Simultaneous Determination of Enantiomeric Purity and Organic Impurities of Dexketoprofen Using Reversed-Phase Liquid Chromatography—Enhancing Enantioselectivity through Hysteretic Behavior and Temperature-Dependent Enantiomer Elution Order Reversal on Polysaccharide Chiral Stationary Phases
Abstract
:1. Introduction
2. Results and Discussion
2.1. Scouting Phase
2.2. Method Development
2.3. Method Validation and Application
2.4. The Role of Eluent in Mixture in Chiral Recognition—Hysteresis Phenomenon in PO and Reversed-Phase Mode
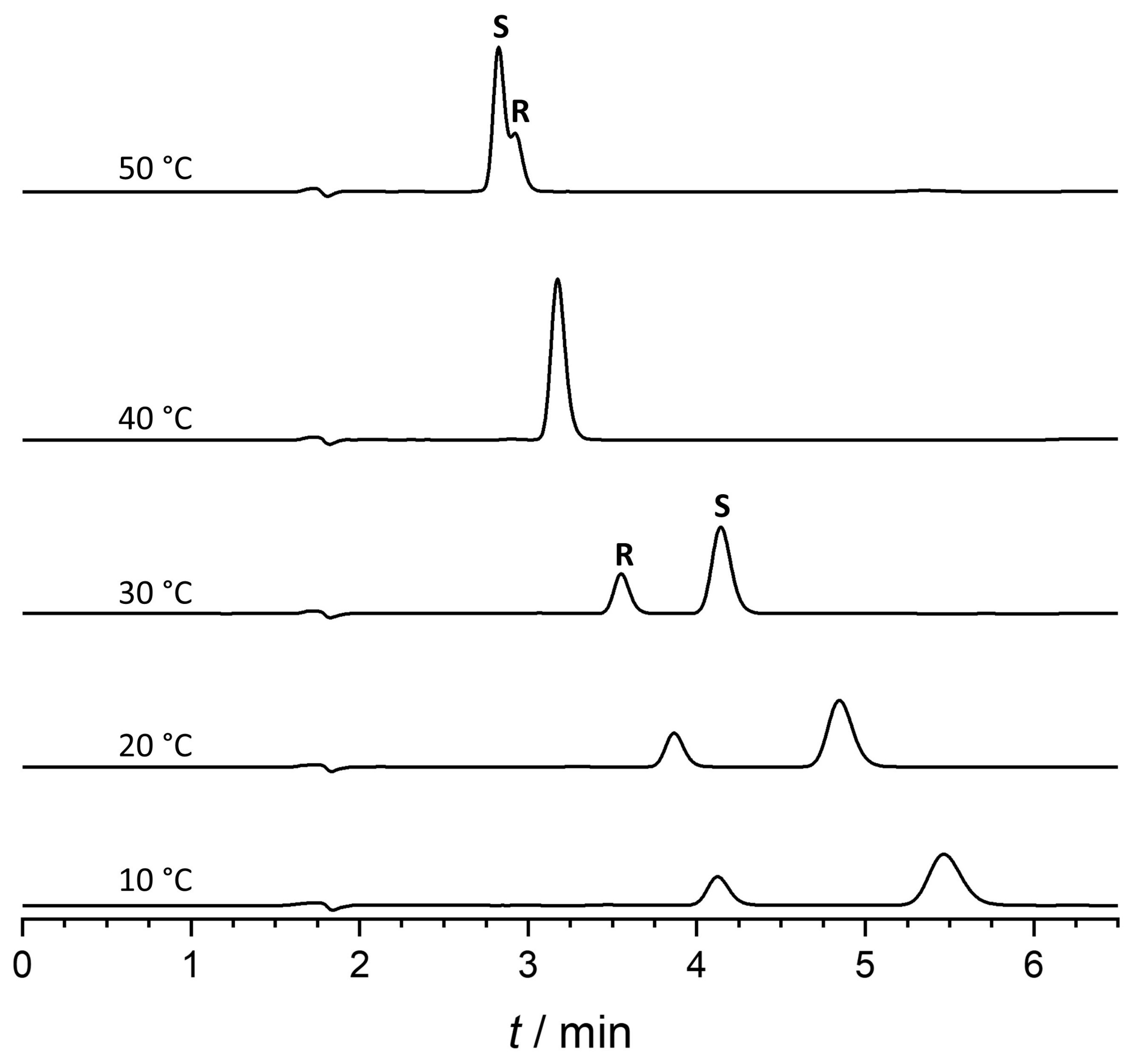
2.5. Thermodynamic Study—Temperature-Dependent Elution Order Reversal
3. Materials and Methods
3.1. Materials
3.2. HPLC Analysis
3.3. Analysis of Pharmaceutical Formulation
3.4. Thermodynamic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hancu, G.; Modroiu, A. Chiral Switch: Between Therapeutical Benefit and Marketing Strategy. Pharmaceuticals 2022, 15, 240. [Google Scholar] [CrossRef]
- Calcaterra, A.; D’Acquarica, I. The Market of Chiral Drugs: Chiral Switches versus de Novo Enantiomerically Pure Compounds. J. Pharm. Biomed. Anal. 2018, 147, 323–340. [Google Scholar] [CrossRef]
- Agranat, I.; Caner, H.; Caldwell, J. Putting Chirality to Work: The Strategy of Chiral Switches. Nat. Rev. Drug Discov. 2002, 1, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Olbe, L.; Carlsson, E.; Lindberg, P. A Proton-Pump Inhibitor Expedition: The Case Histories of Omeprazole and Esomeprazole. Nat. Rev. Drug Discov. 2003, 2, 132–139. [Google Scholar] [CrossRef]
- Hardikar, M.S. Chiral Non-Steroidal Anti-Inflammatory Drugs—A Review. J. Indian Med. Assoc. 2008, 106, 615–618, 622, 624. [Google Scholar]
- Beltrán, J.; Martín-Mola, E.; Figueroa, M.; Granados, J.; Sanmartí, R.; Artigas, R.; Torres, F.; Forns, M.; Mauleón, D. Comparison of Dexketoprofen Trometamol and Ketoprofen in the Treatment of Osteoarthritis of the Knee. J. Clin. Pharmacol. 1998, 38, 74S–80S. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Barden, J. Systematic Review of Dexketoprofen in Acute and Chronic Pain. BMC Clin. Pharmacol. 2008, 8, 11. [Google Scholar] [CrossRef]
