Integrated Physiologic and Proteomic Analyses Reveal the Molecular Mechanism of Navicula sp. in Response to Ultraviolet Irradiation Stress
Abstract
:1. Introduction
2. Results
2.1. Effect of UV Irradiation Dose on Chlorophyll Content in Navicula sp.
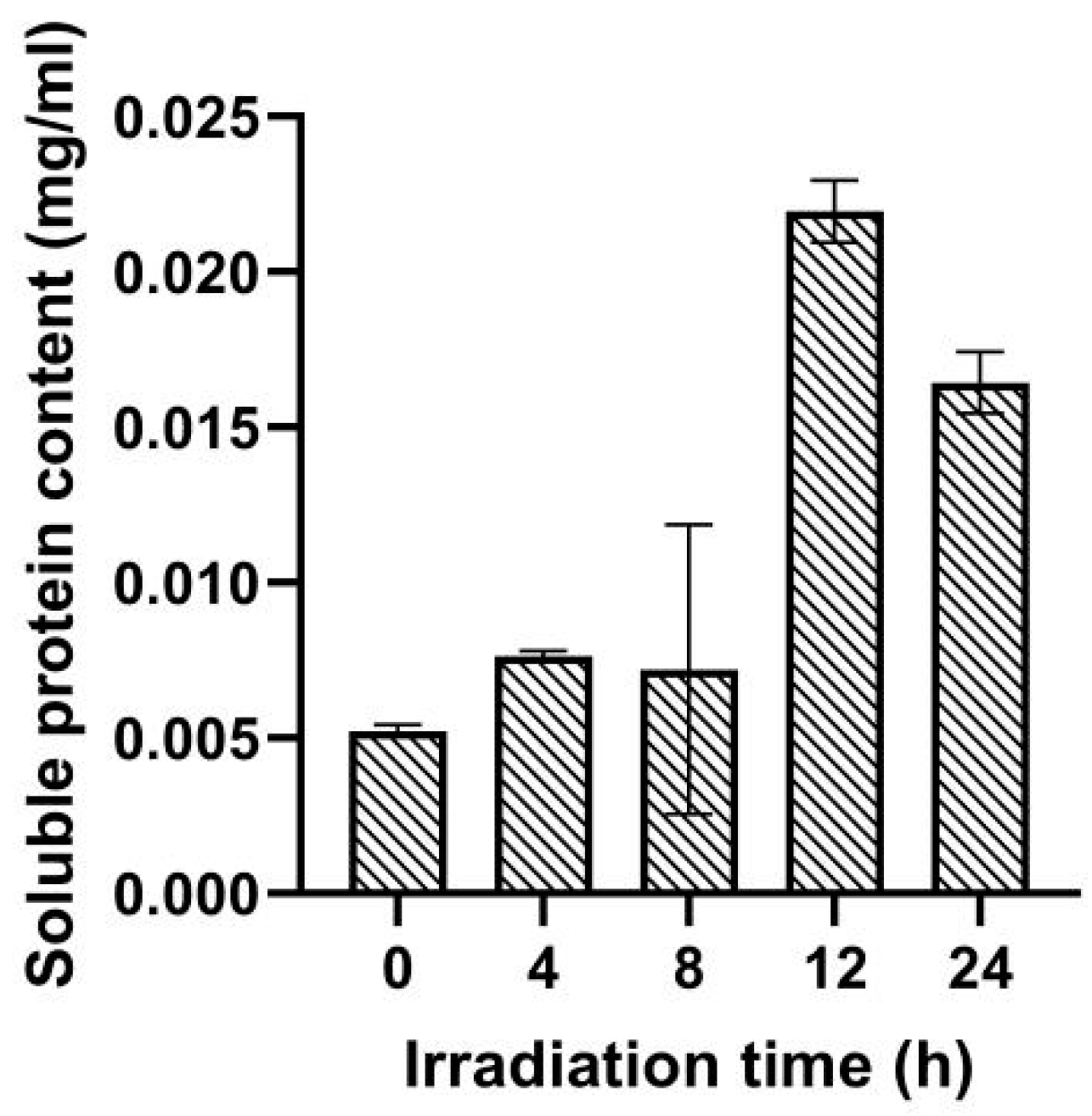
2.2. Effect of UV Irradiation Dose on Soluble Protein Content
2.3. Effect of UV Irradiation Dose on CAT Activity
2.4. Effect of UV Irradiation Dose on SOD Activity
2.5. Effect of UV Irradiation on Proteomics
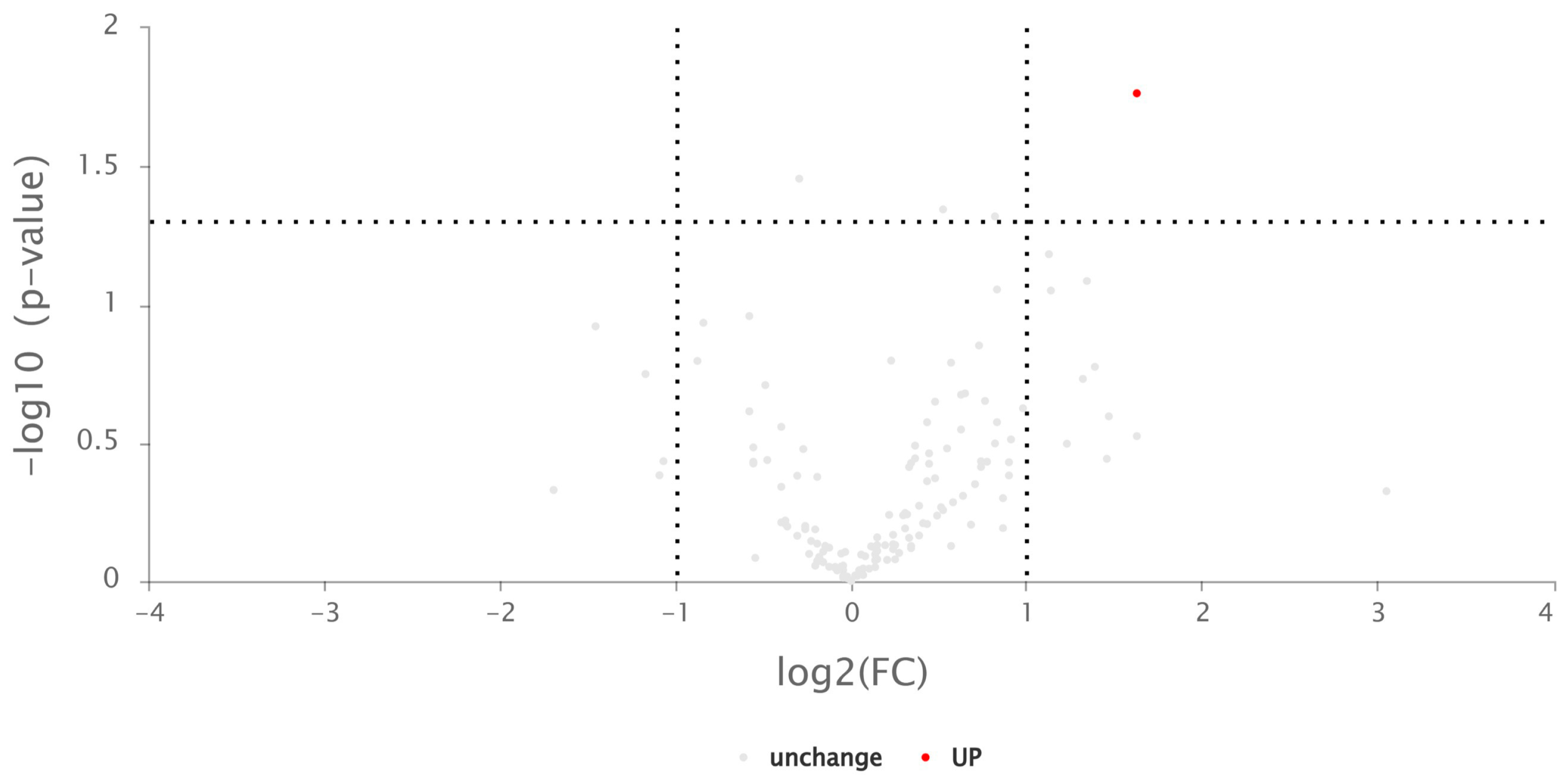
2.5.1. Differentially Expressed Proteins
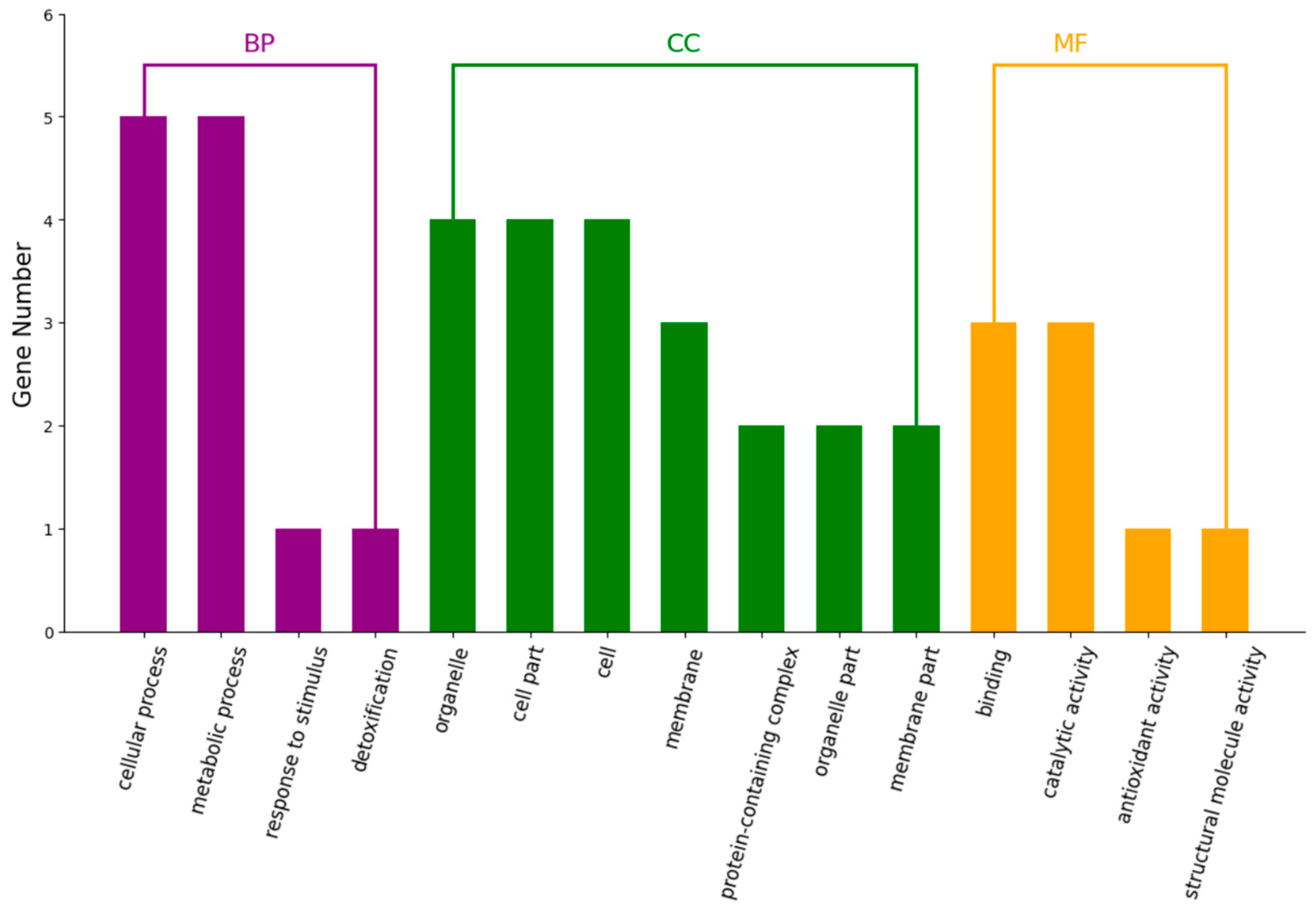
2.5.2. Differential Proteins in GO Analysis
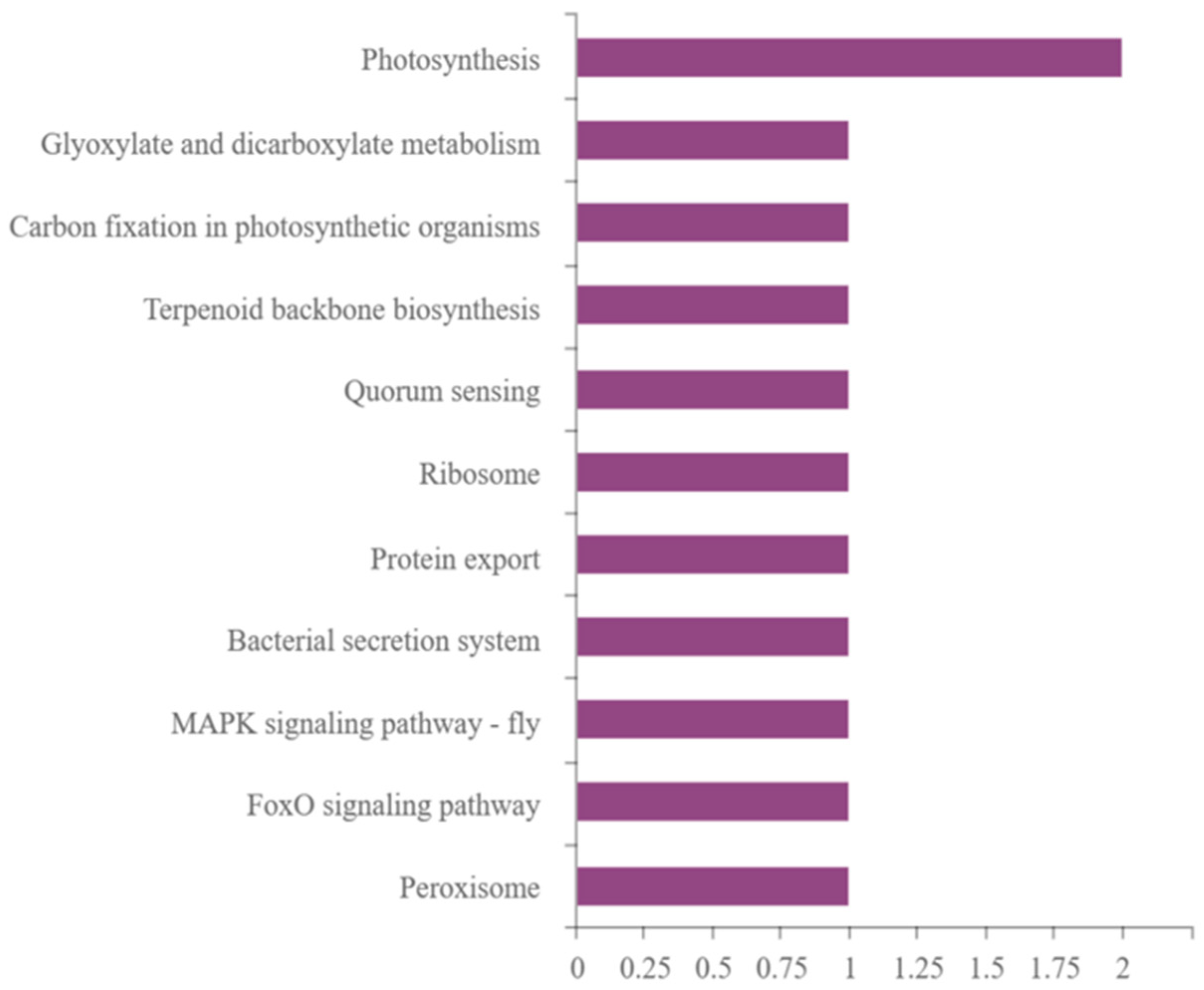
2.5.3. Differential Protein KEGG Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Experimental Methods
4.2.1. Pre-Treatment before Irradiation
4.2.2. Irradiation Treatment
4.2.3. Treatment and Analysis after Irradiation Process
4.3. Assay Methods
4.3.1. Chlorophyll a, Chlorophyll b, and Total Chlorophyll Content
4.3.2. Soluble Protein Content
4.3.3. CAT Activity
4.3.4. SOD Activity
4.3.5. Proteomics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kock, A.; Glanville, H.; Law, A.; Stanton, T.; Carter, L.; Taylor, J.C. Emerging challenges of the impacts of pharmaceuticals on aquatic ecosystems: A diatom perspective. Sci. Total Environ. 2023, 878, 162939. [Google Scholar] [CrossRef]
- Marella, T.K.; López-Pacheco, I.Y.; Parra-Saldívar, R.; Dixit, S.; Tiwari, A. Wealth from waste: Diatoms as tools for phycoremediation of wastewater and for obtaining value from the biomass. Sci. Total Environ. 2020, 724, 137960. [Google Scholar] [CrossRef]
- Alexandrov, S. Algal research in space: History, current status and future prospects. J. Life Sci. 2016, 4, 1–4. [Google Scholar]
