Adult Neurogenesis of the Medial Geniculate Body: In Vitro and Molecular Genetic Analyses Reflect the Neural Stem Cell Capacity of the Rat Auditory Thalamus over Time
Abstract
1. Introduction
2. Results
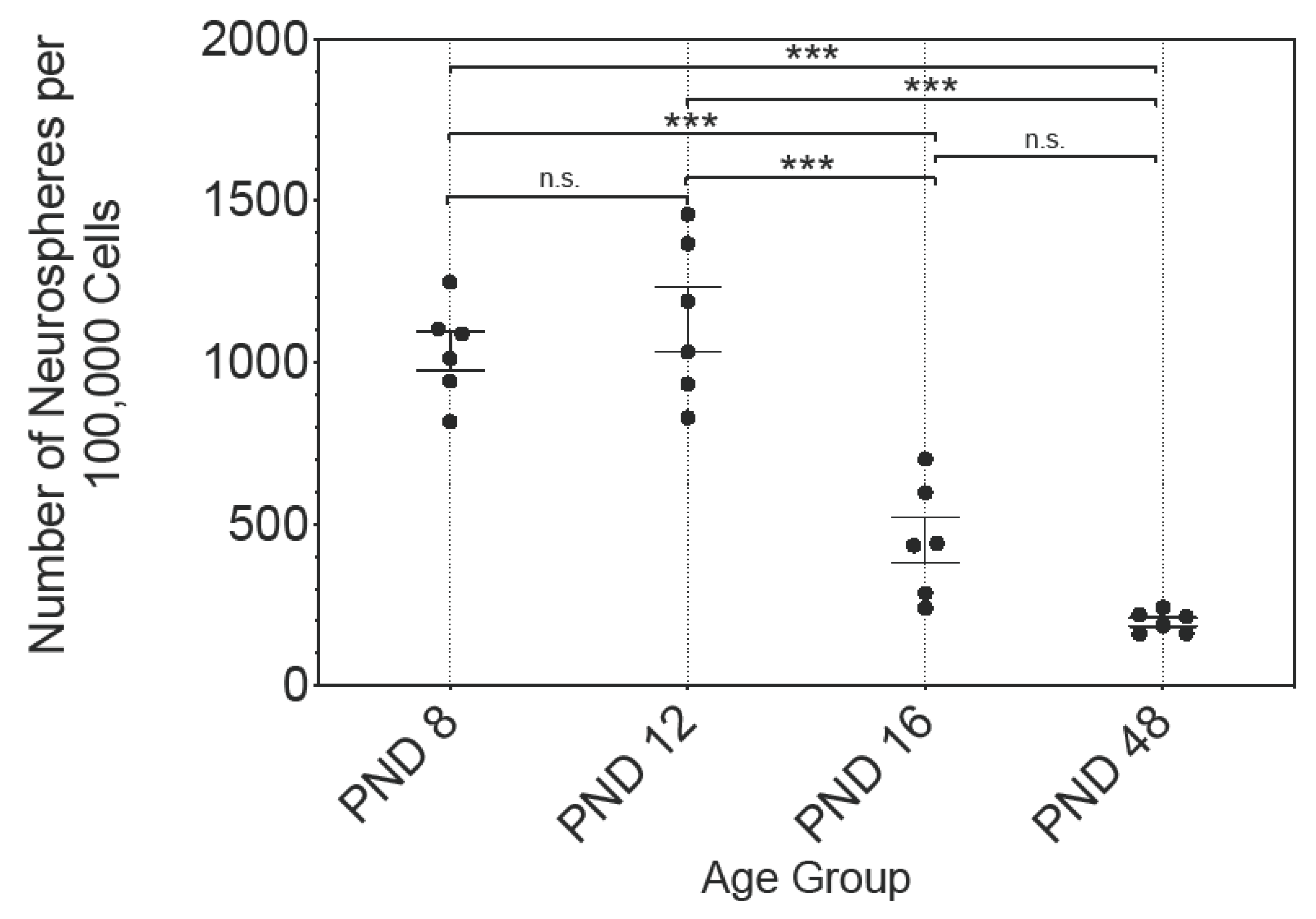
2.1. Cells of the MGB Form Neural Stem and Progenitor Cell Marker Positive Neurospheres with Decreasing Proliferative Capacity up to the Adult Stage
2.2. The Differentiation Capacity of the MGB NSCs Reveals Age-Specific Characteristics
2.3. mRNA Abundance of Neurogenic Factors with High Relevance for MGB Neurogenesis Displays Age-Specific Patterns
3. Discussion
3.1. The Maturing MGB Harbors a NSC Niche up to the Adult Stage with Decreasing Neurogenic Potential with Increasing Age
3.2. The Capacities to Form Progenitor Cells and to Differentiate into Neurons and Glial Cells of MGB NSCs Differs with Increasing Age
3.3. mRNA Levels of Prominent Neurogenic Factors Reflect the NSC Potential of the MGB
3.4. Limitations of the Study
4. Materials and Methods
4.1. Animal Preparation, Neurosphere Assay, and Passaging
4.2. Plating of Neurospheres and Differentiated Single Cells
4.3. Fixation and Immunocytochemistry
4.4. Digital Images and Cytological Analysis
4.5. RNA Extraction and cDNA Synthesis
4.6. Rat Neurogenesis RT2 ProfilerTM PCR Array
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chadha, S.; Kamenov, K.; Cieza, A. The world report on hearing, 2021. Bull. World Health Organ. 2021, 99, 242. [Google Scholar] [CrossRef]
- Kleinlogel, S.; Vogl, C.; Jeschke, M.; Neef, J.; Moser, T. Emerging Approaches for Restoration of Hearing and Vision. Physiol. Rev. 2020, 100, 1467–1525. [Google Scholar] [CrossRef]
- Smith, R.J.; Bale, J.F., Jr.; White, K.R. Sensorineural hearing loss in children. Lancet 2005, 365, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Hatano, M.; Hattori, T.; Takarada-Iemata, M.; Shinozaki, T.; Sugimoto, H.; Ito, M.; Yoshizaki, T.; Hori, O. Microglial activation in the cochlear nucleus after early hearing loss in rats. Auris Nasus Larynx 2019, 46, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, R.K.; Hardie, N.A. Deafness-induced changes in the auditory pathway: Implications for cochlear implants. Audiol. Neurootol. 2001, 6, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, S.; Toyoda, M.; Umezawa, A.; Ogawa, K. Application of Mesenchymal Stem Cell Therapy and Inner Ear Regeneration for Hearing Loss: A Review. Int. J. Mol. Sci. 2020, 21, 5764. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.; Holmstrom, N.; Carlen, M.; Cassidy, R.; Lundberg, C.; Frisen, J. Gene delivery to adult neural stem cells. Exp. Cell Res. 2002, 279, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Ottoboni, L.; von Wunster, B.; Martino, G. Therapeutic Plasticity of Neural Stem Cells. Front. Neurol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Lee, I.S.; Jung, K.; Kim, I.S.; Lee, H.; Kim, M.; Yun, S.; Hwang, K.; Shin, J.E.; Park, K.I. Human neural stem cells alleviate Alzheimer-like pathology in a mouse model. Mol. Neurodegener. 2015, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef]