- Ferencz, E.; Kovács, B.; Boda, F.; Foroughbakhshfasaei, M.; Kelemen, É.K.; Tóth, G.; Szabó, Z.I. Simultaneous Determination of Chiral and Achiral Impurities of Ivabradine on a Cellulose Tris(3-Chloro-4-Methylphenylcarbamate) Chiral Column Using Polar Organic Mode. J. Pharm. Biomed. Anal. 2020, 177, 112851. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Bartalis-Fábián, Á.; Tóth, G. Simultaneous Determination of Escitalopram Impurities Including the R-Enantiomer on a Cellulose Tris(3,5-Dimethylphenylcarbamate)-Based Chiral Column in Reversed-Phase Mode. Molecules 2022, 27, 9022. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, C.; La Regina, G.; Ferretti, R.; Silvestri, R.; Cirilli, R. Single-Run Chemo- and Enantio-Selective High-Performance Liquid Chromatography Separation of Tramadol and Its Principal Metabolite, O-Desmethyltramadol, Using a Chlorinated Immobilized Amylose-Based Chiral Stationary Phase under Multimodal Elution Conditions. Sep. Sci. Plus 2022, 5, 99–104. [Google Scholar] [CrossRef]
- Mammone, F.R.; Rotundo, P.; Ferretti, R.; Puxeddu, M.; Silvestri, R.; Cirilli, R. Chemo- and Enantio-Selective Reversed-Phase HPLC Analysis of Rosuvastatin Using a Cellulose-Based Chiral Stationary Phase in Gradient Elution Mode. J. Pharm. Biomed. Anal. 2023, 225, 115239. [Google Scholar] [CrossRef]
- Chankvetadze, B. Recent Trends in Preparation, Investigation and Application of Polysaccharide-Based Chiral Stationary Phases for Separation of Enantiomers in High-Performance Liquid Chromatography. TrAC Trends Anal. Chem. 2020, 122, 115709. [Google Scholar] [CrossRef]
- Peluso, P.; Mamane, V.; Dallocchio, R.; Dessì, A.; Cossu, S. Noncovalent Interactions in High-Performance Liquid Chromatography Enantioseparations on Polysaccharide-Based Chiral Selectors. J. Chromatogr. A 2020, 1623, 461202. [Google Scholar] [CrossRef]
- Kucerova, G.; Kalikova, K.; Tesarova, E. Enantioselective Potential of Polysaccharide-Based Chiral Stationary Phases in Supercritical Fluid Chromatography. Chirality 2017, 29, 239–246. [Google Scholar] [CrossRef]
- Vyas, R.; Bhushan, R.; Nagar, H.; Sharma, A. Reversed-Phase-HPLC Enantioseparation and Control of Enantiomeric Purity of Duloxetine Using a New Chiral Reagent and Recovery of Enantiomers. Biomed. Chromatogr. 2021, 35, e5228. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, K.; Ohnishi, A. Reversed-Phase Liquid Chromatographic Separation of Enantiomers on Polysaccharide Type Chiral Stationary Phases. J. Chromatogr. A 2001, 906, 127–154. [Google Scholar] [CrossRef]
