Dexamethasone Inhibits White Adipose Tissue Browning
Abstract
1. Introduction
2. Results
2.1. DXM Treatment Effects in Body Weight, Caloric Intake and Metabolic Parameters
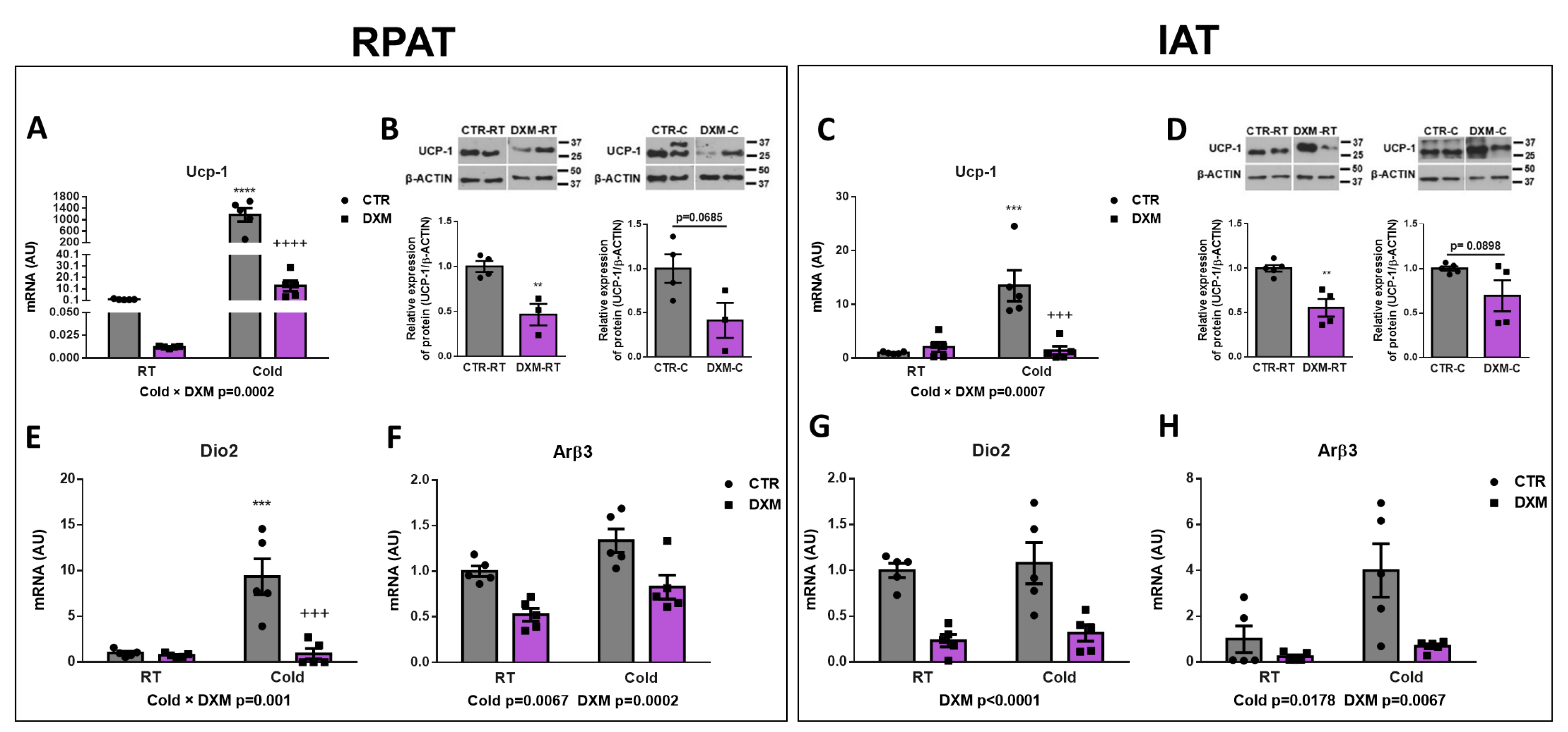
2.2. DXM Inhibited Browning of IAT and RPAT
2.3. DXM Decreased Mitochondrial Content in RPAT and IAT
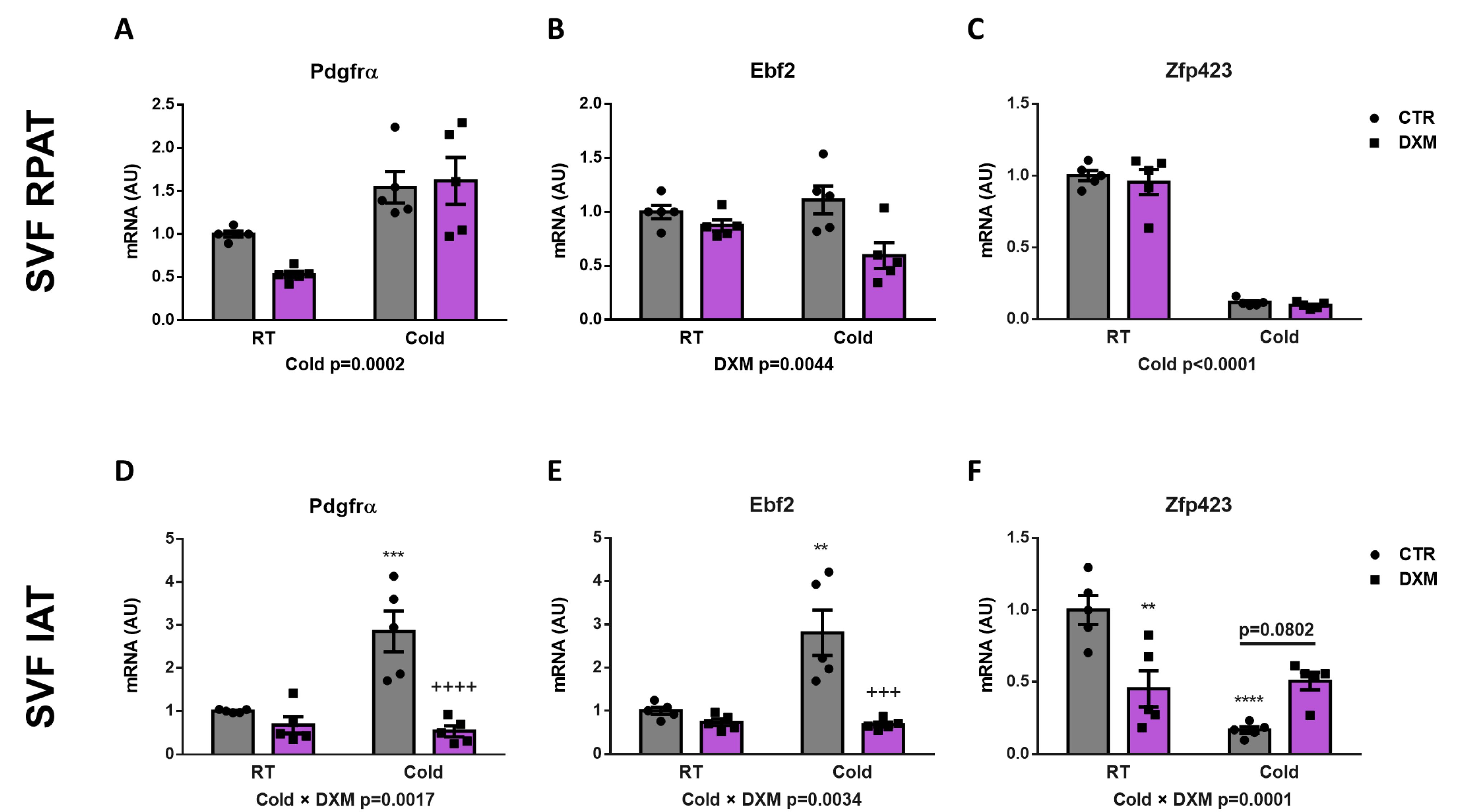
2.4. Effect of DXM on Gene Expression from Stromal Vascular Fraction (SVF) Cells
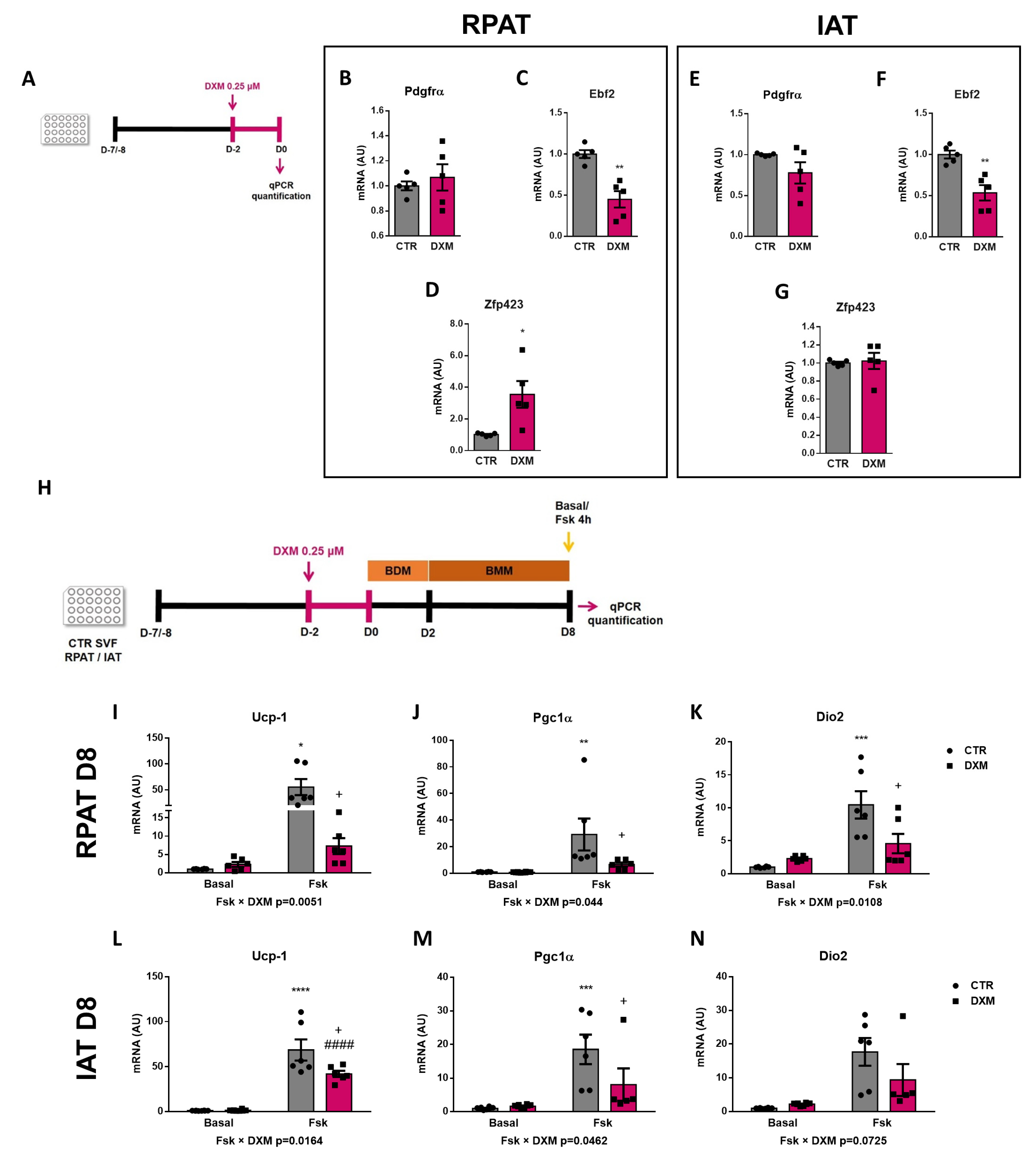
2.5. DXM Alters the Expression Pattern of Beige Precursor Cells
2.6. DXM Decreased Thermogenic Capacity on Mature Beige Adipocytes
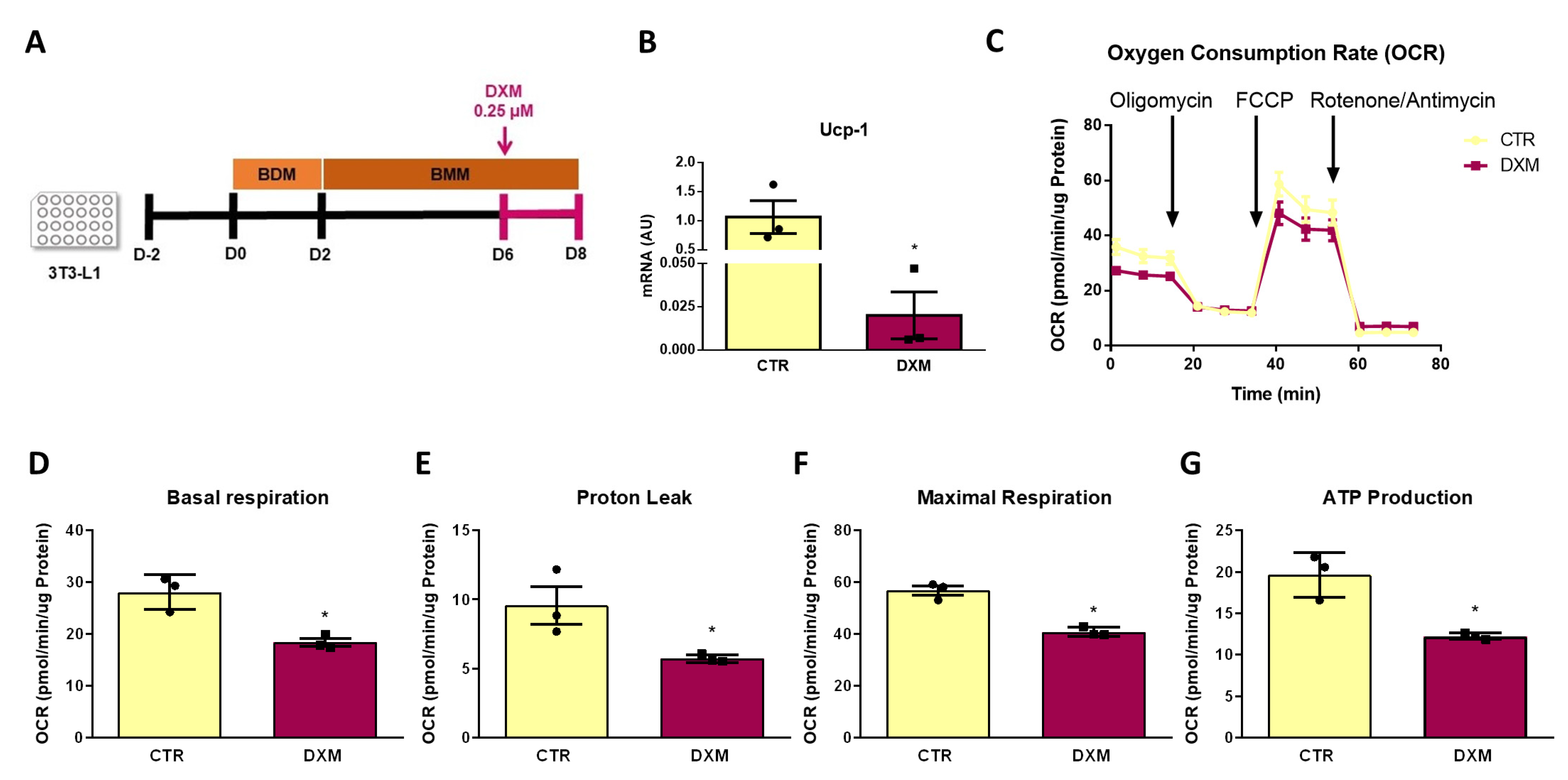
2.7. DXM Impacts OCR Profile in 3T3-L1 Adipocytes
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Metabolic Parameter Measurements
4.3. Liver Lipid Content
4.4. Histological Analysis
4.5. RPAT and IAT Stromal Vascular Fraction Cell Isolation
4.6. RNA Isolation and Quantitative Real-Time PCR (qPCR)
4.7. Western Blotting Assays
4.8. Mitochondrial DNA Quantification
4.9. Gene Expression Analysed in 3T3-L1 Cells
4.10. Oxygen Consumption Rate (OCR) Determination
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef] [PubMed]
- Kalinovich, A.V.; de Jong, J.M.A.; Cannon, B.; Nedergaard, J. UCP1 in adipose tissues: Two steps to full browning. Biochimie 2017, 134, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Cousin, B.; Cinti, S.; Morroni, M.; Raimbault, S.; Ricquier, D.; Pénicaud, L.; Casteilla, L. Occurrence of brown adipocytes in rat white adipose tissue: Molecular and morphological characterization. J. Cell Sci. 1992, 103 Pt 4, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Audet-Walsh, É.; Manteghi, S.; Dufour, C.R.; Walker, B.; Baba, M.; St-Pierre, J.; Giguère, V.; Pause, A. Chronic AMPK activation via loss of FLCN induces functional beige adipose tissue through PGC-1α/ERRα. Genes Dev. 2016, 30, 1034–1046. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Seale, P.; Conroe, H.M.; Estall, J.; Kajimura, S.; Frontini, A.; Ishibashi, J.; Cohen, P.; Cinti, S.; Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Investig. 2011, 121, 96–105. [Google Scholar] [CrossRef]