- Kempermann, G.; Gage, F.H. New nerve cells for the adult brain. Sci. Am. 1999, 280, 48–53. [Google Scholar] [CrossRef]
- Kempermann, G.; Wiskott, L.; Gage, F.H. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004, 14, 186–191. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Rietze, R.L. Neural stem cells and neurospheres—re-evaluating the relationship. Nat. Methods 2005, 2, 333–336. [Google Scholar] [CrossRef]
- Engert, J.; Rak, K.; Bieniussa, L.; Scholl, M.; Hagen, R.; Voelker, J. Evaluation of the Neurogenic Potential in the Rat Inferior Colliculus from Early Postnatal Days Until Adulthood. Mol. Neurobiol. 2021, 58, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tao, L.; Deng, M. Postnatal Changes of Neural Stem Cells in the Mammalian Auditory Cortex. Int. J. Mol. Sci. 2021, 22, 1550. [Google Scholar] [CrossRef] [PubMed]
- Rak, K.; Volker, J.; Frenz, S.; Scherzed, A.; Radeloff, A.; Hagen, R.; Mlynski, R. Dynamic changes of the neurogenic potential in the rat cochlear nucleus during post-natal development. Exp. Brain Res. 2013, 226, 393–406. [Google Scholar] [CrossRef]
- Rask-Andersen, H.; Bostrom, M.; Gerdin, B.; Kinnefors, A.; Nyberg, G.; Engstrand, T.; Miller, J.M.; Lindholm, D. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear. Res. 2005, 203, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Grimm, C.M.; Corrales, C.E.; Senn, P.; Martinez Monedero, R.; Geleoc, G.S.; Edge, A.; Holt, J.R.; Heller, S. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J. Assoc. Res. Otolaryngol. 2007, 8, 18–31. [Google Scholar] [CrossRef]
- Engert, J.; Spahn, B.; Bieniussa, L.; Hagen, R.; Rak, K.; Voelker, J. Neurogenic Stem Cell Niche in the Auditory Thalamus: In Vitro Evidence of Neural Stem Cells in the Rat Medial Geniculate Body. Life 2023, 13, 1188. [Google Scholar] [CrossRef]
- Webster, D.B.; Fay, R.R. The Mammalian Auditory Pathway: Neuroanatomy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 1. [Google Scholar]
- Winer, J.A. The Functional Architecture of the Medial Geniculate Body and the Primary Auditory Cortex. In The Mammalian Auditory Pathway: Neuroanatomy; Webster, D.B., Popper, A.N., Fay, R.R., Eds.; Springer: New York, NY, USA, 1992; pp. 222–409. [Google Scholar]
- Winer, J.A.; Wenstrup, J.J.; Larue, D.T. Patterns of GABAergic immunoreactivity define subdivisions of the mustached bat’s medial geniculate body. J. Comp. Neurol. 1992, 319, 172–190. [Google Scholar] [CrossRef]
- Devos, J.V.P.; Smit, J.V.; George, E.L.J.; Leue, C.; Ackermans, L.; Temel, Y.; Janssen, M.L.F. Effective treatment of refractory tinnitus by bilateral deep brain stimulation of the medial geniculate body of the thalamus: A case report. Brain Stimul. 2023, 16, 1322–1324. [Google Scholar] [CrossRef]