- Sharma, G.C.; Nandan, R. Reliability modeling and analysis of environmental control and life support systems of space stations: A literature survey. Acta Astronaut. 2019, 155, 238–246. [Google Scholar] [CrossRef]
- Han, B.P.; Han, Z.G.; Fu, X. Mechanism and Model of Algal Photosynthesis; Science Press: Beijing, China, 2003. [Google Scholar]
- Niederwieser, T.; Kociolek, P.; Klaus, D. Spacecraft cabin environment effects on the growth and behavior of Chlorella vulgaris for life support applications. Life Sci. Space Res. 2018, 16, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Nan, G.N.; Zhang, Q.S.; Sheng, Z.T.; Zhang, D. Coordination between xanthophyll cycle and antioxidant system in Sargassum thunbergii (Sargassaceae, Phaeophyta) in response to high light and dehydration stresses. J. Appl. Phycol. 2016, 28, 2587–2596. [Google Scholar] [CrossRef]
- Chen, S.W.; Wu, B.X. Algal responses to enhanced UV-B and its mechanism on molecular level. J. Jinan Univ. Nat. Sci. Med. Ed. 2000, 5, 88–94. [Google Scholar]
- Wu, H.; Cockshutt, A.M.; McCarthy, A.; Campbell, D.A. Distinctive photosystem II photoinactivation and protein dynamics in marine diatoms. Plant Physiol. 2011, 156, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.I.; Inaba, M.; Yokota, A.; Murata, N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001, 20, 5587–5594. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Sinha, R.P.; Kannaujiya, V.K. Effects of ultraviolet and photosynthetically active radiation on morphogenesis, antioxidants and photoprotective defense mechanism in a hot-spring cyanobacterium Nostoc sp. strain VKB02. Res. Microbiol. