FLASH Radiotherapy: Expectations, Challenges, and Current Knowledge
Abstract
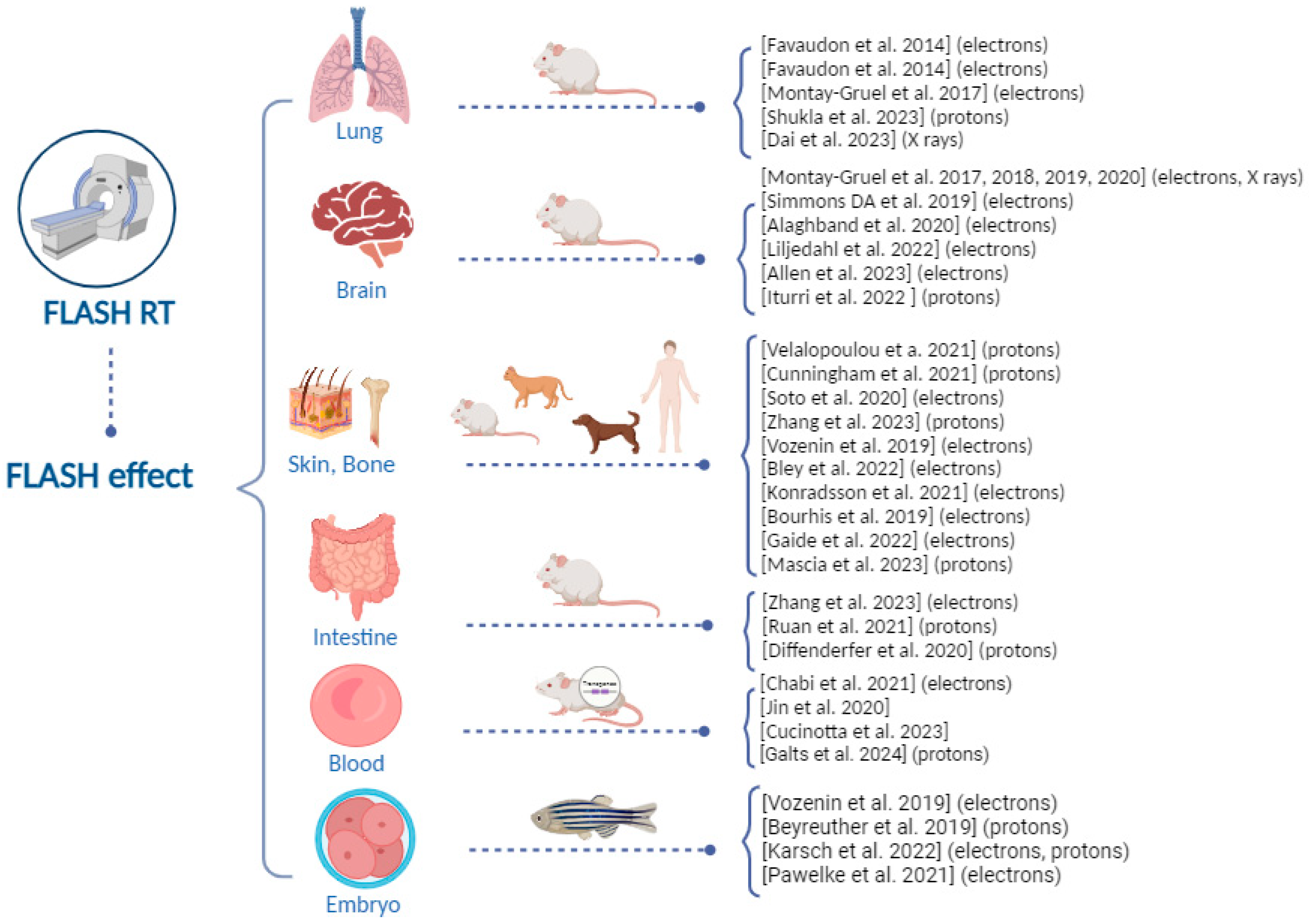
1. Introduction
2. FLASH Radiotherapy: Tumor and Normal Tissue Responses
2.1. Lung Tissue
2.2. Brain Tissue
2.3. Skin Tissue
2.4. Intestine Tissue
2.5. Blood Tissue
2.6. Zebrafish as an Emerging Model System
3. Treatment of Human Patients and First Clinical Trials
4. Biological Mechanisms behind the FLASH Effect: The Role of DNA Damage
5. Technologies for FLASH Radiation Beams
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Favaudon, V.; Caplier, L.; Monceau, V.; Pouzoulet, F.; Sayarath, M.; Fouillade, C.; Poupon, M.F.; Brito, I.; Hupé, P.; Bourhis, J.; et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci. Transl. Med. 2014, 6, 245ra93. [Google Scholar] [CrossRef]
- Farr, J.B.; Parodi, K.; Carlson, D.J. FLASH: Current status and the transition to clinical use. Med. Phys. 2022, 49, 1972–1973. [Google Scholar] [CrossRef]
- Vozenin, M.-C.; Hendry, J.H.; Limoli, C.L. Biological Benefits of Ultra-High Dose Rate FLASH Radiotherapy: Sleeping Beauty Awoken. Clin. Oncol. 2019, 31, 407–415. [Google Scholar] [CrossRef]
- Limoli, C.L.; Vozenin, M.-C. Reinventing radiobiology in the light of FLASH radiotherapy. Annu. Rev. Cancer Biol. 2023, 7, 1–21. [Google Scholar] [CrossRef]
- Friedl, A.A.; Prise, K.M.; Butterworth, K.T.; Montay-Gruel, P.; Favaudon, V. Radiobiology of the FLASH effect. Med. Phys. 2022, 49, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Kacem, H.; Almeida, A.; Cherbuin, N.; Vozenin, M.C. Understanding the FLASH effect to unravel the potential of ultra-high dose rate irradiation. Int. J/ Radiat. Biol. 2022, 98, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, E.; Macaeva, E.; Isebaert, S.; Haustermans, K. Potential Molecular Mechanisms behind the Ultra-High Dose Rate “FLASH” Effect. Int. J. Mol. Sci. 2022, 23, 12109. [Google Scholar] [CrossRef]
- Hageman, E.; Pei-Pei, C.; Dahele, M.; Slotman, B.J.; Sminia, P. Radiobiological Aspects of FLASH Radiotherapy. Rev. Biomol. 2022, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Borghini, A.; Vecoli, C.; Labate, L.; Panetta, D.; Andreassi, M.G.; Gizzi, L.A. FLASH ultra-high dose rates in radiotherapy: Preclinical and radiobiological evidence. Int. J. Radiat. Biol. 2022, 98, 127–135. [Google Scholar] [CrossRef]
- Vozenin, M.C.; Bourhis, J.; Durante, M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 2022, 19, 791–803. [Google Scholar] [CrossRef]
- Dewey, D.L.; Boag, J.W. Modification of the oxygen effect when bacteria are given large pulses of radiation. Nature 1959, 183, 1450–1451. [Google Scholar] [CrossRef]
- Town, C.D. Effect of High Dose-Rates on Survival of Mammalian Cells. Nature 1967, 215, 847–848. [Google Scholar] [CrossRef]