- Dhanasingh, A.; Hochmair, I. Drug delivery in cochlear implantation. Acta Otolaryngol. 2021, 141, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Sohur, U.S.; Emsley, J.G.; Mitchell, B.D.; Macklis, J.D. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos. Trans. R Soc. Lond. B Biol. Sci. 2006, 361, 1477–1497. [Google Scholar] [CrossRef] [PubMed]
- Crowley, D.E.; Hepp-Reymond, M.-C. Development of cochlear function in the ear of the infant rat. J. Comp. Physiol. Psychol. 1966, 62, 427–432. [Google Scholar] [CrossRef]
- Uziel, A.; Romand, R.; Marot, M. Development of cochlear potentials in rats. Audiology 1981, 20, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Blatchley, B.J.; Cooper, W.A.; Coleman, J.R. Development of auditory brainstem response to tone pip stimuli in the rat. Dev. Brain Res. 1987, 32, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.R.; Price, E.O. Sexual maturation and fecundity of wild and domestic Norway rats (Rattus norvegicus). J. Reprod. Fertil. 1981, 63, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Charrier, C.; Coronas, V.; Fombonne, J.; Roger, M.; Jean, A.; Krantic, S.; Moyse, E. Characterization of neural stem cells in the dorsal vagal complex of adult rat by in vivo proliferation labeling and in vitro neurosphere assay. Neuroscience 2006, 138, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, H.; Heller, S. Pluripotent stem cells from the adult mouse inner ear. Nat. Med. 2003, 9, 1293–1299. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Elliott, K.L.; Pavlinkova, G.; Chizhikov, V.V.; Yamoah, E.N.; Fritzsch, B. Development in the Mammalian Auditory System Depends on Transcription Factors. Int. J. Mol. Sci. 2021, 22, 4189. [Google Scholar] [CrossRef]
- Maricich, S.M.; Xia, A.; Mathes, E.L.; Wang, V.Y.; Oghalai, J.S.; Fritzsch, B.; Zoghbi, H.Y. Atoh1-lineal neurons are required for hearing and for the survival of neurons in the spiral ganglion and brainstem accessory auditory nuclei. J. Neurosci. 2009, 29, 11123–11133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev. Neurosci. 2004, 26, 148–165. [Google Scholar] [CrossRef]
- Francis, F.; Koulakoff, A.; Boucher, D.; Chafey, P.; Schaar, B.; Vinet, M.C.; Friocourt, G.; McDonnell, N.; Reiner, O.; Kahn, A.; et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron 1999, 23, 247–256. [Google Scholar] [CrossRef]
- Lindsey, B.W.; Di Donato, S.; Kaslin, J.; Tropepe, V. Sensory-specific modulation of adult neurogenesis in sensory structures is associated with the type of stem cell present in the neurogenic niche of the zebrafish brain. Eur. J. Neurosci. 2014, 40, 3591–3607. [Google Scholar] [CrossRef]