- Horváth, S.; Németh, G. Hysteresis of Retention and Enantioselectivity on Amylose Tris(3,5-Dimethylphenylcarbamate) Chiral Stationary Phases in Mixtures of 2-Propanol and Methanol. J. Chromatogr. A 2018, 1568, 149–159. [Google Scholar] [CrossRef]
- Horváth, S.; Eke, Z.; Németh, G. Utilization of the Hysteresis Phenomenon for Chiral High-Performance Liquid Chromatographic Method Selection in Polar Organic Mode. J. Chromatogr. A 2020, 1625, 461280. [Google Scholar] [CrossRef] [PubMed]
- Foroughbakhshfasaei, M.; Dobó, M.; Boda, F.; Szabó, Z.I.; Tóth, G. Comparative Chiral Separation of Thalidomide Class of Drugs Using Polysaccharide-type Stationary Phases with Emphasis on Elution Order and Hysteresis in Polar Organic Mode. Molecules 2022, 27, 111. [Google Scholar] [CrossRef]
- Wang, X.; Jameson, C.J.; Murad, S. Modeling Enantiomeric Separations as an Interfacial Process Using Amylose Tris(3,5-Dimethylphenyl Carbamate) (ADMPC) Polymers Coated on Amorphous Silica. Langmuir 2020, 36, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Dobó, M.; Foroughbakhshfasaei, M.; Horváth, P.; Szabó, Z.I.; Tóth, G. Chiral Separation of Oxazolidinone Analogues by Liquid Chromatography on Polysaccharide Stationary Phases Using Polar Organic Mode. J. Chromatogr. A 2022, 1662, 462741. [Google Scholar] [CrossRef]
- Jibuti, G.; Mskhiladze, A.; Takaishvili, N.; Karchkhadze, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. HPLC Separation of Dihydropyridine Derivatives Enantiomers with Emphasis on Elution Order Using Polysaccharide-Based Chiral Columns. J. Sep. Sci. 2012, 35, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Mosiashvili, L.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. On the Effect of Basic and Acidic Additives on the Separation of the Enantiomers of Some Basic Drugs with Polysaccharide-Based Chiral Selectors and Polar Organic Mobile Phases. J. Chromatogr. A 2013, 1317, 167–174. [Google Scholar] [CrossRef]
- Mskhiladze, A.; Karchkhadze, M.; Dadianidze, A.; Fanali, S.; Farkas, T.; Chankvetadze, B. Enantioseparation of Chiral Antimycotic Drugs by HPLC with Polysaccharide-Based Chiral Columns and Polar Organic Mobile Phases with Emphasis on Enantiomer Elution Order. Chromatographia 2013, 76, 1449–1458. [Google Scholar] [CrossRef]
- Gegenava, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. Enantioseparation of Selected Chiral Sulfoxides in High-Performance Liquid Chromatography with Polysaccharide-Based Chiral Selectors in Polar Organic Mobile Phases with Emphasis on Enantiomer Elution Order. J. Sep. Sci. 2014, 37, 1083–1088. [Google Scholar] [CrossRef]