- Berry, R.J.; Hall, E.J.; Forster, D.W.; Storr, T.H.; Goodman, M.J. Survival of Mammalian Cells Exposed to X Rays At Ultra-High Dose-Rates. Br. J. Radiol. 1969, 42, 102–107. [Google Scholar] [CrossRef]
- Montay-Gruel, P.; Petersson, K.; Jaccard, M.; Boivin, G.; Germond, J.F.; Petit, B.; Doenlen, R.; Favaudon, V.; Bochud, F.; Bailat, C.; et al. Irradiation in a flash: Unique sparing of memory in mice after whole brain irradiation with dose rates above 100 Gy/s. Radiother. Oncol. 2017, 124, 365–369. [Google Scholar] [CrossRef]
- Favaudon, V.; Fouillade, C.; Vozenin, M.C. Ultrahigh dose-rate, “flash” irradiation minimizes the side-effects of radiotherapy. Cancer Radiother. 2015, 19, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Saha, T.; Rama, T.; Acharya, A.; Le, T.; Bian, F.; Donovan, J.; Tan, L.A.; Vatner, R.; Kalinichenk, K.; et al. Ultra-high dose-rate proton FLASH improves tumor control. Radiother. Oncol. 2023, 186, 109741. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liang, R.; Wang, J.; Zhang, J.; Wu, D.; Zhao, R.; Liu, Z.; Chen, F. Fractionated FLASH radiation in xenografted lung tumors induced FLASH effect at a split dose of 2 Gy. Int. J. Radiat. Biol. 2023, 99, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Bouchet, A.; Jaccard, M.; Patin, D.; Serduc, R.; Aim, W.; Petersson, K.; Petit, B.; Bailat, C.; Bourhis, J.; et al. X-rays can trigger the FLASH effect: Ultra-high dose-rate synchrotron light source prevents normal brain injury after whole brain irradiation in mice. Radiother. Oncol. 2018, 129, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Petersson, K.; Alikhani, L.; Yakkala, C.; Allen, B.D.; Ollivier, J.; Petit, B.; Jorge, P.G.; Syage, A.R.; et al. Long-term neurocognitive benefits of FLASH radiotherapy driven by reduced reactive oxygen species. Proc. Natl. Acad. Sci. USA 2019, 116, 10943–10951. [Google Scholar] [CrossRef] [PubMed]
- Montay-Gruel, P.; Acharya, M.M.; Gonçalves Jorge, P.; Petit, B.; Petridis, I.G.; Fuchs, P.; Leavitt, R.; Petersson, K.; Gondré, M.; Ollivier, J.; et al. Hypo-Fractionated FLASH-RT as an Effective Treatment Against Glioblastoma That Reduces Neurocognitive Side Effects in Mice. Clin. Cancer Res. 2020, 27, 3. [Google Scholar]
- Simmons, D.A.; Lartey, F.M.; Schüler, E.; Rafat, M.; King, G.; Kim, A.; Ko, R.; Semaan, S.; Gonzalez, S.; Jenkins, M.; et al. Reduced cognitive deficits after FLASH irradiation of whole mouse brain are associated with less hippocampal dendritic spine loss and neuroinflammation. Radiother. Oncol. 2019, 139, 4–10. [Google Scholar] [CrossRef]
- Alaghband, Y.; Cheeks, S.N.; Allen, B.D.; Montay-Gruel, P.; Doan, N.L.; Petit, B.; Jorge, P.G.; Giedzinski, E.; Acharya, M.M.; Vozenin, M.C.; et al. Neuroprotection of radiosensitive juvenile mice by ultra-high dose rate FLASH irradiation. Cancers 2020, 12, 1671. [Google Scholar] [CrossRef] [PubMed]