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, T.V.; Björn, L.O.; Chernov, Y.; Chapin, T.; Christensen, T.R.; Huntley, B.; Ims, R.A.; Johansson, M.; Jolly, D.; Jonasson, S.; et al. Responses to projected changes in climate and UV-B at the species level. Ambio 2004, 33, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Figueroa, F.L.; Vega, J.; Avilés, A.; Horta, P.A.; Korbee, N.; Bonomi-Barufi, J. Effects of UV–visible radiation on growth, photosynthesis, pigment accumulation and UV-absorbing compounds in the red macroalga Gracilaria cornea (Gracilariales, Rhodophyta). Algal Res. 2022, 64, 102702. [Google Scholar] [CrossRef]
- Zeeshan, M.; Prasad, S.M. Differential response of growth, photosynthesis, antioxidant enzymes and lipid peroxidation to UV-B radiation in three cyanobacteria. S. Afr. J. Bot. 2009, 75, 466–474. [Google Scholar] [CrossRef]
- Lu, P.Y.; Zang, Y.; Xue, S.; Sun, Y.; Zhu, M.L.; Liang, S.; Tang, X.X. Gender-specific responses of Mougeotia’s reactive oxygen species scavenging system to enhanced UV-B radiation. J. Ocean Univ. China 2022, 52, 52–59. (In Chinese) [Google Scholar]
- Pfendler, S.; Munch, T.; Bousta, F.; Alaoui-Sosse, L.; Aleya, L.; Alaoui-Sossé, B. Bleaching of biofilm-forming algae induced by UV-C treatment: A preliminary study on chlorophyll degradation and its optimization for an application on cultural heritage. Environ. Sci. Pollut. Res. 2018, 25, 14097–14105. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, Z.; Du, W.; Gao, K. Physiological response of marine centric diatoms to ultraviolet radiation, with special reference to cell size. J. Photochem. Photobiol. B. 2015, 153, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Korkaric, M.; Behra, R.; Fischer, B.B.; Junghans, M.; Eggen, R.I. Multiple stressor effects in Chlamydomonas reinhardtii—Toward understanding mechanisms of interaction between effects of ultraviolet radiation and chemical pollutants. Aquat. Toxicol. 