Matrix Metalloproteinases in the Periodontium—Vital in Tissue Turnover and Unfortunate in Periodontitis
Abstract
:1. Introduction
2. The Extracellular Matrix
3. Matrix Metalloproteinases
3.1. Types of MMPs
3.2. MMP Structure
3.3. Expression and Activity Regulation
4. A Brief Glance at the Role of Matrix Metalloproteinases in the Development and Diseases of the Oral Cavity
5. The Extracellular Matrix of the Periodontium and the Role of Matrix Metalloproteinases in Periodontitis
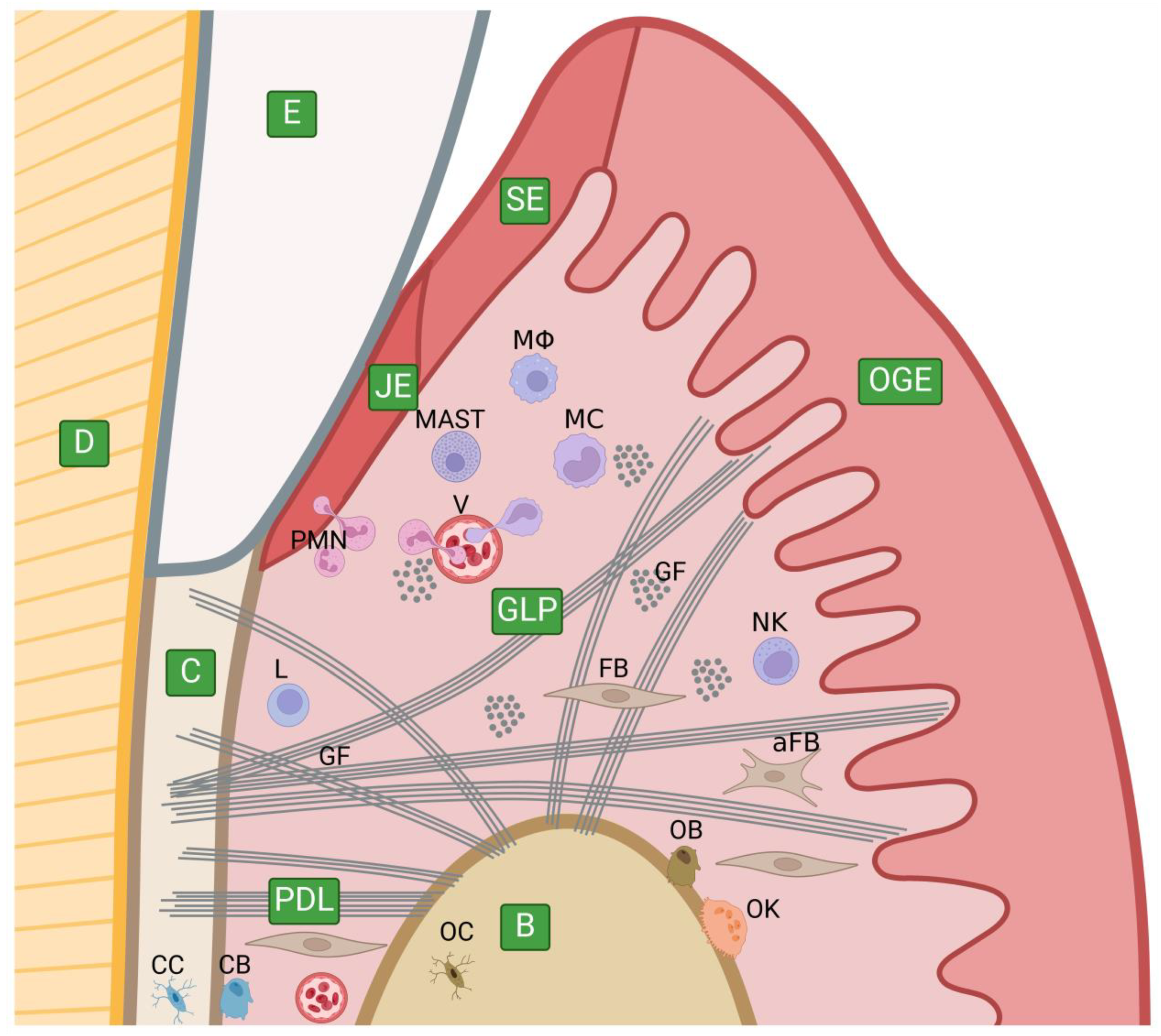
5.1. The Anatomy of the Periodontium and Its Extracellular Matrix
5.2. A Brief Discussion of Periodontitis
5.3. The Role of Matrix Metalloproteinases in the Periodontium and Periodontitis
6. A Few Words about MMP Inhibitors in the Therapy of Periodontitis as a Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Słotwińska, S.M. The Immunologic Aspects of Periodontal Disease. Cent. Eur. J. Immunol. 2011, 36, 279–283. [Google Scholar]
- Van Dyke, T.E.; Bartold, P.M.; Reynolds, E.C. The Nexus Between Periodontal Inflammation and Dysbiosis. Front. Immunol. 2020, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Franco, C.; Patricia, H.-R.; Timo, S.; Claudia, B.; Marcela, H. Matrix Metalloproteinases as Regulators of Periodontal Inflammation. Int. J. Mol. Sci. 2017, 18, 440. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Owczarek, K.; Lewandowska, U. The Role of Metalloproteinases and Their Tissue Inhibitors on Ocular Diseases: Focusing on Potential Mechanisms. Int. J. Mol. Sci. 2022, 23, 4256. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, T.; Stankova, T.; Bivolarska, A.; Vlaykova, T. Matrix Metalloproteinases in Oral Health-Special Attention on MMP-8. Biomedicines 2023, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Monea, M.; Pop, A.M. The Use of Salivary Levels of Matrix Metalloproteinases as an Adjuvant Method in the Early Diagnosis of Oral Squamous Cell Carcinoma: A Narrative Literature Review. Curr. Issues Mol. Biol. 2022, 44, 6306–6322. [Google Scholar] [CrossRef]
- Wright, J.T.; Hart, T.C.; Hart, P.S.; Simmons, D.; Suggs, C.; Daley, B.; Simmer, J.; Hu, J.; Bartlett, J.D.; Li, Y.; et al. Human and Mouse Enamel Phenotypes Resulting from Mutation or Altered Expression of AMEL, ENAM, MMP20 and KLK4. Cells Tissues Organs 2009, 189, 224–229. [Google Scholar] [CrossRef]
- Naruishi, K. Biological Roles of Fibroblasts in Periodontal Diseases. Cells 2022, 11, 3345. [Google Scholar] [CrossRef]
- Yuan, G.-H.; Yang, G.-B.; Wu, L.-A.; Chen, Z.; Chen, S. Potential Role of Dentin Sialoprotein by Inducing Dental Pulp Mesenchymal Stem Cell Differentiation and Mineralization for Dental Tissue Repair. Dent. Hypotheses 2010, 1, 69–75. [Google Scholar] [CrossRef]
- Butler, W.T. Dentin Matrix Proteins. Eur. J. Oral Sci. 1998, 106 (Suppl. S1), 204–210. [Google Scholar] [CrossRef]
- Fiorino, A.; Marturano, A.; Placella, G.; Staderini, E.; Domingo, L.I.; Cerulli, G.G.; Tiribuzi, R.; Blasi, P. Amelogenin-Derived Peptides in Bone Regeneration: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9224. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular Matrix Structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Janulaityte, I.; Januskevicius, A.; Rimkunas, A.; Palacionyte, J.; Vitkauskiene, A.; Malakauskas, K. Asthmatic Eosinophils Alter the Gene Expression of Extracellular Matrix Proteins in Airway Smooth Muscle Cells and Pulmonary Fibroblasts. Int. J. Mol. Sci. 2022, 23, 4086. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M. Connective Tissues of the Periodontium. Research and Clinical Implications. Aust. Dent. J. 1991, 36, 255–268. [Google Scholar] [CrossRef]
- Gresham, R.C.H.; Bahney, C.S.; Leach, J.K. Growth Factor Delivery Using Extracellular Matrix-Mimicking Substrates for Musculoskeletal Tissue Engineering and Repair. Bioact. Mater. 2021, 6, 1945–1956. [Google Scholar] [CrossRef]
- Maher, M.K.; White, J.F.; Glattauer, V.; Yue, Z.; Hughes, T.C.; Ramshaw, J.A.M.; Wallace, G.G. Variation in Hydrogel Formation and Network Structure for Telo-, Atelo- and Methacrylated Collagens. Polymers 2022, 14, 1775. [Google Scholar] [CrossRef]
- Leggieri, A.; Attanasio, C.; Palladino, A.; de Girolamo, P.; Lucini, C.; D’Angelo, L. Neuronal Phenotype of col4a1 and col25a1: An Intriguing Hypothesis in Vertebrates Brain Aging. Int. J. Mol. Sci. 2022, 23, 1778. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Caimi, G.; Hopps, E.; Montana, M.; Urso, C.; Carollo, C.; Canino, B.; Lo Presti, R. The Function of Matrix Metalloproteinase-9 (MMP-9) and Its Tissue Inhibitor (TIMP-1) in Several Clinical Conditions: Results and Analysis of Our Survey. Clin. Hemorheol. Microcirc. 2021, 78, 401–416. [Google Scholar] [CrossRef]
- Sekiguchi, R.; Yamada, K.M. Basement Membranes in Development and Disease. Curr. Top. Dev. Biol. 2018, 130, 143–191. [Google Scholar] [CrossRef]
- Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int. J. Mol. Sci. 2021, 22, 12690. [Google Scholar] [CrossRef]
- Poole, J.J.A.; Mostaço-Guidolin, L.B. Optical Microscopy and the Extracellular Matrix Structure: A Review. Cells 2021, 10, 1760. [Google Scholar] [CrossRef]
- Kozlova, N.; Grossman, J.E.; Iwanicki, M.P.; Muranen, T. The Interplay of the Extracellular Matrix and Stromal Cells as a Drug Target in Stroma-Rich Cancers. Trends Pharmacol. Sci. 2020, 41, 183–198. [Google Scholar] [CrossRef]
- Lam, D.; Enright, H.A.; Cadena, J.; Peters, S.K.G.; Sales, A.P.; Osburn, J.J.; Soscia, D.A.; Kulp, K.S.; Wheeler, E.K.; Fischer, N.O. Tissue-Specific Extracellular Matrix Accelerates the Formation of Neural Networks and Communities in a Neuron-Glia Co-Culture on a Multi-Electrode Array. Sci. Rep. 2019, 9, 4159. [Google Scholar] [CrossRef] [PubMed]
- Leclech, C.; Natale, C.F.; Barakat, A.I. The Basement Membrane as a Structured Surface-Role in Vascular Health and Disease. J. Cell Sci. 2020, 133, jcs239889. [Google Scholar] [CrossRef] [PubMed]
- Usman, K.; Hsieh, A.; Hackett, T.-L. The Role of miRNAs in Extracellular Matrix Repair and Chronic Fibrotic Lung Diseases. Cells 2021, 10, 1706. [Google Scholar] [CrossRef] [PubMed]
- Pompili, S.; Latella, G.; Gaudio, E.; Sferra, R.; Vetuschi, A. The Charming World of the Extracellular Matrix: A Dynamic and Protective Network of the Intestinal Wall. Front. Med. 2021, 8, 610189. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.K.; Basu, S.; Sharma, R.I. The Extracellular Matrix: Structure, Composition, Age-Related Differences, Tools for Analysis and Applications for Tissue Engineering. J. Tissue Eng. 2014, 5, 2041731414557112. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.E. Fibrosis Is a Basement Membrane-Related Disease in the Cornea: Injury and Defective Regeneration of Basement Membranes May Underlie Fibrosis in Other Organs. Cells 2022, 11, 309. [Google Scholar] [CrossRef]
- Indik, Z.; Yeh, H.; Ornstein-Goldstein, N.; Kucich, U.; Abrams, W.; Rosenbloom, J.C.; Rosenbloom, J. Structure of the Elastin Gene and Alternative Splicing of Elastin mRNA: Implications for Human Disease. Am. J. Med. Genet. 1989, 34, 81–90. [Google Scholar] [CrossRef]
- Chavrier, C. The Elastic System Fibres in Healthy Human Gingiva. Arch. Oral Biol. 1990, 35, 223S–225S. [Google Scholar] [CrossRef]
- Leblond, C.P.; Inoue, S. Structure, Composition, and Assembly of Basement Membrane. Am. J. Anat. 1989, 185, 367–390. [Google Scholar] [CrossRef]
- Kalluri, R. Basement Membranes: Structure, Assembly and Role in Tumour Angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Khalilgharibi, N.; Mao, Y. To Form and Function: On the Role of Basement Membrane Mechanics in Tissue Development, Homeostasis and Disease. Open Biol. 2021, 11, 200360. [Google Scholar] [CrossRef] [PubMed]
- Devergne, O.; Tsung, K.; Barcelo, G.; Schüpbach, T. Polarized Deposition of Basement Membrane Proteins Depends on Phosphatidylinositol Synthase and the Levels of Phosphatidylinositol 4,5-Bisphosphate. Proc. Natl. Acad. Sci. USA 2014, 111, 7689–7694. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The Vascular Basement Membrane in the Healthy and Pathological Brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef]
- Mao, M.; Labelle-Dumais, C.; Tufa, S.F.; Keene, D.R.; Gould, D.B. Elevated TGFβ Signaling Contributes to Ocular Anterior Segment Dysgenesis in Col4a1 Mutant Mice. Matrix Biol. 2022, 110, 151–173. [Google Scholar] [CrossRef]
- Kuan, M.I.; Jaeger, H.K.; Balemba, O.B.; O’Dowd, J.M.; Duricka, D.; Hannemann, H.; Marx, E.; Teissier, N.; Gabrielli, L.; Bonasoni, M.P.; et al. Human Cytomegalovirus Interactions with the Basement Membrane Protein Nidogen 1. J. Virol. 2021, 95, e01506-20. [Google Scholar] [CrossRef] [PubMed]
- Arikawa-Hirasawa, E. Impact of the Heparan Sulfate Proteoglycan Perlecan on Human Disease and Health. Am. J. Physiol. Cell Physiol. 2022, 322, C1117–C1122. [Google Scholar] [CrossRef]
- Ozols, M.; Eckersley, A.; Platt, C.I.; Stewart-McGuinness, C.; Hibbert, S.A.; Revote, J.; Li, F.; Griffiths, C.E.M.; Watson, R.E.B.; Song, J.; et al. Predicting Proteolysis in Complex Proteomes Using Deep Learning. Int. J. Mol. Sci. 2021, 22, 3071. [Google Scholar] [CrossRef]
- Boudko, S.P.; Danylevych, N.; Hudson, B.G.; Pedchenko, V.K. Basement Membrane Collagen IV: Isolation of Functional Domains. Methods Cell Biol. 2018, 143, 171–185. [Google Scholar] [CrossRef]
- El-Domyati, M.; Abdel-Wahab, H.; Ahmad, H. Immunohistochemical Localization of Basement Membrane Laminin 5 and Collagen IV in Adult Linear IgA Disease. Int. J. Dermatol. 2015, 54, 922–928. [Google Scholar] [CrossRef]
- Walko, G.; Castañón, M.J.; Wiche, G. Molecular Architecture and Function of the Hemidesmosome. Cell Tissue Res. 2015, 360, 363–378. [Google Scholar] [CrossRef]