- King, A.J.; Nelken, I. Unraveling the principles of auditory cortical processing: Can we learn from the visual system? Nat. Neurosci. 2009, 12, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Caceres, A. Expression of the class III beta-tubulin isotype in developing neurons in culture. J. Neurosci. Res. 1992, 32, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Compston, A.; Zajicek, J.; Sussman, J.; Webb, A.; Hall, G.; Muir, D.; Shaw, C.; Wood, A.; Scolding, N. Glial lineages and myelination in the central nervous system. J. Anat. 1997, 190 (Pt 2), 161–200. [Google Scholar] [CrossRef]
- Campos, L.S. Neurospheres: Insights into neural stem cell biology. J. Neurosci. Res. 2004, 78, 761–769. [Google Scholar] [CrossRef]
- Joseph, G.; Orme, R.P.; Kyriacou, T.; Fricker, R.A.; Roach, P. Effects of Surface Chemistry Interaction on Primary Neural Stem Cell Neurosphere Responses. ACS Omega 2021, 6, 19901–19910. [Google Scholar] [CrossRef]
- Lee, M.K.; Rebhun, L.I.; Frankfurter, A. Posttranslational modification of class III beta-tubulin. Proc. Natl. Acad. Sci. USA 1990, 87, 7195–7199. [Google Scholar] [CrossRef]
- Encinas, J.M.; Michurina, T.V.; Peunova, N.; Park, J.H.; Tordo, J.; Peterson, D.A.; Fishell, G.; Koulakov, A.; Enikolopov, G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 2011, 8, 566–579. [Google Scholar] [CrossRef]
- Obernier, K.; Cebrian-Silla, A.; Thomson, M.; Parraguez, J.I.; Anderson, R.; Guinto, C.; Rodas Rodriguez, J.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Adult Neurogenesis Is Sustained by Symmetric Self-Renewal and Differentiation. Cell Stem Cell 2018, 22, 221–234. [Google Scholar] [CrossRef]
- Xing, Y.; Samuvel, D.J.; Stevens, S.M.; Dubno, J.R.; Schulte, B.A.; Lang, H. Age-related changes of myelin basic protein in mouse and human auditory nerve. PLoS ONE 2012, 7, e34500. [Google Scholar] [CrossRef]
- Merkle, F.T.; Fuentealba, L.C.; Sanders, T.A.; Magno, L.; Kessaris, N.; Alvarez-Buylla, A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 2014, 17, 207–214. [Google Scholar] [CrossRef]
- Landgren, H.; Curtis, M.A. Locating and labeling neural stem cells in the brain. J. Cell Physiol. 2011, 226, 1–7. [Google Scholar] [CrossRef]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural stem cell niche heterogeneity. Semin. Cell Dev. Biol. 2019, 95, 42–53. [Google Scholar] [CrossRef]
- Guo, R.; Liao, M.; Ma, X.; Hu, Y.; Qian, X.; Xiao, M.; Gao, X.; Chai, R.; Tang, M. Cochlear implant-based electric-acoustic stimulation modulates neural stem cell-derived neural regeneration. J. Mater. Chem. B 2021, 9, 7793–7804. [Google Scholar] [CrossRef]
- Ryugo, D. Auditory neuroplasticity, hearing loss and cochlear implants. Cell Tissue Res. 2015, 361, 251–269. [Google Scholar] [CrossRef]
- Sinden, J.D.; Hicks, C.; Stroemer, P.; Vishnubhatla, I.; Corteling, R. Human Neural Stem Cell Therapy for Chronic Ischemic Stroke: Charting Progress from Laboratory to Patients. Stem Cells Dev. 2017, 26, 933–947. [Google Scholar] [CrossRef]