- Chankvetadze, L.; Ghibradze, N.; Karchkhadze, M.; Peng, L.; Farkas, T.; Chankvetadze, B. Enantiomer Elution Order Reversal of Fluorenylmethoxycarbonyl-Isoleucine in High-Performance Liquid Chromatography by Changing the Mobile Phase Temperature and Composition. J. Chromatogr. A 2011, 1218, 6554–6560. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M. Reversal of Elution Order during the Chiral Separation in High Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 2002, 27, 401–407. [Google Scholar] [CrossRef]
- Yang, X.; Su, L.; Hou, X.; Ding, S.; Xu, W.; Wang, B.; Fang, H. High-Performance Liquid Chromatographic Enantioseparation of 3,5-Disubstituted Hydantoins Analogs and Temperature-Induced Reversals of Elution Orders on a Polysaccharide-Based Chiral Stationary Phase. J. Chromatogr. A 2014, 1355, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Sardella, R.; Lisanti, A.; Carotti, A.; Blasi, P.; Lindner, W.; Natalini, B. Ketoprofen Enantioseparation with a Cinchona Alkaloid Based Stationary Phase: Enantiorecognition Mechanism and Release Studies. J. Sep. Sci. 2014, 37, 2696–2703. [Google Scholar] [CrossRef]
- Tang, Y. Significance of Mobile Phase Composition in Enantioseparation of Chiral Drugs by HPLC on a Cellulose-Based Chiral Stationary Phase. Chirality 1996, 8, 136–142. [Google Scholar] [CrossRef]
- Tok, K.C.; Gumustas, M.; Jibuti, G.; Suzen, H.S.; Ozkan, S.A.; Chankvetadze, B. The Effect of Enantiomer Elution Order on the Determination of Minor Enantiomeric Impurity in Ketoprofen and Enantiomeric Purity Evaluation of Commercially Available Dexketoprofen Formulations. Molecules 2020, 25, 5865. [Google Scholar] [CrossRef]
- Li, M.; Liang, X.; Guo, X.; Di, X.; Jiang, Z. Enantiomeric Separation and Enantioselective Determination of Some Representive Non-Steroidal Anti-Inflammatory Drug Enantiomers in Fish Tissues by Using Chiral Liquid Chromatography Coupled with Tandem Mass Spectrometry. Microchem. J. 2020, 153, 104511. [Google Scholar] [CrossRef]
- Menzel-Soglowek, S.; Geisslinger, G.; Brune, K. Stereoselective High-Performance Liquid Chromatographic Determination of Ketoprofen, Ibuprofen and Fenoprofen in Plasma Using a Chiral A1-Acid Glycoprotein Column. J. Chromatogr. B Biomed. Sci. Appl. 1990, 532, 295–303. [Google Scholar] [CrossRef]
