Enhancement of Mass Transfer Process for Photocatalytic Reduction in Cr(VI) by Electric Field Assistance
Abstract
1. Introduction
2. Results and Discussion
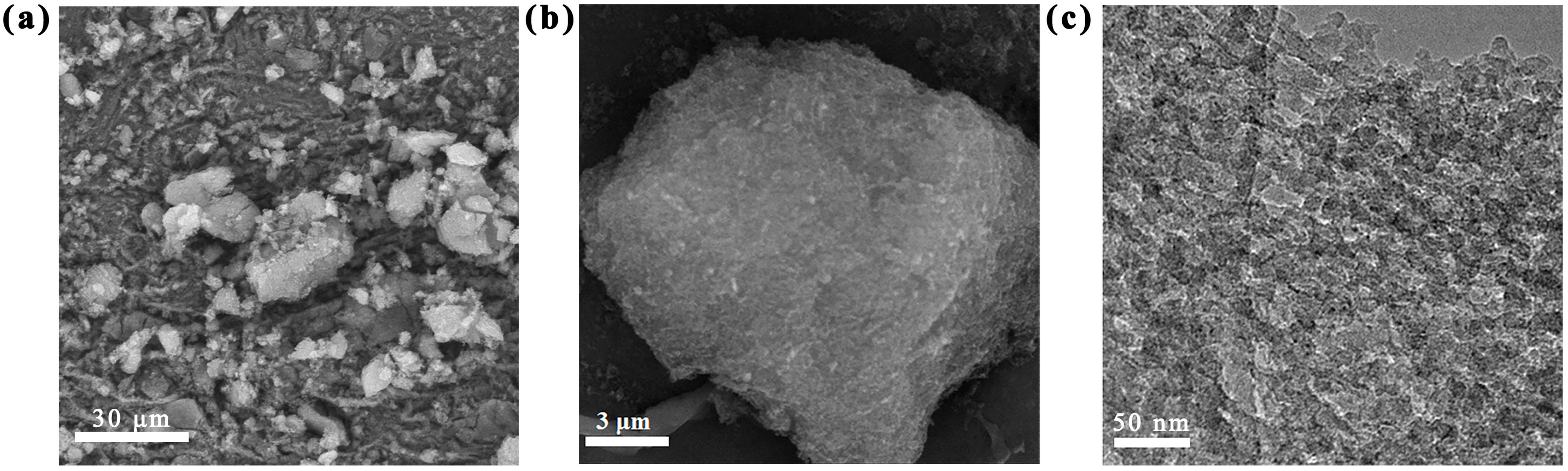