- Liljedahl, E.; Konradsson, E.; Gustafsson, E.; Jonsson, K.F.; Olofsson, J.K.; Ceberg, C.; Redebrandt, H.N. Long-term anti-tumor effects following both conventional radiotherapy and FLASH in fully immunocompetent animals with glioblastoma. Sci. Rep. 2022, 12, 12285. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.B.; Alaghband, Y.; Kramár, E.A.; Ru, N.; Petit, B.; Grilj, V.; Petronek, M.S.; Pulliam, C.F.; Kim, R.Y.; Doan, N.-L.; et al. Elucidating the neurological mechanism of the FLASH effect in juvenile mice exposed to hypofractionated radiotherapy. Neuro. Oncol. 2023, 25, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Iturri, L.; Bertho, A.; Lamirault, C.; Juchaux, M.; Gilbert, C.; Espenon, J.; Sebrie, C.; Jourdain, L.; Pouzoulet, F.; Verrelle, P.; et al. Proton FLASH Radiation Therapy and Immune Infiltration: Evaluation in an Orthotopic Glioma Rat Model. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Velalopoulou, A.; Karagounis, I.V.; Cramer, G.M.; Kim, M.M.; Skoufos, G.G.; Goia, D.; Hagan, S.; Verginadis, I.I.; Shoniyozov, K.; Chiango, J.; et al. FLASH Proton Radiotherapy Spares Normal Epithelial and Mesenchymal Tissues While Preserving Sarcoma Response. Cancer Res. 2021, 81, 4808–4821. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; McCauley, S.; Vairamani, K.; Speth, J.; Girdhani, S.; Abel, E.; Sharma, R.A.; Perentesi, J.P.; Wells, S.I.; Mascia, A.; et al. FLASH Proton Pencil Beam Scanning Irradiation Minimizes Radiation-Induced Leg Contracture and Skin Toxicity in Mice. Cancers 2021, 13, 1012. [Google Scholar] [CrossRef] [PubMed]
- Soto, L.A.; Casey, K.M.; Wang, J.; Blaney, A.; Manjappa, R.; Breitkreutz, D.; Skinner, L.; Dutt, S.; Ko, R.B.; Bush, K.; et al. FLASH Irradiation Results in Reduced Severe Skin Toxicity Compared to Conventional-Dose-Rate Irradiation. Radiat. Res. 2020, 195, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gerweck, L.E.; Cascio, E.; Yang, Q.; Huang, P.; Niemierko, A.; Bertolet, A.; Nesteruk, K.P.; McNamara, A.; Schuemann, J. Proton FLASH effects on mouse skin at different oxygen tensions. Phys Med. Biol. 2023, 68, 5. [Google Scholar] [CrossRef]
- Vozenin, M.C.; De Fornel, P.; Petersson, K.; Favaudon, V.; Jaccard, M.; Germond, J.F.; Petit, B.; Burki, M.; Ferrand, G.; Patin, D.; et al. The Advantage of FLASH Radiotherapy Confirmed in Mini-pig and Cat-cancer Patients. Clin. Cancer Res. 2019, 25, 35–42. [Google Scholar] [CrossRef]
- Bley, C.R.; Wolf, F.; Jorge, P.G.; Grilj, V.; Petridis, I.; Petit, B.; Bohlen, T.T.; Moeckli, R.; Limoli, C.; Bourhis, J.; et al. Dose- and Volume-Limiting Late Toxicity of FLASH Radiotherapy in Cats with Squamous Cell Carcinoma of the Nasal Planum and in Mini Pigs. Clin. Cancer Res. 2022, 28, 3814–3823. [Google Scholar] [CrossRef]