- Németi, G.; Berkecz, R.; Le, T.M.; Szakonyi, Z.; Péter, A.; Ilisz, I. High-Performance Liquid Chromatographic Enantioseparation of Azole Analogs of Monoterpene Lactones and Amides Focusing on the Separation Characteristics of Polysaccharide-Based Chiral Stationary Phases. J. Chromatogr. A 2024, 1717, 464660. [Google Scholar] [CrossRef] [PubMed]
- Panella, C.; Ferretti, R.; Casulli, A.; Cirilli, R. Temperature and Eluent Composition Effects on Enantiomer Separation of Carvedilol by High-Performance Liquid Chromatography on Immobilized Amylose-Based Chiral Stationary Phases. J. Pharm. Anal. 2019, 9, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Suhail, M.; Asnin, L.; Aboul-Enein, H.Y. Effect of Various Parameters and Mechanism of Reversal Order of Elution in Chiral HPLC. Curr. Anal. Chem. 2020, 16, 59–78. [Google Scholar] [CrossRef]
- Stringham, R.W.; Blackwell, J.A. “Entropically Driven” Chiral Separations in Supercritical Fluid Chromatography. Confirmation of Isoelution Temperature and Reversal of Elution Order. Anal. Chem. 1996, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Matarashvili, I.; Kobidze, G.; Chelidze, A.; Dolidze, G.; Beridze, N.; Jibuti, G.; Farkas, T.; Chankvetadze, B. The Effect of Temperature on the Separation of Enantiomers with Coated and Covalently Immobilized Polysaccharide-Based Chiral Stationary Phases. J. Chromatogr. A 2019, 1599, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yao, Y.; Deng, M.; Jiang, Z.; Li, Q. Enantioselective Separation of Twelve Pairs of Enantiomers on Polysaccharide-Based Chiral Stationary Phases and Thermodynamic Analysis of Separation Mechanism. Electrophoresis 2018, 39, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.-I.; Foroughbakhshfasaei, M.; Gál, R.; Horváth, P.; Komjáti, B.; Noszál, B.; Tóth, G. Chiral Separation of Lenalidomide by Liquid Chromatography on Polysaccharide-Type Stationary Phases and by Capillary Electrophoresis Using Cyclodextrin Selectors. J. Sep. Sci. 2018, 41, 1414–1423. [Google Scholar] [CrossRef]
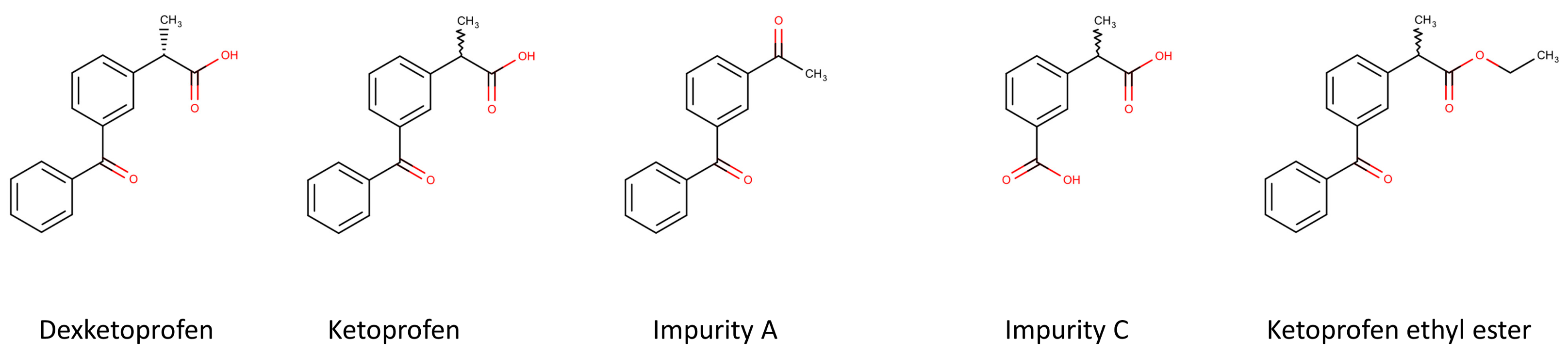


| Column | Mobile Phase * | k1 | Rs |
|---|---|---|---|
| Lux Amylose-1 | MeOH | 0.26 | 0 |
| ACN | 0.19 | 0 | |
| MeOH/water 90:10 | 0.61 | 0 | |
| MeOH/water 70:30 | 3.55 | 0.67 | |
| ACN/water 90:10 | 0.13 | 0 | |
| ACN/water 70:30 | 0.33 | 0 | |
| ACN/water 50:50 | 1.23 | 0 | |
| Lux Amylose-2 | MeOH | 0.29 | 0 |
| ACN | 0.75 | 0 | |
