Similarities in Structure and Function of UDP-Glycosyltransferase Homologs from Human and Plants
Abstract
1. Introduction
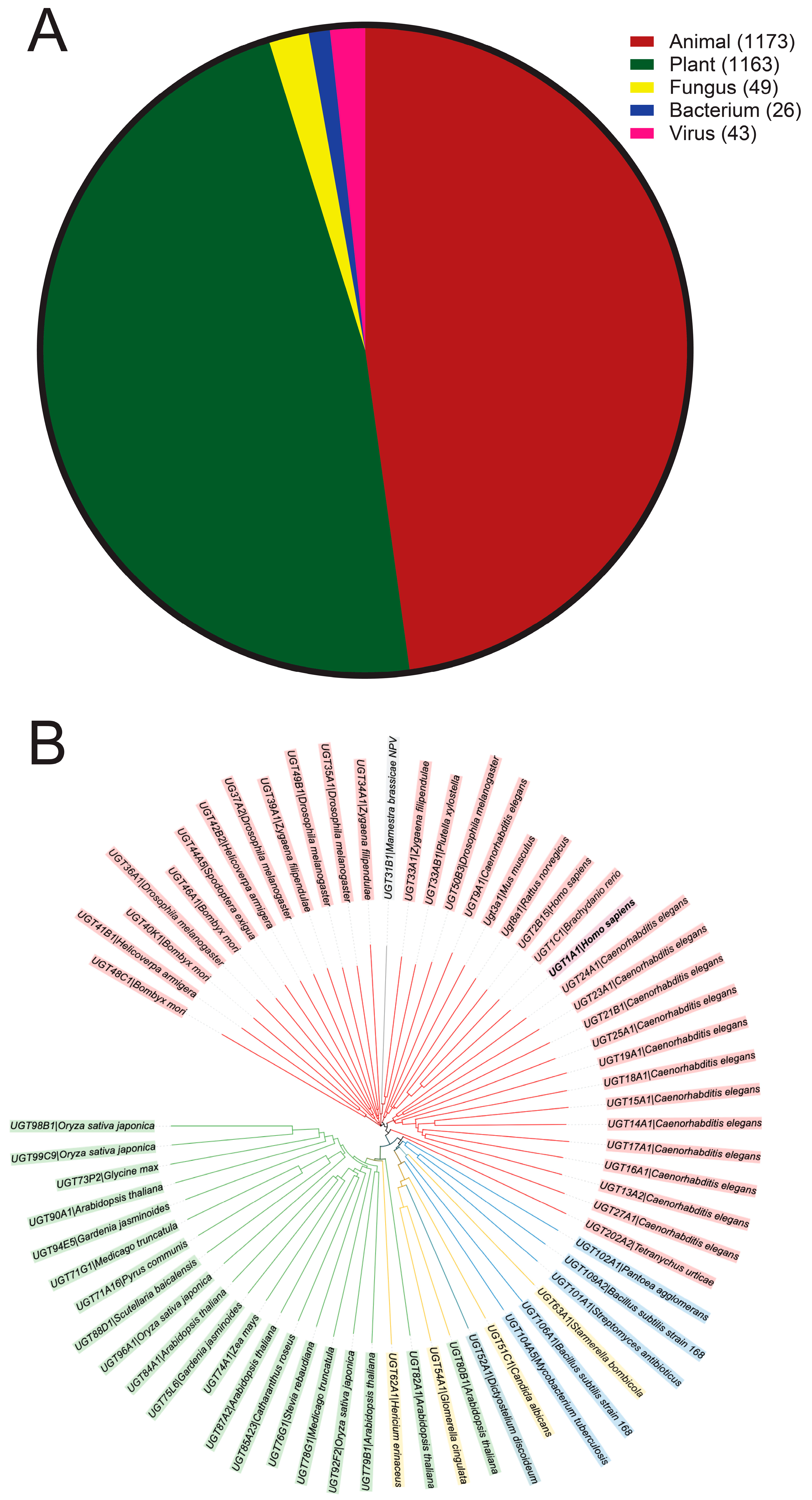
2. Classification of UGT Homologs
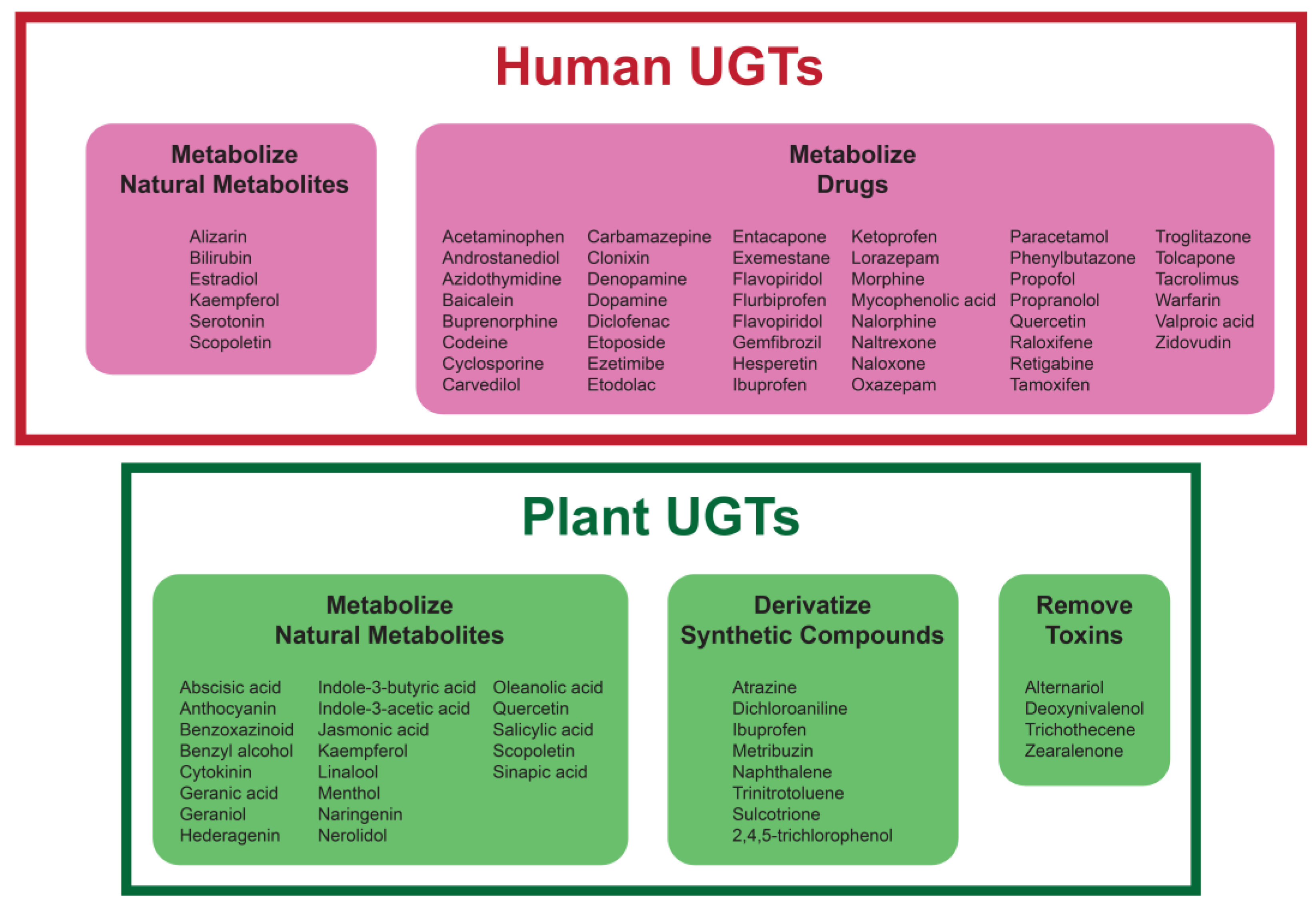
3. Substrate Recognition and Biological Activities of UGTs
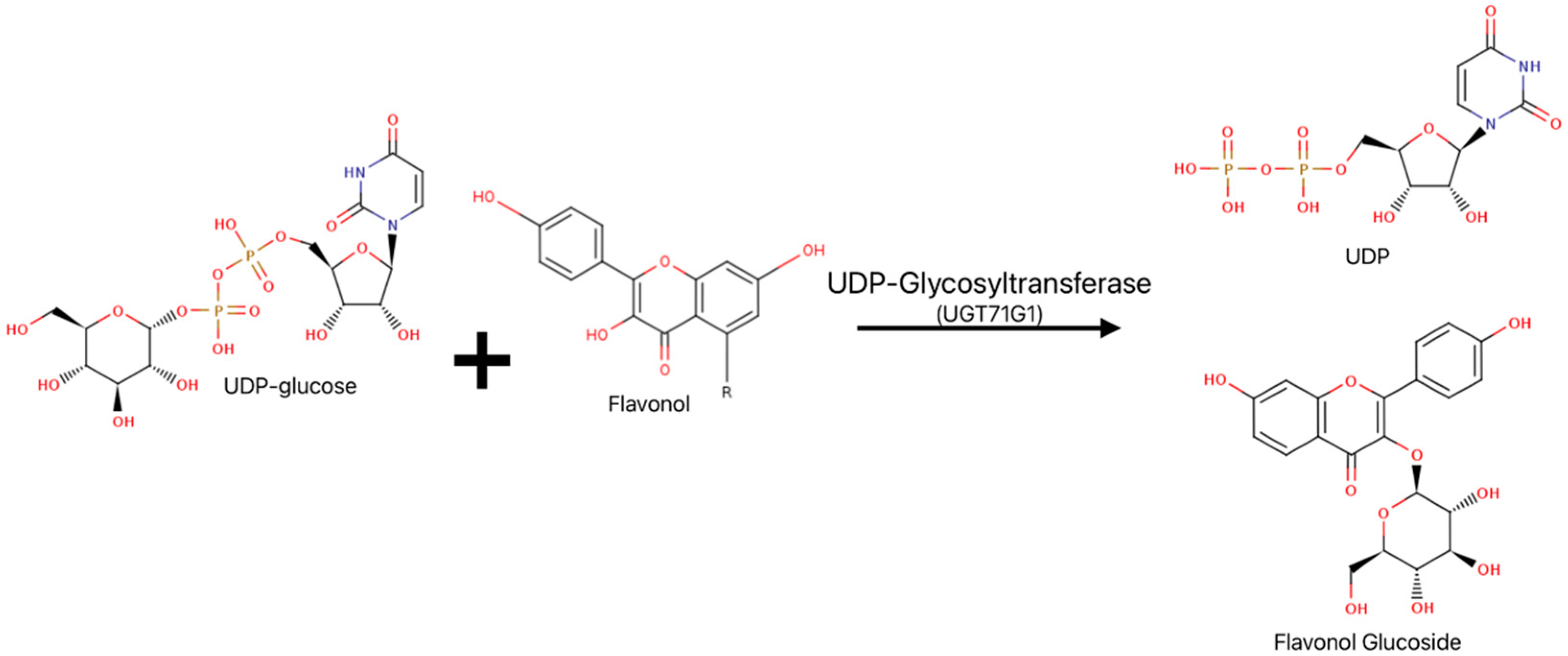
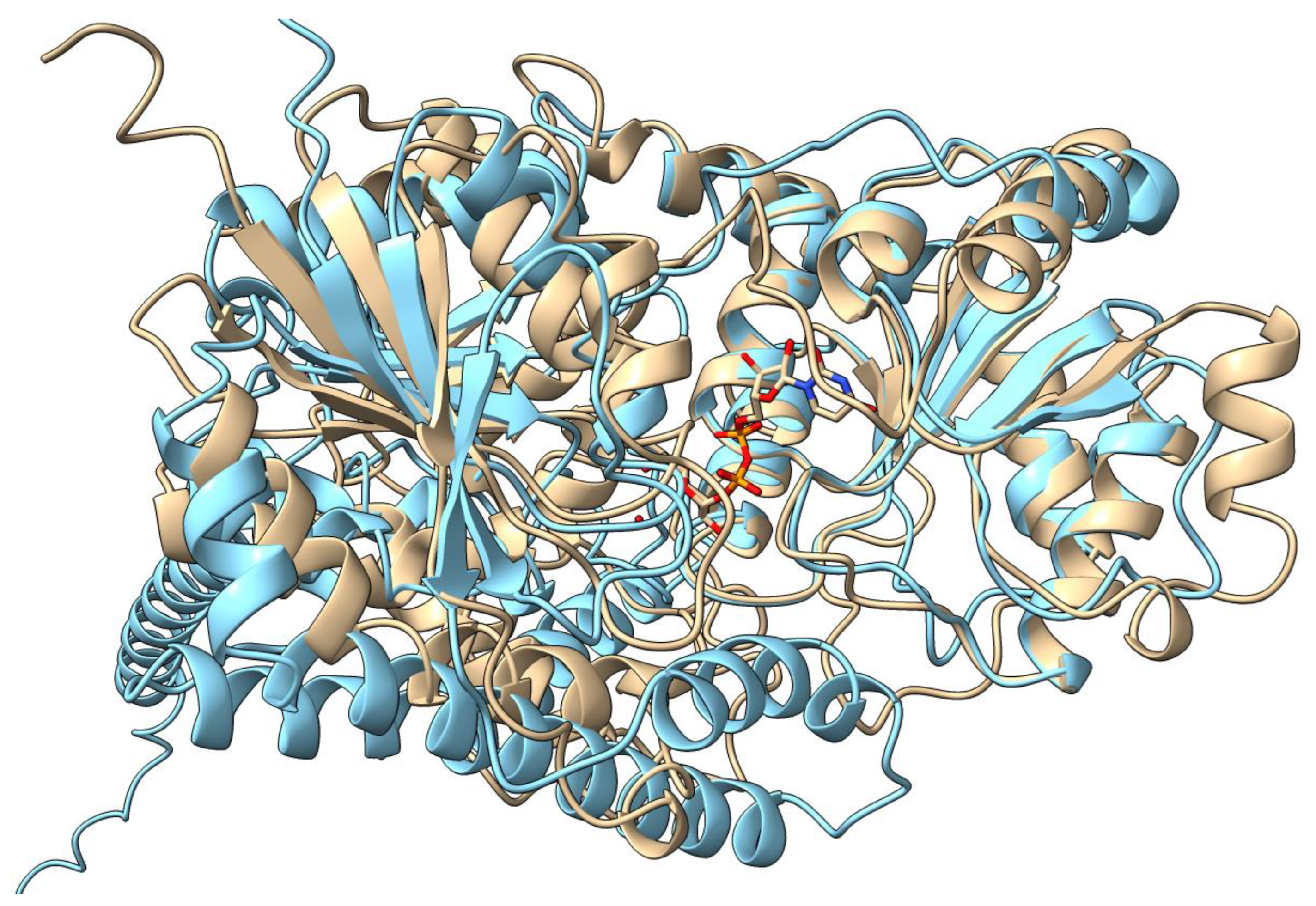
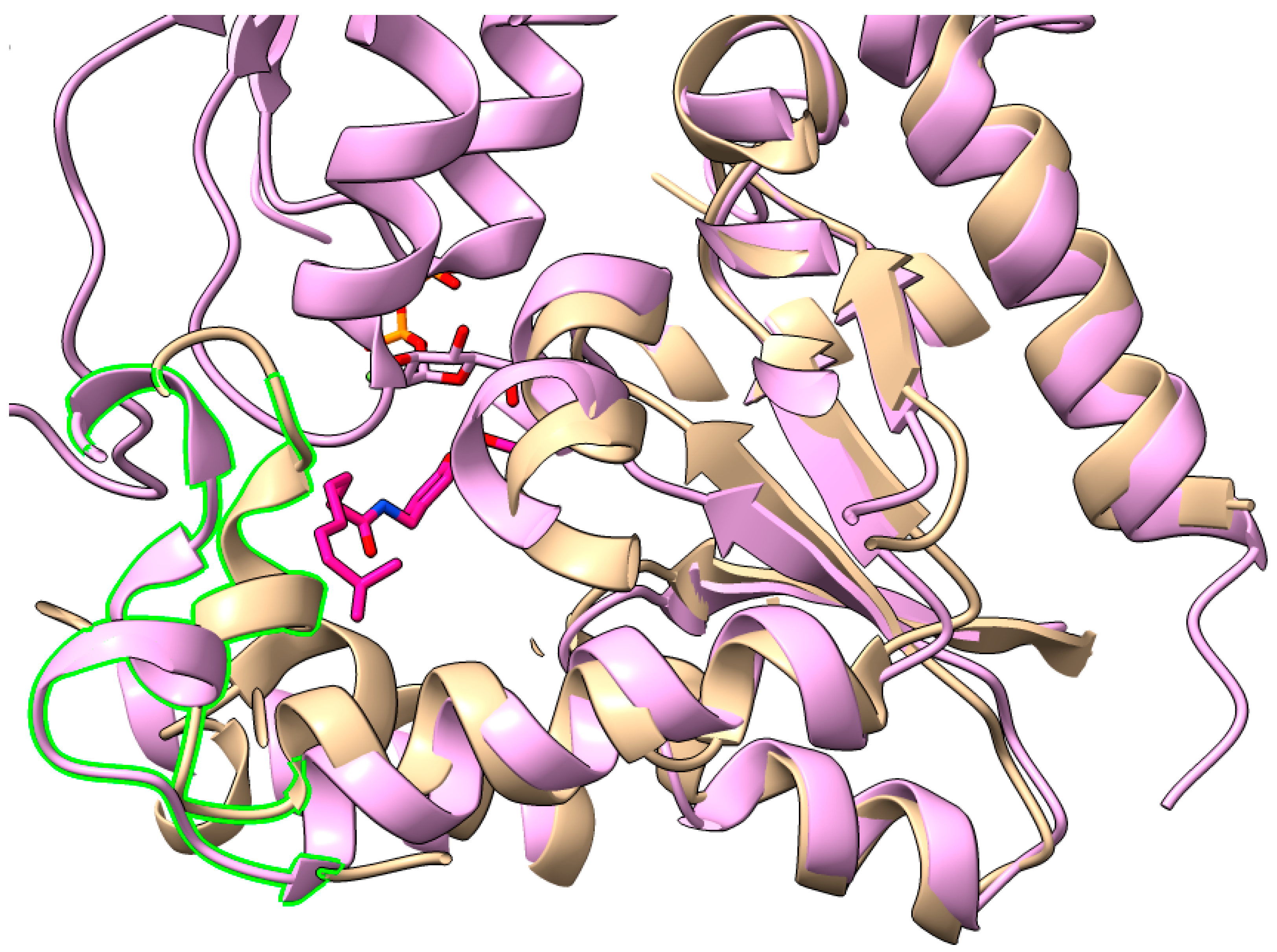
4. Reactions of Plant and Human UGTs
5. UGT1A1 Diseases Related to Dysfunctions of UGTs
6. Potential of Exogenous UGT Homologs for Medical Applications
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shao, H.; He, X.; Achnine, L.; Blount, J.W.; Dixon, R.A.; Wang, X. Crystal structures of a multifunctional triterpene/flavonoid glycosyltransferase from Medicago truncatula. Plant Cell 2005, 17, 3141–3154. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Hanada, K. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 2011, 66, 182–193. [Google Scholar] [CrossRef]
- Wang, X. Structure, mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett. 2009, 583, 3303–3309. [Google Scholar] [CrossRef]
- Ross, J.; Li, Y.; Lim, E.; Bowles, D.J. Higher plant glycosyltransferases. Genome Biol. 2001, 2, REVIEWS3004. [Google Scholar] [CrossRef]
- Meech, R.; Miners, J.O.; Lewis, B.C.; Mackenzie, P.I. The glycosidation of xenobiotics and endogenous compounds: Versatility and redundancy in the UDP glycosyltransferase superfamily. Pharmacol. Ther. 2012, 134, 200–218. [Google Scholar] [CrossRef]
- Tiwari, P.; Sangwan, R.S.; Sangwan, N.S. Plant secondary metabolism linked glycosyltransferases: An update on expanding knowledge and scopes. Biotechnol. Adv. 2016, 34, 714–739. [Google Scholar] [CrossRef]
- Mackenzie, P.I.; Owens, I.S.; Burchell, B.; Bock, K.W.; Bairoch, A.; Belanger, A.; Fournel-Gigleux, S.; Green, M.; Hum, D.W.; Iyanagi, T.; et al. The UDP glycosyltransferase gene superfamily: Recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 1997, 7, 255–269. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Hiromoto, T.; Honjo, E.; Noda, N.; Tamada, T.; Kazuma, K.; Suzuki, M.; Blaber, M.; Kuroki, R. Structural basis for acceptor-substrate recognition of UDP-glucose: Anthocyanidin 3-O-glucosyltransferase from Clitoria ternatea. Protein Sci. 2015, 24, 395–407. [Google Scholar] [CrossRef]
- Lee, S.G.; Salomon, E.; Yu, O.; Jez, J.M. Molecular basis for branched steviol glucoside biosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 13131–13136. [Google Scholar] [CrossRef]
- Cheng, W.; Fang, X.; Guan, Z.; Yao, Y.; Xu, Z.; Bi, Y.; Ren, K.; Li, J.; Chen, F.; Chen, X.; et al. Functional characterization and structural basis of a reversible glycosyltransferase involves in plant chemical defence. Plant Biotechnol. J. 2023, 21, 2611–2624. [Google Scholar] [CrossRef]
- Maharjan, R.; Fukuda, Y.; Shimomura, N.; Nakayama, T.; Okimoto, Y.; Kawakami, K.; Nakayama, T.; Hamada, H.; Inoue, T.; Ozaki, S.I. An Ambidextrous Polyphenol Glycosyltransferase PaGT2 from Phytolacca americana. Biochemistry 2020, 59, 2551–2561. [Google Scholar] [CrossRef]