- Jin, K.; Wang, X.; Xie, L.; Mao, X.O.; Zhu, W.; Wang, Y.; Shen, J.; Mao, Y.; Banwait, S.; Greenberg, D.A. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA 2006, 103, 13198–13202. [Google Scholar] [CrossRef]
- Tramontina, F.; Conte, S.; Goncalves, D.; Gottfried, C.; Portela, L.V.; Vinade, L.; Salbego, C.; Goncalves, C.A. Developmental changes in S100B content in brain tissue, cerebrospinal fluid, and astrocyte cultures of rats. Cell. Mol. Neurobiol. 2002, 22, 373–378. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Srivatsa, S.; Nityanandam, A.; Tarabykin, V. Ntf3 acts downstream of Sip1 in cortical postmitotic neurons to control progenitor cell fate through feedback signaling. Development 2014, 141, 3324–3330. [Google Scholar] [CrossRef]
- Yan, W.; Chen, Z.Y.; Chen, J.Q.; Chen, H.M. BMP2 promotes the differentiation of neural stem cells into dopaminergic neurons in vitro via miR-145-mediated upregulation of Nurr1 expression. Am. J. Transl. Res. 2016, 8, 3689–3699. [Google Scholar]
- Stover, T.; Nam, Y.; Gong, T.L.; Lomax, M.I.; Altschuler, R.A. Glial cell line-derived neurotrophic factor (GDNF) and its receptor complex are expressed in the auditory nerve of the mature rat cochlea. Hear Res. 2001, 155, 143–151. [Google Scholar] [CrossRef]
- Conover, J.C.; Doetsch, F.; Garcia-Verdugo, J.M.; Gale, N.W.; Yancopoulos, G.D.; Alvarez-Buylla, A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nat. Neurosci. 2000, 3, 1091–1097. [Google Scholar] [CrossRef]
- Couillard-Despres, S.; Winner, B.; Schaubeck, S.; Aigner, R.; Vroemen, M.; Weidner, N.; Bogdahn, U.; Winkler, J.; Kuhn, H.G.; Aigner, L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005, 21, 1–14. [Google Scholar] [CrossRef]
- Elliott, K.L.; Pavlinkova, G.; Chizhikov, V.V.; Yamoah, E.N.; Fritzsch, B. Neurog1, Neurod1, and Atoh1 are essential for spiral ganglia, cochlear nuclei, and cochlear hair cell development. Fac. Rev. 2021, 10, 47. [Google Scholar] [CrossRef]
- Ma, Q.; Anderson, D.J.; Fritzsch, B. Neurogenin 1 null mutant ears develop fewer, morphologically normal hair cells in smaller sensory epithelia devoid of innervation. J. Assoc. Res. Otolaryngol. 2000, 1, 129–143. [Google Scholar] [CrossRef]
- Han, S.; Dennis, D.J.; Balakrishnan, A.; Dixit, R.; Britz, O.; Zinyk, D.; Touahri, Y.; Olender, T.; Brand, M.; Guillemot, F.; et al. A non-canonical role for the proneural gene Neurog1 as a negative regulator of neocortical neurogenesis. Development 2018, 145, dev157719. [Google Scholar] [CrossRef]
- Gee, J.R.; Keller, J.N. Astrocytes: Regulation of brain homeostasis via apolipoprotein E. Int. J. Biochem. Cell Biol. 2005, 37, 1145–1150. [Google Scholar] [CrossRef]
- Jalali, A.; Bassuk, A.G.; Kan, L.; Israsena, N.; Mukhopadhyay, A.; McGuire, T.; Kessler, J.A. HeyL promotes neuronal differentiation of neural progenitor cells. J. Neurosci. Res. 2011, 89, 299–309. [Google Scholar] [CrossRef]