| MeOH/water 90:10 | 0.53 | 0 | |
| MeOH/water 70:30 | 2.49 | 0 | |
| ACN/water 90:10 | 0.51 | 0 | |
| ACN/water 70:30 | 0.93 | 0 | |
| ACN/water 50:50 | 1.77 | 3.32 | |
| Lux Cellulose-1 | MeOH | 0.63 | 0 |
| ACN | 0.93 | 0 | |
| MeOH/water 90:10 | 0.94 | 0 | |
| MeOH/water 70:30 | 4.01 | 0 | |
| ACN/water 90:10 | 0.33 | 0 | |
| ACN/water 70:30 | 0.62 | 0 | |
| ACN/water 50:50 | 1.98 | 0 | |
| Lux Cellulose-2 | MeOH | 0.74 | 0 |
| ACN | 1.43 | 0 | |
| MeOH/water 90:10 | 1.03 | 0 | |
| MeOH/water 70:30 | 3.92 | 0 | |
| ACN/water 90:10 | 0.63 | 0 | |
| ACN/water 70:30 | 1.13 | 0 | |
| ACN/water 50:50 | 3.57 | 0 |
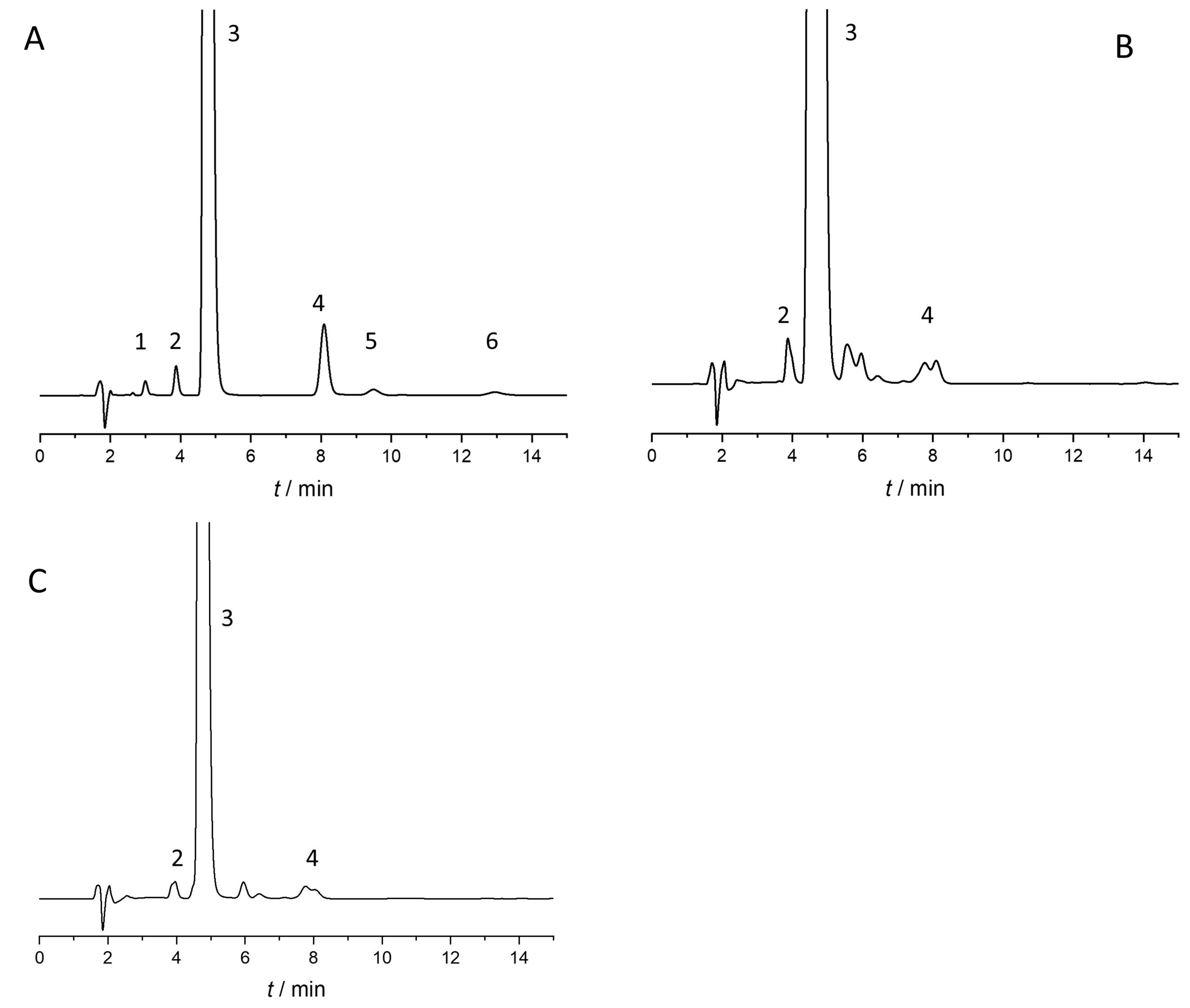
| Run No. | ACN in Water (%) * (Factor 1) | Temperature (°C) (Factor 2) | Analysis Time (min) | Rs1 ** | Rs2 ** | Rs3 ** | Rs4 ** | Elution Order *** |
|---|---|---|---|---|---|---|---|---|
| 1 | 70 | 20 | 4.1 | 1.44 | 0 | 4.78 | 0.87 | C > R = S > A > ester |
| 2 | 60 | 30 | 5.5 | 0 | 2.02 | 7.27 | 1.79 | C = R > S > A > ester |
| 3 | 50 | 10 | 13.9 | 4.55 | 3.46 | 7.56 | 4.42 | C > R > S > A > ester |
| 4 | 50 | 20 | 12.5 | 5.07 | 3.32 | 8.91 | 4.64 | C > R > S > A > ester |
| 5 | 70 | 40 | 3.5 | 1.16 | 0 | 4.85 | 0.8 | C > R = S > A > ester |
| 6 | 60 | 20 | 6.3 | 1.89 | 2.42 | 6.63 | 2.41 | C > R > S > A > ester |
| 7 | 60 | 10 | 7.0 | 1.88 | 2.72 | 06.01 | 2.30 | C > R > S > A > ester |
| 8 | 70 | 10 | 4.4 | 1.94 | 0 | 4.39 | 0.91 | C > R = S > A > ester |
| 9 | 50 | 30 | 10.3 | 3.45 | 2.48 | 9.97 | 3.91 | C > R > S > A > ester |
| 10 | 50 | 40 | 7.9 | 3.30 | 0 | 11.30 | 1.64 | C > R = S > A > ester |
| 11 | 60 | 40 | 5.1 | 1.36 | 0 | 7.31 | 2.55 | C > R = S > A > ester |
| 12 | 70 | 30 | 3.6 | 1.3 | 0 | 4.58 | 0 | R > C = S > A = ester |
| 13 | 70 | 30 | 3.7 | 1.33 | 0.8 | 4.71 | 0 | R > C = S > A = ester |
| 14 | 70 | 40 | 3.5 | 1.14 | 0 | 4.87 | 0.79 | C > R = S > A > ester |
| 15 | 60 | 30 | 5.5 | 0 | 2,17 | 7.27 | 1.8 | C = R > S > A > ester |
| 16 | 60 | 20 | 6.3 | 1.88 | 2.4 | 6.67 | 2.38 | C > R > S > A > ester |
| 17 | 60 | 10 | 7.0 | 1.91 | 2.7 | 5.98 | 2.30 | C > R > S > A > ester |
| 18 | 70 | 10 | 4.4 | 1.97 | 0 | 4.39 | 0.91 | C > R = S > A > ester |
| 19 | 50 | 20 | 12.4 | 5.04 | 3.32 | 8.86 | 4.59 | C > R > S > A > ester |
| 20 | 50 | 40 | 7.8 | 3.04 | 0 | 11.35 | 1.47 | C > R = S > A > ester |
| 21 | 50 | 10 | 13.9 | 4.62 | 3.46 | 7.59 | 4.42 | C > R > S > A > ester |