- Li, J.; Qu, G.; Shang, N.; Chen, P.; Men, Y.; Liu, W.D.; Mei, Z.L.; Sun, Y.X.; Sun, Z.T. Near-perfect control of the regioselective glucosylation enabled by rational design of glycosyltransferases. Green Synth. Catal. 2021, 2, 45–53. [Google Scholar] [CrossRef]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Page, B.D.G.; Collier, A.C. Coughtrie MWH: Homology Modeling of Human Uridine-5’-diphosphate-glucuronosyltransferase 1A6 Reveals Insights into Factors Influencing Substrate and Cosubstrate Binding. ACS Omega 2020, 5, 6872–6887. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.; Chen, S.; Zeng, S. Glucuronidation of flavonoids by recombinant UGT1A3 and UGT1A9. Biochem. Pharmacol. 2008, 76, 416–425. [Google Scholar] [CrossRef]
- He, X.Z.; Li, W.S.; Blount, J.W.; Dixon, R.A. Regioselective synthesis of plant (iso)flavone glycosides in Escherichia coli. Appl. Microbiol. Biotechnol. 2008, 80, 253–260. [Google Scholar] [CrossRef]
- Kubo, A.; Arai, Y.; Nagashima, S.; Yoshikawa, T. Alteration of sugar donor specificities of plant glycosyltransferases by a single point mutation. Arch. Biochem. Biophys. 2004, 429, 198–203. [Google Scholar] [CrossRef]
- Meech, R.; Mubarokah, N.; Shivasami, A.; Rogers, A.; Nair, P.C.; Hu, D.G.; McKinnon, R.A.; Mackenzie, P.I. A novel function for UDP glycosyltransferase 8: Galactosidation of bile acids. Mol. Pharmacol. 2015, 87, 442–450. [Google Scholar] [CrossRef]
- Nomura, Y.; Seki, H.; Suzuki, T.; Ohyama, K.; Mizutani, M.; Kaku, T.; Tamura, K.; Ono, E.; Horikawa, M.; Sudo, H.; et al. Functional specialization of UDP-glycosyltransferase 73P12 in licorice to produce a sweet triterpenoid saponin, glycyrrhizin. Plant J. 2019, 99, 1127–1143. [Google Scholar] [CrossRef]
- Kadakol, A.; Ghosh, S.S.; Sappal, B.S.; Sharma, G.; Chowdhury, J.R.; Chowdhury, N.R. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: Correlation of genotype to phenotype. Hum. Mutat. 2000, 16, 297–306. [Google Scholar] [CrossRef]
- McGraphery, K.; Schwab, W. Comparative Analysis of High-Throughput Assays of Family-1 Plant Glycosyltransferases. Int. J. Mol. Sci. 2020, 21, 2208. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Singh, R.; Liu, Z.; Hu, M. Structure and concentration changes affect characterization of UGT isoform-specific metabolism of isoflavones. Mol. Pharm. 2009, 6, 1466–1482. [Google Scholar] [CrossRef] [PubMed]
- Achnine, L.; Huhman, D.V.; Farag, M.A.; Sumner, L.W.; Blount, J.W.; Dixon, R.A. Genomics-based selection and functional characterization of triterpene glycosyltransferases from the model legume Medicago truncatula. Plant J. 2005, 41, 875–887. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.; Fukuda, Y.; Nakayama, T.; Nakayama, T.; Hamada, H.; Ozaki, S.I.; Inoue, T. Structural basis for substrate recognition in the Phytolacca americana glycosyltransferase PaGT3. Acta Crystallogr. D Struct. Biol. 2022, 78, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Wenning, L.A.; Petry, A.S.; Kost, J.T.; Jin, B.; Breidinger, S.A.; DeLepeleire, I.; Carlini, E.J.; Young, S.; Rushmore, T.; Wagner, F.; et al. Pharmacokinetics of raltegravir in individuals with UGT1A1 polymorphisms. Clin. Pharmacol. Ther. 2009, 85, 623–627. [Google Scholar] [CrossRef]