- Gartner, L.P.; Hiatt, J.L. 4—Extracellular Matrix. In Concise Histology; Gartner, L.P., Hiatt, J.L., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 40–47. ISBN 9780702031144. [Google Scholar]
- Yurchenco, P.D. Basement Membranes: Cell Scaffoldings and Signaling Platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Streuli, C.H. Integrins and Epithelial Cell Polarity. J. Cell Sci. 2014, 127, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Wojdas, M.; Dąbkowska, K.; Winsz-Szczotka, K. Alterations of Extracellular Matrix Components in the Course of Juvenile Idiopathic Arthritis. Metabolites 2021, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Lulińska, E.; Gibbon, A.; Kaczmarczyk, M.; Maciejewska-Skrendo, A.; Ficek, K.; Leońska-Duniec, A.; Wilk, M.; Leźnicka, K.; Michałowska-Sawczyn, M.; Humińska-Lisowska, K.; et al. Matrix Metalloproteinase Genes (MMP1, MMP10, MMP12) on Chromosome 11q22 and the Risk of Non-Contact Anterior Cruciate Ligament Ruptures. Genes 2020, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix Metalloproteinases Participation in the Metastatic Process and Their Diagnostic and Therapeutic Applications in Cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, M. Enzyme-Responsive Hydrogels as Potential Drug Delivery Systems-State of Knowledge and Future Prospects. Int. J. Mol. Sci. 2022, 23, 4421. [Google Scholar] [CrossRef] [PubMed]
- Marino, G.; Funk, C. Matrix Metalloproteinases in Plants: A Brief Overview. Physiol. Plant. 2012, 145, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Corre, M.-H.; Bachmann, V.; Kohn, T. Bacterial Matrix Metalloproteases and Serine Proteases Contribute to the Extra-Host Inactivation of Enteroviruses in Lake Water. ISME J. 2022, 16, 1970–1979. [Google Scholar] [CrossRef]
- Cerdà-Costa, N.; Gomis-Rüth, F.X. Architecture and Function of Metallopeptidase Catalytic Domains. Protein Sci. 2014, 23, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Marino-Puertas, L.; Goulas, T.; Gomis-Rüth, F.X. Matrix Metalloproteinases Outside Vertebrates. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2026–2035. [Google Scholar] [CrossRef]
- Kruszewska, J.; Cudnoch-Jedrzejewska, A.; Czarzasta, K. Remodeling and Fibrosis of the Cardiac Muscle in the Course of Obesity—Pathogenesis and Involvement of the Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 4195. [Google Scholar] [CrossRef]
- Checchi, V.; Maravic, T.; Bellini, P.; Generali, L.; Consolo, U.; Breschi, L.; Mazzoni, A. The Role of Matrix Metalloproteinases in Periodontal Disease. Int. J. Environ. Res. Public Health 2020, 17, 4923. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How Matrix Metalloproteinases Regulate Cell Behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Sood, A.; Cherian, L.M.; Heera, R.; Sathyan, S.; Banerjee, M. Association between Matrix Metalloproteinases-2 and -9 Gene Polymorphism with Basement Membrane Disruption in Oral Lichen Planus: A Case-Control Pilot Study. J. Oral Biol. Craniofac. Res. 2022, 12, 258–262. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, L.; Feng, J.; Yang, G.; Ni, Q.; Xu, X.; Wan, C.; Lindsey, M.; Donly, K.J.; MacDougall, M.; et al. Dentin Sialoprotein Is a Novel Substrate of Matrix Metalloproteinase 9 in Vitro and in Vivo. Sci. Rep. 2017, 7, 42449. [Google Scholar] [CrossRef]
- Boelen, G.-J.; Boute, L.; d’Hoop, J.; EzEldeen, M.; Lambrichts, I.; Opdenakker, G. Matrix Metalloproteinases and Inhibitors in Dentistry. Clin. Oral Investig. 2019, 23, 2823–2835. [Google Scholar] [CrossRef]
- Maskos, K. Crystal Structures of MMPs in Complex with Physiological and Pharmacological Inhibitors. Biochimie 2005, 87, 249–263. [Google Scholar] [CrossRef]
- Binder, M.J.; Ward, A.C. The Role of the Metzincin Superfamily in Prostate Cancer Progression: A Systematic-Like Review. Int. J. Mol. Sci. 2021, 22, 3608. [Google Scholar] [CrossRef]
- Arza, B.; De Maeyer, M.; Félez, J.; Collen, D.; Lijnen, H.R. Critical Role of Glutamic Acid 202 in the Enzymatic Activity of Stromelysin-1 (MMP-3). Eur. J. Biochem. 2001, 268, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, L.; Geronimo, B.D.; Ortín, I.; Coderch, C.; Zapico, J.M.; Ramos, A.; de Pascual-Teresa, B. Molecular Imaging Probes Based on Matrix Metalloproteinase Inhibitors (MMPIs). Molecules 2019, 24, 2982. [Google Scholar] [CrossRef] [PubMed]
- Galea, C.A.; Nguyen, H.M.; George Chandy, K.; Smith, B.J.; Norton, R.S. Domain Structure and Function of Matrix Metalloprotease 23 (MMP23): Role in Potassium Channel Trafficking. Cell. Mol. Life Sci. 2014, 71, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- Singh, W.; Fields, G.B.; Christov, C.Z.; Karabencheva-Christova, T.G. Importance of the Linker Region in Matrix Metalloproteinase-1 Domain Interactions. RSC Adv. 2016, 6, 23223–23232. [Google Scholar] [CrossRef]
- Van Doren, S.R. Matrix Metalloproteinase Interactions with Collagen and Elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef]
- Bertini, I.; Fragai, M.; Luchinat, C.; Melikian, M.; Toccafondi, M.; Lauer, J.L.; Fields, G.B. Structural Basis for Matrix Metalloproteinase 1-Catalyzed Collagenolysis. J. Am. Chem. Soc. 2012, 134, 2100–2110. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases: Structure, Function, and Biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Han, Y.-P.; Yan, C.; Garner, W.L. Proteolytic Activation of Matrix Metalloproteinase-9 in Skin Wound Healing Is Inhibited by Alpha-1-Antichymotrypsin. J. Investig. Dermatol. 2008, 128, 2334–2342. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Fadnes, B.; Sylte, I.; Uhlin-Hansen, L.; Winberg, J.-O. Regulation of Matrix Metalloproteinase Activity in Health and Disease. FEBS J. 2011, 278, 28–45. [Google Scholar] [CrossRef]
- Ismail, A.A.; Shaker, B.T.; Bajou, K. The Plasminogen-Activator Plasmin System in Physiological and Pathophysiological Angiogenesis. Int. J. Mol. Sci. 2021, 23, 337. [Google Scholar] [CrossRef]
- Hu, Q.; Ecker, M. Overview of MMP-13 as a Promising Target for the Treatment of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 1742. [Google Scholar] [CrossRef]
- Butkiewicz, D.; Gdowicz-Kłosok, A.; Krześniak, M.; Rutkowski, T.; Łasut-Szyszka, B.; Składowski, K. Germline Variants in Angiogenesis-Related Genes Contribute to Clinical Outcome in Head and Neck Squamous Cell Carcinoma. Cancers 2022, 14, 1844. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef] [PubMed]
- Sandholm, L. Proteases and Their Inhibitors in Chronic Inflammatory Periodontal Disease. J. Clin. Periodontol. 1986, 13, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.C.M.; Thomas, D.M.; Choong, P.F.M.; Dass, C.R. RECK--a Newly Discovered Inhibitor of Metastasis with Prognostic Significance in Multiple Forms of Cancer. Cancer Metastasis Rev. 2007, 26, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Di Pasqua, L.G.; Croce, A.C.; Ferrigno, A.; Vairetti, M. Recent Updates on the Therapeutic Prospects of Reversion-Inducing Cysteine-Rich Protein with Kazal Motifs (RECK) in Liver Injuries. Int. J. Mol. Sci. 2023, 24, 17407. [Google Scholar] [CrossRef] [PubMed]
- Mendes, S.R.; Amo-Maestro, L.D.; Marino-Puertas, L.; de Diego, I.; Goulas, T.; Gomis-Rüth, F.X. Analysis of the Inhibiting Activity of Reversion-Inducing Cysteine-Rich Protein with Kazal Motifs (RECK) on Matrix Metalloproteinases. Sci. Rep. 2020, 10, 6317. [Google Scholar] [CrossRef]
- Bernardo, M.M.; Fridman, R. TIMP-2 (tissue Inhibitor of Metalloproteinase-2) Regulates MMP-2 (matrix Metalloproteinase-2) Activity in the Extracellular Environment after pro-MMP-2 Activation by MT1 (membrane Type 1)-MMP. Biochem. J. 2003, 374, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Zajgla, J.; Del Pozo, L.; Ceballos, G.; Maldonado, V. Tissue Inhibitor of Metalloproteinases-4. The Road Less Traveled. Mol. Cancer 2008, 7, 85. [Google Scholar] [CrossRef]
- Morgunova, E.; Tuuttila, A.; Bergmann, U.; Tryggvason, K. Structural Insight into the Complex Formation of Latent Matrix Metalloproteinase 2 with Tissue Inhibitor of Metalloproteinase 2. Proc. Natl. Acad. Sci. USA 2002, 99, 7414–7419. [Google Scholar] [CrossRef]
- Raeeszadeh-Sarmazdeh, M.; Do, L.D.; Hritz, B.G. Metalloproteinases and Their Inhibitors: Potential for the Development of New Therapeutics. Cells 2020, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Charzewski, Ł.; Krzyśko, K.A.; Lesyng, B. Structural Characterisation of Inhibitory and Non-Inhibitory MMP-9-TIMP-1 Complexes and Implications for Regulatory Mechanisms of MMP-9. Sci. Rep. 2021, 11, 13376. [Google Scholar] [CrossRef]
- Fanchon, S.; Bourd, K.; Septier, D.; Everts, V.; Beertsen, W.; Menashi, S.; Goldberg, M. Involvement of Matrix Metalloproteinases in the Onset of Dentin Mineralization. Eur. J. Oral Sci. 2004, 112, 171–176. [Google Scholar] [CrossRef]
- Prajapati, S.; Ruan, Q.; Mukherjee, K.; Nutt, S.; Moradian-Oldak, J. The Presence of MMP-20 Reinforces Biomimetic Enamel Regrowth. J. Dent. Res. 2018, 97, 84–90. [Google Scholar] [CrossRef]
- Mukhtar, U.; Goyal, A.; Luthra-Guptasarma, M.; Gauba, K.; Kapur, A.; Thakur, A.K. Label-Free Quantitative Proteomics Reveals Molecular Correlates of Altered Biomechanical Properties in Molar Incisor Hypomineralization (MIH): An in Vitro Study. Eur. Arch. Paediatr. Dent. 2022, 23, 179–191. [Google Scholar] [CrossRef]