- Wu, J.; Cohen, P.; Spiegelman, B.M. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes Dev. 2013, 27, 234–250. [Google Scholar] [CrossRef]
- Frontini, A.; Vitali, A.; Perugini, J.; Murano, I.; Romiti, C.; Ricquier, D.; Guerrieri, M.; Cinti, S. White-to-brown transdifferentiation of omental adipocytes in patients affected by pheochromocytoma. Biochim. Biophys. Acta 2013, 1831, 950–959. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef]
- Wang, W.; Kissig, M.; Rajakumari, S.; Huang, L.; Lim, H.-W.; Won, K.-J.; Seale, P. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl. Acad. Sci. USA 2014, 111, 14466–14471. [Google Scholar] [CrossRef]
- Stine, R.R.; Shapira, S.N.; Lim, H.-W.; Ishibashi, J.; Harms, M.; Won, K.-J.; Seale, P. EBF2 promotes the recruitment of beige adipocytes in white adipose tissue. Mol. Metab. 2016, 5, 57–65. [Google Scholar] [CrossRef]
- Lee, Y.H.; Petkova, A.P.; Mottillo, E.P.; Granneman, J.G. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 2012, 15, 480–491. [Google Scholar] [CrossRef]
- Shao, M.; Ishibashi, J.; Kusminski, C.M.; Wang, Q.A.; Hepler, C.; Vishvanath, L.; MacPherson, K.A.; Spurgin, S.B.; Sun, K.; Holland, W.L.; et al. Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab. 2016, 23, 1167–1184. [Google Scholar] [CrossRef]
- Shao, M.; Zhang, Q.; Truong, A.; Shan, B.; Vishvanath, L.; Li, L.; Seale, P.; Gupta, R.K. ZFP423 controls EBF2 coactivator recruitment and PPARγ occupancy to determine the thermogenic plasticity of adipocytes. Genes Dev. 2021, 35, 1461–1474. [Google Scholar] [CrossRef]
- Kuo, T.; McQueen, A.; Chen, T.-C.; Wang, J.-C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [PubMed]
