The Effect of Oxidative Stress on the Human Voice
Abstract
:1. Introduction
2. Role of Oxidative Stress on Vocal Pathologies
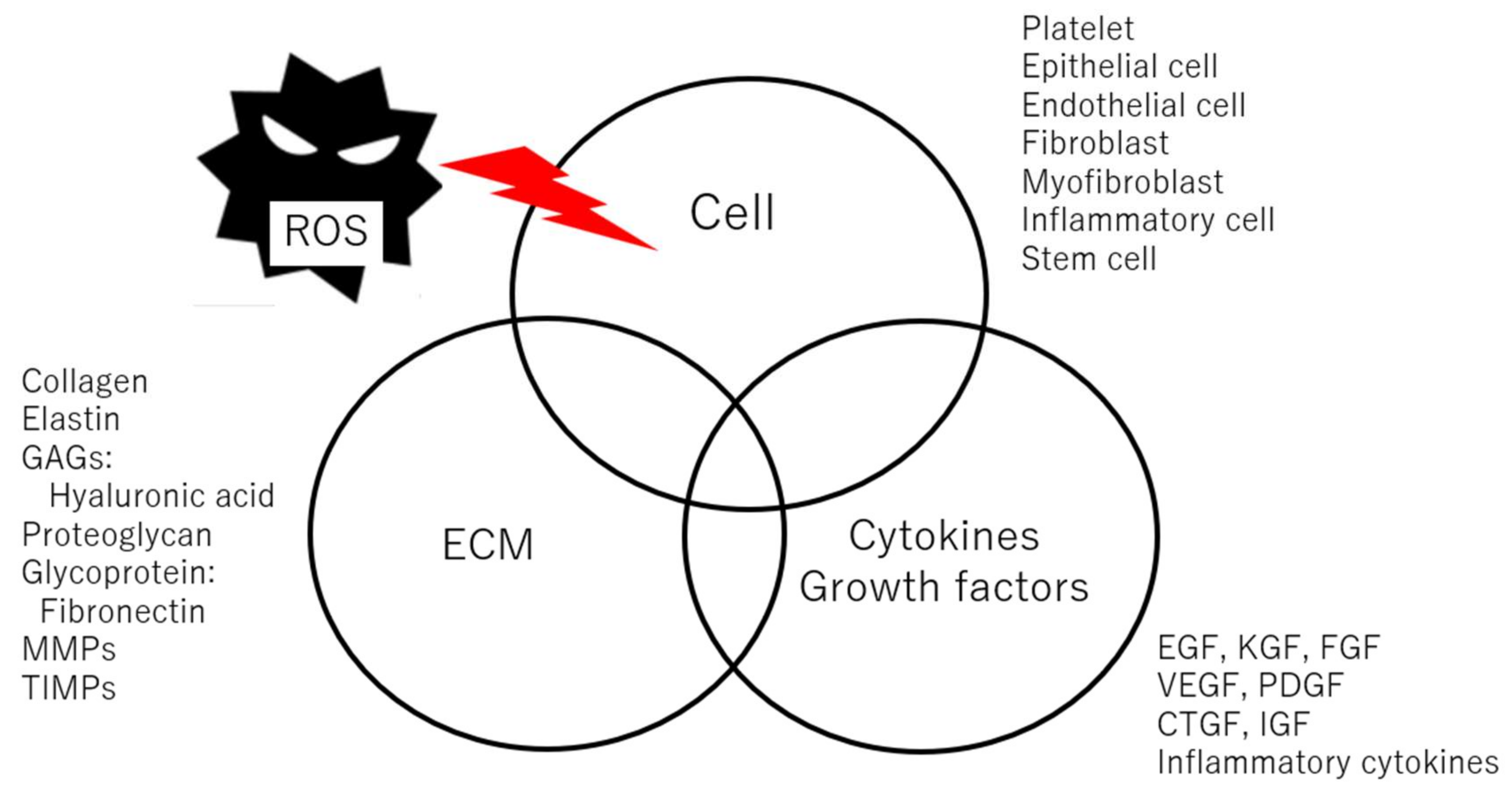
3. Vocal Fold Wound Healing and Scarring
4. The Role of Oxidative Stress and Antioxidants on Vocal Fold Wound Healing
5. Aging of Vocal Fold and Voice
6. Role of Oxidative Stress and Antioxidant on Aging Voice
7. Antioxidant for Maintenance of Voice
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hirano, M. Morphological structure of the vocal cord as a vibrator and its variations. Folia Phoniatr. Logop. 1976, 26, 89–94. [Google Scholar] [CrossRef]
- Hirano, M.; Kakita, Y. Cover-body theory of vocal fold vibration. In Speech Science; Daniloff, R.G., Ed.; College-Hill Press: San Diego, CA, USA, 1985; pp. 1–46. [Google Scholar]
- Hirano, M. Phonosurgery. Basic and clinical investigations. Otologia 1975, 21, 239–440. [Google Scholar]
- Kurita, S.; Nagata, K.; Hirano, M. A comparative study of the layer structure of the vocal fold. In Vocal Fold Physiology; Bless, D.M., Abbs, J.H., Eds.; College-Hill Press: San Diego, CA, USA, 1981; pp. 3–21. [Google Scholar]
- Hirano, M.; Kurita, S.; Nakashima, T. Growth, development, and aging of human vocal folds. In Vocal Fold Physiology; Bless, D.M., Abbs, J.H., Eds.; College Hill Press: San Diego, CA, USA, 1983; pp. 22–43. [Google Scholar]
- Sataloff, R.T. The effects of age on the voice. In Professional Voice: The Science and Art of Clinical Care; Sataloff, R.T., Ed.; Raven Press: New York, NY, USA, 1991. [Google Scholar]
- Kahane, J.C. Growth of the human prepubertal and pubertal larynx. J. Speech Lang. Hear. Res. 1982, 25, 446–455. [Google Scholar] [CrossRef]
- Gray, S.D.; Titze, I.R.; Chan, R.; Hammond, T.H. Vocal fold proteoglycans and their influence on biomechanics. Laryngoscope 1999, 109, 845–854. [Google Scholar] [CrossRef]
- Hahn, M.S.; Jao, C.Y.; Faquin, W.; Grande-Allen, K.J. Glycosaminoglycan composition of the vocal fold lamina propria in relation to function. Ann. Otol. Rhinol. Laryngol. 2008, 117, 371–381. [Google Scholar] [CrossRef]
- Hansen, J.K.; Thibeault, S.L. Current Understanding and Review of the Literature: Vocal Fold Scarring. J. Voice 2006, 20, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Kumai, Y. Pathophysiology of Fibrosis in the Vocal Fold: Current Research, Future Treatment Strategies, and Obstacles to Restoring Vocal Fold Pliability. Int. J. Mol. Sci. 2019, 20, 2551. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Gray, S.D.; Mammond, E.; Hanson, D.F. Benign pathologic responses of the larynx. Ann. Otol. Rhinol. Laryngol. 1995, 104, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Courey, M.S.; Shohet, J.A.; Scott, M.A.; Ossoff, R.H. Immunohistochemical characterization of benign laryngeal lesions. Ann. Otol. Rhinol. Laryngol. 1996, 105, 525–531. [Google Scholar] [CrossRef]
- Dikkers, F.G.; Nikkels, P.G.J. Benign lesions of the vocal folds: Histopathology and phonotrauma. Ann. Otol. Rhinol. Laryngol. 1996, 104, 698–703. [Google Scholar] [CrossRef]
