Sequential Co-Immobilization of Enzymes on Magnetic Nanoparticles for Efficient l-Xylulose Production
Abstract
1. Introduction
2. Results and Discussion
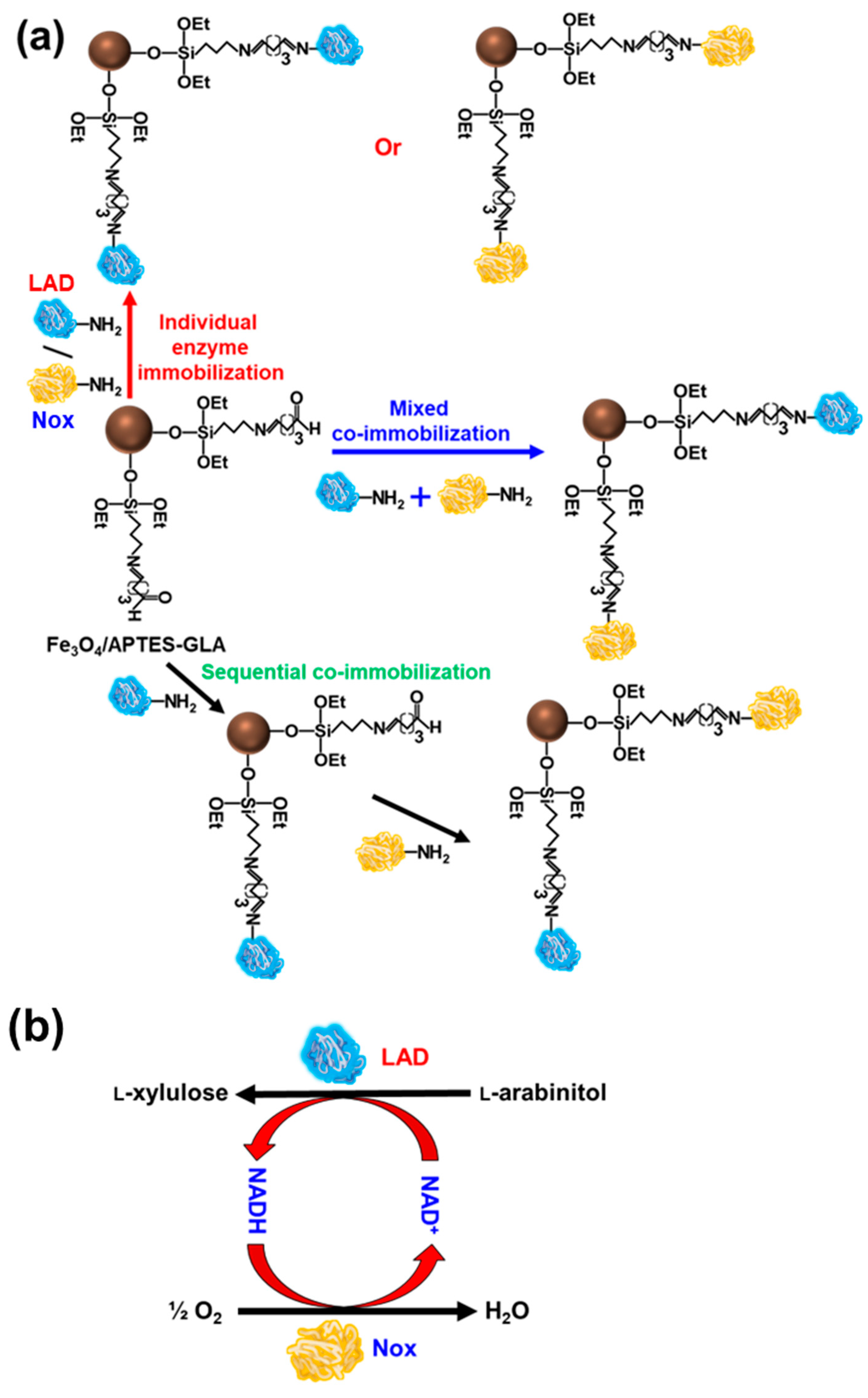
2.1. Immobilization of LAD or Nox on Magnetic Nanoparticles
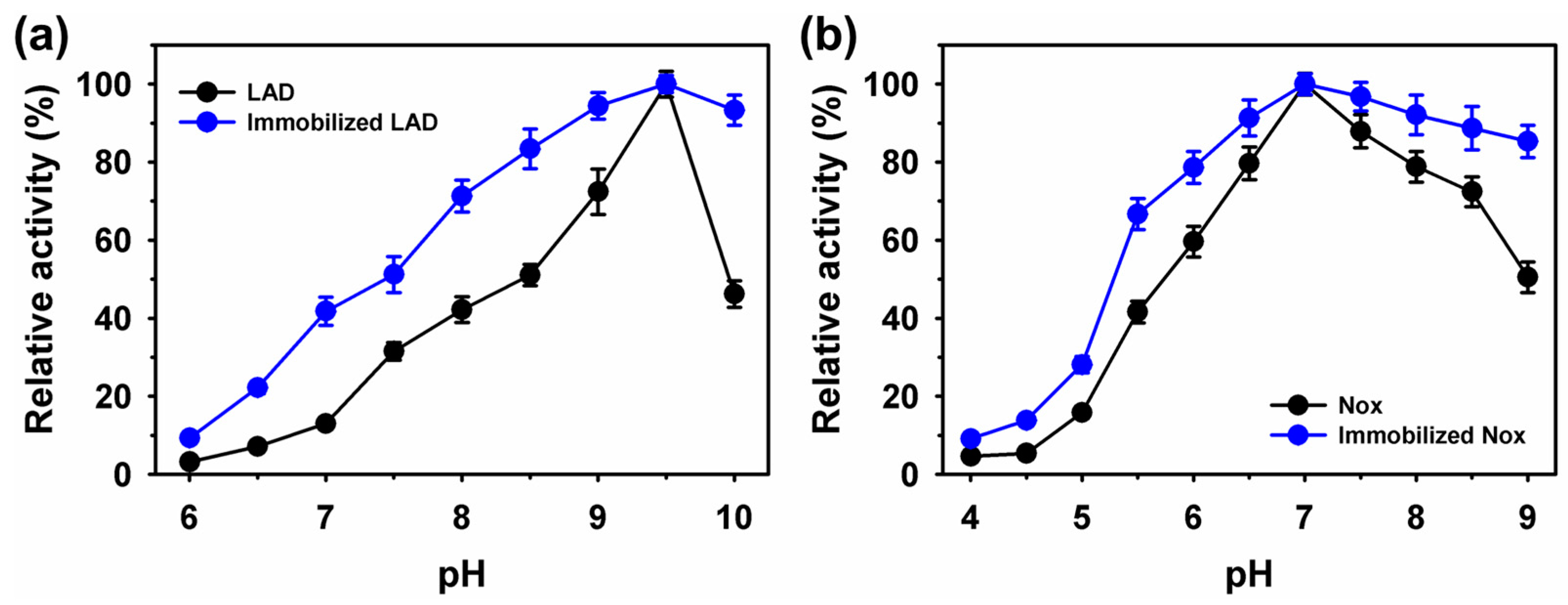
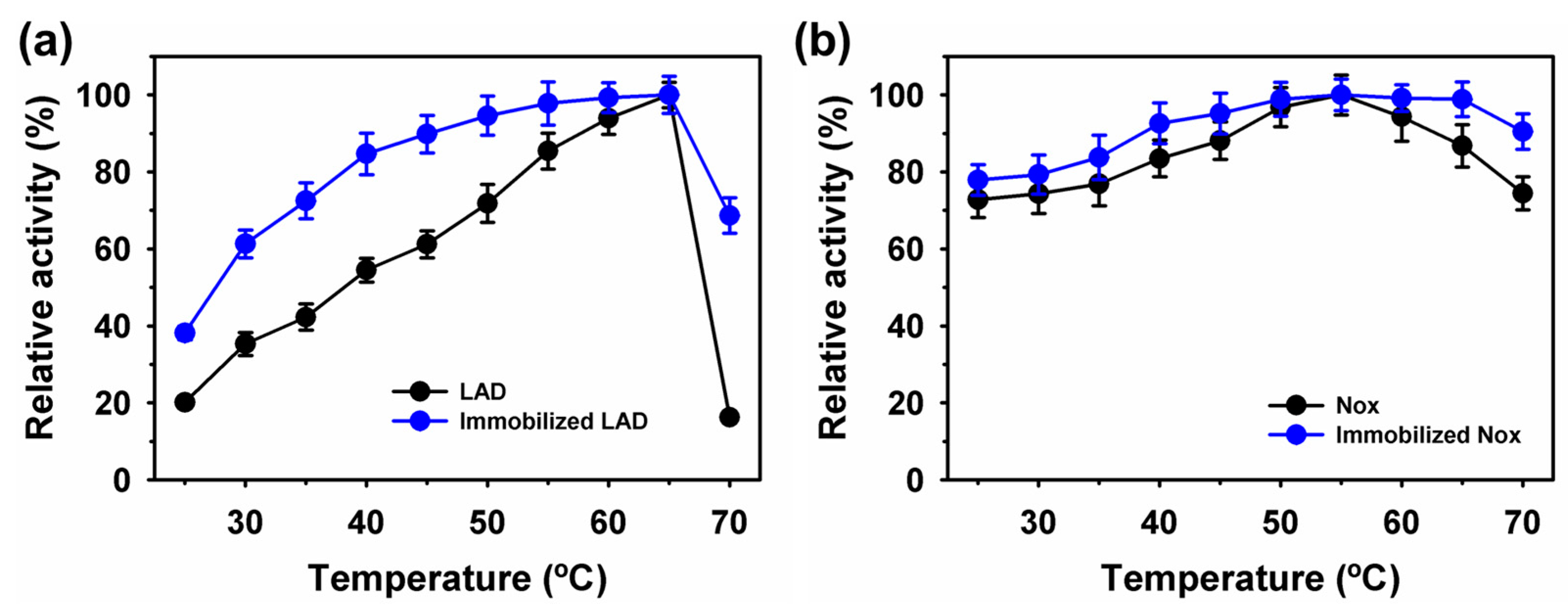
2.2. Characterization of Immobilized LAD or Nox on Fe3O4 Nanoparticles
2.3. Mixed and Sequential Co-Immobilization of LAD and Nox on Fe3O4 Nanoparticles
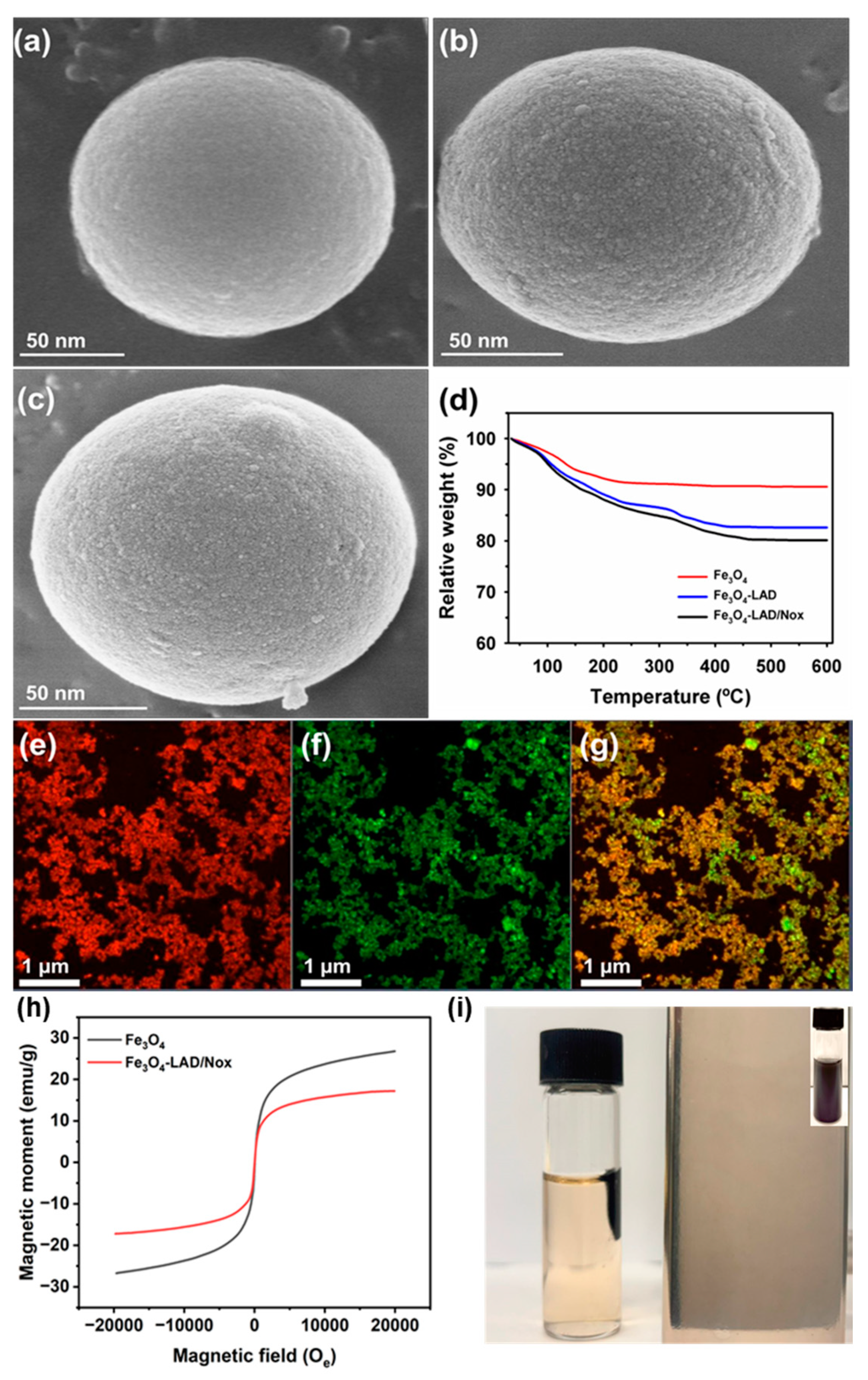
2.4. Analysis of Co-Immobilized Enzymes on Fe3O4 Nanoparticles
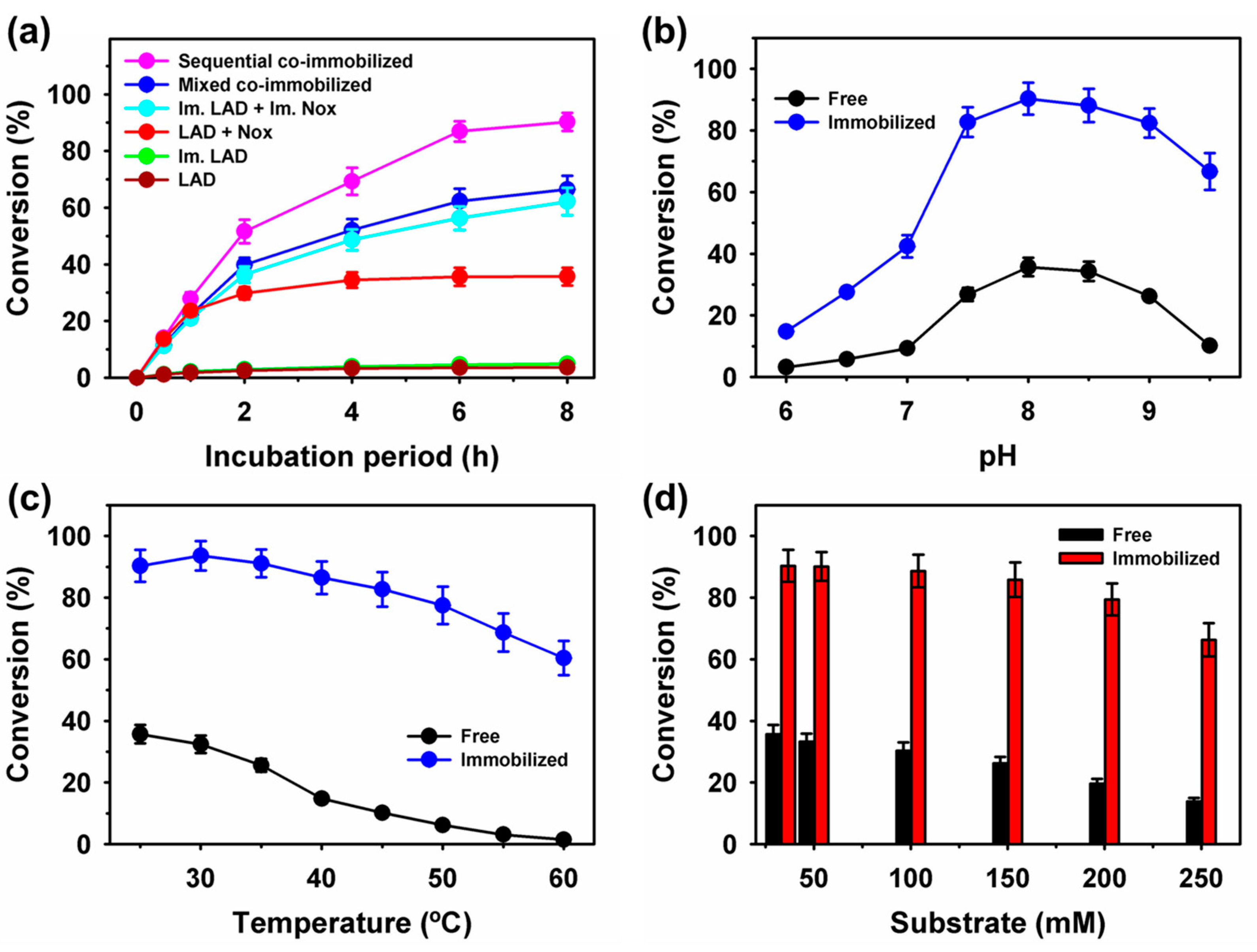
2.5. Production of l-Xylulose by Free and Immobilized Enzymes on Fe3O4 Nanoparticles
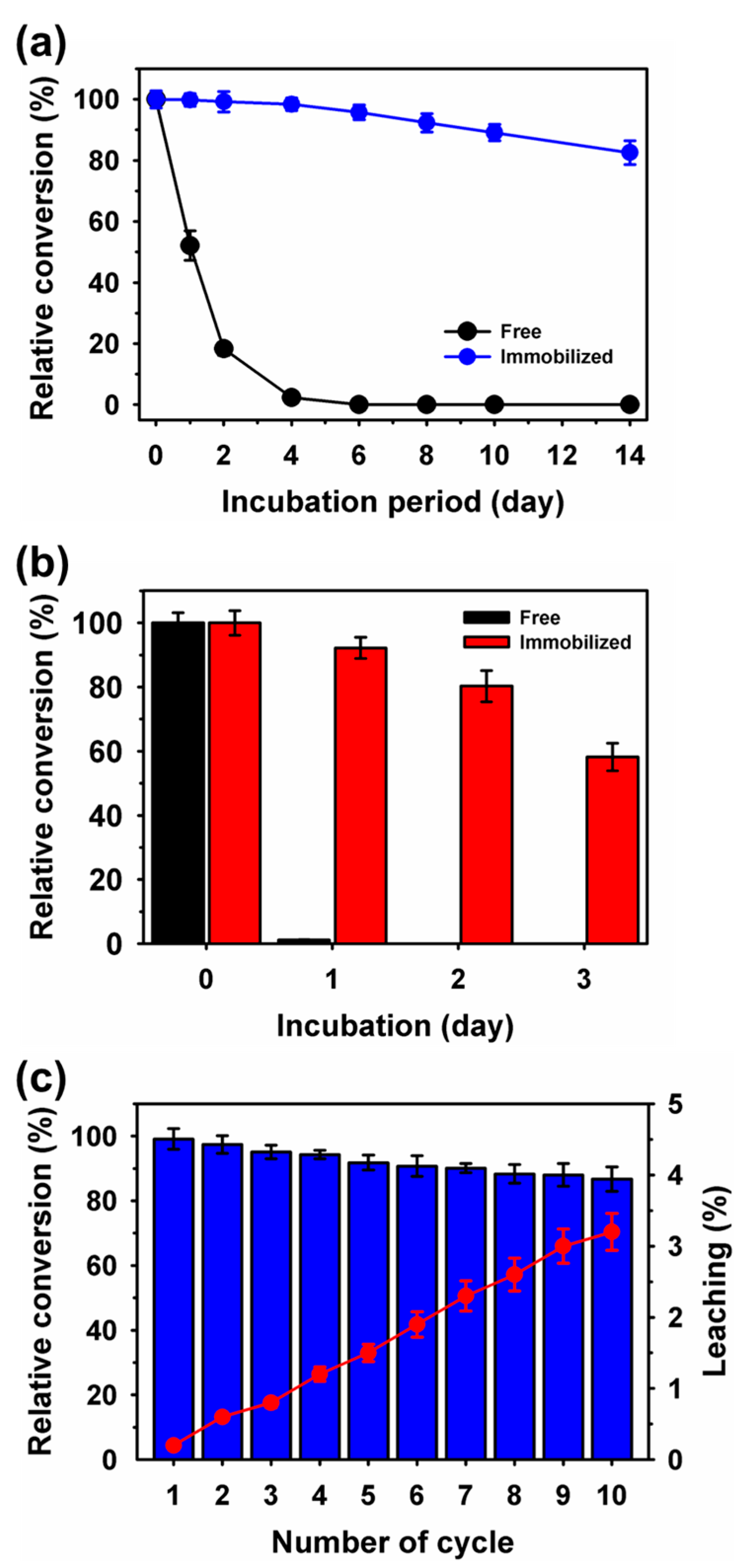
2.6. Storage Stability, Reusability and Leaching Measurements
3. Materials and Methods
3.1. Materials and Reagents
3.2. Cell Culture and Protein Purification
3.3. Functionalization of Nanoparticles and Immobilization of LAD or Nox
3.4. Activity Measurements
3.5. Characterization of Immobilized LAD or Nox
3.6. Mixed and Sequential Co-Immobilization of LAD and Nox on Fe3O4 Nanoparticles for l-Xylulose Production
3.7. Storage Stability and Reusability of Sequential Co-Immobilized Enzymes on Fe3O4 Nanoparticles for l-Xylulose Production
3.8. Instrumental Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, L.-J.; Li, R.-F.; Li, X.-Y.; Zhang, Y.-W. One-step selective affinity purification and immobilization of His-tagged enzyme by recyclable magnetic nanoparticles. Eng. Life Sci. 2021, 21, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Pan, L.; Li, Q.; Zhang, Y.; Mou, F.; Liu, Z.; Zhang, Y.; Duan, L.; Qin, B.; Hu, Z. The isolation, identification and immobilization method of three novel enzymes with diosgenin-producing activity derived from an Aspergillus flavus. Int. J. Mol. Sci. 2023, 24, 17611. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Ouyang, Y.; Kong, W.; Ma, T.; Zhao, H.; Jiang, Y.; Gao, J.; Ma, L. One pot purification and co-immobilization of His-tagged old yellow enzyme and glucose dehydrogenase for asymmetric hydrogenation. Enzyme Microb. Technol. 2022, 156, 110001. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Berenguer-Murcia, A.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Choi, S.H.; Kang, Y.C.; Lee, J.-K. Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk–shell particles: A promising support for enzyme immobilization. Nanoscale 2016, 8, 6728. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Gupta, R.K.; Kim, I.-W.; Lee, J.-K. Coriolus versicolor laccase-based inorganic protein hybrid synthesis for application in biomass saccharification to enhance biological production of hydrogen and ethanol. Enzyme Microb Technol. 2023, 170, 110301. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, R.; Liu, M.; Ma, L.; Zhang, W. Co-immobilization of lipases with different specificities for efficient and recyclable biodiesel production from waste oils: Optimization using response surface methodology. Int. J. Mol. Sci. 2023, 24, 4726. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Li, K.; Wang, J.; Ghide, M.K.; Yan, Y. Co-immobilization of Rhizopus oryzae and Candida rugosa lipases onto mMWCNTs@4-arm-PEG-NH2—A novel magnetic nanotube–polyethylene glycol amine composite—and its applications for biodiesel production. Int. J. Mol. Sci. 2021, 22, 11956. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Multienzymatic catalysis and Enzyme Co-Immobilization. Catalysts 2023, 13, 1488. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Otari, S.V.; Kang, Y.C.; Lee, J.-K. Protein–inorganic hybrid system for efficient histagged enzymes immobilization and its application in L-xylulose production. RSC Adv. 2017, 7, 3488–3494. [Google Scholar] [CrossRef]
- Ye, Q.; Jin, X.; Gao, H.; Wei, N. Site-specific and tunable co-immobilization of proteins onto magnetic nanoparticles via spy chemistry. ACS Appl. Bio Mater. 2022, 5, 5665–5674. [Google Scholar] [CrossRef]