- Leimeister, C.; Externbrink, A.; Klamt, B.; Gessler, M. Hey genes: A novel subfamily of hairy- and Enhancer of split related genes specifically expressed during mouse embryogenesis. Mech. Dev. 1999, 85, 173–177. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Jarrett, J.; Chlon, T.; Kessler, J.A. HeyL regulates the number of TrkC neurons in dorsal root ganglia. Dev. Biol. 2009, 334, 142–151. [Google Scholar] [CrossRef]
- Fischer, A.; Gessler, M. Delta-Notch—and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007, 35, 4583–4596. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, J.; Zhou, L.; Pollard, S.M.; Smith, A. Interplay between FGF2 and BMP controls the self-renewal, dormancy and differentiation of rat neural stem cells. J. Cell Sci. 2011, 124, 1867–1877. [Google Scholar] [CrossRef]
- Dayer, A.G.; Jenny, B.; Sauvain, M.O.; Potter, G.; Salmon, P.; Zgraggen, E.; Kanemitsu, M.; Gascon, E.; Sizonenko, S.; Trono, D.; et al. Expression of FGF-2 in neural progenitor cells enhances their potential for cellular brain repair in the rodent cortex. Brain 2007, 130, 2962–2976. [Google Scholar] [CrossRef]
- Nakashima, K.; Yanagisawa, M.; Arakawa, H.; Taga, T. Astrocyte differentiation mediated by LIF in cooperation with BMP2. FEBS Lett. 1999, 457, 43–46. [Google Scholar] [CrossRef]
- Kamegai, M.; Niijima, K.; Kunishita, T.; Nishizawa, M.; Ogawa, M.; Araki, M.; Ueki, A.; Konishi, Y.; Tabira, T. Interleukin 3 as a trophic factor for central cholinergic neurons in vitro and in vivo. Neuron 1990, 4, 429–436. [Google Scholar] [CrossRef]
- Snapyan, M.; Lemasson, M.; Brill, M.S.; Blais, M.; Massouh, M.; Ninkovic, J.; Gravel, C.; Berthod, F.; Gotz, M.; Barker, P.A.; et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J. Neurosci. 2009, 29, 4172–4188. [Google Scholar] [CrossRef]
- Gerhardt, H.; Ruhrberg, C.; Abramsson, A.; Fujisawa, H.; Shima, D.; Betsholtz, C. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev. Dyn. 2004, 231, 503–509. [Google Scholar] [CrossRef]
- Lopez-Bendito, G.; Flames, N.; Ma, L.; Fouquet, C.; Di Meglio, T.; Chedotal, A.; Tessier-Lavigne, M.; Marin, O. Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J. Neurosci. 2007, 27, 3395–3407. [Google Scholar] [CrossRef]
- El-Husseini, A.E.; Schnell, E.; Chetkovich, D.M.; Nicoll, R.A.; Bredt, D.S. PSD-95 involvement in maturation of excitatory synapses. Science 2000, 290, 1364–1368. [Google Scholar] [CrossRef]
- Johnson, G.V.; Jope, R.S. The role of microtubule-associated protein 2 (MAP-2) in neuronal growth, plasticity, and degeneration. J. Neurosci. Res. 1992, 33, 505–512. [Google Scholar] [CrossRef]
- Trigueiros-Cunha, N.; Renard, N.; Humbert, G.; Tavares, M.A.; Eybalin, M. Catecholamine-independent transient expression of tyrosine hydroxylase in primary auditory neurons is coincident with the onset of hearing in the rat cochlea. Eur. J. Neurosci. 2003, 18, 2653–2662. [Google Scholar] [CrossRef]