- Kotby, M.N.; Nassar, A.M.; Seif, E.I.; Helal, E.H.; Saleh, M.M. Ultrastructural features of vocal fold nodules and polyps. Acta Otolaryngol. 1988, 105, 477–482. [Google Scholar] [CrossRef]
- Branski, R.C.; Zhou, H.; Kraus, D.H.; Sivasankar, M. The effects of cigarette smoke condensate on vocal fold transepithelial resistance and inflammatory signaling in vocal fold fibroblasts. Laryngoscope 2011, 121, 601–605. [Google Scholar] [CrossRef]
- Alper, R.; Fu, X.; Erickson-Levendoski, E.; Zheng, W.; Sivasankar, M. Acute stress to excised vocal fold epithelium from reactive oxygen species. Laryngoscope 2011, 121, 2180–2184. [Google Scholar] [CrossRef]
- Gugatschka, M.; Darnhofer, B.; Grossmann, T.; Schittmayer, M.; Hortobagyi, D.; Kirsch, A.; Karpf, E.; Brcic, L.; Birner-Gruenberger, R.; Karbiener, M. Proteomic Analysis of Vocal Fold Fibroblasts Exposed to Cigarette Smoke Extract: Exploring the Pathophysiology of Reinke’s Edema. Mol. Cell. Proteom. 2019, 18, 1511–1525. [Google Scholar] [CrossRef]
- Joo, Y.H.; Lee, S.S.; Han, K.; Park, K.H. Association between chronic laryngitis and particulate matter based on the Korea National Health and Nutrition Examination Survey 2008–2012. PLoS ONE 2015, 10, e0133180. [Google Scholar] [CrossRef]
- Choi, H.; Kim, C.S. Polycyclic Aromatic Hydrocarbons from Fine Particulate Matter Induce Oxidative Stress and the Inflammatory Response in Human Vocal Fold Fibroblast Cells. Oxid. Med. Cell. Longev. 2021, 2021, 5530390. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Minamiguchi, S.; Yamashita, M.; Ohno, T.; Kanemaru, S.; Kitamura, M. Histologic characterization of human scarred vocal folds. J. Voice 2009, 23, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Bouchayer, M.; Cornut, G.; Loire, R.; Witzig, E.; Roch, J.B.; Bastian, R.W. Epidermoid cysts, sulci, and mucosal bridges of the true vocal cord: A report of 157 cases. Laryngoscope 1985, 95, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.; Inagi, K.; Khidr, A.; Bless, D.M.; Gilchrist, K.W. Sulcus vocalis: A rational analytical approach to diagnosis and management. Ann. Otol. Rhinol. Laryngol. 1996, 105, 189–200. [Google Scholar] [CrossRef]
- Nakayama, M.; Ford, C.N.; Brandenburg, J.H.; Bless, D.M. Sulcus vocalis in laryngeal cancer: A histopathologic study. Laryngoscope 1994, 104, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hirano, M. Electron microscopic investigation of sulcus vocalis. Ann. Otol. Rhinol. Laryngol. 1998, 107, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Peled, Z.M.; Chin, G.S.; Liu, W.; Galliano, R.; Longaker, M.T. Response to tissue injury. Clin. Plast. Surg. 2000, 27, 489–500. [Google Scholar] [CrossRef]
- Martin, P. Wound healing -Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Parks, W.C. Matrix metalloproteinases in repair. Wound Repair Regen. 1999, 7, 423–432. [Google Scholar] [CrossRef]
- Branski, R.C.; Rosen, C.A.; Verdolini, K.; Hebda, P.A. Acute vocal fold wound healing in a rabbit model. Ann. Otol. Rhinol. Laryngol. 2005, 114, 19–24. [Google Scholar] [CrossRef]