- Peng, F.; Ou, X.-Y.; Guo, Z.-W.; Zeng, Y.-J.; Zong, M.-H.; Lou, W.-Y. Co-immobilization of multiple enzymes by self-assembly and chemical crosslinking for cofactor regeneration and robust biocatalysis. Int. J. Biol. Macromol. 2020, 162, 445–453. [Google Scholar] [CrossRef]
- Zdarta, J.; Kołodziejczak-Radzimska, A.; Bachosz, K.; Rybarczyk, A.; Bilal, M.; Iqbal, H.M.N.; Buszewski, B.; Jesionowski, T. Nanostructured supports for multienzyme co-immobilization for biotechnological applications: Achievements, challenges and prospects. Adv. Colloid Interface Sci. 2023, 315, 102889. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Q.; Dong, X.; Sun, Y. Co-Immobilization of laccase and mediator into Fe-doped ZIF-8 significantly enhances the degradation of organic pollutants. Molecules 2024, 29, 307. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, V.G.; Bronstein, L.M. Magnetic nanoparticle containing supports as carriers of immobilized enzymes: Key factors influencing the biocatalyst performance. Nanomaterials 2021, 11, 2257. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, I.A.T.; de Oliveira, P.C.O.; Wegermann, C.A.; de Moraes, M.C. Magnetic particles for enzyme immobilization: A versatile support for ligand screening. J. Pharm. Biomed. Anal. 2021, 204, 114286. [Google Scholar]
- Gao, H.; Li, J.; Sivakumar, D.; Kim, T.-S.; Patel, S.K.S.; Kalia, V.C.; Kim, I.-W.; Zhang, Y.-W.; Lee, J.-K. NADH oxidase from Lactobacillus reuteri: A versatile enzyme for oxidized cofactor regeneration. Int. J. Biol. Macromol. 2019, 123, 629–636. [Google Scholar] [CrossRef]
- Ran, G.; Tan, D.; Zhao, J.; Fan, F.; Zhang, Q.; Wu, X.; Fan, P.; Fang, X.; Lu, X. Functionalized polyhydroxyalkanoate nano-beads as a stable biocatalyst for cost-effective production of the rare sugar D-allulose. Bioresour. Technol. 2019, 289, 121673. [Google Scholar] [CrossRef]