- Munck, A.; Guyre, P.M.; Holbrook, N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984, 5, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Yeager, M.P.; Guyre, P.M.; Munck, A.U. Glucocorticoid regulation of the inflammatory response to injury. Acta Anaesthesiol. Scand. 2004, 48, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, D.P.; Forbes, S.; Walker, B.R. Glucocorticoids and fatty acid metabolism in humans: Fuelling fat redistribution in the metabolic syndrome. J. Endocrinol. 2008, 197, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. Molecular regulation of adipogenesis. Annu. Rev. Cell Dev. Biol. 2000, 16, 145–171. [Google Scholar] [CrossRef] [PubMed]
- Assis, A.P.; Silva, K.E.; Lautherbach, N.; Morgan, H.J.N.; Garófalo, M.A.R.; Zanon, N.M.; Navegantes, L.C.C.; Chaves, V.E.; do Carmo Kettelhut, I. Glucocorticoids decrease thermogenic capacity and increase triacylglycerol synthesis by glycerokinase activation in the brown adipose tissue of rats. Lipids 2022, 57, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Poggioli, R.; Ueta, C.B.; Drigo, R.A.E.; Castillo, M.; Fonseca, T.L.; Bianco, A.C. Dexamethasone reduces energy expenditure and increases susceptibility to diet-induced obesity in mice. Obesity 2013, 21, E415–E420. [Google Scholar] [CrossRef] [PubMed]
- Soumano, K.; Desbiens, S.; Rabelo, R.; Bakopanos, E.; Camirand, A.; Silva, J.E. Glucocorticoids inhibit the transcriptional response of the uncoupling protein-1 gene to adrenergic stimulation in a brown adipose cell line. Mol. Cell. Endocrinol. 2000, 165, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Glantschnig, C.; Koenen, M.; Lozano, M.G.; Karbiener, M.; Pickrahn, I.; Williams-Dautovich, J.; Patel, R.; Cummins, C.L.; Giroud, M.; Hartleben, G.; et al. A miR-29a–driven negative feedback loop regulates peripheral glucocorticoid receptor signaling. FASEB J. 2019, 33, 5924–5941. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.-F.; Yu, J.; Sheng, Y.-L.; Huang, M.; Kong, X.-C.; Di, W.-J.; Liu, J.; Zhou, H.; Liang, H.; Ding, G.-X. Glucocorticoids Suppress the Browning of Adipose Tissue via miR-19b in Male Mice. Endocrinology 2018, 159, 310–322. [Google Scholar] [CrossRef]
