontal and occipital scalp of 10 male patients with AGA | NA | NA | NA | NA | Illumina HiSeq 2500 | NA/GSE212301 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Diaz Duran, R.; Martinez-Ledesma, E.; Garcia-Garcia, M.; Bajo Gauzin, D.; Sarro-Ramírez, A.; Gonzalez-Carrillo, C.; Rodríguez-Sardin, D.; Fuentes, A.; Cardenas-Lopez, A. The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia. Int. J. Mol. Sci. 2024, 25, 2542. https://doi.org/10.3390/ijms25052542
Cuevas-Diaz Duran R, Martinez-Ledesma E, Garcia-Garcia M, Bajo Gauzin D, Sarro-Ramírez A, Gonzalez-Carrillo C, Rodríguez-Sardin D, Fuentes A, Cardenas-Lopez A. The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia. International Journal of Molecular Sciences. 2024; 25(5):2542. https://doi.org/10.3390/ijms25052542
Chicago/Turabian StyleCuevas-Diaz Duran, Raquel, Emmanuel Martinez-Ledesma, Melissa Garcia-Garcia, Denisse Bajo Gauzin, Andrea Sarro-Ramírez, Carolina Gonzalez-Carrillo, Denise Rodríguez-Sardin, Alejandro Fuentes, and Alejandro Cardenas-Lopez. 2024. "The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia" International Journal of Molecular Sciences 25, no. 5: 2542. https://doi.org/10.3390/ijms25052542
APA StyleCuevas-Diaz Duran, R., Martinez-Ledesma, E., Garcia-Garcia, M., Bajo Gauzin, D., Sarro-Ramírez, A., Gonzalez-Carrillo, C., Rodríguez-Sardin, D., Fuentes, A., & Cardenas-Lopez, A. (2024). The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia. International Journal of Molecular Sciences, 25(5), 2542. https://doi.org/10.3390/ijms25052542