- Jiajun, C.; Hao, W.; Wenli, Z.; Wanmeng, M. Recent advances in properties, production, and applications of L-ribulose. Appl. Microbiol. Biotechnol. 2020, 104, 5663–5672. [Google Scholar]
- Rai, S.K.; Kaur, H.; Kauldhar, B.S.; Yadav, S.K. Dual-enzyme metal hybrid crystal for direct transformation of whey lactose into a high-value rare sugar D-tagatose: Synthesis, characterization, and a sustainable process. ACS Biomater. Sci. Eng. 2020, 6, 6661–6670. [Google Scholar] [CrossRef] [PubMed]
- Beerens, K.; Desmet, T.; Soetaert, W. Enzymes for the biocatalytic production of rare sugars. J. Ind. Microbiol. Biotechnol. 2012, 39, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Angaw, T.M.; Xin, W.; Huibin, L.; Yujie, L.; Can, L.; Jianqiang, L.; Jianqun, L. Efficient L-xylulose production using whole-cell biocatalyst with NAD+ regeneration system through co-expression of xylitol dehydrogenase and NADH oxidase in Escherichia coli. Biochem. Eng. J. 2021, 175, 108137. [Google Scholar]
- Morita, M.; Sawa, E.; Yamaji, K.; Sakai, T.; Natori, T.; Koezuka, Y.; Fukushima, H.; Akimoto, K. Practical total synthesis of (2S,3S,4R)-1-O-(α-D-Galactopyranosyl)-N-hexacosanoyl-2-amino-1,3,4-octadecanetriol, the antitumorial and immunostimulatory α-Galactosylcer-amide, KRN7000. Biosci. Biotechnol. Biochem. 1996, 60, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-Y.; Zhu, Y.-H.; Zhou, H.-P.; Xu, Y.-Y.; Gao, J.; Zhang, Y.-W. Cloning, expression, and characterization of an arabitol dehydrogenase and coupled with NADH oxidase for effective production of L-xylulose. Prep. Biochem. Biotechnol. 2022, 52, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Peng, J. Immobilization of glycerol dehydrogenase and NADH oxidase for enzymatic synthesis of 1,3-dihydroxyacetone with in situ cofactor regeneration. J. Nanosci. Nanotechnol. 2018, 18, 3888–3892. [Google Scholar] [CrossRef] [PubMed]
- Fuzhi, L.; Wei, X.; Wenli, Z.; Cuie, G.; Wanmeng, M. Polyol dehydrogenases: Intermediate role in the bioconversion of rare sugars and alcohols. Appl. Microbiol. Biotechnol. 2019, 103, 6473–6481. [Google Scholar]