- Kong, X.; Yu, J.; Bi, J.; Qi, H.; Di, W.; Wu, L.; Wang, L.; Zha, J.; Lv, S.; Zhang, F.; et al. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes 2015, 64, 393–404. [Google Scholar] [CrossRef]
- Chondronikola, M.; Volpi, E.; Børsheim, E.; Porter, C.; Annamalai, P.; Enerbäck, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef]
- Aru, M.; Alev, K.; Pehme, A.; Purge, P.; Õnnik, L.; Ellam, A.; Kaasik, P.; Seene, T. Changes in Body Composition of Old Rats at Different Time Points After Dexamethasone Administration. Curr. Aging Sci. 2019, 11, 255–260. [Google Scholar] [CrossRef]
- Filippopoulou, F.; Habeos, G.I.; Rinotas, V.; Sophocleous, A.; Sykiotis, G.P.; Douni, E.; Chartoumpekis, D.V. Dexamethasone Administration in Mice Leads to Less Body Weight Gain over Time, Lower Serum Glucose, and Higher Insulin Levels Independently of NRF2. Antioxidants 2021, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Malkawi, A.K.; Alzoubi, K.H.; Jacob, M.; Matic, G.; Ali, A.; Al Faraj, A.; Almuhanna, F.; Dasouki, M.; Abdel Rahman, A.M. Metabolomics Based Profiling of Dexamethasone Side Effects in Rats. Front. Pharmacol. 2018, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.L.; Leinung, M.C. Exogenous Cushing’s syndrome and glucocorticoid withdrawal. Endocrinol. Metab. Clin. N. Am. 2005, 34, 371–384. [Google Scholar] [CrossRef]
- Kopecky, J.; Clarke, G.; Enerbäck, S.; Spiegelman, B.; Kozak, L.P. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J. Clin. Investig. 1995, 96, 2914–2923. [Google Scholar] [CrossRef]
- Van Den Beukel, J.C.; Grefhorst, A.; Hoogduijn, M.J.; Steenbergen, J.; Mastroberardino, P.G.; Dor, F.J.M.F.; Themmen, A.P.N. Women have more potential to induce browning of perirenal adipose tissue than men. Obesity 2015, 23, 1671–1679. [Google Scholar] [CrossRef]