2015, 162, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Ahmad, I.Z. Evaluation of the effect of UV-B radiation on growth, photosynthetic pigment, and antioxidant enzymes of some cyanobacteria. Environ. Res. 2023, 218, 114943. [Google Scholar] [CrossRef]
- Arena, C.; Graham, T.; Legué, V.; Paradiso, R. Editorial: Higher plants, algae and cyanobacteria in space environments. Front. Plant Sci. 2021, 12, 629014. [Google Scholar] [CrossRef]
- Borderie, F.; Tête, N.; Cailhol, D.; Alaoui-Sehmer, L.; Bousta, F.; Rieffel, D.; Aleya, L.; Alaoui-Sossé, B. Factors driving epilithic algal colonization in show caves and new insights into combating biofilm development with UV-C treatments. Sci. Total Environ. 2014, 484, 43–52. [Google Scholar] [CrossRef]
- Marangoni, R.; Messina, N.; Gioffré, D.; Colombetti, G. Effects of UV-B irradiation on a marine icroecosystem. Photochem. Photobiol. 2004, 80, 78. [Google Scholar]
- Zhong, Z.; Liu, S.; Zhu, W.; Ou, Y.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Tian, J.; Komatsu, S. Phosphoproteomics Reveals the Biosynthesis of Secondary Metabolites in Catharanthus roseus under Ultraviolet-B Radiation. J. Proteome Res. 2019, 18, 3328–3341. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, K.; Chen, L.; Hu, C.; Zhang, D.; Liu, Y. The involvement of the antioxidant system in protection of desert cyanobacterium Nostoc sp. against UV-B radiation and the effects of exogenous antioxidants. Ecotoxicol. Environ. Saf. 2008, 69, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.; Eriksson, M.J.; Öquist, G.; Gustafsson, P.; Clarke, A.K. The cyanobacterium