- Konradsson, E.; Arendt, M.L.; Bastholm Jensen, K.; Børresen, B.; Hansen, A.E.; Bäck, S.; Kristensen, A.T.; Munck af Rosenschöld, P.; Ceberg, C.; Petersson, K. Establishment and Initial Experience of Clinical FLASH Radiotherapy in Canine Cancer Patients. Front. Oncol. 2021, 11, 658004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gerweck, L.E.; Cascio, E.; Gu, L.; Yang, Q.; Dong, X.; Huang, P.; Bertolet, A.; Nesteruk, K.P.; Sung, W.; et al. Absence of Tissue-Sparing Effects in Partial Proton FLASH Irradiation in Murine Intestine. Cancers 2023, 15, 2269. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.L.; Lee, C.; Wouters, S.; Tullis, I.D.C.; Verslegers, M.; Mysara, M.; Then, C.K.; Smart, S.C.; Hill, M.A.; Ruth, J.M.; et al. Irradiation at Ultra-High (FLASH) Dose Rates Reduces Acute Normal Tissue Toxicity in the Mouse Gastrointestinal System. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1250–1261. [Google Scholar] [CrossRef]
- Kim, M.M.; Verginadis, I.I.; Goia, D.; Haertter, A.; Shoniyozov, K.; Zou, W.; Maity, A.; Busch, T.M.; Metz, J.M.; Cengel, K.A.; et al. Comparison of FLASH Proton Entrance and the Spread-Out Bragg Peak Dose Regions in the Sparing of Mouse Intestinal Crypts and in a Pancreatic Tumor Model. Cancers 2021, 13, 4244. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xie, D.; Yang, Y.; Huang, S.; Gao, X.; Peng, Y.; Wang, B.; Wang, J.; Xiao, D.; Wu, D.; et al. Radioprotective effect of X-ray abdominal FLASH irradiation: Adaptation to oxidative damage and inflammatory response may be benefiting factors. Med. Phys. 2022, 49, 4812–4822. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Yang, Y.; Zhang, W.; Wang, J.; Xiao, D.; Ren, H.; Wang, T.; Gao, F.; Liu, Z.; Zhou, K.; et al. FLASH X-ray spares intestinal crypts from pyroptosis initiated by cGAS-STING activation upon radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2022, 119, e2208506119. [Google Scholar] [CrossRef] [PubMed]
- Diffenderfer, E.S.; Verginadis, I.I.; Kim, M.M.; Shoniyozov, K.; Velalopoulou, A.; Goia, D.; Putt, M.; Hagan, S.; Avery, S.; Teo, K.; et al. Design, implementation, and in vivo validation of a novel proton FLASH radiation therapy system. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 440–448. [Google Scholar] [CrossRef]
- Chabi, S.; To, T.H.; Leavitt, R.; Poglio, S.; Jorge, P.G.; Jaccard, M.; Petersson, K.; Petit, B.; Roméo, P.H.; Pflumio, F.; et al. Ultra-high-dose-rate FLASH and Conventional-Dose-Rate Irradiation Differentially Affect Human Acute Lymphoblastic Leukemia and Normal Hematopoiesis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 819–829. [Google Scholar] [CrossRef]
- Jin, J.Y.; Gu, A.; Wang, W.; Oleinick, N.L.; Machtay, M.; Spring Kong, F.M. Ultra-high dose rate effect on circulating immune cells: A potential mechanism for FLASH effect? Radiother. Oncol. 2020, 149, 55–62. [Google Scholar] [CrossRef]
- Cucinotta, F.A.; Smirnova, E.A. Effects of Flash Radiotherapy on Blood Lymphocytes in Humans and Small Laboratory Animals. Radiat. Res. 2023, 199, 240–251. [Google Scholar]