- Wetterhorn, K.M.; Gabardi, K.; Michlmayr, H.; Malachova, A.; Busman, M.; McCormick, S.P.; Berthiller, F.; Adam, G.; Rayment, I. Determinants and Expansion of Specificity in a Trichothecene UDP-Glucosyltransferase from Oryza sativa. Biochemistry 2017, 56, 6585–6596. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Agati, G.; Matteini, P.; Goti, A.; Tattini, M. Chloroplast-located flavonoids can scavenge singlet oxygen. New Phytol. 2007, 174, 77–89. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Vanderauwera, S.; Zimmermann, P.; Rombauts, S.; Vandenabeele, S.; Langebartels, C.; Gruissem, W.; Inze, D.; Van Breusegem, F. Genome-wide analysis of hydrogen peroxide-regulated gene expression in Arabidopsis reveals a high light-induced transcriptional cluster involved in anthocyanin biosynthesis. Plant Physiol. 2005, 139, 806–821. [Google Scholar] [CrossRef]
- Behr, M.; Speeckaert, N.; Kurze, E.; Morel, O.; Prevost, M.; Mol, A.; Mahamadou Adamou, N.; Barage, M.; Renaut, J.; Schwab, W.; et al. Leaf necrosis resulting from downregulation of poplar glycosyltransferase UGT72A2. Tree Physiol. 2022, 42, 1084–1099. [Google Scholar] [CrossRef]
- Osbourn, A.E. Saponins in cereals. Phytochemistry 2003, 62, 1–4. [Google Scholar] [CrossRef]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-pass metabolism via UDP-glucuronosyltransferase: A barrier to oral bioavailability of phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef] [PubMed]
- Tukey, R.H.; Strassburg, C.P. Human UDP-glucuronosyltransferases: Metabolism, expression, and disease. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 581–616. [Google Scholar] [CrossRef] [PubMed]
- Sugatani, J.; Kojima, H.; Ueda, A.; Kakizaki, S.; Yoshinari, K.; Gong, Q.H.; Owens, I.S.; Negishi, M.; Sueyoshi, T. The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology 2001, 33, 1232–1238. [Google Scholar] [CrossRef] [PubMed]
- Lepine, J.; Bernard, O.; Plante, M.; Tetu, B.; Pelletier, G.; Labrie, F.; Belanger, A.; Guillemette, C. Specificity and regioselectivity of the conjugation of estradiol, estrone, and their catecholestrogen and methoxyestrogen metabolites by human uridine diphospho-glucuronosyltransferases expressed in endometrium. J. Clin. Endocrinol. Metab. 2004, 89, 5222–5232. [Google Scholar] [CrossRef]