- Anshida, V.P.; Kumari, R.A.; Murthy, C.S.; Samuel, A. Extracellular Matrix Degradation by Host Matrix Metalloproteinases in Restorative Dentistry and Endodontics: An Overview. J. Oral Maxillofac. Pathol. 2020, 24, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Amano, K.; Iohara, K.; Ito, M.; Imabayashi, K.; Into, T.; Matsushita, K.; Nakamura, H.; Nakashima, M. Matrix Metalloproteinase-3 Accelerates Wound Healing Following Dental Pulp Injury. Am. J. Pathol. 2009, 175, 1905–1914. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.; Faria, M.; Giraldi, G.; Bastos, L.; Oliveira, L.; Conci, A. Classification of Approximal Caries in Bitewing Radiographs Using Convolutional Neural Networks. Sensors 2021, 21, 5192. [Google Scholar] [CrossRef] [PubMed]
- Opydo-Szymaczek, J.; Borysewicz-Lewicka, M.; Andrysiak, K.; Witkowska, Z.; Hoffmann-Przybylska, A.; Przybylski, P.; Walicka, E.; Gerreth, K. Clinical Consequences of Dental Caries, Parents’ Perception of Child’s Oral Health and Attitudes towards Dental Visits in a Population of 7-Year-Old Children. Int. J. Environ. Res. Public Health 2021, 18, 5844. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-R.; Nam, S.-H. A Randomized, Double-Blind, Placebo-Controlled Clinical Trial of a Mouthwash Containing Glycyrrhiza Uralensis Extract for Preventing Dental Caries. Int. J. Environ. Res. Public Health 2021, 19, 242. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.; Smales, R. Oral Diagnosis and Treatment Planning: Part 2. Dental Caries and Assessment of Risk. Br. Dent. J. 2012, 213, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Ren, Q.; Li, Z.; Zhang, L. Chitosan-Based Biomimetically Mineralized Composite Materials in Human Hard Tissue Repair. Molecules 2020, 25, 4785. [Google Scholar] [CrossRef] [PubMed]
- Zarif, M.E.; Yehia, S.A.; Biță, B.; Sătulu, V.; Vizireanu, S.; Dinescu, G.; Holban, A.M.; Marinescu, F.; Andronescu, E.; Grumezescu, A.M.; et al. Atmospheric Pressure Plasma Activation of Hydroxyapatite to Improve Fluoride Incorporation and Modulate Bacterial Biofilm. Int. J. Mol. Sci. 2021, 22, 13103. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization. Front. Biosci. 2011, 3, 711–735. [Google Scholar] [CrossRef]
- Mazzoni, A.; Tjäderhane, L.; Checchi, V.; Di Lenarda, R.; Salo, T.; Tay, F.R.; Pashley, D.H.; Breschi, L. Role of Dentin MMPs in Caries Progression and Bond Stability. J. Dent. Res. 2015, 94, 241–251. [Google Scholar] [CrossRef]
- Radzki, D.; Wilhelm-Węglarz, M.; Pruska, K.; Kusiak, A.; Ordyniec-Kwaśnica, I. A Fresh Look at Mouthwashes—What Is Inside and What Is It For? Int. J. Environ. Res. Public Health 2022, 19, 3926. [Google Scholar] [CrossRef]
- Karki, S.; Alaraudanjoki, V.; Päkkilä, J.; Laitala, M.-L.; Anttonen, V. Different Risk Factors for Erosive Tooth Wear in Rural and Urban Nepal: A National Study. Int. J. Environ. Res. Public Health 2021, 18, 7766. [Google Scholar] [CrossRef]
- Freitas, P.H.; André, C.B.; Fronza, B.M.; Giannini, M.; Rosalen, P.L.; Consani, S.; França, R. Physicochemical Properties, Metalloproteinases Inhibition, and Antibiofilm Activity of Doxycycline-Doped Dental Adhesive. J. Dent. 2021, 104, 103550. [Google Scholar] [CrossRef]
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The Role of Matrix Metalloproteinases (MMPs) in Human Caries. J. Dent. Res. 2006, 85, 22–32. [Google Scholar] [CrossRef]
- Kritikou, K.; Greabu, M.; Imre, M.; Miricescu, D.; Ripszky Totan, A.; Burcea, M.; Stanescu-Spinu, I.-I.; Spinu, T. ILs and MMPs Levels in Inflamed Human Dental Pulp: A Systematic Review. Molecules 2021, 26, 4129. [Google Scholar] [CrossRef]
- Linde, A. The Extracellular Matrix of the Dental Pulp and Dentin. J. Dent. Res. 1985, 64, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.-Y.; Li, L.; Liu, L.-S.; Jiang, C.-M.; Zhang, H.-Z.; Wang, J.-X. Expression of Matrix Metalloproteinases and Tissue Inhibitor of Matrix Metalloproteinases during Apical Periodontitis Development. J. Endod. 2021, 47, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Hu, J.; Quan, H.-Y. Abnormal Expression of FAK and Paxillin Correlates with Oral Cancer Invasion and Metastasis. Acta Biochim. Pol. 2021, 68, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, M.G.; Min, K.-W.; Jung, U.S.; Kim, D.-H. High MMP-11 Expression Associated with Low CD8+ T Cells Decreases the Survival Rate in Patients with Breast Cancer. PLoS ONE 2021, 16, e0252052. [Google Scholar] [CrossRef] [PubMed]
- Podstawski, P.; Ropka-Molik, K.; Semik-Gurgul, E.; Samiec, M.; Skrzyszowska, M.; Podstawski, Z.; Szmatoła, T.; Witkowski, M.; Pawlina-Tyszko, K. Assessment of BPV-1 Mediated Matrix Metalloproteinase Genes Deregulation in the In Vivo and In Vitro Models Designed to Explore Molecular Nature of Equine Sarcoids. Cells 2022, 11, 1268. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielińska, W.; Krajewski, A.; Hałas-Wiśniewska, M.; Mikołajczyk, K.; Gagat, M.; Grzanka, A. Downregulation of MMP-9 Enhances the Anti-Migratory Effect of Cyclophosphamide in MDA-MB-231 and MCF-7 Breast Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 12783. [Google Scholar] [CrossRef] [PubMed]
- Radzki, D.; Kusiak, A.; Ordyniec-Kwaśnica, I.; Bondarczuk, A. Human Papillomavirus and Leukoplakia of the Oral Cavity: A Systematic Review. Postepy Dermatol. Alergol. 2021, 39, 594–600. [Google Scholar] [CrossRef]
- Miguel, A.F.P.; Embaló, B.; Alves Dias, H.B.; Rivero, E.R.C. Immunohistochemical Expression of MMP-9, TIMP-1, and Vimentin and Its Correlation with Inflammatory Reaction and Clinical Parameters in Oral Epithelial Dysplasia. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Radzki, D.; Baiduk, K.; Burzyńska, M.; Lisai, A.; Pańszczyk, K.; Bochniak, M.; Kusiak, A. Rola Brodawczaka Ludzkiego W Patologii Jamy Ustnej—Przegląd Piśmiennictwa. Dent. Forum 2018, 46, 67–74. [Google Scholar] [CrossRef]
- Visalli, G.; Di Pietro, A.; Currò, M.; Pruiti Ciarello, M.; D’Andrea, F.; Nunnari, G.; Pellicanò, G.F.; Facciolà, A. How Much Does HIV Positivity Affect the Presence of Oral HPV? A Molecular Epidemiology Survey. Int. J. Environ. Res. Public Health 2021, 18, 8999. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Oczko, M.; Owczarek-Drabińska, J.; Szczygielska, A.; Szczepaniak, M.; Duś-Ilnicka, I. Salivary HPV Infection in Healthy People. Postępy Hig. Med. Dośw. 2022, 76, 143–148. [Google Scholar] [CrossRef]
- Prosper, B.; Florencia, D.W.; Mahoukèdè, Z.T.; Dorcas, O.-Y.; Alima, T.E.M.; Karim, O.A.; Clarisse, O.T.-W.; Toyin, B.S.O.; Ayaovi, S.M.; Angèle, T.I.M.; et al. Polymorphism of MMP1 and MMP3 Promoter Regions and HR-HPV Infection in Women from Burkina Faso and Côte d’Ivoire. Biomol. Concepts 2020, 11, 116–124. [Google Scholar] [CrossRef]
- Lepetsos, P.; Pampanos, A.; Kanavakis, E.; Tzetis, M.; Korres, D.; Papavassiliou, A.G.; Efstathopoulos, N. Association of MMP-1 -1607 1G/2G (rs1799750) Polymorphism with Primary Knee Osteoarthritis in the Greek Population. J. Orthop. Res. 2014, 32, 1155–1160. [Google Scholar] [CrossRef]
- Zanetta, P.; Ormelli, M.; Amoruso, A.; Pane, M.; Azzimonti, B.; Squarzanti, D.F. Probiotics as Potential Biological Immunomodulators in the Management of Oral Lichen Planus: What’s New? Int. J. Mol. Sci. 2022, 23, 3489. [Google Scholar] [CrossRef]
- Romano, F.; Arduino, P.G.; Maggiora, M.; Curmei, E.; Manavella, V.; Broccoletti, R.; Aimetti, M. Effect of a Structured Plaque Control on MMP-1 and MMP-9 Crevicular Levels in Patients with Desquamative Gingivitis Associated with Oral Lichen Planus. Clin. Oral Investig. 2019, 23, 2651–2658. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-García, J.Z.; Munguía-Robledo, S.; Estrada-Orozco, J.J.; Licéaga-Escalera, C.; Rodríguez, M.A. Expression Level and Proteolytic Activity of MMP-2 and MMP-9 in Dental Follicles, Dentigerous Cysts, Odontogenic Keratocysts and Unicystic Ameloblastomas. J. Oral Biol. Craniofac. Res. 2022, 12, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yu, Y.; Ruan, H.; Luo, Y.; Guo, X. Morphological and Functional Characteristics of Human Gingival Junctional Epithelium. BMC Oral Health 2014, 14, 30. [Google Scholar] [CrossRef]
- Schroeder, H.E.; Listgarten, M.A. The Junctional Epithelium: From Strength to Defense. J. Dent. Res. 2003, 82, 158–161. [Google Scholar] [CrossRef]
- Ko, Y.K.; Hong, S.; Kim, H.M.; Liu, M.; Moon, E.; Kim, P.; Choi, Y. Characterization of Junctional Structures in the Gingival Epithelium as Barriers against Bacterial Invasion. J. Periodontal Res. 2022, 57, 799–810. [Google Scholar] [CrossRef]
- Skrypnyk, M.; Petrushanko, T.; Neporada, K.; Vynnyk, N.; Petrushanko, V.; Skrypnyk, R. Colonization Resistance of Oral Mucosa in Individuals with Diverse Body Mass Index. Czas. Stomatol. 2022, 75, 171–175. [Google Scholar] [CrossRef]
- Fischer, N.G.; Aparicio, C. Junctional Epithelium and Hemidesmosomes: Tape and Rivets for Solving the “Percutaneous Device Dilemma” in Dental and Other Permanent Implants. Bioact. Mater. 2022, 18, 178–198. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, Q.; Han, X.; Bai, D.; Li, D.; Tian, Y. Proteoglycans in the Periodontium: A Review with Emphasis on Specific Distributions, Functions, and Potential Applications. J. Periodontal Res. 2021, 56, 617–632. [Google Scholar] [CrossRef]
- Bartold, P.M.; Wiebkin, O.W.; Thonard, J.C. Proteoglycans in Human Gingiva: Molecular Size Distribution in Epithelium and in Connective Tissue. Arch. Oral Biol. 1982, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.; Gossen, M.; Lendlein, A. Designing Cardiovascular Implants Taking in View the Endothelial Basement Membrane. Int. J. Mol. Sci. 2021, 22, 13120. [Google Scholar] [CrossRef] [PubMed]