- Strack, A.M.; Bradbury, M.J.; Dallman, M.F. Corticosterone decreases nonshivering thermogenesis and increases lipid storage in brown adipose tissue. Am. J. Physiol. 1995, 268, R183–R191. [Google Scholar] [CrossRef]
- Luijten, I.H.N.; Brooks, K.; Boulet, N.; Shabalina, I.G.; Jaiprakash, A.; Carlsson, B.; Fischer, A.W.; Cannon, B.; Nedergaard, J. Glucocorticoid-Induced Obesity Develops Independently of UCP1. Cell Rep. 2019, 27, 1686–1698.e5. [Google Scholar] [CrossRef] [PubMed]
- Ramage, L.E.; Akyol, M.; Fletcher, A.M.; Forsythe, J.; Nixon, M.; Carter, R.N.; van Beek, E.J.R.R.; Morton, N.M.; Walker, B.R.; Stimson, R.H. Glucocorticoids Acutely Increase Brown Adipose Tissue Activity in Humans, Revealing Species-Specific Differences in UCP-1 Regulation. Cell Metab. 2016, 24, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Martín, F.M.; Alzamendi, A.; Harnichar, A.E.; Castrogiovanni, D.; Zubiría, M.G.; Spinedi, E.; Giovambattista, A. Role of glucocorticoid receptor (GR) in white adipose tissue beiging. Life Sci. 2023, 322, 121681. [Google Scholar] [CrossRef]
- Van Den Beukel, J.C.; Boon, M.R.; Steenbergen, J.; Rensen, P.C.N.; Meijer, O.C.; Themmen, A.P.N.; Grefhorst, A. Cold Exposure Partially Corrects Disturbances in Lipid Metabolism in a Male Mouse Model of Glucocorticoid Excess. Endocrinology 2015, 156, 4115–4128. [Google Scholar] [CrossRef]
- Harnichar, A.E.; Zubiría, M.G.; Giordano, A.P.; Miguel, I.; Rey, M.A.; Spinedi, E.; Giovambattista, A. Inhibitory effect of androgens on white adipose tissue thermogenic capacity. Mol. Cell. Endocrinol. 2022, 543, 111542. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef]
- Roh, H.C.; Tsai, L.T.Y.; Shao, M.; Tenen, D.; Shen, Y.; Kumari, M.; Lyubetskaya, A.; Jacobs, C.; Dawes, B.; Gupta, R.K.; et al. Warming Induces Significant Reprogramming of Beige, but Not Brown, Adipocyte Cellular Identity. Cell Metab. 2018, 27, 1121–1137.e5. [Google Scholar] [CrossRef] [PubMed]