- Hu, D.G.; Selth, L.A.; Tarulli, G.A.; Meech, R.; Wijayakumara, D.; Chanawong, A.; Russell, R.; Caldas, C.; Robinson, J.L.; Carroll, J.S.; et al. Androgen and Estrogen Receptors in Breast Cancer Coregulate Human UDP-Glucuronosyltransferases 2B15 and 2B17. Cancer Res. 2016, 76, 5881–5893. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.P.; Bhadauriya, A.; Patil, A.; Sangamwar, A.T. Substrate selectivity of human intestinal UDP-glucuronosyltransferases (UGTs): In silico and in vitro insights. Drug Metab. Rev. 2013, 45, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Keegstra, K.; Raikhel, N. Plant glycosyltransferases. Curr. Opin. Plant Biol. 2001, 4, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.Y.; Shiu, T.Y.; Huang, S.M.; Lin, H.H.; Lee, T.C.; Chen, P.J.; Chu, H.C.; Chang, W.K.; Jeng, K.S.; Lai, M.M.; et al. Molecular pathogenesis of Gilbert’s syndrome: Decreased TATA-binding protein binding affinity of UGT1A1 gene promoter. Pharm. Genom. 2007, 17, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Nixon, J.C.; Monahan, G.J. Gilbert’s disease and the bilirubin tolerance test. Can. Med. Assoc. J. 1967, 96, 370–373. [Google Scholar] [PubMed]
- Lankisch, T.O.; Moebius, U.; Wehmeier, M.; Behrens, G.; Manns, M.P.; Schmidt, R.E.; Strassburg, C.P. Gilbert’s disease and atazanavir: From phenotype to UDP-glucuronosyltransferase haplotype. Hepatology 2006, 44, 1324–1332. [Google Scholar] [CrossRef]
- Strassburg, C.P. Pharmacogenetics of Gilbert’s syndrome. Pharmacogenomics 2008, 9, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Seppen, J.; Bosma, P.J.; Goldhoorn, B.G.; Bakker, C.T.; Chowdhury, J.R.; Chowdhury, N.R.; Jansen, P.L.; Oude Elferink, R.P. Discrimination between Crigler-Najjar type I and II by expression of mutant bilirubin uridine diphosphate-glucuronosyltransferase. J. Clin. Investig. 1994, 94, 2385–2391. [Google Scholar] [CrossRef]
- Jansen, P.L.; Mulder, G.J.; Burchell, B.; Bock, K.W. New developments in glucuronidation research: Report of a workshop on “glucuronidation, its role in health and disease”. Hepatology 1992, 15, 532–544. [Google Scholar] [CrossRef]
- Petit, F.; Gajdos, V.; Capel, L.; Parisot, F.; Myara, A.; Francoual, J.; Labrune, P. Crigler-Najjar type II syndrome may result from several types and combinations of mutations in the UGT1A1 gene. Clin. Genet. 2006, 69, 525–527. [Google Scholar] [CrossRef]
- D’Antiga, L.; Beuers, U.; Ronzitti, G.; Brunetti-Pierri, N.; Baumann, U.; Di Giorgio, A.; Aronson, S.; Hubert, A.; Romano, R.; Junge, N.; et al. Gene Therapy in Patients with the Crigler-Najjar Syndrome. N. Engl. J. Med. 2023, 389, 620–631. [Google Scholar] [CrossRef]
- Belanger, A.; Hum, D.W.; Beaulieu, M.; Levesque, E.; Guillemette, C.; Tchernof, A.; Belanger, G.; Turgeon, D.; Dubois, S. Characterization and regulation of UDP-glucuronosyltransferases in steroid target tissues. J. Steroid. Biochem. Mol. Biol. 1998, 65, 301–310. [Google Scholar] [CrossRef]
- Cavalieri, E.L.; Rogan, E.G. Unbalanced metabolism of endogenous estrogens in the etiology and prevention of human cancer. J. Steroid. Biochem. Mol. Biol. 2011, 125, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Coffman, B.L.; Rios, G.R.; King, C.D.; Tephly, T.R. Human UGT2B7 catalyzes morphine glucuronidation. Drug Metab. Dispos. 1997, 25, 1–4. [Google Scholar] [PubMed]