Synechococcus resists UV-B by exchanging photosystem II reaction-center D1 proteins. Proc. Natl. Acad. Sci. USA 1998, 95, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Mindrebo, J.T.; Nartey, C.M.; Seto, Y.; Burkart, M.D.; Noel, J.P. Unveiling the functional diversity of the alpha/beta hydrolase superfamily in the plant kingdom. Curr. Opin. Struct. Biol. 2016, 41, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Huarancca Reyes, T.; Mariotti, L.; Chiellini, C.; Guglielminetti, L.; Fonseca, G.G. UV-B irradiation effect on microalgae performance in the remediation of effluent derived from the cigarette butt cleaning process. Plants 2022, 11, 2356. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Khalid, M.F.; Vincent, C.; Morillon, R.; Anjum, M.A.; Ahmad, S.; Hussain, S. Different strategies lead to a common outcome: Different water-deficit scenarios highlight physiological and biochemical strategies of water-deficit tolerance in diploid versus tetraploid Volkamer lemon. Tree Physiol. 2021, 41, 2359–2374. [Google Scholar] [CrossRef]
- Malea, P.; Kokkinidi, D.; Kevrekidou, A.; Adamakis, I.S. The Enzymatic and non-enzymatic antioxidant system response of the seagrass Cymodocea nodosa to bisphenol-A Toxicity. Int. J. Mol. Sci. 2022, 23, 1348. [Google Scholar] [CrossRef]
- Saber, H.; El-Sheekh, M.M.; Ibrahim, A.; Alwaleed, E.A. Effect of UV-B radiation on amino acids profile, antioxidant enzymes and lipid peroxidation of some cyanobacteria and green algae. Int. J. Radiat. Biol. 2020, 96, 1192–1206. [Google Scholar] [CrossRef]
- Koochak, H.; Puthiyaveetil, S.; Mullendore, D.L.; Li, M.; Kirchhoff, H. The structural and functional domains of plant thylakoid membranes. Plant J. 2019, 97, 412–429. [Google Scholar] [CrossRef] [PubMed]