- Smukler, H.; Dreyer, C.J. Principal Fibres of the Periodontium. J. Periodontal Res. 1969, 4, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yamanouchi, K.; Tsuruga, E.; Oka, K.; Sawa, Y.; Ishikawa, H. Fibrillin-1 and Fibrillin-2 Are Essential for Formation of Thick Oxytalan Fibers in Human Nonpigmented Ciliary Epithelial Cells in Vitro. Connect. Tissue Res. 2012, 53, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, E.; Irie, K.; Yajima, T. Fibrillin-2 Degradation by Matrix Metalloproteinase-2 in Periodontium. J. Dent. Res. 2007, 86, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Sugawara, Y.; Asai, T.; Aida, N.; Yanagisawa, T.; Ohta, K.; Inoue, S. Immunohistochemical Characterization of Elastic System Fibers in Rat Molar Periodontal Ligament. J. Histochem. Cytochem. 2006, 54, 1095–1103. [Google Scholar] [CrossRef]
- Yamalik, N.; Kilinç, K.; Caglayan, F.; Eratalay, K.; Caglayan, G. Molecular Size Distribution Analysis of Human Gingival Proteoglycans and Glycosaminoglycans in Specific Periodontal Diseases. J. Clin. Periodontol. 1998, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M. Proteoglycans of the Periodontium: Structure, Role and Function. J. Periodontal Res. 1987, 22, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Page, R.C. Proteoglycans Synthesized by Cultured Fibroblasts Derived from Normal and Inflamed Human Gingiva. Vitr. Cell. Dev. Biol. 1986, 22, 407–417. [Google Scholar] [CrossRef]
- Sterczała, B.; Chwiłkowska, A.; Szwedowicz, U.; Kobielarz, M.; Chwiłkowski, B.; Dominiak, M. Impact of APRF+ in Combination with Autogenous Fibroblasts on Release Growth Factors, Collagen, and Proliferation and Migration of Gingival Fibroblasts: An In Vitro Study. Materials 2022, 15, 796. [Google Scholar] [CrossRef]
- Steinsvoll, S.; Helgeland, K.; Schenck, K. Mast Cells--a Role in Periodontal Diseases? J. Clin. Periodontol. 2004, 31, 413–419. [Google Scholar] [CrossRef]
- Laurell, L.; Rylander, H.; Sundin, Y. Histologic Characteristics of Clinically Healthy Gingiva in Adolescents. Scand. J. Dent. Res. 1987, 95, 456–462. [Google Scholar] [CrossRef]
- Seidel, A.; Seidel, C.L.; Weider, M.; Junker, R.; Gölz, L.; Schmetzer, H. Influence of Natural Killer Cells and Natural Killer T Cells on Periodontal Disease: A Systematic Review of the Current Literature. Int. J. Mol. Sci. 2020, 21, 9766. [Google Scholar] [CrossRef]
- Meghil, M.M.; Cutler, C.W. Oral Microbes and Mucosal Dendritic Cells, “Spark and Flame” of Local and Distant Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 1643. [Google Scholar] [CrossRef] [PubMed]
- Mohebichamkhorami, F.; Fattahi, R.; Niknam, Z.; Aliashrafi, M.; Khakpour Naeimi, S.; Gilanchi, S.; Zali, H. Periodontal Ligament Stem Cells as a Promising Therapeutic Target for Neural Damage. Stem Cell Res. Ther. 2022, 13, 273. [Google Scholar] [CrossRef]
- De Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The Intricate Anatomy of the Periodontal Ligament and Its Development: Lessons for Periodontal Regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef]
- Queiroz, A.; Albuquerque-Souza, E.; Gasparoni, L.M.; de França, B.N.; Pelissari, C.; Trierveiler, M.; Holzhausen, M. Therapeutic Potential of Periodontal Ligament Stem Cells. World J. Stem Cells 2021, 13, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Aghanashini, S.; Mundinamane, D.B.; Bhat, D.; Nadiger, S.; Mallikarjunappa, A.S.; George, S. Collagen—The Skeleton of the Periodontium: A Review. J. Sci. Dent. 2021, 11, 31–36. [Google Scholar] [CrossRef]
- Kaku, M.; Yamauchi, M. Mechano-Regulation of Collagen Biosynthesis in Periodontal Ligament. J. Prosthodont. Res. 2014, 58, 193–207. [Google Scholar] [CrossRef]
- Manabe, Y.; Shiga, M.; Kometani-Gunjigake, K.; Nakao-Kuroishi, K.; Mizuhara, M.; Toyono, T.; Seta, Y.; Kawamoto, T. Fibrillin-1 Regulates Periostin Expression during Maintenance of Periodontal Homeostasis. J. Dent. Sci. 2022, 17, 1714–1721. [Google Scholar] [CrossRef]
- Imhof, T.; Balic, A.; Heilig, J.; Chiquet-Ehrismann, R.; Chiquet, M.; Niehoff, A.; Brachvogel, B.; Thesleff, I.; Koch, M. Pivotal Role of Tenascin-W (-N) in Postnatal Incisor Growth and Periodontal Ligament Remodeling. Front. Immunol. 2020, 11, 608223. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Nie, E.-M.; Deng, G.; Lai, L.-Z.; Sun, F.-Y.; Tian, H.; Fang, F.-C.; Zou, Y.-G.; Wu, B.-L.; Ou-Yang, J. Periostin Is Essential for Periodontal Ligament Remodeling during Orthodontic Treatment. Mol. Med. Rep. 2017, 15, 1800–1806. [Google Scholar] [CrossRef]
- Barczyk, M.; Bolstad, A.I.; Gullberg, D. Role of Integrins in the Periodontal Ligament: Organizers and Facilitators. Periodontology 2000 2013, 63, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Schröder, A.; Nazet, U.; Neubert, P.; Jantsch, J.; Spanier, G.; Proff, P.; Kirschneck, C. Sodium-Chloride-Induced Effects on the Expression Profile of Human Periodontal Ligament Fibroblasts with Focus on Simulated Orthodontic Tooth Movement. Eur. J. Oral Sci. 2019, 127, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Tomokiyo, A.; Wada, N.; Maeda, H. Periodontal Ligament Stem Cells: Regenerative Potency in Periodontium. Stem Cells Dev. 2019, 28, 974–985. [Google Scholar] [CrossRef] [PubMed]
- Diekwisch, T.G. The Developmental Biology of Cementum. Int. J. Dev. Biol. 2001, 45, 695–706. [Google Scholar] [PubMed]
- Foster, B.L. On the Discovery of Cementum. J. Periodontal Res. 2017, 52, 666–685. [Google Scholar] [CrossRef]
- Nowak-Wachol, A.; Korytkowska-Wałach, A.; Chmiela, B.; Wachol, K.; Łopaciński, M.; Wyszyńska, M.; Al-Dulaimi, Y.; Skucha-Nowak, M. Yttrium Trifluoride as a Marker of Infiltration Rate of Decalcified Root Cementum: An In Vitro Study. Polymers 2022, 14, 780. [Google Scholar] [CrossRef]
- Gonçalves, P.F.; Sallum, E.A.; Sallum, A.W.; Casati, M.Z.; de Toledo, S.; Junior, F.H.N. Dental Cementum Reviewed: Development, Structure, Composition, Regeneration and Potential Functions. Braz. J. Oral Sci. 2005, 4, 651–658. [Google Scholar]
- Ho, S.P.; Balooch, M.; Goodis, H.E.; Marshall, G.W.; Marshall, S.J. Ultrastructure and Nanomechanical Properties of Cementum Dentin Junction. J. Biomed. Mater. Res. A 2004, 68, 343–351. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Selvig, K.A. Dental Cementum: The Dynamic Tissue Covering of the Root. Periodontol. 2000 1997, 13, 41–75. [Google Scholar] [CrossRef]
- Arzate, H.; Zeichner-David, M.; Mercado-Celis, G. Cementum Proteins: Role in Cementogenesis, Biomineralization, Periodontium Formation and Regeneration. Periodontology 2000 2015, 67, 211–233. [Google Scholar] [CrossRef]
- Saito, M.; Iwase, M.; Maslan, S.; Nozaki, N.; Yamauchi, M.; Handa, K.; Takahashi, O.; Sato, S.; Kawase, T.; Teranaka, T.; et al. Expression of Cementum-Derived Attachment Protein in Bovine Tooth Germ during Cementogenesis. Bone 2001, 29, 242–248. [Google Scholar] [CrossRef]
- Yonemura, K.; Raines, E.W.; Ahn, N.G.; Narayanan, A.S. Mitogenic Signaling Mechanisms of Human Cementum-Derived Growth Factors. J. Biol. Chem. 1993, 268, 26120–26126. [Google Scholar] [CrossRef]
- Nagasaki, K.; Chavez, M.B.; Nagasaki, A.; Taylor, J.M.; Tan, M.H.; Ma, M.; Ralston, E.; Thew, M.E.; Kim, D.-G.; Somerman, M.J.; et al. The Bone Sialoprotein RGD Domain Modulates and Maintains Periodontal Development. J. Dent. Res. 2022, 101, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Ababneh, K.T.; Hall, R.C.; Embery, G. The Proteoglycans of Human Cementum: Immunohistochemical Localization in Healthy, Periodontally Involved and Ageing Teeth. J. Periodontal Res. 1999, 34, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.P.; Sulyanto, R.M.; Marshall, S.J.; Marshall, G.W. The Cementum-Dentin Junction Also Contains Glycosaminoglycans and Collagen Fibrils. J. Struct. Biol. 2005, 151, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hasegawa, T.; Yamamoto, T.; Hongo, H.; Amizuka, N. Histology of Human Cementum: Its Structure, Function, and Development. Jpn. Dent. Sci. Rev. 2016, 52, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P.M.; du Toit, N. CHAPTER 5-Dental Anatomy. In Equine Dentistry, 3rd ed.; Easley, J., Dixon, P.M., Schumacher, J., Eds.; W.B. Saunders: Edinburgh, UK, 2011; pp. 51–76. ISBN 9780702029806. [Google Scholar]
- Goldberg, M. The Alveolar Bone Provides Support to Teeth and Other Functions: A Review. Med. Sci. Monit. 2022, 3, 1–17. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.-Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone Structure and Formation: A New Perspective. Mater. Sci. Eng. R Rep. 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Feng, X. Chemical and Biochemical Basis of Cell-Bone Matrix Interaction in Health and Disease. Curr. Chem. Biol. 2009, 3, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Robey, P.G. Chapter 14-Bone Matrix Proteoglycans and Glycoproteins. In Principles of Bone Biology, 2nd ed.; Bilezikian, J.P., Raisz, L.G., Rodan, G.A., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 225–237. ISBN 9780120986521. [Google Scholar]
- Robey, P.G.; Fedarko, N.S.; Hefferan, T.E.; Bianco, P.; Vetter, U.K.; Grzesik, W.; Friedenstein, A.; Van der Pluijm, G.; Mintz, K.P.; Young, M.F. Structure and Molecular Regulation of Bone Matrix Proteins. J. Bone Miner. Res. 1993, 8 (Suppl. 2), S483–S487. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Muszyński, S.; Hułas-Stasiak, M.; Leszczyński, N.; Mielnik-Błaszczak, M.; Donaldson, J.; Dobrowolski, P. Prenatal Fumonisin Exposure Impairs Bone Development via Disturbances in the OC/Leptin and RANKL/RANK/OPG Systems in Weaned Rat Offspring. Int. J. Mol. Sci. 2023, 24, 8743. [Google Scholar] [CrossRef]
- Alkhayal, Z.; Shinwari, Z.; Gaafar, A.; Alaiya, A. Carbonic Anhydrase II Activators in Osteopetrosis Treatment: A Review. Curr. Issues Mol. Biol. 2023, 45, 1373–1386. [Google Scholar] [CrossRef]