- Ohno, S.; Kawana, K.; Nakajin, S. Contribution of UDP-glucuronosyltransferase 1A1 and 1A8 to morphine-6-glucuronidation and its kinetic properties. Drug Metab. Dispos. 2008, 36, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Court, M.H.; Duan, S.X.; von Moltke, L.L.; Greenblatt, D.J.; Patten, C.J.; Miners, J.O.; Mackenzie, P.I. Interindividual variability in acetaminophen glucuronidation by human liver microsomes: Identification of relevant acetaminophen UDP-glucuronosyltransferase isoforms. J. Pharmacol. Exp. Ther. 2001, 299, 998–1006. [Google Scholar]
- Zhu, B.T.; Liehr, J.G. Inhibition of catechol O-methyltransferase-catalyzed O-methylation of 2- and 4-hydroxyestradiol by quercetin. Possible role in estradiol-induced tumorigenesis. J. Biol. Chem. 1996, 271, 1357–1363. [Google Scholar] [CrossRef]
- Jiang, H.M.; Fang, Z.Z.; Cao, Y.F.; Hu, C.M.; Sun, X.Y.; Hong, M.; Yang, L.; Ge, G.B.; Liu, Y.; Zhang, Y.Y.; et al. New insights for the risk of bisphenol A: Inhibition of UDP-glucuronosyltransferases (UGTs). Chemosphere 2013, 93, 1189–1193. [Google Scholar] [CrossRef]
- Akin, L.; Kendirci, M.; Narin, F.; Kurtoglu, S.; Saraymen, R.; Kondolot, M.; Kocak, S.; Elmali, F. The endocrine disruptor bisphenol A may play a role in the aetiopathogenesis of polycystic ovary syndrome in adolescent girls. Acta Paediatr. 2015, 104, e171–e177. [Google Scholar] [CrossRef]
- Guida, M.; Troisi, J.; Ciccone, C.; Granozio, G.; Cosimato, C.; Di Spiezio Sardo, A.; Ferrara, C.; Guida, M.; Nappi, C.; Zullo, F.; et al. Bisphenol A and congenital developmental defects in humans. Mutat. Res. 2015, 774, 33–39. [Google Scholar] [CrossRef]
- Matthews, J.B.; Twomey, K.; Zacharewski, T.R. In vitro and in vivo interactions of bisphenol A and its metabolite, bisphenol A glucuronide, with estrogen receptors alpha and beta. Chem. Res. Toxicol. 2001, 14, 149–157. [Google Scholar] [CrossRef]
- Hanioka, N.; Naito, T.; Narimatsu, S. Human UDP-glucuronosyltransferase isoforms involved in bisphenol A glucuronidation. Chemosphere 2008, 74, 33–36. [Google Scholar] [CrossRef]
- Sun, Y.; Ji, K.; Liang, B.; Du, Y.; Jiang, L.; Wang, J.; Kai, W.; Zhang, Y.; Zhai, X.; Chen, P.; et al. Suppressing ABA uridine diphosphate glucosyltransferase (SlUGT75C1) alters fruit ripening and the stress response in tomato. Plant J. 2017, 91, 574–589. [Google Scholar] [CrossRef]
- Chen, T.T.; Liu, F.F.; Xiao, D.W.; Jiang, X.Y.; Li, P.; Zhao, S.M.; Hou, B.K.; Li, Y.J. The Arabidopsis UDP-glycosyltransferase75B1, conjugates abscisic acid and affects plant response to abiotic stresses. Plant Mol. Biol. 2020, 102, 389–401. [Google Scholar] [CrossRef]
- Magnone, M.; Sturla, L.; Guida, L.; Spinelli, S.; Begani, G.; Bruzzone, S.; Fresia, C.; Zocchi, E. Abscisic Acid: A Conserved Hormone in Plants and Humans and a Promising Aid to Combat Prediabetes and the Metabolic Syndrome. Nutrients 2020, 12, 1724. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.; Hontecillas, R.; Tubau-Juni, N.; Zoccoli-Rodriguez, V.; Goodpaster, B.; Bassaganya-Riera, J. Abscisic acid enriched fig extract promotes insulin sensitivity by decreasing systemic inflammation and activating LANCL2 in skeletal muscle. Sci. Rep. 2020, 10, 10463. [Google Scholar] [CrossRef]