- Gisriel, C.J.; Shen, G.; Ho, M.; Kurashov, V.; Flesher, D.A.; Wang, J.; Armstrong, W.H.; Golbeck, J.H.; Gunner, M.R.; Vinyard, D.J.; et al. Structure of a monomeric photosystem II core complex from a cyanobacterium acclimated to far-red light reveals the functions of chlorophylls d and f. J. Biol. Chem. 2022, 298, 101424. [Google Scholar] [CrossRef] [PubMed]
- Boekema, E.J.; Hankamer, B.; Bald, D.; Kruip, J.; Nield, J.; Boonstra, A.F.; Barber, J.; Rögner, M. Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc. Natl. Acad. Sci. USA 1995, 92, 175–179. [Google Scholar] [CrossRef]
- Singh, R.D.; Sethy, S.; Ghosh, S.; Srivastava, A.K. UV and γ-radiation induced molecular changes for rapid lipid accumulation in Chlorella sorokiniana. Biomass Bioenergy 2022, 163, 106493. [Google Scholar] [CrossRef]
- Wang, B.; Ye, T.; Li, C.; Li, X.; Chen, L.; Wang, G. Cell damage repair mechanism in a desert green algae Chlorella sp. against UV-B radiation. Ecotoxicol. Environ. Saf. 2022, 242, 113916. [Google Scholar] [CrossRef]
- Yadav, K.N.; Semchonok, D.A.; Nosek, L.; Kouřil, R.; Fucile, G.; Boekema, E.J.; Eichacker, L.A. Supercomplexes of plant photosystem I with cytochrome b6f, light-harvesting complex II and NDH. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 12–20. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, W.; Peng, M.; Tan, G.; Qaseem, M.F.; Li, H.; Wu, A. Physiological and transcriptomic responses to magnesium deficiency in Neolamarckia cadamba. Plant Physiol. Biochem. 2023, 197, 107645. [Google Scholar] [CrossRef] [PubMed]
- Vonlanthen, M.; Cuétara-Guadarrama, F.; Sorroza-Martínez, K.; González-Méndez, I.; Estrada-Montaño, A.S.; Rivera, E. A review of porphyrin dendrimers as light-harvesting versatile platforms. Dyes Pigm. 2024, 222, 111873. [Google Scholar] [CrossRef]