- Majdzadeh, N.; Wang, L.; Morrison, B.E.; Bassel-Duby, R.; Olson, E.N.; D’Mello, S.R. HDAC4 inhibits cell-cycle progression and protects neurons from cell death. Dev. Neurobiol. 2008, 68, 1076–1092. [Google Scholar] [CrossRef]
- Tang, C.; Wang, M.; Wang, P.; Wang, L.; Wu, Q.; Guo, W. Neural Stem Cells Behave as a Functional Niche for the Maturation of Newborn Neurons through the Secretion of PTN. Neuron 2019, 101, 32–44.e6. [Google Scholar] [CrossRef]
- Etheridge, S.L.; Ray, S.; Li, S.; Hamblet, N.S.; Lijam, N.; Tsang, M.; Greer, J.; Kardos, N.; Wang, J.; Sussman, D.J.; et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS Genet. 2008, 4, e1000259. [Google Scholar] [CrossRef]
- Bond, J.F.; Farmer, S.R. Regulation of tubulin and actin mRNA production in rat brain: Expression of a new beta-tubulin mRNA with development. Mol. Cell. Biol. 1983, 3, 1333–1342. [Google Scholar] [CrossRef]
- Micheva, K.D.; Vallee, A.; Beaulieu, C.; Herman, I.M.; Leclerc, N. beta-Actin is confined to structures having high capacity of remodelling in developing and adult rat cerebellum. Eur. J. Neurosci. 1998, 10, 3785–3798. [Google Scholar] [CrossRef]
- Datta, S.; Chakrabarti, N. Age related rise in lactate and its correlation with lactate dehydrogenase (LDH) status in post-mitochondrial fractions isolated from different regions of brain in mice. Neurochem. Int. 2018, 118, 23–33. [Google Scholar] [CrossRef]
- Lai, K.; Kaspar, B.K.; Gage, F.H.; Schaffer, D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003, 6, 21–27. [Google Scholar] [CrossRef]
- Han, Y.G.; Spassky, N.; Romaguera-Ros, M.; Garcia-Verdugo, J.M.; Aguilar, A.; Schneider-Maunoury, S.; Alvarez-Buylla, A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008, 11, 277–284. [Google Scholar] [CrossRef]
- Ahn, S.; Joyner, A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 2005, 437, 894–897. [Google Scholar] [CrossRef]
- Palma, V.; Lim, D.A.; Dahmane, N.; Sanchez, P.; Brionne, T.C.; Herzberg, C.D.; Gitton, Y.; Carleton, A.; Alvarez-Buylla, A.; Ruiz i Altaba, A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 2005, 132, 335–344. [Google Scholar] [CrossRef]
- Hiramoto, T.; Kanda, Y.; Satoh, Y.; Takishima, K.; Watanabe, Y. Dopamine D2 receptor stimulation promotes the proliferation of neural progenitor cells in adult mouse hippocampus. Neuroreport 2007, 18, 659–664. [Google Scholar] [CrossRef]
- Hoglinger, G.U.; Rizk, P.; Muriel, M.P.; Duyckaerts, C.; Oertel, W.H.; Caille, I.; Hirsch, E.C. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 2004, 7, 726–735. [Google Scholar] [CrossRef]
- Houlihan, S.L.; Lanctot, A.A.; Guo, Y.; Feng, Y. Upregulation of neurovascular communication through filamin abrogation promotes ectopic periventricular neurogenesis. Elife 2016, 5, e17823. [Google Scholar] [CrossRef]