- Ling, C.; Yamashita, M.; Waselchuk, E.A.; Raasch, J.L.; Bless, D.M.; Welham, N.V. Alteration in cellular morphology, density and distribution in rat vocal fold mucosa following injury. Wound Repair Regen. 2010, 18, 89–97. [Google Scholar] [CrossRef]
- King, S.N.; Guille, J.; Thibeault, S.L. Characterization of the Leukocyte Response in Acute Vocal Fold Injury. PLoS ONE 2015, 10, e0139260. [Google Scholar] [CrossRef] [PubMed]
- Kaba, S.; Nakamura, R.; Yamashita, M.; Katsuno, T.; Suzuki, R.; Tateya, I.; Kishimoto, Y.; Omori, K. Alterations in macrophage polarization in injured murine vocal folds. Laryngoscope 2019, 129, E135–E142. [Google Scholar] [CrossRef] [PubMed]
- Tateya, T.; Tateya, I.; Sohn, J.H.; Bless, D.M. Histological study of acute vocal fold injury in a rat model. Ann. Otol. Rhinol. Laryngol. 2006, 115, 285–292. [Google Scholar] [CrossRef]
- Lim, X.; Tateya, I.; Tateya, T.; Muñoz-Del-Río, A.; Bless, D.M. Immediate inflammatory response and scar formation in wounded vocal folds. Ann. Otol. Rhinol. Laryngol. 2006, 115, 921–929. [Google Scholar] [CrossRef]
- Welham, N.V.; Lim, X.; Tateya, I.; Bless, D.M. Inflammatory factor profiles one hour following vocal fold injury. Ann. Otol. Rhinol. Laryngol. 2008, 117, 145–152. [Google Scholar] [CrossRef]
- Ohno, T.; Hirano, S.; Rousseau, B. Gene expression of transforming growth factor-b1 and hepatocyte growth factor during wound healing of injured rat vocal fold. Laryngosocpe 2009, 119, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.; Sohn, J.; Montequin, D.W.; Tateya, I.; Bless, D.M. Functional outcomes of reduced hyaluronan in acute vocal fold scar. Ann. Otol. Rhinol. Laryngol. 2004, 113, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Nakamura, T. Hepatocyte growth factor (HGF) as a tissue organizer for organogenesis and regeneration. Biochem. Biophys. Res. Commun. 1997, 239, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P.; Thomas, D.W. The cellular proliferative phase of the wound repair process. J. Wound Care 2002, 11, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Vanlis, J.M.; Kalssbeek, G.L. Glycosaminoglycans in human skin. Br. J. Dermatol. 1973, 88, 355–361. [Google Scholar]
- Ronnov-Jessen, L.; Petersen, O.W. Induction of alpha-smooth muscle actin by transforming grwoth factor beta 1 in quiescent human breast gland fibroblasts. Implications for myofibroblast generation in breast neoplasia. Lab. Investig. 1993, 68, 696–707. [Google Scholar] [PubMed]
- Parsons, S.L.; Watson, S.A.; Brown, P.D.; Collins, H.M.; Steele, R.J. Matrix metalloproteinases. Br. J. Surg. 1997, 84, 160–166. [Google Scholar]
- Tateya, T.; Tateya, I.; Sohn, J.; Bless, D.M. Histologic characterization of rat vocal fold scarring. Ann. Otol. Rhinol. Laryngol. 2005, 114, 183–191. [Google Scholar] [CrossRef]
- Rousseau, B.; Hirano, S.; Chan, R.W.; Welham, N.V.; Thibeault, S.L.; Ford, C.N.; Bless, D.M. Characterization of Chronic Vocal Fold Scarring in a Rabbit Model. J. Voice 2004, 18, 116–124. [Google Scholar] [CrossRef]