- Zubiría, M.G.; Giordano, A.P.; Gambaro, S.E.; Alzamendi, A.; Frontini-López, Y.R.; Moreno, G.; Spinedi, E.; Giovambattista, A. Dexamethasone primes adipocyte precursor cells for differentiation by enhancing adipogenic competency. Life Sci. 2020, 261, 118363. [Google Scholar] [CrossRef]
- Quinn, M.; Ramamoorthy, S.; Cidlowski, J.A. Sexually dimorphic actions of glucocorticoids: Beyond chromosomes and sex hormones. Ann. N. Y. Acad. Sci. 2014, 1317, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Duma, D.; Collins, J.B.; Chou, J.W.; Cidlowski, J.A. Sexually dimorphic actions of glucocorticoids provide a link to inflammatory diseases with gender differences in prevalence. Sci. Signal. 2010, 3, ra74. [Google Scholar] [CrossRef]
- Thangavel, C.; Boopathi, E.; Shapiro, B.H. Intrinsic sexually dimorphic expression of the principal human CYP3A4 correlated with suboptimal activation of GH/glucocorticoid-dependent transcriptional pathways in men. Endocrinology 2011, 152, 4813–4824. [Google Scholar] [CrossRef] [PubMed]
- Kaikaew, K.; Grefhorst, A.; Visser, J.A. Sex Differences in Brown Adipose Tissue Function: Sex Hormones, Glucocorticoids, and Their Crosstalk. Front. Endocrinol. 2021, 12, 4813–4824. [Google Scholar] [CrossRef]
- Gómez-García, I.; Trepiana, J.; Fernández-Quintela, A.; Giralt, M.; Portillo, M.P. Sexual Dimorphism in Brown Adipose Tissue Activation and White Adipose Tissue Browning. Int. J. Mol. Sci. 2022, 23, 8250. [Google Scholar] [CrossRef]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic Mechanisms of Glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Buttgereit, F.; Bijlsma, J.W.J.; Strehl, C. Will we ever have better glucocorticoids? Clin. Immunol. 2018, 186, 64–66. [Google Scholar] [CrossRef]
- Chanson, P.; Salenave, S. Metabolic syndrome in Cushing’s syndrome. Neuroendocrinology 2010, 92 (Suppl. S1), 96–101. [Google Scholar] [CrossRef]