| Number | Component | Concentration | Content |
|---|---|---|---|
| 1 | Ca(NO3)2·4H2O | 15 g/100 mL dH2O | 1 mL/L |
| 2 | KNO3 | 10 g/100 mL dH2O | 1 mL/L |
| 3 | MgSO4·7H2O | 4 g/100 mL dH2O | 1 mL/L |
| 4 | β-Na2 glycerophosphate·5H2O | 2.5 g/100 mL dH2O | 1 mL/L |
| 5 | Vitamin B12 | 0.1 µg/L | |
| 6 | Biotin | 0.1 µg/L | |
| 7 | Thiamine HCl | 10 µg/L | |
| 8 | HEPES | 0.5 g/L | |
| 9 | Na2SiO3·9H2O | 0.1 g/L | |
| 10 | Soil extract * | 30 mL/L | |
| 11 (PIV) | Na2EDTA | 0.75 g/L dH2O | 6 mL/L |
| MnCl2·4H2O | 0.041 g/L dH2O | ||
| ZnCl2·7H2O | 0.005 g/L dH2O | ||
| Na2MoO4·2H2O | 0.004 g/L dH2O | ||
| FeCl3·6H2O | 0.097 g/L dH2O | ||
| CoCl2·6H2O | 0.002 g/L dH2O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, S.; Pan, P.; Meng, X.; Zhang, Y.; Xu, H.; Hu, H.; Cheng, X.; Yan, Q. Integrated Physiologic and Proteomic Analyses Reveal the Molecular Mechanism of Navicula sp. in Response to Ultraviolet Irradiation Stress. Int. J. Mol. Sci. 2024, 25, 2747. https://doi.org/10.3390/ijms25052747
Gong S, Pan P, Meng X, Zhang Y, Xu H, Hu H, Cheng X, Yan Q. Integrated Physiologic and Proteomic Analyses Reveal the Molecular Mechanism of Navicula sp. in Response to Ultraviolet Irradiation Stress. International Journal of Molecular Sciences. 2024; 25(5):2747. https://doi.org/10.3390/ijms25052747
Chicago/Turabian StyleGong, Siyu, Pan Pan, Xiangying Meng, Yuxin Zhang, Hanli Xu, Honggang Hu, Xiyu Cheng, and Qiong Yan. 2024. "Integrated Physiologic and Proteomic Analyses Reveal the Molecular Mechanism of Navicula sp. in Response to Ultraviolet Irradiation Stress" International Journal of Molecular Sciences 25, no. 5: 2747. https://doi.org/10.3390/ijms25052747