- Boachon, B.; Gamir, J.; Pastor, V.; Erb, M.; Dean, J.V.; Flors, V.; Mauch-Mani, B. Role of two UDP-Glycosyltransferases from the L group of arabidopsis in resistance against pseudomonas syringae. Eur. J. Plant Pathol. 2014, 139, 707–720. [Google Scholar] [CrossRef]
- Tezuka, D.; Matsuura, H.; Saburi, W.; Mori, H.; Imai, R. A Ubiquitously Expressed UDP-Glucosyltransferase, UGT74J1, Controls Basal Salicylic Acid Levels in Rice. Plants-Basel 2021, 10, 1875. [Google Scholar] [CrossRef] [PubMed]
- Yeasmin, F.; Choi, H.W. Natural Salicylates and Their Roles in Human Health. Int. J. Mol. Sci. 2020, 21, 9049. [Google Scholar] [CrossRef]
- Li, Q.; Yu, H.M.; Meng, X.F.; Lin, J.S.; Li, Y.J.; Hou, B.K. Ectopic expression of glycosyltransferase UGT76E11 increases flavonoid accumulation and enhances abiotic stress tolerance in Arabidopsis. Plant Biol. 2018, 20, 10–19. [Google Scholar] [CrossRef]
- Dong, N.Q.; Sun, Y.; Guo, T.; Shi, C.L.; Zhang, Y.M.; Kan, Y.; Xiang, Y.H.; Zhang, H.; Yang, Y.B.; Li, Y.C.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef]
- Poppenberger, B.; Berthiller, F.; Lucyshyn, D.; Sieberer, T.; Schuhmacher, R.; Krska, R.; Kuchler, K.; Glossl, J.; Luschnig, C.; Adam, G. Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. J. Biol. Chem. 2003, 278, 47905–47914. [Google Scholar] [CrossRef]
- Pasquet, J.C.; Changenet, V.; Macadre, C.; Boex-Fontvieille, E.; Soulhat, C.; Bouchabke-Coussa, O.; Dalmais, M.; Atanasova-Penichon, V.; Bendahmane, A.; Saindrenan, P.; et al. A Brachypodium UDP-Glycosyltransferase Confers Root Tolerance to Deoxynivalenol and Resistance to Fusarium Infection. Plant Physiol. 2016, 172, 559–574. [Google Scholar] [CrossRef]
- Schweiger, W.; Steiner, B.; Ametz, C.; Siegwart, G.; Wiesenberger, G.; Berthiller, F.; Lemmens, M.; Jia, H.; Adam, G.; Muehlbauer, G.J.; et al. Transcriptomic characterization of two major Fusarium resistance quantitative trait loci (QTLs), Fhb1 and Qfhs.ifa-5A, identifies novel candidate genes. Mol. Plant Pathol. 2013, 14, 772–785. [Google Scholar] [CrossRef] [PubMed]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; You, L.; Wang, X.; Wu, W.; Kuca, K.; Wu, Q.; Wei, W. Deoxynivalenol: Emerging Toxic Mechanisms and Control Strategies, Current and Future Perspectives. J. Agric. Food Chem. 2023, 71, 10901–10915. [Google Scholar] [CrossRef] [PubMed]
- Kovalsky Paris, M.P.; Schweiger, W.; Hametner, C.; Stuckler, R.; Muehlbauer, G.J.; Varga, E.; Krska, R.; Berthiller, F.; Adam, G. Zearalenone-16-O-glucoside: A new masked mycotoxin. J. Agric. Food Chem. 2014, 62, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Tognetti, V.B.; Van Aken, O.; Morreel, K.; Vandenbroucke, K.; van de Cotte, B.; De Clercq, I.; Chiwocha, S.; Fenske, R.; Prinsen, E.; Boerjan, W.; et al. Perturbation of indole-3-butyric acid homeostasis by the UDP-glucosyltransferase UGT74E2 modulates Arabidopsis architecture and water stress tolerance. Plant Cell 2010, 22, 2660–2679. [Google Scholar] [CrossRef]