- Hepziba Suganthi, S.; Swathi, K.V.; Biswas, R.; Basker, S.; Ramani, K. Co-immobilization of multiple enzymes onto surface-functionalized magnetic nanoparticle for the simultaneous hydrolysis of multiple substrates containing industrial wastes. Appl. Nanosci. 2019, 9, 1439–1457. [Google Scholar] [CrossRef]
- Han, J.; Luo, P.; Wang, L.; Wu, J.; Li, C.; Wang, Y. Construction of a multienzymatic cascade reaction system of coimmobilized hybrid nanoflowers for efficient conversion of starch into gluconic acid. ACS Appl. Mater. Interfaces 2020, 12, 15023–15033. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, K.; Tae, G.; Kwon, I. Nano-entrapping multiple oxidoreductases and cofactor for all-in-one nanoreactors. ACS Sustainable Chem. Eng. 2021, 9, 6741–6747. [Google Scholar] [CrossRef]
- Kalyana Sundaram, S.D.; Hossain, M.M.; Rezki, M.; Ariga, K.; Tsujimura, S. Enzyme cascade electrode reactions with nanomaterials and their applicability towards biosensor and biofuel cells. Biosensors 2023, 13, 1018. [Google Scholar] [CrossRef]
- Zhuang, M.-Y.; Jiang, X.-P.; Ling, X.-M.; Xu, M.-Q.; Zhu, Y.-H.; Zhang, Y.-W. Immobilization of glycerol dehydrogenase and NADH oxidase for enzymatic synthesis of 1,3-dihydroxyacetone with in situ cofactor regeneration. J. Chem. Technol. Biotechnol. 2018, 93, 2351–2358. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Anwar, M.Z.; Kumar, A.K.; Otari, S.V.; Pagolu, R.T.; Kim, S.-Y.; Kim, I.-W.; Lee, J.-K. Fe2O3 yolk-shell particle-based laccase biosensor for efficient detection of 2, 6-dimethoxyphenol. Biochem. Eng. J. 2018, 132, 1–8. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251. [Google Scholar] [CrossRef]