- Wagner, J.M.; Reinkemeier, F.; Wallner, C.; Dadras, M.; Dittfeld, S.; Drysch, M.; Sogorski, A.; von Glinski, M.; Lehnhardt, M.; Behr, B.; et al. Inhibition of Pathological Increased Matrix Metalloproteinase (MMP) Activity for Improvement of Bone Regeneration in Diabetes. Life 2022, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog. Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar] [CrossRef] [PubMed]
- Saftig, P.; Hunziker, E.; Everts, V.; Jones, S.; Boyde, A.; Wehmeyer, O.; Suter, A.; von Figura, K. Functions of Cathepsin K in Bone Resorption. Lessons from Cathepsin K Deficient Mice. Adv. Exp. Med. Biol. 2000, 477, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Khattri, S.; Kaushik, M.; Sharma, S. Periodontal Ligament: Health, Disease and Regeneration. IJPHRD 2022, 13, 296–301. [Google Scholar] [CrossRef]
- Nahian, A.; Davis, D.D. Histology, Osteoprogenitor Cells; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Matsubara, T.; Suardita, K.; Ishii, M.; Sugiyama, M.; Igarashi, A.; Oda, R.; Nishimura, M.; Saito, M.; Nakagawa, K.; Yamanaka, K.; et al. Alveolar Bone Marrow as a Cell Source for Regenerative Medicine: Differences between Alveolar and Iliac Bone Marrow Stromal Cells. J. Bone Miner. Res. 2005, 20, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Kadlub, N.; Debelmas, A.; Dallard, J.; Picard, A.; Boisson, J. Modeling of the Human Mandibular Periosteum Material Properties and Comparison with the Calvarial Periosteum. Biomech. Model. Mechanobiol. 2020, 19, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Lin, Z.; Fateh, A.; Salem, D.M.; Intini, G. Periosteum: Biology and Applications in Craniofacial Bone Regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef]
- Al-Qtaitat, A.; Shore, R.C.; Aaron, J.E. Structural Changes in the Ageing Periosteum Using Collagen III Immuno-Staining and Chromium Labelling as Indicators. J. Musculoskelet. Neuronal Interact. 2010, 10, 112–123. [Google Scholar]
- Listgarten, M.A. Pathogenesis of Periodontitis. J. Clin. Periodontol. 1986, 13, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M.; Van Dyke, T.E. An Appraisal of the Role of Specific Bacteria in the Initial Pathogenesis of Periodontitis. J. Clin. Periodontol. 2019, 46, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Meyle, J.; Chapple, I. Molecular Aspects of the Pathogenesis of Periodontitis. Periodontology 2000 2015, 69, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, A.A.; Singh, S.; Saha, S.; Molnar, J.; Arora, R. The Prevalence and Incidence of Coronary Heart Disease Is Significantly Increased in Periodontitis: A Meta-Analysis. Am. Heart J. 2007, 154, 830–837. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; da Cruz, S.S.; Trindade, S.C.; Passos-Soares, J.d.S.; Carvalho-Filho, P.C.; Figueiredo, A.C.M.G.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and Respiratory Diseases: A Systematic Review with Meta-Analysis. Oral Dis. 2020, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Khader, Y.S.; Dauod, A.S.; El-Qaderi, S.S.; Alkafajei, A.; Batayha, W.Q. Periodontal Status of Diabetics Compared with Nondiabetics: A Meta-Analysis. J. Diabetes Complicat. 2006, 20, 59–68. [Google Scholar] [CrossRef]
- Schmitt, A.; Carra, M.C.; Boutouyrie, P.; Bouchard, P. Periodontitis and Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2015, 42, 977–987. [Google Scholar] [CrossRef]
- Botero, J.E.; Rodríguez-Medina, C.; Jaramillo-Echeverry, A.; Contreras, A. Association between Human Cytomegalovirus and Periodontitis: A Systematic Review and Meta-Analysis. J. Periodontal Res. 2020, 55, 551–558. [Google Scholar] [CrossRef]
- Karimbux, N.Y.; Saraiya, V.M.; Elangovan, S.; Allareddy, V.; Kinnunen, T.; Kornman, K.S.; Duff, G.W. Interleukin-1 Gene Polymorphisms and Chronic Periodontitis in Adult Whites: A Systematic Review and Meta-Analysis. J. Periodontol. 2012, 83, 1407–1419. [Google Scholar] [CrossRef]
- Hussain, S.B.; Leira, Y.; Zehra, S.A.; Botelho, J.; Machado, V.; Ciurtin, C.; D’Aiuto, F.; Orlandi, M. Periodontitis and Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. J. Periodontal Res. 2022, 57, 1–10. [Google Scholar] [CrossRef]
- Rosário-Dos-Santos, H.L.; Miranda, S.S.; Gomes-Filho, I.S.; da Cruz, S.S.; Figueiredo, A.C.M.G.; Souza, E.S.; Hintz, A.M.; Loomer, P.M.; Passos-Soares, J.d.S. Periodontitis Severity Relationship with Metabolic Syndrome: A Systematic Review with Meta-Analysis. Oral Dis. 2022, 29, 2512–2520. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Y.; Cao, Z.; Chen, Y.; Si, C.; Sun, X.; Huang, S. The Role of Mitochondrial Dysfunction in Periodontitis: From Mechanisms to Therapeutic Strategy. J. Periodontal Res. 2023, 58, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Cai, G.; Ni, Q.; Geng, Y.; Wang, T.; Bao, C.; Ruan, X.; Wang, H.; Sun, W. Roles of Trained Immunity in the Pathogenesis of Periodontitis. J. Periodontal Res. 2023, 58, 864–873. [Google Scholar] [CrossRef]
- Wielento, A.; Lagosz-Cwik, K.B.; Potempa, J.; Grabiec, A.M. The Role of Gingival Fibroblasts in the Pathogenesis of Periodontitis. J. Dent. Res. 2023, 102, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Menashi, S.; Lu, H.; Soria, C.; Legrand, Y. Endothelial Cell Proteases: Physiological Role and Regulation. Baillieres. Clin. Haematol. 1993, 6, 559–576. [Google Scholar] [CrossRef]
- Kajanne, R.; Miettinen, P.; Mehlem, A.; Leivonen, S.-K.; Birrer, M.; Foschi, M.; Kähäri, V.-M.; Leppä, S. EGF-R Regulates MMP Function in Fibroblasts through MAPK and AP-1 Pathways. J. Cell. Physiol. 2007, 212, 489–497. [Google Scholar] [CrossRef]
- Rifas, L.; Fausto, A.; Scott, M.J.; Avioli, L.V.; Welgus, H.G. Expression of Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Human Osteoblast-like Cells: Differentiation Is Associated with Repression of Metalloproteinase Biosynthesis. Endocrinology 1994, 134, 213–221. [Google Scholar] [CrossRef]
- Everts, V.; Korper, W.; Jansen, D.C.; Steinfort, J.; Lammerse, I.; Heera, S.; Docherty, A.J.; Beertsen, W. Functional Heterogeneity of Osteoclasts: Matrix Metalloproteinases Participate in Osteoclastic Resorption of Calvarial Bone but Not in Resorption of Long Bone. FASEB J. 1999, 13, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Quiding-Järbrink, M.; Smith, D.A.; Bancroft, G.J. Production of Matrix Metalloproteinases in Response to Mycobacterial Infection. Infect. Immun. 2001, 69, 5661–5670. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent Impact of Periodontitis and Cardiovascular Disease on Elevated Soluble Urokinase-Type Plasminogen Activator Receptor (suPAR) Levels. J. Periodontol. 2021, 92, 896–906. [Google Scholar] [CrossRef]
- Yin, X.; Bunn, C.L.; Bartold, P.M. Detection of Tissue Plasminogen Activator (t-PA) and Plasminogen Activator Inhibitor 2(PAI-2) in Gingival Crevicular Fluid from Healthy, Gingivitis and Periodontitis Patients. J. Clin. Periodontol. 2000, 27, 149–156. [Google Scholar] [CrossRef]
- Hirayama, A.; Awano, S.; Seta, Y.; Ansai, T. ADAM17 Regulates TNF-α Expression upon Lipopolysaccharide Stimulation in Oral Keratinocytes. Biomed. Res. 2017, 38, 157–165. [Google Scholar] [CrossRef]
- Fernandes, D.; Khambata, R.S.; Massimo, G.; Ruivo, E.; Gee, L.C.; Foster, J.; Goddard, A.; Curtis, M.; Barnes, M.R.; Wade, W.G.; et al. Local Delivery of Nitric Oxide Prevents Endothelial Dysfunction in Periodontitis. Pharmacol. Res. 2023, 188, 106616. [Google Scholar] [CrossRef]
- Aurer, A.; Aleksić, J.; Ivić-Kardum, M.; Aurer, J.; Culo, F. Nitric Oxide Synthesis Is Decreased in Periodontitis. J. Clin. Periodontol. 2001, 28, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.H.; Morgan, M.J.; Whawell, S.A.; Atkin, P.; Roblin, P.; Furness, J.; Speight, P.M. Metalloproteinase Expression in Normal and Malignant Oral Keratinocytes: Stimulation of MMP-2 and -9 by Scatter Factor. Eur. J. Oral Sci. 2000, 108, 281–291. [Google Scholar] [CrossRef]
- Tandara, A.A.; Mustoe, T.A. MMP- and TIMP-Secretion by Human Cutaneous Keratinocytes and Fibroblasts--Impact of Coculture and Hydration. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, 108–116. [Google Scholar] [CrossRef]
- Hiyoshi, T.; Domon, H.; Maekawa, T.; Tamura, H.; Isono, T.; Hirayama, S.; Sasagawa, K.; Takizawa, F.; Tabeta, K.; Terao, Y. Neutrophil Elastase Aggravates Periodontitis by Disrupting Gingival Epithelial Barrier via Cleaving Cell Adhesion Molecules. Sci. Rep. 2022, 12, 8159. [Google Scholar] [CrossRef] [PubMed]
- Dutzan, N.; Vernal, R.; Hernandez, M.; Dezerega, A.; Rivera, O.; Silva, N.; Aguillon, J.C.; Puente, J.; Pozo, P.; Gamonal, J. Levels of Interferon-Gamma and Transcription Factor T-Bet in Progressive Periodontal Lesions in Patients with Chronic Periodontitis. J. Periodontol. 2009, 80, 290–296. [Google Scholar] [CrossRef]
- Lagosz-Cwik, K.B.; Wielento, A.; Lipska, W.; Kantorowicz, M.; Darczuk, D.; Kaczmarzyk, T.; Gibbs, S.; Potempa, J.; Grabiec, A.M. hTERT-Immortalized Gingival Fibroblasts Respond to Cytokines but Fail to Mimic Primary Cell Responses to Porphyromonas Gingivalis. Sci. Rep. 2021, 11, 10770. [Google Scholar] [CrossRef] [PubMed]
- Khudan, R.; Krynytska, I.; Marushchak, M.; Korda, M. The Influence of Chronic Hyper-Homocysteinemia on Characteristics of Peripheral Neutrophils’ Programmed Death in Lipo-Polysaccharide-Induced Periodontitis. Czas. Stomatol. 2022, 75, 155–162. [Google Scholar] [CrossRef]
- Manor, A.; Lebendiger, M.; Shiffer, A.; Tovel, H. Bacterial Invasion of Periodontal Tissues in Advanced Periodontitis in Humans. J. Periodontol. 1984, 55, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C.; Fox, J.W. A Comprehensive View of the Structural and Functional Alterations of Extracellular Matrix by Snake Venom Metalloproteinases (SVMPs): Novel Perspectives on the Pathophysiology of Envenoming. Toxins 2016, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Bartold, P.M. Turnover in Periodontal Connective Tissues: Dynamic Homeostasis of Cells, Collagen and Ground Substances. Oral Dis. 1995, 1, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Tanaka, J.; Aizawa, R.; Yajima-Himuro, S.; Seki, T.; Tanaka, K.; Yamada, A.; Ogawa, M.; Kamijo, R.; Tsuji, T.; et al. Visualization of Junctional Epithelial Cell Replacement by Oral Gingival Epithelial Cells over a Life Time and after Gingivectomy. Sci. Rep. 2019, 9, 7640. [Google Scholar] [CrossRef]