- Galts, A.; Hammi, A. FLASH radiotherapy sparing effect on the circulating lymphocytes in pencil beam scanning proton therapy: Impact of hypofractionation and dose rate. Phys. Med. Biol. 2024, 69, 2. [Google Scholar] [CrossRef] [PubMed]
- Beyreuther, E.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Leßmann, E.; Schürer, M.; Szabó, E.R.; Pawelke, J. Feasibility of proton FLASH effect tested by zebrafish embryo irradiation. Radiother. Oncol. 2019, 139, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Karsch, L.; Pawelke, J.; Brand, M.; Hans, S.; Hideghéty, K.; Jansen, J.; Lessmann, E.; Löck, S.; Schürer, M.; Schurig, R.; et al. Beam Pulse Structure and Dose Rate as Determinants for the Flash Effect Observed in Zebrafish Embryo. Radiother. Oncol. 2022, 173, 49–54. [Google Scholar] [CrossRef]
- Pawelke, J.; Brand, M.; Hans, S.; Hideghéty, K.; Karsch, L.; Lessmann, E.; Löck, S.; Schürer, M.; Szabó, E.R.; Beyreuther, E. Electron Dose Rate and Oxygen Depletion Protect Zebrafish Embryos from Radiation Damage. Radiother. Oncol. 2021, 158, 7–12. [Google Scholar] [CrossRef]
- Ghannam, Y.; Chiavassa, S.; Saade, G.; Koumeir, C.; Blain, G.; Delpon, G.; Evin, M.; Haddad, F.; Maigne, L.; Mouchard, Q.; et al. First evidence of in vivo effect of FLASH radiotherapy with helium ions in zebrafish embryos. Radiother. Oncol. 2023, 187, 109820. [Google Scholar] [CrossRef]
- Bourhis, J.; Sozzi, W.J.; Jorge, P.G.; Gaide, O.; Bailat, C.; Duclos, F.; Patin, D.; Ozsahin, M.; Bochud, F.; Germond, J.F.; et al. Treatment of a first patient with FLASH-radiotherapy. Radiother. Oncol. 2019, 139, 18–22. [Google Scholar] [CrossRef]
- Gaide, O.; Herrera, F.; Sozzi, J.W.; Jorge, P.G.; Kinj, R.; Bailat, C.; Duclos, F.; Bochud, F.; Germond, J.F.; Gondré, M.; et al. Comparison of ultra-high versus conventional dose rate radiotherapy in a patient with cutaneous lymphoma. Radiother. Oncol. 2022, 174, 87–91. [Google Scholar] [CrossRef]
- Daugherty, E.C.; Mascia, A.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; McCann, C.; Russell, K.; Levine, L.; et al. FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases (FAST-01): Protocol for the First Prospective Feasibility Study. JMIR. Res. Protoc. 2023, 12, e41812. [Google Scholar] [CrossRef]
- Mascia, A.E.; Daugherty, E.C.; Zhang, Y.; Lee, E.; Xiao, Z.; Sertorio, M.; Woo, J.; Backus, L.R.; McDonald, J.M.; McCann, C.; et al. Proton FLASH Radiotherapy for the Treatment of Symptomatic Bone Metastases: The FAST-01 Nonrandomized Trial. JAMA Oncol. 2023, 9, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Search of: FLASH Radiotherapy—List Results—ClinicalTrials.Gov. Available online: https://www.clinicaltrials.gov/ct2/results?cond=&term=FLASH+Radiotherapy&cntry=&state=&city&dist (accessed on 29 August 2022).