- Sueda, R.; Imayoshi, I.; Harima, Y.; Kageyama, R. High Hes1 expression and resultant Ascl1 suppression regulate quiescent vs. active neural stem cells in the adult mouse brain. Genes Dev. 2019, 33, 511–523. [Google Scholar] [CrossRef]
- Alexson, T.O.; Hitoshi, S.; Coles, B.L.; Bernstein, A.; van der Kooy, D. Notch signaling is required to maintain all neural stem cell populations—irrespective of spatial or temporal niche. Dev. Neurosci. 2006, 28, 34–48. [Google Scholar] [CrossRef]
- Ninkovic, J.; Gotz, M. Signaling in adult neurogenesis: From stem cell niche to neuronal networks. Curr. Opin. Neurobiol. 2007, 17, 338–344. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Sun, J.; Sun, J.; Ming, G.L.; Song, H. Epigenetic regulation of neurogenesis in the adult mammalian brain. Eur. J. Neurosci. 2011, 33, 1087–1093. [Google Scholar] [CrossRef]
- Kusumoto, D.; Yuasa, S. The application of convolutional neural network to stem cell biology. Inflamm. Regen. 2019, 39, 14. [Google Scholar] [CrossRef]
- Pinto, L.H.; Vitaterna, M.H.; Shimomura, K.; Siepka, S.M.; Balannik, V.; McDearmon, E.L.; Omura, C.; Lumayag, S.; Invergo, B.M.; Glawe, B.; et al. Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis. Neurosci. 2007, 24, 111–123. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Plante, T.B.; Cushman, M. Choosing color palettes for scientific figures. Res. Pract. Thromb. Haemost. 2020, 4, 176–180. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Engert, J.; Spahn, B.; Sommerer, S.; Ehret Kasemo, T.; Hackenberg, S.; Rak, K.; Voelker, J. Adult Neurogenesis of the Medial Geniculate Body: In Vitro and Molecular Genetic Analyses Reflect the Neural Stem Cell Capacity of the Rat Auditory Thalamus over Time. Int. J. Mol. Sci. 2024, 25, 2623. https://doi.org/10.3390/ijms25052623
Engert J, Spahn B, Sommerer S, Ehret Kasemo T, Hackenberg S, Rak K, Voelker J. Adult Neurogenesis of the Medial Geniculate Body: In Vitro and Molecular Genetic Analyses Reflect the Neural Stem Cell Capacity of the Rat Auditory Thalamus over Time. International Journal of Molecular Sciences. 2024; 25(5):2623. https://doi.org/10.3390/ijms25052623
Chicago/Turabian StyleEngert, Jonas, Bjoern Spahn, Sabine Sommerer, Totta Ehret Kasemo, Stephan Hackenberg, Kristen Rak, and Johannes Voelker. 2024. "Adult Neurogenesis of the Medial Geniculate Body: In Vitro and Molecular Genetic Analyses Reflect the Neural Stem Cell Capacity of the Rat Auditory Thalamus over Time" International Journal of Molecular Sciences 25, no. 5: 2623. https://doi.org/10.3390/ijms25052623
APA StyleEngert, J., Spahn, B., Sommerer, S., Ehret Kasemo, T., Hackenberg, S., Rak, K., & Voelker, J. (2024). Adult Neurogenesis of the Medial Geniculate Body: In Vitro and Molecular Genetic Analyses Reflect the Neural Stem Cell Capacity of the Rat Auditory Thalamus over Time. International Journal of Molecular Sciences, 25(5), 2623. https://doi.org/10.3390/ijms25052623