- Sandoval-Cárdenas, D.I.; Feregrino-Pérez, A.A.; Favela-Camacho, S.E.; Arredondo-Ochoa, T.; Escamilla-Garcia, M.; Regalado, C.; Amaro-Reyes, A. Potential antioxidant activity of multienzymatically hydrolyzed corncob. Biologia 2022, 77, 803–813. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Haw, J.-R.; Lee, J.-K. Immobilization of L-arabinitol dehydrogenase on aldehyde-functionalized silicon oxide nanoparticles for L-xylulose production. Appl. Microbiol. Biotechnol. 2014, 98, 1095–1104. [Google Scholar] [CrossRef]
- García-Bofill, M.; Sutton, P.W.; Guillén, M.; Alvaro, G. Enzymatic synthesis of a statin precursor by immobilised alcohol dehydrogenase with NADPH oxidase as cofactor regeneration system. Appl. Catal. A-Gen. 2021, 609, 117909. [Google Scholar] [CrossRef]
- Patil, S.S.; Rathod, V.K. Combined effect of enzyme co-immobilized magnetic nanoparticles (MNPs) and ultrasound for effective extraction and purification of curcuminoids from Curcuma longa. Ind. Crop. Prod. 2022, 177, 114385. [Google Scholar] [CrossRef]
- Dik, G.; Bakar, B.; Ulu, A.; Ates, B. Propelling of enzyme activity by using different triggering strategies: Applications and perspectives. Ind. Eng. Chem. Res. 2023, 62, 14111–14129. [Google Scholar] [CrossRef]
- Bachosz, K.; Synoradzki, K.; Staszak, M.; Pinelo, M.; Meyer, A.S.; Zdarta, J.; Jesionowski, T. Bioconversion of xylose to xylonic acid via co-immobilized dehydrogenases for conjunct cofactor regeneration. Bioorg. Chem. 2019, 93, 102747. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, Y.; Jiao, Z.; Chen, Z.; Wang, J.; Yang, C.; Li, D.; Ma, K.; Shi, R. A system of co-immobilized dual-enzyme and coenzyme for in-situ coenzyme regeneration. Mol. Catal. 2022, 530, 112570. [Google Scholar] [CrossRef]
- Zadeh, P.S.N.; do Valle Gomes, M.Z.; Akerman, B.; Palmqvist, A.E.C. Förster resonance energy transfer study of the improved biocatalytic conversion of CO2 to formaldehyde by coimmobilization of enzymes in siliceous mesostructured cellular foams. ACS Catal. 2018, 8, 7251–7260. [Google Scholar] [CrossRef]
- Subramani, I.G.; Perumal, V.; Gopinath, S.C.; Mohamed, N.M.; Joshi, N.; Ovinis, M.; Sze, L.L. 3D nanoporous hybrid nanoflower for enhanced non-faradaic redox-free electrochemical impedimetric biodetermination. J. Taiwan Inst. Chem. Eng. 2020, 116, 26–35. [Google Scholar] [CrossRef]