- Cao, X.; Zhang, R.; Esipova, T.V.; Allu, S.R.; Ashraf, R.; Rahman, M.; Gunn, J.R.; Bruza, P.; Gladstone, D.J.; Williams, B.B.; et al. Quantification of Oxygen Depletion During FLASH Irradiation In Vitro and In Vivo. Int.J. Radiat. Oncol. Biol. Phys. 2021, 111, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Knoll, J.; Beyreuther, E.; Pawelke, J.; Skuza, R.; Hanley, R.; Brons, S.; Pagliari, F.; Seco, J. Does FLASH deplete oxygen? Experimental evaluation for photons, protons, and carbon ions. Med. Phys. 2021, 48, 3982–3990. [Google Scholar] [CrossRef]
- El Khatib, M.; Van Slyke, A.L.; Velalopoulou, A.; Kim, M.M.; Shoniyozov, K.; Allu, S.R.; Diffenderfer, E.E.; Busch, T.M.; Wiersma, R.D.; Koch, C.J.; et al. Ultrafast Tracking of Oxygen Dynamics During Proton FLASH. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.C.; van der Kogel, A.J. Basic Clinical Radiobiology; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef]
- Gizzi, L.A.; Andreassi, M.G. Ready for tanslational research. Nat. Phys. 2022, 18, 237–238. [Google Scholar] [CrossRef]
- Kumaravel, T.S.; Jha, A.N. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res. 2006, 605, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Celeste, A.; Fernandez-Capetillo, O.; Kruhlak, M.J.; Pilch, D.R.; Staudt, D.W.; Lee, A.; Bonner, R.F.; Bonner, W.M.; Nussenzweig, A. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat. Cell. Biol. 2003, 5, 675–679. [Google Scholar] [CrossRef]
- Leatherbarrow, E.L.; Harper, J.V.; Cucinotta, F.A.; O’Neill, P. Induction and quantification of gamma-H2AX foci following low and high LET-irradiation. Int. J. Radiat. Biol. 2006, 82, 111–118. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Cytogenetic Dosimetry: Applications in Preparedness for and Response to Radiation Emergencies; IAEA: Vienna, Austria, 2011. [Google Scholar]
- Buonanno, M.; Grilj, V.; Brenner, D.J. Biological effects in normal cells exposed to FLASH dose rate protons. Radiother. Oncol. 2019, 139, 51–55. [Google Scholar] [CrossRef]
- Fouillade, C.; Curras-Alonso, S.; Giuranno, L.; Quelennec, E.; Heinrich, S.; Bonnet-Boissinot, S.; Beddok, A.; Leboucher, S.; Karakurt, H.U.; Bohec, M.; et al. FLASH Irradiation Spares Lung Progenitor Cells and Limits the Incidence of Radio-Induced Senescence. Clin. Cancer Res. 2020, 26, 1497–1506. [Google Scholar] [CrossRef]
- Bayart, E.; Flacco, A.; Delmas, O.; Pommarel, L.; Levy, D.; Cavallone, M.; Megnin-Chanet, F.; Deutsch, E.; Malka, V. Fast dose fractionation using ultra-short laser accelerated proton pulses can increase cancer cell mortality, which relies on functional PARP1 protein. Sci. Rep. 2019, 9, 1. [Google Scholar] [CrossRef]
- Raschke, S.; Spickermann, S.; Toncian, T.; Swantusch, M.; Boeker, J.; Giesen, U.; Iliakis, G.; Willi, O.; Boege, F. Ultra-short laser-accelerated proton pulses have similar DNA-damaging effectiveness but produce less immediate nitroxidative stress than conventional proton beams. Sci. Rep. 2016, 6, 1. [Google Scholar] [CrossRef]
- Konishi, T.; Kusumoto, T.; Hiroyama, Y.; Kobayashi, A.; Mamiya, T.; Kodaira, S. Induction of DNA strand breaks and oxidative base damages in plasmid DNA by ultra-high dose rate proton irradiation. Int. J. Radiat. Biol. 2023, 99, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, D.; Hiroyama, Y.; Kobayashi, A.; Kusumoto, T.; Kitamura, H.; Hojo, S.; Kodaira, S.; Konishi, T. DNA strand break induction of aqueous plasmid DNA exposed to 30 MeV protons at ultra-high dose rate. Radiat. Res. 2022, 63, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Perstin, A.; Poirier, Y.; Sawant, A.; Tambasco, M. Quantifying the DNA-damaging Effects of FLASH Irradiation With Plasmid DNA. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.R.; Jones, D.; Jones, G.; Petersson, K. FLASH irradiation induces lower levels of DNA damage ex vivo, an effect modulated by oxygen tension, dose, and dose rate. Br. J. Radiol. 2022, 95, 20211150. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.R.; Jones, D.; Jones, G.; Petersson, K. Comet Assay Profiling of FLASH-Induced Damage: Mechanistic Insights into the Effects of FLASH Irradiation. Int. J. Mol. Sci. 2023, 24, 7195. [Google Scholar] [CrossRef] [PubMed]