- Rousseau, B.; Hirano, S.; Scheidt, T.D.; Welham, N.V.; Thibeault, S.L.; Chan, R.W.; Bless, D.M. Characterization of Vocal Fold Scarring in a Canine Model. Laryngoscope 2003, 113, 620–627. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Hirano, S.; Tateya, I.; Kanemaru, S.; Ito, J. Temporal changes in vocal functions of human scarred vocal folds after cordectomy. Laryngoscope 2010, 120, 1597–1601. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kumin, A.; Huber, C.; Rulicke, T.; Wolf, E.; Werner, S. Peroxiredoxin 6 is a potent cytoprotective enzyme in the epidermis. Am. J. Pathol. 2006, 169, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Hirano, S.; Ohno, S.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Ito, J. Expression of reactive oxygen species during wound healing of vocal folds in a rat model. Ann. Otol. Rhinol. Laryngol. 2012, 12, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, M.; Hirano, S.; Hiwatashi, N.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Ito, J. The effect of astaxanthin on vocal fold wound healing. Laryngoscope 2014, 124, E1–E7. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.G.; Fernandez, M.L. Potential of dietary non-provitamin A carotenoids in the prevention and treatment of diabetic microvascular complications. Adv. Nutr. 2016, 7, 14–24. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H. Astaxanthin Modulation of Signaling Pathways That Regulate Autophagy. Mar. Drugs 2019, 17, 546. [Google Scholar] [CrossRef] [PubMed]
- Manciula, L.G.; Berce, C.; Tabaran, F.; Trombitaș, V.; Albu, S. The Effects of Postoperative Astaxanthin Administration on Nasal Mucosa Wound Healing. J. Clin. Med. 2019, 8, 1941. [Google Scholar] [CrossRef] [PubMed]
- Meephansan, J.; Rungjang, A.; Yingmema, W.; Deenonpoe, R.; Ponnikorn, S. Effect of astaxanthin on cutaneous wound healing. Clin. Cosmet. Investig. Dermatol. 2017, 10, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.G.M.; Freire, M.C.L.C.; Oliveira, V.D.S.; Solisio, C.; Converti, A.; de Lima, Á.A.N. Astaxanthin Delivery Systems for Skin Application: A Review. Mar. Drugs 2021, 19, 511. [Google Scholar] [CrossRef]
- Golub, J.S.; Chen, P.H.; Otto, K.J.; Hapner, E.; Johns, M.M. Prevalence of perceived dysphonia in a geriatric population. J. Am. Geriatr. Soc. 2006, 54, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Stemple, J.; Merrill, R.M.; Thomas, L. Epidemiology of voice disorders in the elderly: Preliminary findings. Laryngoscope 2007, 117, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Verdonck-de Leeuw, I.; Mahieu, H. Vocal aging and the impact on daily life: A longitudinal study. J. Voice 2004, 18, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.E.; Hammond, T.H.; Gray, S.D. Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope 2001, 111, 907–911. [Google Scholar] [CrossRef]
- Hirano, M.; Kurita, S.; Sakaguchi, S. Ageing of the vibratory tissue of human vocal folds. Acta Otolaryngol. 1989, 107, 428–433. [Google Scholar] [CrossRef]
- Sato, K.; Hirano, M. Age-related changes of elastic fibers in the superficial layer of the lamina propria of vocal folds. Ann. Otol. Rhinol. Laryngol. 1997, 106, 44–48. [Google Scholar] [CrossRef]