- Skendrović, D.; Švarc, A.; Rezić, T.; Chernev, A.; Rađenović, A.; Presečki, A.V. Improvement of DERA activity and stability in the synthesis of statin precursors by immobilization on magnetic nanoparticles. React. Chem. Eng. 2024, 9, 82–90. [Google Scholar] [CrossRef]
- Hwang, E.T.; Lee, S. Multienzymatic cascade reactions via enzyme complex by immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Cheng, P.; Tang, M.; Chen, Z.; Liu, W.; Jiang, X.; Pei, X.; Su, W. Dual-enzyme and NADPH co-embedded organic–inorganic hybrid nanoflowers prepared using biomimetic mineralization for the asymmetric synthesis of (R)-(−)-pantolactone. React. Chem. Eng. 2020, 5, 973–980. [Google Scholar] [CrossRef]
- Dedania, S.R.; Patel, V.K.; Soni, S.S.; Patel, D.H. Immobilization of Agrobacterium tumefaciens D-psicose 3-epimerase onto titanium dioxide for bioconversion of rare sugar. Enzyme Microb. Technol. 2020, 140, 109605. [Google Scholar] [CrossRef]
- Chen, D.; Jiajun, C.; Xiaoyong, L.; Cuie, G.; Wenli, Z.; Wanmeng, M. Biochemical identification of a hyperthermostable L-ribulose 3-epimerase from Labedella endophytica and its application for D-allulose bioconversion. Int. J. Biol. Macromol. 2021, 189, 214–222. [Google Scholar] [CrossRef]
| Nanoparticles | LAD | Nox | ||
|---|---|---|---|---|
| Immobilization Yield (IY, %) | Relative Activity (RA, %) | IY % | RA (%) a | |
| Fe3O4 | 10.2 ± 0.8 | 23.4 ± 1.9 | 8.6 ± 1.2 | 20.8 ± 1.6 |
| Fe3O4GLA | 85.6 ± 4.8 | 95.3 ± 6.5 | 87.3 ± 3.7 | 92.9 ± 7.8 |
| Fe3O4APTES | 48.5 ± 4.4 | 67.6 ± 4.9 | 57.2 ± 4.6 | 71.3 ± 6.6 |
| Fe3O4/APTES-GLA | 91.4 ± 4.8 | 98.8 ± 7.2 | 92.1 ± 3.8 | 97.2 ± 7.0 |
| SrFe12O19 | 9.5 ± 0.8 | 18.7 ± 1.5 | 8.1 ± 0.7 | 19.2 ± 1.6 |
| SrFe12O19/GLA | 73.2 ± 6.1 | 85.4 ± 6.4 | 74.8 ± 1.2 | 82.4 ± 6.9 |
| SrFe12O19/APTES | 52.1 ± 3.7 | 60.5 ± 4.4 | 48.7 ± 4.2 | 65.1 ± 5.4 |