- Barghouth, P.G.; Melemenidis, S.; Montay-Gruel, P.; Ollivier, J.; Viswanathan, V.; Jorge, P.G.; Soto, L.A.; Lau, B.C.; Sadeghi, C.; Edlabadkar, A.; et al. FLASH-RT does not affect chromosome translocations and junction structures beyond that of CONV-RT dose-rates. Radiother. Oncol. 2023, 188, 109906. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Bhat, N.N.; Praveen, J.; Sanjeev, G.; Sreedevi, B.; Narayan, Y. Dose rate effect on micronuclei induction in human blood lymphocytes exposed to single pulse and multiple pulses of electrons. Radiat. Environ. Biophys. 2011, 50, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.G.; Borghini, A.; Pulignani, S.; Baffigi, F.; Fulgentini, L.; Koester, P.; Cresci, M.; Vecoli, C.; Lamia, D.; Russo, G.; et al. Radiobiological Effectiveness of Ultrashort Laser-Driven Electron Bunches: Micronucleus Frequency, Telomere Shortening and Cell Viability. Radiat. Res. 2016, 186, 245–253. [Google Scholar] [CrossRef]
- Kam, W.W.; Banatia, R.B. Effects of ionizing radiation on mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef]
- Guo, Z.; Buonanno, M.; Harken, A.; Zhou, G.; Hei, T.K. Mitochondrial Damage Response and Fate of Normal Cells Exposed to FLASH Irradiation with Protons. Radiat. Res. 2022, 197, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Mei, Z.; Lu, C.; Qian, J.; Liang, Y.; Sun, X.; Pan, Z.; Kong, D.; Xu, S.; Liu, Z.; et al. Ultra-High Dose Rate FLASH Irradiation Induced Radio-Resistance of Normal Fibroblast Cells Can Be Enhanced by Hypoxia and Mitochondrial Dysfunction Resulting From Loss of Cytochrome C. Front. Cell Dev. Biol. 2021, 9, 672929. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Beyreuther, E.; García-Calderón, D.; Karsch, L.; Knoll, J.; Pawelke, J.; Schürer, M.; Seco, J. Changes in Radical Levels as a Cause for the FLASH effect: Impact of beam structure parameters at ultra-high dose rates on oxygen depletion in water. Radiother. Oncol. 2022, 175, 193–196. [Google Scholar] [CrossRef]
- Sunnerberg, J.P.; Zhang, R.; Gladstone, D.J.; Swartz, H.M.; Gui, J.; Pogue, B.P. Mean dose rate in ultra-high dose rate electron irradiation is a significant predictor for O2 consumption and H2O2 yield. Phys. Med. Biol. 2023, 68, 165014. [Google Scholar] [CrossRef]
- Miles, D.; Sforza, D.; Wong, J.W.; Gabrielson, K.; Aziz, K.; Mahesh, M.; Coulter, J.B.; Siddiqui, I.; Tran, P.T.; Viswanathan, A.N.; et al. FLASH Effects Induced by Orthovoltage X-rays. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 1018–1027. [Google Scholar] [CrossRef]
- Almeida, A.; Togno, M.; Ballesteros-Zebadua, P.; Franco-Perez, J.; Geyer, R.; Schaefer, R.; Petit, B.; Grilj, V.; Meer, D.; Safai, S.; et al. Dosimetric and biologic intercomparison between electron and proton FLASH beams. Radiother. Oncol. 2023, 13, 109953. [Google Scholar] [CrossRef]
- Farr, J.; Grilj, V.; Malka, V.; Sudharsan, S.; Schippers, M. Ultra-high dose rate radiation production and delivery systems intended for FLASH. Med. Phys. 2022, 49, 4875–4911. [Google Scholar] [CrossRef] [PubMed]
- Schulte, R.; Johnstone, C.; Boucher, S.; Esarey, E.; Geddes, C.G.R.; Kravchenko, M.; Kutsaev, S.; Loo, B.W., Jr.; Méot, F.; Mustapha, B.; et al. Transformative Technology for FLASH Radiation Therapy. Appl. Sci. 2023, 13, 5021. [Google Scholar] [CrossRef]