- Sato, K.; Hirano, M.; Nakashima, T. Age-related changes of collagenous fibers in the human vocal fold mucosa. Ann. Otol. Rhinol. Laryngol. 2002, 111, 15–20. [Google Scholar] [CrossRef]
- Hammond, T.H.; Gray, S.D.; Butler, J.; Zhou, R.; Hammond, E. A study of age and gender related elastin distribution changes in human vocal folds. Otolaryngol. Head Neck Surg. 1998, 119, 314–322. [Google Scholar] [CrossRef]
- Hammond, T.H.; Gray, S.D.; Butler, J.E. Age- and gender-related collagen distribution in human vocal folds. Ann. Otol. Rhinol. Laryngol. 2000, 109, 913–920. [Google Scholar] [CrossRef]
- Ohno, T.; Hirano, S.; Rousseau, B. Age-associated changes in the expression and deposition of vocal fold collagen and hyaluronan. Ann. Otol. Rhinol. Laryngol. 2009, 118, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Rodeno, M.T.; Sanchez-Fernandez, J.M.; Rivera-Pomar, J.M. Histochemical and morphometrical ageing changes in human vocal cord muscles. Acta Otolaryngol. 1993, 113, 445–449. [Google Scholar] [CrossRef]
- Sato, K.; Hirano, M. Age-related changes of the macula flava of the human vocal fold. Ann. Otol. Rhinol. Laryngol. 1995, 104, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Sato, K.; Nakashima, T. Fibroblasts in geriatric vocal fold mucosa. Acta Otolaryngol. 2000, 120, 336–340. [Google Scholar] [PubMed]
- Ding, H.; Gray, S.D. Senescent expression of genes coding collagens, collagen-degrading metalloproteinases, and tissue inhibitors of metalloproteinases in rat vocal folds: Comparison with skin and lungs. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, B145–B152. [Google Scholar] [CrossRef]
- Ding, H.; Gray, S.D. Senescent expression of genes coding tropoelastin, elastase, lysyl oxidase, and tissue inhibitors of metalloproteinases in rat vocal folds: Comparison with skin and lungs. J. Speech Lang. Hear. Res. 2001, 44, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Bloch, I.; Behrman, A. Quantitative analysis of videostroboscopic images in presbylarynges. Laryngoscope 2001, 111, 2022–2027. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.; Casper, J.; Colton, R.; Brewer, D. Dysphonia in the aging: Physiology versus disease. Laryngoscope 1992, 102, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Honjo, I.; Isshiki, N. Laryngoscopic and voice characteristics of aged persons. Arch Otolaryngol. 1980, 106, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Pontes, P.; Yamasaki, R.; Behlau, M. Morphological and functional aspects of the senile larynx. Folia Phoniatr. Logop. 2006, 58, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ramig, L.O.; Gray, S.; Baker, K.; Buder, E.; Luschei, E.; Coon, H.; Smith, M. The aging voice: A review, treatment data and familial and genetic perspectives. Folia Phoniatr. Logop. 2001, 53, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.D.; Chandran, S.; Lurie, D.; Sataloff, R.T. Voice disorders in the elderly. J. Voice 2012, 26, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.; Ramig, L.; Luschei, E.; Smith, M.E. Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology 1998, 51, 1592–1598. [Google Scholar] [CrossRef]