- Huang, X.X.; Zhu, G.Q.; Liu, Q.; Chen, L.; Li, Y.J.; Hou, B.K. Modulation of Plant Salicylic Acid-Associated Immune Responses via Glycosylation of Dihydroxybenzoic Acids. Plant Physiol. 2018, 176, 3103–3119. [Google Scholar] [CrossRef]
- Langenbach, C.; Campe, R.; Schaffrath, U.; Goellner, K.; Conrath, U. UDP-glucosyltransferase UGT84A2/BRT1 is required for Arabidopsis nonhost resistance to the Asian soybean rust pathogen Phakopsora pachyrhizi. New Phytol. 2013, 198, 536–545. [Google Scholar] [CrossRef]
- Campos, L.; Lopez-Gresa, M.P.; Fuertes, D.; Belles, J.M.; Rodrigo, I.; Lison, P. Tomato glycosyltransferase Twi1 plays a role in flavonoid glycosylation and defence against virus. BMC Plant Biol. 2019, 19, 450. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Phan, H.; Liu, Y.; Cao, S.; Zhang, Z.; Chu, C.; Schlappi, M.R. Glycosyltransferase OsUGT90A1 helps protect the plasma membrane during chilling stress in rice. J. Exp. Bot. 2020, 71, 2723–2739. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.J.; Zhang, F.J.; Zhang, G.Z.; Jiang, X.Y.; Yu, H.M.; Hou, B.K. The Arabidopsis UDP-glycosyltransferases UGT79B2 and UGT79B3, contribute to cold, salt and drought stress tolerance via modulating anthocyanin accumulation. Plant J. 2017, 89, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Fan, Y.; Wei, W.; Wang, P.; Liu, Q.; Wei, Y.; Zhang, L.; Zhao, G.; Yue, J.; Zhou, Z. Production of bioactive ginsenoside compound K in metabolically engineered yeast. Cell Res. 2014, 24, 770–773. [Google Scholar] [CrossRef]
- Nan, W.; Zhao, F.; Zhang, C.; Ju, H.; Lu, W. Promotion of compound K production in Saccharomyces cerevisiae by glycerol. Microb. Cell Fact 2020, 19, 41. [Google Scholar] [CrossRef]
- Li, D.; Wu, Y.; Zhang, C.; Sun, J.; Zhou, Z.; Lu, W. Production of Triterpene Ginsenoside Compound K in the Non-conventional Yeast Yarrowia lipolytica. J. Agric. Food Chem. 2019, 67, 2581–2588. [Google Scholar] [CrossRef]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef]
- Isabella, V.M.; Ha, B.N.; Castillo, M.J.; Lubkowicz, D.J.; Rowe, S.E.; Millet, Y.A.; Anderson, C.L.; Li, N.; Fisher, A.B.; West, K.A.; et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. Nat. Biotechnol. 2018, 36, 857–864. [Google Scholar] [CrossRef]
- Borrero, J.; Chen, Y.; Dunny, G.M.; Kaznessis, Y.N. Modified lactic acid bacteria detect and inhibit multiresistant enterococci. ACS Synth. Biol. 2015, 4, 299–306. [Google Scholar] [CrossRef]
- Mao, N.; Cubillos-Ruiz, A.; Cameron, D.E.; Collins, J.J. Probiotic strains detect and suppress cholera in mice. Sci. Transl. Med. 2018, 10, eaao2586. [Google Scholar] [CrossRef]
- Awad, S.; Al-Ahmer, S.D.; Shahla’a, M.S. Expression in Saccharomyces boulardii of recombinant Toxin-coregulated pilus A subunit (TcpA) of Vibrio cholerae O1. VacciMonitor 2020, 29, 136–142. [Google Scholar]