- Giuliano, L.; Franciosini, G.; Palumbo, L.; Aggar, L.; Dutreix, M.; Faillace, L.; Favaudon, V.; Felici, G.; Galante, F.; Mostacci, A.; et al. Characterization of Ultra-High-Dose Rate Electron Beams with ElectronFlash Linac. Appl. Sci. 2023, 13, 631. [Google Scholar] [CrossRef]
- Wilson, J.D.; Hammond, E.M.; Higgins, G.S.; Petersson, K. Ultra-High Dose Rate (FLASH) radiotherapy: Silver bullet or fool’s gold? Front. Oncol. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- DesRosiers, C.; Moskvin, V.; Bielajew, A.F.; Papiez, L. 150–250 meV electron beams in radiation therapy. Phys Med Biol. 2000, 45, 1781–1805. [Google Scholar] [CrossRef] [PubMed]
- Schüler, E.; Eriksson, K.; Hynning, E.; Hancock, S.L.; Hiniker, S.M.; Bazalova-Carter, M.; Wong, T.; Le, Q.-T.; Loo, B.W.; Maxim, P.G. Very high-energy electron (VHEE) beams in radiation therapy; Treatment plan comparison between VHEE, VMAT, and PPBS. Med. Phys. 2017, 44, 2544–2555. [Google Scholar] [CrossRef] [PubMed]
- Taba, S.T.; Gureyev, T.E.; Alakhras, M.; Lewis, S.; Lockie, D.; Brennan, P.C. X-ray phase-contrast technology in breast imaging: Principles, options, and clinical application. AJR Am. J. Roentgenol. 2018, 211, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, L.; Bosco, F.; Carillo, M.; De Arcangelis, D.; Ficcadenti, L.; Francescone, D.; De Gregorio, A.; Franciosini, G.; Migliorati, M.; Mostacci, A.; et al. Proposal of a VHEE Linac for FLASH radiotherapy. J. Phys. Conf. Ser. 2023, 2420, 012087. [Google Scholar] [CrossRef]
- Wuensch, W. The CHUV-CERN Facility for FLASH Treatment of Large, Deep-Seated Tumors: The DEFT (Deep Electron FLASH Therapy) Facility. In Proceedings of the FLASH Radiotherapy & Particle Therapy Conference, Barcelona, Spain, 1–3 December 2021. [Google Scholar]
- Faure, J.; Glinec, Y.; Pukhov, A.; Kiselev, S.; Gordienko, S.; Lefebvre, E.; Rousseau, J.P.; Burgy, F.; Malka, V. A laser-plasma accelerator producing monoenergetic electron beams. Nature 2004, 431, 541. [Google Scholar] [CrossRef] [PubMed]
- Labate, L.; Palla, D.; Panetta, D.; Avella, F.; Baffigi, F.; Brandi, F.; Di Martino, F.; Fulgentini, L.; Giulietti, A.; Köster, P.; et al. Toward an effective use of laser-driven very high energy electrons for radiotherapy: Feasibility assessment of multi-field and intensity modulation irradiation schemes. Sci. Rep. 2020, 10, 17307. [Google Scholar] [CrossRef]
- Gizzi, A.L.; Mathieu, F.; Mason, P.; Rajeev, P.P. Laser drivers for plasma accelerators. New J. Phys. 2021, 23, 031101. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borghini, A.; Labate, L.; Piccinini, S.; Panaino, C.M.V.; Andreassi, M.G.; Gizzi, L.A. FLASH Radiotherapy: Expectations, Challenges, and Current Knowledge. Int. J. Mol. Sci. 2024, 25, 2546. https://doi.org/10.3390/ijms25052546
Borghini A, Labate L, Piccinini S, Panaino CMV, Andreassi MG, Gizzi LA. FLASH Radiotherapy: Expectations, Challenges, and Current Knowledge. International Journal of Molecular Sciences. 2024; 25(5):2546. https://doi.org/10.3390/ijms25052546
Chicago/Turabian StyleBorghini, Andrea, Luca Labate, Simona Piccinini, Costanza Maria Vittoria Panaino, Maria Grazia Andreassi, and Leonida Antonio Gizzi. 2024. "FLASH Radiotherapy: Expectations, Challenges, and Current Knowledge" International Journal of Molecular Sciences 25, no. 5: 2546. https://doi.org/10.3390/ijms25052546
APA StyleBorghini, A., Labate, L., Piccinini, S., Panaino, C. M. V., Andreassi, M. G., & Gizzi, L. A. (2024). FLASH Radiotherapy: Expectations, Challenges, and Current Knowledge. International Journal of Molecular Sciences, 25(5), 2546. https://doi.org/10.3390/ijms25052546