| 22 | 50 | 30 | 10.4 | 3.64 | 2.5 | 10.01 | 3.97 | C > R > S > A > ester |
| 23 | 60 | 40 | 5.1 | 1.13 | 0 | 7.50 | 2.55 | C > R = S > A > ester |
| 24 | 70 | 20 | 4.1 | 1.48 | 0 | 4.74 | 0.87 | C > R = S > A > ester |
| 25 | 40 | 10 | 45.2 | 11.56 | 4.43 | 8.59 | 08.07 | C > R > S > A > ester |
| 26 | 40 | 10 | 45.3 | 11.23 | 4.38 | 8.64 | 8.01 | C > R > S > A > ester |
| 27 | 40 | 20 | 37.6 | 11.63 | 4.33 | 10.43 | 8.53 | C > R > S > A > ester |
| 28 | 40 | 20 | 37.9 | 11.99 | 4.42 | 10.42 | 08.02 | C > R > S > A > ester |
| 29 | 40 | 30 | 29.6 | 10.09 | 3.44 | 12.56 | 7.44 | C > R > S > A > ester |
| 30 | 40 | 30 | 29.5 | 9.95 | 3.46 | 12.49 | 7.29 | C > R > S > A > ester |
| 31 | 40 | 40 | 18.8 | 7.23 | 0 | 16.24 | 03.08 | C > R = S > A > ester |
| 32 | 40 | 40 | 18.3 | 6.65 | 0 | 16.44 | 2.72 | C > R = S > A > ester |
| 33 | 40 | 50 | 14.4 | 5.10 | 0 | 16.55 | 2.12 | C > R = S > A > ester |
| 34 | 40 | 50 | 14.4 | 4.93 | 0 | 16.47 | 02.06 | C > R = S > A > ester |
| 35 | 70 | 50 | 2.7 | 0 | 1.78 | 1.55 | 1.08 | R = S > C > ester > A |
| 36 | 70 | 50 | 2.7 | 0 | 1.62 | 1.85 | 1.21 | R = S > C > ester > A |
| 37 | 60 | 50 | 3.7 | 0 | 0.85 | 6.96 | 1.62 | R = S > C > ester > A |
| 38 | 60 | 50 | 3.6 | 0 | 0.79 | 6.96 | 1.62 | R = S > C > ester > A |
| 39 | 50 | 50 | 6.2 | 0.85 | 0 | 10.72 | 03.09 | C > R = S > A > ester |
| 40 | 50 | 50 | 6.2 | 0.90 | 0 | 10.72 | 03.09 | C > R = S > A > ester |
| Parameter | Level | IMP-A | R-ket | IMP-C | Ester-a | Ester-b |
|---|---|---|---|---|---|---|
| Range (%) | 0.05–0.3 | 0.05–0.3 | 0.05–0.3 | 0.1–0.3 | 0.1–0.3 | |
| Equation | 743.6x + 6.965 | 654.51x + 1.896 | 1738.2x + 7.822 | 560.43x + 2.108 | 612x + 1.123 | |
| r2 | 0.9988 | 0.9990 | 0.9979 | 0.9991 | 0.9994 | |
| LOD (μg/mL) | 0.45 | 0.37 | 0.13 | 1.12 | 1.15 | |
| LOQ (μg/mL) | 1.50 | 1.23 | 0.43 | 3.73 | 3.83 | |
| Accuracy | I. * | 99.4% | 99.1% | 99.8% | 99.1% | 99.6% |
| II. (0.15%) | 99.9% | 100.3% | 100.2% | 100.3% | 98.8% | |
| III. (0.3%) | 99.6% | 98.2% | 99.6% | 98.2% | 100.1% | |
| Intraday precision (RSD%) | I. * | 0.8% | 0.9% | 0.3% | 0.6% | 1.4% |
| II. (0.15%) | 0.8% | 1.2% | 0.1% | 0.9% | 1.3% | |
| III. (0.3%) | 0.5% | 0.1% | 0.1% | 1.1% | 2.0% | |
| Intermediate precision (RSD%) | I. * | 0.9% | 1.5% | 0.3% | 1.5% | 1.1% |
| II. (0.15%) | 1.3% | 0.2% | 0.2% | 1.8% | 2.0% | |
| III. (0.3%) | 0.6% | 0.2% | 0.2% | 1.5% | 2.1% |