- Andersen, K.K.; Strokappe, N.M.; Hultberg, A.; Truusalu, K.; Smidt, I.; Mikelsaar, R.-H.; Mikelsaar, M.; Verrips, T.; Hammarström, L.; Marcotte, H. Neutralization of Clostridium difficile toxin B mediated by engineered lactobacilli that produce single-domain antibodies. Infect. Immun. 2016, 84, 395–406. [Google Scholar] [CrossRef]
- Wei, P.; Yang, Y.; Liu, Z.; Huang, J.; Gong, Y.; Sun, H. Oral Bifidobacterium longum expressing alpha-melanocyte-stimulating hormone to fight experimental colitis. Drug Deliv. 2016, 23, 2058–2064. [Google Scholar] [CrossRef]
- Yoon, S.W.; Lee, C.H.; Kim, J.Y.; Kim, J.Y.; Sung, M.H.; Poo, H. Lactobacillus casei secreting alpha-MSH induces the therapeutic effect on DSS-induced acute colitis in Balb/c Mice. J. Microbiol. Biotechnol. 2008, 18, 1975–1983. [Google Scholar]
- Liu, S.; Li, Y.; Deng, B.; Xu, Z. Recombinant Lactococcus lactis expressing porcine insulin-like growth factor I ameliorates DSS-induced colitis in mice. BMC Biotechnol. 2016, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Shigemori, S.; Watanabe, T.; Kudoh, K.; Ihara, M.; Nigar, S.; Yamamoto, Y.; Suda, Y.; Sato, T.; Kitazawa, H.; Shimosato, T. Oral delivery of Lactococcus lactis that secretes bioactive heme oxygenase-1 alleviates development of acute colitis in mice. Microb. Cell Fact. 2015, 14, 189. [Google Scholar] [CrossRef] [PubMed]
- Leinwand, J.C.; Paul, B.; Chen, R.; Xu, F.; Sierra, M.A.; Paluru, M.M.; Nanduri, S.; Alcantara, C.G.; Shadaloey, S.A.; Yang, F.; et al. Intrahepatic microbes govern liver immunity by programming NKT cells. J. Clin. Investig. 2022, 132, e151725. [Google Scholar] [CrossRef] [PubMed]
- Din, M.O.; Danino, T.; Prindle, A.; Skalak, M.; Selimkhanov, J.; Allen, K.; Julio, E.; Atolia, E.; Tsimring, L.S.; Bhatia, S.N.; et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature 2016, 536, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Schnabl, B. The Microbiome and the Liver. Gastroenterol. Hepatol. 2014, 10, 519–521. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lethe, M.C.L.; Paris, V.; Wang, X.; Chan, C.T.Y. Similarities in Structure and Function of UDP-Glycosyltransferase Homologs from Human and Plants. Int. J. Mol. Sci. 2024, 25, 2782. https://doi.org/10.3390/ijms25052782
Lethe MCL, Paris V, Wang X, Chan CTY. Similarities in Structure and Function of UDP-Glycosyltransferase Homologs from Human and Plants. International Journal of Molecular Sciences. 2024; 25(5):2782. https://doi.org/10.3390/ijms25052782
Chicago/Turabian StyleLethe, Mary Caroline L., Vincent Paris, Xiaoqiang Wang, and Clement T. Y. Chan. 2024. "Similarities in Structure and Function of UDP-Glycosyltransferase Homologs from Human and Plants" International Journal of Molecular Sciences 25, no. 5: 2782. https://doi.org/10.3390/ijms25052782
APA StyleLethe, M. C. L., Paris, V., Wang, X., & Chan, C. T. Y. (2024). Similarities in Structure and Function of UDP-Glycosyltransferase Homologs from Human and Plants. International Journal of Molecular Sciences, 25(5), 2782. https://doi.org/10.3390/ijms25052782


_Kim.png)