| SrFe12O19/APTES-GLA | 84.6 ± 4.9 | 86.8 ± 4.8 | 83.5 ± 5.3 | 90.1 ± 6.9 |
| Enzyme | Vmax (µmol min−1 mg protein−1) | Km | Kcat/Km |
|---|---|---|---|
| LAD | 93.2 ± 7.7 | 17.8 ± 2.3 mM | 210 ± 17 mM−1 min−1 |
| Immobilized LAD | 92.2 ± 7.8 | 15.5 ± 2.1 mM | 240 ± 18 mM−1 min−1 |
| Nox | 340 ± 31 | 27.1 ± 2.3 µM | 630 ± 48 µM−1 min−1 |
| Immobilized Nox | 330 ± 29 | 23.9 ± 1.8 µM | 690 ± 56 µM−1 min−1 |
| Immobilization Method/System | Immobilization Yield (IY, %) | Loading (mg per g of Support) | Conversion Efficiency (%) | Relative Total Turnover Number (Folds) | |
|---|---|---|---|---|---|
| LAD | Nox | ||||
| Mixed | 87.2 ± 5.3 | 83.8 ± 4.9 | 114 ± 6.8 | 66.5 ± 4.7 | 113 |
| Sequential | 91.4 ± 4.8 | 96.0 ± 2.6 | 122 ± 6.3 | 90.3 ± 3.2 | 153 |
| Free LAD (control) | - a | - | - | 3.6 ± 0.3 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.K.S.; Gupta, R.K.; Karuppanan, K.K.; Kim, I.-W.; Lee, J.-K. Sequential Co-Immobilization of Enzymes on Magnetic Nanoparticles for Efficient l-Xylulose Production. Int. J. Mol. Sci. 2024, 25, 2746. https://doi.org/10.3390/ijms25052746
Patel SKS, Gupta RK, Karuppanan KK, Kim I-W, Lee J-K. Sequential Co-Immobilization of Enzymes on Magnetic Nanoparticles for Efficient l-Xylulose Production. International Journal of Molecular Sciences. 2024; 25(5):2746. https://doi.org/10.3390/ijms25052746
Chicago/Turabian StylePatel, Sanjay K. S., Rahul K. Gupta, Karthikeyan K. Karuppanan, In-Won Kim, and Jung-Kul Lee. 2024. "Sequential Co-Immobilization of Enzymes on Magnetic Nanoparticles for Efficient l-Xylulose Production" International Journal of Molecular Sciences 25, no. 5: 2746. https://doi.org/10.3390/ijms25052746
APA StylePatel, S. K. S., Gupta, R. K., Karuppanan, K. K., Kim, I.-W., & Lee, J.-K. (2024). Sequential Co-Immobilization of Enzymes on Magnetic Nanoparticles for Efficient l-Xylulose Production. International Journal of Molecular Sciences, 25(5), 2746. https://doi.org/10.3390/ijms25052746