- Perelló, M.; Gaillard, R.C.; Chisari, A.; Spinedi, E. Adrenal enucleation in MSG-damaged hyperleptinemic male rats transiently restores adrenal sensitivity to leptin. Neuroendocrinology 2003, 78, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Alzamendi, A.; Castrogiovanni, D.; Gaillard, R.C.; Spinedi, E.; Giovambattista, A. Increased male offspring’s risk of metabolic-neuroendocrine dysfunction and overweight after fructose-rich diet intake by the lactating mother. Endocrinology 2010, 151, 4214–4223. [Google Scholar] [CrossRef]
- Galarraga, M.; Campión, J.; Muñoz-Barrutia, A.; Boqué, N.; Moreno, H.; Martínez, J.A.; Milagro, F.; Ortiz-de-Solórzano, C. Adiposoft: Automated software for the analysis of white adipose tissue cellularity in histological sections. J. Lipid Res. 2012, 53, 2791–2796. [Google Scholar] [CrossRef] [PubMed]
- Alzamendi, A.; Giovambattista, A.; García, M.E.; Rebolledo, O.R.; Gagliardino, J.J.; Spinedi, E. Effect of pioglitazone on the fructose-induced abdominal adipose tissue dysfunction. PPAR Res. 2012, 2012, 259093. [Google Scholar] [CrossRef]
- Giovambattista, A.; Gaillard, R.C.; Spinedi, E. Ghrelin gene-related peptides modulate rat white adiposity. Vitam. Horm. 2008, 77, 171–205. [Google Scholar] [PubMed]
- Divakaruni, A.S.; Paradyse, A.; Ferrick, D.A.; Murphy, A.N.; Jastroch, M. Analysis and Interpretation of Microplate-Based Oxygen Consumption and pH Data. Methods Enzymol. 2014, 547, 309–354. [Google Scholar]
- Wei, J.; Carroll, R.J.; Harden, K.K.; Wu, G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids 2012, 42, 2031–2035. [Google Scholar] [CrossRef]



| Variable | CTR-RT | DXM-RT | CTR-C | DXM-C | 2-Way ANOVA | ||
|---|---|---|---|---|---|---|---|
| Cold | DXM | ColdxDXM | |||||
| Plasma Glu (g/L) | 1.27 ± 0.05 | 1.25 ± 0.02 | 1.33 ± 0.03 | 1.25 ± 0.04 | ns | ns | ns |
| Plasma Tg (g/L) | 1.13 ± 0.10 | 1.72 ± 0.19 | 0.28 ± 0.03 | 0.54 ± 0.09 | p < 0.0001 | p < 0.01 | ns |
| Liver Tg (mg/g Liver) | 5.83 ± 0.96 | 5.77 ± 0.84 | 4.22 ± 0.23 | 5.09 ± 0.19 | ns | ns | ns |
| Cort (ng/dL) | 4.52 ± 0.89 | 2.62 ± 0.37 | 7.79 ± 2.02 | 4.22 ± 0.93 | p < 0.05 | p < 0.05 | ns |
| Primers (5′-3′) | GBAN | bp | |
|---|---|---|---|