- Smith, D.W.; Azadi, A.; Lee, C.-J.; Gardiner, B.S. Spatial Composition and Turnover of the Main Molecules in the Adult Glomerular Basement Membrane. Tissue Barriers 2023, 11, 2110798. [Google Scholar] [CrossRef]
- Page, R.C.; Ammons, W.F. Collagen Turnover in the Gingiva and Other Mature Connective Tissues of the Marmoset Saguinus Oedipus. Arch. Oral Biol. 1974, 19, 651–658. [Google Scholar] [CrossRef]
- Brochado Martins, J.F.; Rodrigues, C.F.D.; Diogo, P.; Paulo, S.; Palma, P.J.; do Vale, F.F. Remodelling Compartment in Root Cementum. Folia Morphol. 2021, 80, 972–979. [Google Scholar] [CrossRef] [PubMed]
- Rippin, J.W. Collagen Turnover in the Periodontal Ligament under Normal and Altered Functional Forces. I. Young Rat Molars. J. Periodontal Res. 1976, 11, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhou, C.; Chen, S.; Huang, D.; Jiang, Y.; Lan, Y.; Zou, S.; Li, Y. Osteoporosis and Alveolar Bone Health in Periodontitis Niche: A Predisposing Factors-Centered Review. Cells 2022, 11, 3380. [Google Scholar] [CrossRef] [PubMed]
- Sodek, J. A Comparison of the Rates of Synthesis and Turnover of Collagen and Non-Collagen Proteins in Adult Rat Periodontal Tissues and Skin Using a Microassay. Arch. Oral Biol. 1977, 22, 655–665. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujihara, C.; Nantakeeratipat, T.; Matsumoto, M.; Noguchi, T.; Kitagawa, M.; Yamada, S.; Takata, T.; Kitaura, H.; Murakami, S. CD40-CD40 Ligand Interaction between Periodontal Ligament Cells and Cementoblasts Enhances Periodontal Tissue Remodeling in Response to Mechanical Stress. J. Periodontal Res. 2023, 58, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Behm, C.; Nemec, M.; Weissinger, F.; Rausch, M.A.; Andrukhov, O.; Jonke, E. MMPs and TIMPs Expression Levels in the Periodontal Ligament during Orthodontic Tooth Movement: A Systematic Review of In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2021, 22, 6967. [Google Scholar] [CrossRef]
- Hathaway-Schrader, J.D.; Novince, C.M. Maintaining Homeostatic Control of Periodontal Bone Tissue. Periodontology 2000 2021, 86, 157–187. [Google Scholar] [CrossRef] [PubMed]
- Creemers, L.B.; Jansen, I.D.; Docherty, A.J.; Reynolds, J.J.; Beertsen, W.; Everts, V. Gelatinase A (MMP-2) and Cysteine Proteinases Are Essential for the Degradation of Collagen in Soft Connective Tissue. Matrix Biol. 1998, 17, 35–46. [Google Scholar] [CrossRef]
- Garlet, T.P.; Coelho, U.; Silva, J.S.; Garlet, G.P. Cytokine Expression Pattern in Compression and Tension Sides of the Periodontal Ligament during Orthodontic Tooth Movement in Humans. Eur. J. Oral Sci. 2007, 115, 355–362. [Google Scholar] [CrossRef]
- Liu, N.; Cao, Y.; Zhu, G. Expression of Matrix Metalloproteinases-2, -9 and Reversion-Inducing Cysteine-Rich Protein with Kazal Motifs in Gingiva in Periodontal Health and Disease. Arch. Oral Biol. 2017, 75, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Parks, W.C. Role of Matrix Metalloproteinases in Epithelial Migration. J. Cell. Biochem. 2009, 108, 1233–1243. [Google Scholar] [CrossRef]
- McGuire, J.K.; Li, Q.; Parks, W.C. Matrilysin (matrix Metalloproteinase-7) Mediates E-Cadherin Ectodomain Shedding in Injured Lung Epithelium. Am. J. Pathol. 2003, 162, 1831–1843. [Google Scholar] [CrossRef]
- Im, N.-R.; Kim, B.; Jung, K.-Y.; Baek, S.-K. Matrix Metalloproteinase-7 Induces E-Cadherin Cleavage in Acid-Exposed Primary Human Pharyngeal Epithelial Cells via the ROS/ERK/c-Jun Pathway. J. Mol. Med. 2022, 100, 313–322. [Google Scholar] [CrossRef]
- Lynch, C.C.; Vargo-Gogola, T.; Matrisian, L.M.; Fingleton, B. Cleavage of E-Cadherin by Matrix Metalloproteinase-7 Promotes Cellular Proliferation in Nontransformed Cell Lines via Activation of RhoA. J. Oncol. 2010, 2010, 530745. [Google Scholar] [CrossRef]
- Ghosh, P.; Muthuraj, T.S.; Bandyopadhyay, P.; Swarnakar, S.; Sarkar, P.; Varatharajan, A. Expression of Matrix Metalloproteinase-9 in Gingival Tissue Biopsy in Patients with Slowly/Moderately and Rapidly Progressing Periodontitis: An Observational Study. J. Indian Soc. Periodontol. 2021, 25, 386–392. [Google Scholar] [CrossRef]
- Cotrim, P.; de Andrade, C.R.; Martelli-Junior, H.; Graner, E.; Sauk, J.J.; Coletta, R.D. Expression of Matrix Metalloproteinases in Cyclosporin-Treated Gingival Fibroblasts Is Regulated by Transforming Growth Factor (TGF)-beta1 Autocrine Stimulation. J. Periodontol. 2002, 73, 1313–1322. [Google Scholar] [CrossRef]
- Trackman, P.C.; Kantarci, A. Connective Tissue Metabolism and Gingival Overgrowth. Crit. Rev. Oral Biol. Med. 2004, 15, 165–175. [Google Scholar] [CrossRef]
- Reynolds, J.J. Collagenases and Tissue Inhibitors of Metalloproteinases: A Functional Balance in Tissue Degradation. Oral Dis. 1996, 2, 70–76. [Google Scholar] [CrossRef]
- Everts, V.; van der Zee, E.; Creemers, L.; Beertsen, W. Phagocytosis and Intracellular Digestion of Collagen, Its Role in Turnover and Remodelling. Histochem. J. 1996, 28, 229–245. [Google Scholar] [CrossRef]
- Lauritano, D.; Palmieri, A.; Lucchese, A.; Di Stasio, D.; Moreo, G.; Carinci, F. Role of Cyclosporine in Gingival Hyperplasia: An In Vitro Study on Gingival Fibroblasts. Int. J. Mol. Sci. 2020, 21, 595. [Google Scholar] [CrossRef]
- Lauritano, D.; Moreo, G.; Vella, F.D.; Palmieri, A.; Carinci, F.; Petruzzi, M. Biology of Drug-Induced Gingival Hyperplasia: In Vitro Study of the Effect of Nifedipine on Human Fibroblasts. NATO Adv. Sci. Inst. Ser. E Appl. Sci. 2021, 11, 3287. [Google Scholar] [CrossRef]
- Warhonowicz, M.; Staszyk, C.; Gasse, H. Immunohistochemical Detection of Matrix Metalloproteinase-1 in the Periodontal Ligament of Equine Cheek Teeth. Tissue Cell 2007, 39, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Melander, M.C.; Jürgensen, H.J.; Madsen, D.H.; Engelholm, L.H.; Behrendt, N. The Collagen Receptor uPARAP/Endo180 in Tissue Degradation and Cancer (Review). Int. J. Oncol. 2015, 47, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Bildt, M.M.; Henneman, S.; Maltha, J.C.; Kuijpers-Jagtman, A.M.; Von den Hoff, J.W. CMT-3 Inhibits Orthodontic Tooth Displacement in the Rat. Arch. Oral Biol. 2007, 52, 571–578. [Google Scholar] [CrossRef]
- Holliday, L.S.; Vakani, A.; Archer, L.; Dolce, C. Effects of Matrix Metalloproteinase Inhibitors on Bone Resorption and Orthodontic Tooth Movement. J. Dent. Res. 2003, 82, 687–691. [Google Scholar] [CrossRef]
- Miyai-Murai, Y.; Okamoto-Shibayama, K.; Sato, T.; Kikuchi, Y.; Kokubu, E.; Potempa, J.; Ishihara, K. Localization and Pathogenic Role of the Cysteine Protease Dentipain in Treponema Denticola. Mol. Oral Microbiol. 2023, 38, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Potempa, J.; Polanowski, A.; Wikstrom, M.; Travis, J. Purification and Characterization of a 50-kDa Cysteine Proteinase (gingipain) from Porphyromonas Gingivalis. J. Biol. Chem. 1992, 267, 18896–18901. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yan, H.; Huang, L. Salivary Matrix Metalloproteinase (MMP)-8 as a Biomarker for Periodontitis: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2018, 97, e9642. [Google Scholar] [CrossRef]
- Vitkov, L.; Singh, J.; Schauer, C.; Minnich, B.; Krunić, J.; Oberthaler, H.; Gamsjaeger, S.; Herrmann, M.; Knopf, J.; Hannig, M. Breaking the Gingival Barrier in Periodontitis. Int. J. Mol. Sci. 2023, 24, 4544. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Yoshimoto, T.; Kajiya, M.; Ouhara, K.; Matsuda, S.; Takemura, T.; Akutagawa, K.; Takeda, K.; Mizuno, N.; Kurihara, H. Regulation of Defensive Function on Gingival Epithelial Cells Can Prevent Periodontal Disease. Jpn. Dent. Sci. Rev. 2018, 54, 66–75. [Google Scholar] [CrossRef]
- Chen, W.A.; Dou, Y.; Fletcher, H.M.; Boskovic, D.S. Local and Systemic Effects of Porphyromonas Gingivalis Infection. Microorganisms 2023, 11, 470. [Google Scholar] [CrossRef]
- Abe-Yutori, M.; Chikazawa, T.; Shibasaki, K.; Murakami, S. Decreased Expression of E-Cadherin by Porphyromonas Gingivalis-Lipopolysaccharide Attenuates Epithelial Barrier Function. J. Periodontal Res. 2017, 52, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Shiba, H.; Komatsuzawa, H.; Mizuno, N.; Uchida, Y.; Ouhara, K.; Asakawa, R.; Kudo, S.; Kawaguchi, H.; Sugai, M.; et al. Syntheses of Prostaglandin E2 and E-Cadherin and Gene Expression of Beta-Defensin-2 by Human Gingival Epithelial Cells in Response to Actinobacillus Actinomycetemcomitans. Inflammation 2003, 27, 341–349. [Google Scholar] [CrossRef]