- Luschei, E.; Ramig, L.; Baker, K.; Smith, M.E. Discharge characteristics of laryngeal single motor units during phonation in young and older adults and in persons with Parkinson disease. J. Neurophysiol. 1999, 81, 2131–2139. [Google Scholar] [CrossRef]
- Mizuta, M.; Hirano, S.; Hiwatashi, N.; Kobayashi, T.; Tateya, I.; Kanemaru, S.; Nakamura, T.; Ito, J. Effect of astaxanthin on age-associated changes of vocal folds in a rat model. Laryngoscope 2014, 124, E411–E417. [Google Scholar] [CrossRef]
- Hirano, S.; Bless, D.M.; Heisey, D.; Ford, C.N. Effect of growth factors on hyaluronan production by canine vocal fold fibroblasts. Ann. Otol. Rhinol. Laryngol. 2003, 112, 617–624. [Google Scholar] [CrossRef]
- Swanson, E.R.; Abdollahian, D.; Ohno, T.; Ge, P.; Zealear, D.L.; Rousseau, B. Characterization of raised phonation in an evoked rabbit phonation model. Laryngoscope 2009, 119, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Van Deusen, M.; Jerome, W.G.; Garrett, C.G.; Sivasankar, M.P.; Novaleski, C.K.; Rousseau, B. Quantification of Acute Vocal Fold Epithelial Surface Damage with Increasing Time and Magnitude Doses of Vibration Exposure. PLoS ONE 2014, 9, e91615. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.; Suehiro, A.; Echemendia, N.; Sivasankar, M. Raised intensity phonation compromises vocal fold epithelial barrier integrity. Laryngoscope 2011, 121, 346–351. [Google Scholar] [CrossRef]
- Kaneko, M.; Kishimoto, Y.; Suzuki, R.; Kawai, Y.; Tateya, I.; Hirano, S. Protective effect of astaxanthin on vocal fold injury and inflammation due to vocal loading: A clinical trial. J. Voice 2017, 31, 352–358. [Google Scholar] [CrossRef]
- Hirano, S.; Inufusa, H.; You, F.; Sugiyama, Y.; Kaneko, M.; Yoshikawa, T. Anti-oxidant, Twendee X, for maintenance of singing voice. Brain Suppl. 2023, 5, 1–7. [Google Scholar]
- You, F.; Harakawa, Y.; Yoshikawa, T.; Inufusa, H. Why Does the Antioxidant Complex Twendee X® Prevent Dementia? Int. J. Mol. Sci. 2023, 24, 13018. [Google Scholar] [CrossRef]
- Tadokoro, K.; Morihara, R.; Ohta, Y.; Hishikawa, N.; Kawano, S.; Sasaki, R.; Matsumoto, N.; Nomura, E.; Nakano, Y.; Takahashi, Y.; et al. Clinical Benefits of Antioxidative Supplement Twendee X for Mild Cognitive Impairment: A Multicenter, Randomized, Double-Blind, and Placebo-Controlled Prospective Interventional Study. J. Alzheimers Dis. 2019, 71, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, S.; Inufusa, H.; You, F. The Effect of Oxidative Stress on the Human Voice. Int. J. Mol. Sci. 2024, 25, 2604. https://doi.org/10.3390/ijms25052604
Hirano S, Inufusa H, You F. The Effect of Oxidative Stress on the Human Voice. International Journal of Molecular Sciences. 2024; 25(5):2604. https://doi.org/10.3390/ijms25052604
Chicago/Turabian StyleHirano, Shigeru, Haruhiko Inufusa, and Fukka You. 2024. "The Effect of Oxidative Stress on the Human Voice" International Journal of Molecular Sciences 25, no. 5: 2604. https://doi.org/10.3390/ijms25052604