| Product | R-Ketoprofen | Impurity A | Sum of All Impurities |
|---|---|---|---|
| Dekenor | 0.32% ± 0.04 | <0.05% | 0.45% ± 0.05 |
| Ketodex | 0.15% ± 0.01 | <0.05% | 0.57% ± 0.07 |
| Temperature Range (°C) | Equation | r2 | Δ(ΔH°) (kJ/mol) | Δ(ΔS°) (J/molK) | Δ(ΔG°) (kJ/mol) | Tiso (°C) |
|---|---|---|---|---|---|---|
| 10–40 | lnα = 1573.7x − 5.017 | 0.9926 | −13.1 | −41.7 | −0.7 | 40.7 |
| 40–55 | lnα = −1239.5x + 3.957 | 0.9997 | 16.3 | 32.9 | 0.5 | 40.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobó, M.; Dombi, G.; Köteles, I.; Fiser, B.; Kis, C.; Szabó, Z.-I.; Tóth, G. Simultaneous Determination of Enantiomeric Purity and Organic Impurities of Dexketoprofen Using Reversed-Phase Liquid Chromatography—Enhancing Enantioselectivity through Hysteretic Behavior and Temperature-Dependent Enantiomer Elution Order Reversal on Polysaccharide Chiral Stationary Phases. Int. J. Mol. Sci. 2024, 25, 2697. https://doi.org/10.3390/ijms25052697
Dobó M, Dombi G, Köteles I, Fiser B, Kis C, Szabó Z-I, Tóth G. Simultaneous Determination of Enantiomeric Purity and Organic Impurities of Dexketoprofen Using Reversed-Phase Liquid Chromatography—Enhancing Enantioselectivity through Hysteretic Behavior and Temperature-Dependent Enantiomer Elution Order Reversal on Polysaccharide Chiral Stationary Phases. International Journal of Molecular Sciences. 2024; 25(5):2697. https://doi.org/10.3390/ijms25052697
Chicago/Turabian StyleDobó, Máté, Gergely Dombi, István Köteles, Béla Fiser, Csenge Kis, Zoltán-István Szabó, and Gergő Tóth. 2024. "Simultaneous Determination of Enantiomeric Purity and Organic Impurities of Dexketoprofen Using Reversed-Phase Liquid Chromatography—Enhancing Enantioselectivity through Hysteretic Behavior and Temperature-Dependent Enantiomer Elution Order Reversal on Polysaccharide Chiral Stationary Phases" International Journal of Molecular Sciences 25, no. 5: 2697. https://doi.org/10.3390/ijms25052697
APA StyleDobó, M., Dombi, G., Köteles, I., Fiser, B., Kis, C., Szabó, Z.-I., & Tóth, G. (2024). Simultaneous Determination of Enantiomeric Purity and Organic Impurities of Dexketoprofen Using Reversed-Phase Liquid Chromatography—Enhancing Enantioselectivity through Hysteretic Behavior and Temperature-Dependent Enantiomer Elution Order Reversal on Polysaccharide Chiral Stationary Phases. International Journal of Molecular Sciences, 25(5), 2697. https://doi.org/10.3390/ijms25052697