| Actβ | se, AGCCATGTACGTAGCCATCC | NM_031144 | 200 |
| as, ACCCTCATAGATGGGCACAG | |||
| Arβ3 | se, AGTGGGACTCCTCGTAATGC | NM_013108 | 110 |
| as, TTACACAGAGCACGTCCACT | |||
| Dio2 | se, CTGGCGCTCTATGACTCGG | NM_031720 | 194 |
| as, ACGTGCACCACACTGGAAT | |||
| Ebf2 | se, TCTTATCCTACATCCCACACCC | NM_001108383.1 | 113 |
| as, TGAGTCTGGTTTCTGTGGTGG | |||
| Pdgfrα | se, AGGCTTGGGGCTCACTTTTT | NM_012802 | 153 |
| as, AAGAGCTGGCAGACGATGAG | |||
| Pgc1α | se, AAAAGCTTGACTGGCGTCAT | NM_031347 | 199 |
| as, ACACCACTTCAATCCACCCAG | |||
| Ucp-1 | se, CCGAAACTGTACAGCGGTCT | NM_012682 | 155 |
| as, GTCATCAAGCCAGCCGAGAT | |||
| Zfp423 | se, CCGCGATCGGTGAAAGTTG | NM_001393718.1 | 121 |
| as, CACGGCTGGTTTTCCGATCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordano, A.P.; Gambaro, S.E.; Alzamendi, A.; Harnichar, A.E.; Rey, M.A.; Ongaro, L.; Spinedi, E.; Zubiría, M.G.; Giovambattista, A. Dexamethasone Inhibits White Adipose Tissue Browning. Int. J. Mol. Sci. 2024, 25, 2714. https://doi.org/10.3390/ijms25052714
Giordano AP, Gambaro SE, Alzamendi A, Harnichar AE, Rey MA, Ongaro L, Spinedi E, Zubiría MG, Giovambattista A. Dexamethasone Inhibits White Adipose Tissue Browning. International Journal of Molecular Sciences. 2024; 25(5):2714. https://doi.org/10.3390/ijms25052714
Chicago/Turabian StyleGiordano, Alejandra Paula, Sabrina Eliana Gambaro, Ana Alzamendi, Alejandro Ezequiel Harnichar, María Amanda Rey, Luisina Ongaro, Eduardo Spinedi, María Guillermina Zubiría, and Andrés Giovambattista. 2024. "Dexamethasone Inhibits White Adipose Tissue Browning" International Journal of Molecular Sciences 25, no. 5: 2714. https://doi.org/10.3390/ijms25052714
APA StyleGiordano, A. P., Gambaro, S. E., Alzamendi, A., Harnichar, A. E., Rey, M. A., Ongaro, L., Spinedi, E., Zubiría, M. G., & Giovambattista, A. (2024). Dexamethasone Inhibits White Adipose Tissue Browning. International Journal of Molecular Sciences, 25(5), 2714. https://doi.org/10.3390/ijms25052714