- Uitto, V.J. Degradation of Basement Membrane Collagen by Proteinases from Human Gingiva, Leukocytes and Bacterial Plaque. J. Periodontol. 1983, 54, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Takarada, H.; Cattoni, M.; Sugimoto, A.; Rose, G.G. Ultrastructural Studies of Human Gingiva. 3. Changes of the Basal Lamina in Chronic Periodontitis. J. Periodontol. 1974, 45, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Ejeil, A.-L.; Igondjo-Tchen, S.; Ghomrasseni, S.; Pellat, B.; Godeau, G.; Gogly, B. Expression of Matrix Metalloproteinases (MMPs) and Tissue Inhibitors of Metalloproteinases (TIMPs) in Healthy and Diseased Human Gingiva. J. Periodontol. 2003, 74, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Nomura, T.; Takahashi, T.; Hara, K. Expression of mRNA for Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Periodontitis-Affected Human Gingival Tissue. Arch. Oral Biol. 1996, 41, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.D.R.; Oliveira, G.; Hurtado, P.A.; Feitosa, A.; Takiya, C.M.; Granjeiro, J.M.; Trackman, P.C.; Otazú, I.; Feres-Filho, E.J. Expression of Metalloproteinases and Their Tissue Inhibitors in Inflamed Gingival Biopsies. J. Periodontal Res. 2008, 43, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Dahan, M.; Nawrocki, B.; Elkaïm, R.; Soell, M.; Bolcato-Bellemin, A.L.; Birembaut, P.; Tenenbaum, H. Expression of Matrix Metalloproteinases in Healthy and Diseased Human Gingiva. J. Clin. Periodontol. 2001, 28, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Uitto, V.J.; Suomalainen, K.; Sorsa, T. Salivary Collagenase. Origin, Characteristics and Relationship to Periodontal Health. J. Periodontal Res. 1990, 25, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Uitto, V.J.; Haapasalo, M.; Lounatmaa, K.; Konttinen, Y.T.; Salo, T.; Grenier, D.; Sorsa, T. Membrane Components of Treponema Denticola Trigger Proteinase Release from Human Polymorphonuclear Leukocytes. J. Dent. Res. 1996, 75, 1986–1993. [Google Scholar] [CrossRef]
- Wang, X.; Rojas-Quintero, J.; Wilder, J.; Tesfaigzi, Y.; Zhang, D.; Owen, C.A. Tissue Inhibitor of Metalloproteinase-1 Promotes Polymorphonuclear Neutrophil (PMN) Pericellular Proteolysis by Anchoring Matrix Metalloproteinase-8 and -9 to PMN Surfaces. J. Immunol. 2019, 202, 3267–3281. [Google Scholar] [CrossRef]
- Sorsa, T.; Mäntylä, P.; Rönkä, H.; Kallio, P.; Kallis, G.B.; Lundqvist, C.; Kinane, D.F.; Salo, T.; Golub, L.M.; Teronen, O.; et al. Scientific Basis of a Matrix Metalloproteinase-8 Specific Chair-Side Test for Monitoring Periodontal and Peri-Implant Health and Disease. Ann. N. Y. Acad. Sci. 1999, 878, 130–140. [Google Scholar] [CrossRef]
- Kumar, M.S.; Vamsi, G.; Sripriya, R.; Sehgal, P.K. Expression of Matrix Metalloproteinases (MMP-8 and -9) in Chronic Periodontitis Patients with and without Diabetes Mellitus. J. Periodontol. 2006, 77, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Mäntylä, P.; Stenman, M.; Kinane, D.; Salo, T.; Suomalainen, K.; Tikanoja, S.; Sorsa, T. Monitoring Periodontal Disease Status in Smokers and Nonsmokers Using a Gingival Crevicular Fluid Matrix Metalloproteinase-8-Specific Chair-Side Test. J. Periodontal Res. 2006, 41, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Lähteenmäki, H.; Tervahartiala, T.; Räisänen, I.T.; Pärnänen, P.; Mauramo, M.; Gupta, S.; Sampson, V.; Rathnayake, N.; Heikkinen, A.-M.; Alassiri, S.; et al. Active MMP-8 Point-of-Care (PoC)/chairside Enzyme-Test as an Adjunctive Tool for Early and Real-Time Diagnosis of Peri-Implantitis. Clin. Exp. Dent. Res. 2022, 8, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J.; Peppin, G.; Ortiz, X.; Ragsdale, C.; Test, S.T. Oxidative Autoactivation of Latent Collagenase by Human Neutrophils. Science 1985, 227, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Nwhator, S.O.; Sakellari, D.; Grigoriadis, A.; Umeizudike, K.A.; Brandt, E.; Keskin, M.; Tervahartiala, T.; Pärnänen, P.; Gupta, S.; et al. aMMP-8 Oral Fluid PoC Test in Relation to Oral and Systemic Diseases. Front. Oral Health 2022, 3, 897115. [Google Scholar] [CrossRef] [PubMed]
- Mäntylä, P.; Stenman, M.; Kinane, D.F.; Tikanoja, S.; Luoto, H.; Salo, T.; Sorsa, T. Gingival Crevicular Fluid Collagenase-2 (MMP-8) Test Stick for Chair-Side Monitoring of Periodontitis. J. Periodontal Res. 2003, 38, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Räisänen, I.T.; Aji, N.R.A.S.; Sakellari, D.; Grigoriadis, A.; Rantala, I.; Pätilä, T.; Heikkilä, P.; Gupta, S.; Sorsa, T. Active Matrix Metalloproteinase-8 (aMMP-8) Versus Total MMP-8 in Periodontal and Peri-Implant Disease Point-of-Care Diagnostics. Biomedicines 2023, 11, 2885. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Alassiri, S.; Grigoriadis, A.; Räisänen, I.T.; Pärnänen, P.; Nwhator, S.O.; Gieselmann, D.-R.; Sakellari, D. Active MMP-8 (aMMP-8) as a Grading and Staging Biomarker in the Periodontitis Classification. Diagnostics 2020, 10, 61. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef]
- Leppilahti, J.M.; Ahonen, M.-M.; Hernández, M.; Munjal, S.; Netuschil, L.; Uitto, V.-J.; Sorsa, T.; Mäntylä, P. Oral Rinse MMP-8 Point-of-Care Immuno Test Identifies Patients with Strong Periodontal Inflammatory Burden. Oral Dis. 2011, 17, 115–122. [Google Scholar] [CrossRef]
- Kallio, E.; Puolakkainen, T.; Tervahartiala, T.; Snäll, J.; Marttila, E.; Sorsa, T.; Uittamo, J. Applicability of an Active Matrix Metalloproteinase-8 Point-of-Care Test in an Oral and Maxillofacial Surgery Clinic: A Pilot Study. Odontology 2023, 112, 250–255. [Google Scholar] [CrossRef]
- Nędzi-Góra, M.; Górska, R.; Górski, B. The Utility of Gingival Crevicular Fluid Matrix Metalloproteinase-8 Provides Site-Specific Diagnostic Value for Periodontal Grading. Cent. Eur. J. Immunol. 2021, 46, 236–243. [Google Scholar] [CrossRef]
- Räisänen, I.T.; Heikkinen, A.M.; Nwhator, S.O.; Umeizudike, K.A.; Tervahartiala, T.; Sorsa, T. On the Diagnostic Discrimination Ability of Mouthrinse and Salivary aMMP-8 Point-of-Care Testing Regarding Periodontal Health and Disease. Diagn. Microbiol. Infect. Dis. 2019, 95, 114871. [Google Scholar] [CrossRef]
- Waligóra, J.; Kuśnierz-Cabala, B.; Pawlica-Gosiewska, D.; Gawlik, K.; Chomyszyn-Gajewska, M.; Pytko-Polończyk, J. Salivary Matrix Metalloproteinase-9 (MMP-9) as a Biomarker of Periodontitis in Pregnant Patients with Diabetes. Dent. Med. Probl. 2023, 60, 35–45. [Google Scholar] [CrossRef]
- Luchian, I.; Moscalu, M.; Goriuc, A.; Nucci, L.; Tatarciuc, M.; Martu, I.; Covasa, M. Using Salivary MMP-9 to Successfully Quantify Periodontal Inflammation during Orthodontic Treatment. J. Clin. Med. Res. 2021, 10, 379. [Google Scholar] [CrossRef]
- Smith, P.C.; Muñoz, V.C.; Collados, L.; Oyarzún, A.D. In Situ Detection of Matrix Metalloproteinase-9 (MMP-9) in Gingival Epithelium in Human Periodontal Disease. J. Periodontal Res. 2004, 39, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J Immunol Res 2015, 2015, 615486. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Shastri, V.P. Matrix-Metalloproteinase-9 Is Cleaved and Activated by Cathepsin K. BMC Res. Notes 2015, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tang, Y.; Li, X.-Y.; Keller, E.T.; Yang, J.; Cho, J.-S.; Feinberg, T.Y.; Weiss, S.J. Osteoclast-Mediated Bone Resorption Is Controlled by a Compensatory Network of Secreted and Membrane-Tethered Metalloproteinases. Sci. Transl. Med. 2020, 12, eaaw6143. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Chen, W.; Tang, C.-Y.; McVicar, A.; Edwards, D.; Wang, J.; McConnell, M.; Yang, S.; Li, Y.; Chang, Z.; et al. Knockout and Double Knockout of Cathepsin K and Mmp9 Reveals a Novel Function of Cathepsin K as a Regulator of Osteoclast Gene Expression and Bone Homeostasis. Int. J. Biol. Sci. 2022, 18, 5522–5538. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Ishibashi, O.; Origane, Y.; Fujimori, K.; Kokubo, T.; Nakajima, M. Matrix Metalloproteinases and Lysosomal Cysteine Proteases in Osteoclasts Contribute to Bone Resorption through Distinct Modes of Action. Biochem. Biophys. Res. Commun. 1999, 258, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Delaissé, J.-M.; Andersen, T.L.; Engsig, M.T.; Henriksen, K.; Troen, T.; Blavier, L. Matrix Metalloproteinases (MMP) and Cathepsin K Contribute Differently to Osteoclastic Activities. Microsc. Res. Tech. 2003, 61, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Punj, V.; Kim, J.-M.; Lee, S.; Ulmer, T.S.; Lu, W.; Rice, J.C.; An, W. MMP-9 Facilitates Selective Proteolysis of the Histone H3 Tail at Genes Necessary for Proficient Osteoclastogenesis. Genes Dev. 2016, 30, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, A.; Polak, D.; Houri-Haddad, Y.; Shapira, L. The Role of RgpA in the Pathogenicity of Porphyromonas Gingivalis in the Murine Periodontitis Model. J. Clin. Periodontol. 2013, 40, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Maisetta, G.; Brancatisano, F.L.; Esin, S.; Campa, M.; Batoni, G. Gingipains Produced by Porphyromonas Gingivalis ATCC49417 Degrade Human-β-Defensin 3 and Affect Peptide’s Antibacterial Activity in Vitro. Peptides 2011, 32, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Miyamoto, Y.; Yoshimura, K.; Yamada, A.; Takami, M.; Suzawa, T.; Hoshino, M.; Imamura, T.; Akiyama, C.; Yasuhara, R.; et al. Porphyromonas Gingivalis-Derived Lysine Gingipain Enhances Osteoclast Differentiation Induced by Tumor Necrosis Factor-α and Interleukin-1β but Suppresses That by Interleukin-17A: Importance of Proteolytic Degradation of Osteoprotegerin by Lysine Gingipain. J. Biol. Chem. 2014, 289, 15621–15630. [Google Scholar] [CrossRef]
- Guo, Y.; Nguyen, K.-A.; Potempa, J. Dichotomy of Gingipains Action as Virulence Factors: From Cleaving Substrates with the Precision of a Surgeon’s Knife to a Meat Chopper-like Brutal Degradation of Proteins. Periodontology 2000 2010, 54, 15–44. [Google Scholar] [CrossRef]
- Carlsson, J.; Herrmann, B.F.; Höfling, J.F.; Sundqvist, G.K. Degradation of the Human Proteinase Inhibitors Alpha-1-Antitrypsin and Alpha-2-Macroglobulin by Bacteroides Gingivalis. Infect. Immun. 1984, 43, 644–648. [Google Scholar] [CrossRef]
- Kantyka, T.; Latendorf, T.; Wiedow, O.; Bartels, J.; Gläser, R.; Dubin, G.; Schröder, J.-M.; Potempa, J.; Meyer-Hoffert, U. Elafin Is Specifically Inactivated by RgpB from Porphyromonas Gingivalis by Distinct Proteolytic Cleavage. Biol. Chem. 2009, 390, 1313–1320. [Google Scholar] [CrossRef]
- Potempa, J.; Mikolajczyk-Pawlinska, J.; Brassell, D.; Nelson, D.; Thøgersen, I.B.; Enghild, J.J.; Travis, J. Comparative Properties of Two Cysteine Proteinases (gingipains R), the Products of Two Related but Individual Genes of Porphyromonas Gingivalis. J. Biol. Chem. 1998, 273, 21648–21657. [Google Scholar] [CrossRef]
- Grenier, D.; Mayrand, D. Inactivation of Tissue Inhibitor of Metalloproteinases-1 (TIMP-1) by Porphyromonas Gingivalis. FEMS Microbiol. Lett. 2001, 203, 161–164. [Google Scholar] [CrossRef]
- Prucsi, Z.; Zimny, A.; Płonczyńska, A.; Zubrzycka, N.; Potempa, J.; Sochalska, M. Porphyromonas Gingivalis Peptidyl Arginine Deiminase (PPAD) in the Context of the Feed-Forward Loop of Inflammation in Periodontitis. Int. J. Mol. Sci. 2023, 24, 12922. [Google Scholar] [CrossRef]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil Apoptosis: Relevance to the Innate Immune Response and Inflammatory Disease. J. Innate Immun. 2010, 2, 216–227. [Google Scholar] [CrossRef]
- Luo, S.; Xu, T.; Zheng, Q.; Jiang, A.; Zhao, J.; Ying, Y.; Liu, N.; Pan, Y.; Zhang, D. Mitochondria: An Emerging Unavoidable Link in the Pathogenesis of Periodontitis Caused by Porphyromonas Gingivalis. Int. J. Mol. Sci. 2024, 25, 737. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Filho, P.C.; Moura-Costa, L.F.; Pimentel, A.C.M.; Lopes, M.P.P.; Freitas, S.A.; Miranda, P.M.; Costa, R.S.; Figueirêdo, C.A.V.; Meyer, R.; Gomes-Filho, I.S.; et al. Apoptosis Transcriptional Profile Induced by Porphyromonas Gingivalis HmuY. Mediators Inflamm. 2019, 2019, 6758159. [Google Scholar] [CrossRef]
- Sochalska, M.; Potempa, J. Manipulation of Neutrophils by Porphyromonas Gingivalis in the Development of Periodontitis. Front. Cell. Infect. Microbiol. 2017, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulou, P.G.; Galicia, J.C.; Benakanakere, M.R.; Garcia, C.A.; Potempa, J.; Kinane, D.F. Porphyromonas Gingivalis Induce Apoptosis in Human Gingival Epithelial Cells through a Gingipain-Dependent Mechanism. BMC Microbiol. 2009, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Elkington, P.T.G.; O’Kane, C.M.; Friedland, J.S. The Paradox of Matrix Metalloproteinases in Infectious Disease. Clin. Exp. Immunol. 2005, 142, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.J.; Meikle, M.C. Mechanisms of Connective Tissue Matrix Destruction in Periodontitis. Periodontology 2000 1997, 14, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Nawrot-Hadzik, I.; Matkowski, A.; Kubasiewicz-Ross, P.; Hadzik, J. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis-Immunomodulatory Effects, Animal and Clinical Studies. Nutrients 2021, 13, 239. [Google Scholar] [CrossRef]
- Palaska, I.; Papathanasiou, E.; Theoharides, T.C. Use of Polyphenols in Periodontal Inflammation. Eur. J. Pharmacol. 2013, 720, 77–83. [Google Scholar] [CrossRef]
- Steckiewicz, K.P.; Cieciórski, P.; Barcińska, E.; Jaśkiewicz, M.; Narajczyk, M.; Bauer, M.; Kamysz, W.; Megiel, E.; Inkielewicz-Stepniak, I. Silver Nanoparticles as Chlorhexidine and Metronidazole Drug Delivery Platforms: Their Potential Use in Treating Periodontitis. Int. J. Nanomed. 2022, 17, 495–517. [Google Scholar] [CrossRef]
- Gendron, R.; Grenier, D.; Sorsa, T.; Mayrand, D. Inhibition of the Activities of Matrix Metalloproteinases 2, 8, and 9 by Chlorhexidine. Clin. Diagn. Lab. Immunol. 1999, 6, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Potempa, J. Strategies for the Inhibition of Gingipains for the Potential Treatment of Periodontitis and Associated Systemic Diseases. J. Oral Microbiol. 2014, 6, 24800. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, K.; Ishihara, K.; Kimizuka, R.; Okuda, K. Arg-Gingipain A DNA Vaccine Prevents Alveolar Bone Loss in Mice. J. Dent. Res. 2007, 86, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Wilensky, A.; Potempa, J.; Houri-Haddad, Y.; Shapira, L. Vaccination with Recombinant RgpA Peptide Protects against Porphyromonas Gingivalis-Induced Bone Loss. J. Periodontal Res. 2017, 52, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, P.S.; O’Brien-Simpson, N.M.; Slakeski, N.; Hoffmann, B.; Reynolds, E.C. Immunization with the RgpA-Kgp Proteinase-Adhesin Complexes of Porphyromonas Gingivalis Protects against Periodontal Bone Loss in the Rat Periodontitis Model. Infect. Immun. 2002, 70, 2480–2486. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Shimomura, E.; Yin, G.; Tran, C.; Sato, A.; Steiner, A.; Heibeck, T.; Tam, M.; Fairman, J.; Gibson, F.C., 3rd. Immunization with Cell-Free-Generated Vaccine Protects from Porphyromonas Gingivalis-Induced Alveolar Bone Loss. J. Clin. Periodontol. 2019, 46, 197–205. [Google Scholar] [CrossRef]




| Group | MMP | Name | Common Extracellular Matrix Substrates |
|---|---|---|---|
| Collagenases | MMP-1 | collagenase 1, interstitial collagenase | collagen: I, II, III, VII, VIII, X, and XI; gelatin; pro-MMP-1, -2, and -9; fibronectin; laminin; tenascin; entactin/nidgoen |
| MMP-8 | collagenase 2, neutrophil collagenase | collagen: I, II, III, V, VII, VIII, and X; aggrecan; fibronectin; fibrinogen; gelatin; laminin; elastin | |
| MMP-13 | collagenase 3 | collagen: I, II, III, IV, VI, IX, X, and XIV; collagen telopeptides; gelatin; fibronectin; tenascin-C; aggrecan; fibrinogen; pro-MMP-9 | |
| Gelatinases | MMP-2 | gelatinase A, 72kDa gelatinase | denatured collagens: I, II, III, IV, V, VII, X, and XI; aggrecan; elastin; fibronectin; gelatin (hydrolyzed collagen); laminin; entactin/nidgoen; pro-MMP-1, -2, -9, and -13 |
| MMP-9 | gelatinase B, 92kDa gelatinase | denatured collagens: IV, V, XI, and XIV; aggrecan; gelatin (hydrolyzed collagen); elastin; fibronectin; laminin; dentin sialoprotein | |
| Stromelysins | MMP-3 | stromelysin 1 | collagen: II, III, IV, V, VII, IX, X, and XI; collagen telopeptides; aggrecan; decorin; elastin; fibronectin; fibrinogen; gelatin; laminin; perlecan; entactin/nidgoen; versican; pro-MMP-1, -3, -7, -8, -9, and -13 |
| MMP-10 | stromelysin 2 | collagen: III, IV, V, VII, IX, X, and XI; collagen telopeptides; gelatin; elastin; fibronectin; laminin; aggrecan; decorin; perlecan; versican; fibrinogen; pro-MMP-1, -3, -7, -8, -9, and -13 | |
| MMP-11 | stromelysin 3 | collagen: IV; gelatin; fibronectin | |
| Matrilysins | MMP-7 | matrilysin 1 | collagen: I, IV, and X; aggrecan; elastin; fibronectin; gelatin; laminin; entactin/nidgoen; pro-MMP-1, -2, -7, and -9 |
| MMP-26 | matrilysin 2 | collagen: IV; gelatin; fibronectin; gelatin; pro-MMP-9 | |
| Membrane-type | MMP-14 | MT1-MMP | collagen: I, II, and III; aggrecan; elastin; fibronectin; entactin/nidgoen; fibrinogen; gelatin; laminin; pro-MMP-2, -13, and -20 |
| MMP-15 | MT2-MMP | collagen: I; aggrecan; fibronectin; gelatin; laminin; tenascin; pro-MMP-2 | |
| MMP-16 | MT3-MMP | collagen: I and III; gelatin; fibronectin; laminin; pro-MMP-2 | |
| MMP-17 | MT4-MMP | type I gelatin; fibrin; fibrinogen | |
| MMP-24 | MT5-MMP | fibrin; gelatin; pro-MMP-2 | |
| MMP-25 | MT6-MMP | collagen: IV; gelatin; fibronectin; laminin; fibrin; fibrinogen; pro-MMP-2 | |
| Others | MMP-12 | metalloelastase | collagen: I, IV, and V; aggrecan; elastin; gelatin; elastin; entactin/nidgoen; fibronectin; laminin; fibrinogen |
| MMP-19 | RASI-1 | collagen: IV; fibronectin; gelatin; aggrecan; cartilage oligomeric matrix protein; laminin; fibrinogen | |
| MMP-20 | enamelysin | collagen: IV and V; amelogenin; fibronectin; laminin; tenascin-C | |
| MMP-21 | - | a1-antitrypsin | |
| MMP-23 | cysteine array (CA)-MMP, femalysin | gelatin | |
| MMP-27 | - | gelatin | |
| MMP-28 | epilysin | unknown |
| Group | Subgroup | MMPs |
|---|---|---|
| Secreted | Minimal Domain MMPs | MMP-7 and MMP-26 |
| Simple Hemopexin Domain-Containing MMPs | MMP-1, MMP-3, MMP-8, MMP-10, MMP-12, MMP-13, MMP-18, MMP-19, MMP-22, MMP-20 and MMP-27 | |
| Gelatin-binding MMPs | MMP-2 and MMP-9 | |
| Furin-activated MMPs | MMP-11 and MMP-28 | |
| Vitronectin-like Insert Linker-less MMPs | MMP-21 | |
| Membrane-type | Transmembrane MMPs (with cytoplasmic domain) | MMP-14, MMP-15, MMP-16 and MMP-24 |
| Glycosylphosphatidylinositol (GPI)-anchored MMPs | MMP-17 and MMP-25 | |
| Cysteine/Proline-Rich IL-1 Receptor-like Domain MMPs | MMP-23 |
| Periodontitis Grade | Rate of Progression | ||||
|---|---|---|---|---|---|
| Grade A Slow | Grade B Moderate | Grade C Rapid | |||
| Grade modifiers | Risk factors | Smoking | Non-smoker | Smoker <10 cigarettes/day | Smoker ≥10 cigarettes/day |
| Diabetes | Normoglycemic/no diagnosis of diabetes | HbA1c <7.0% in patients with diabetes | HbA1c ≥7.0% in patients with diabetes | ||
| Risk of systemic impact of periodontitis | Inflammatory burden | High sensitivity CRP | <1 mg/L | 1 to 3 mg/L | >3 mg/L |
| (*) Biomarkers | Indicators of CAL/bone loss or collagen destruction | Mouthrinse and aMMP-8 level | <10 ng/mL (none) or 10 to 20 ng/mL (slow) | 20 to 30 ng/mL | >30 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzki, D.; Negri, A.; Kusiak, A.; Obuchowski, M. Matrix Metalloproteinases in the Periodontium—Vital in Tissue Turnover and Unfortunate in Periodontitis. Int. J. Mol. Sci. 2024, 25, 2763. https://doi.org/10.3390/ijms25052763
Radzki D, Negri A, Kusiak A, Obuchowski M. Matrix Metalloproteinases in the Periodontium—Vital in Tissue Turnover and Unfortunate in Periodontitis. International Journal of Molecular Sciences. 2024; 25(5):2763. https://doi.org/10.3390/ijms25052763
Chicago/Turabian StyleRadzki, Dominik, Alessandro Negri, Aida Kusiak, and Michał Obuchowski. 2024. "Matrix Metalloproteinases in the Periodontium—Vital in Tissue Turnover and Unfortunate in Periodontitis" International Journal of Molecular Sciences 25, no. 5: 2763. https://doi.org/10.3390/ijms25052763