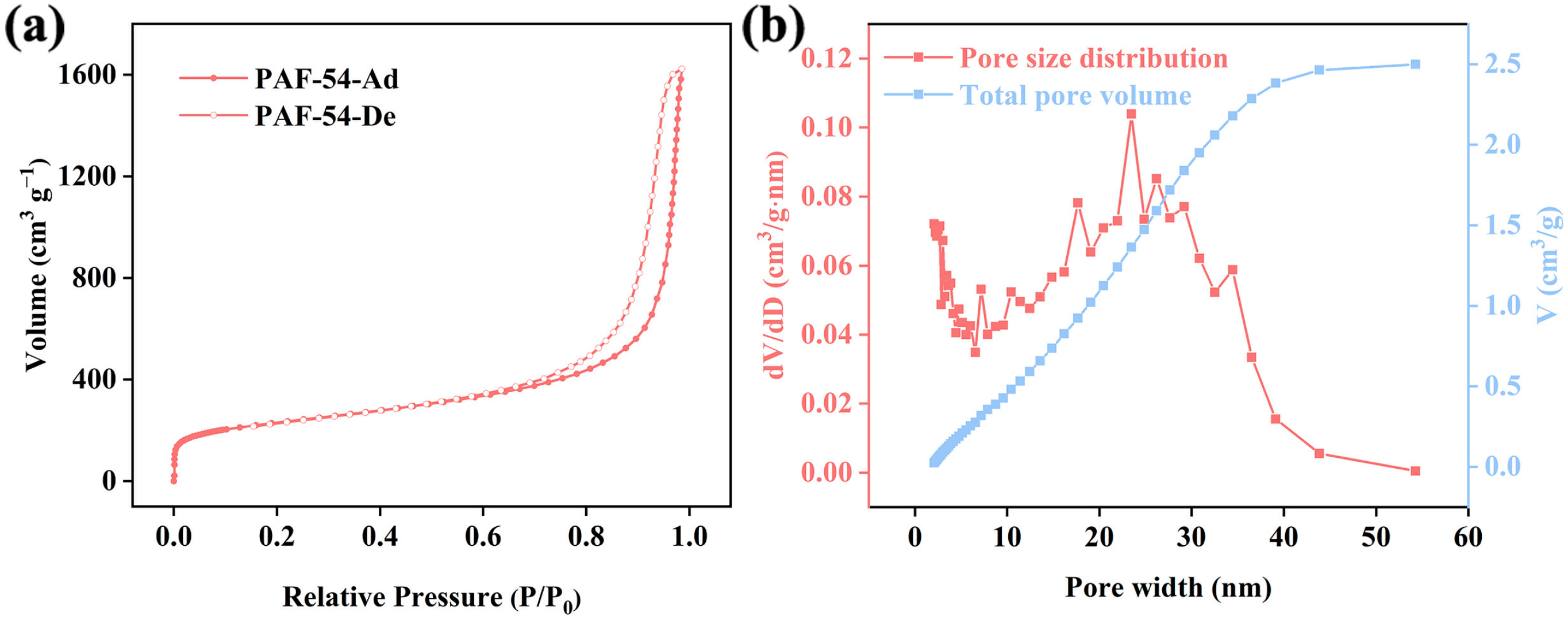
2.1. Structural Characterization
2.2. Adsorption Behavior and Mechanism
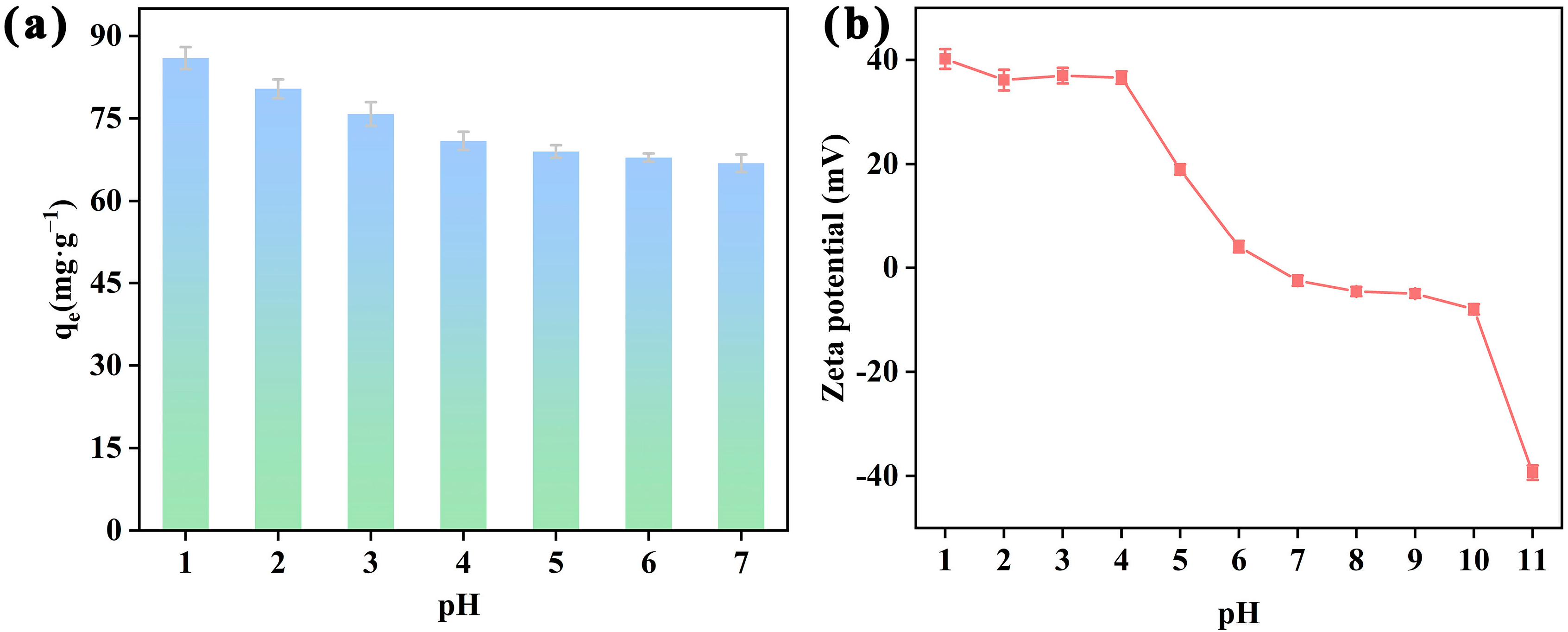
2.2.1. Influence of pH toward Structure and Adsorption
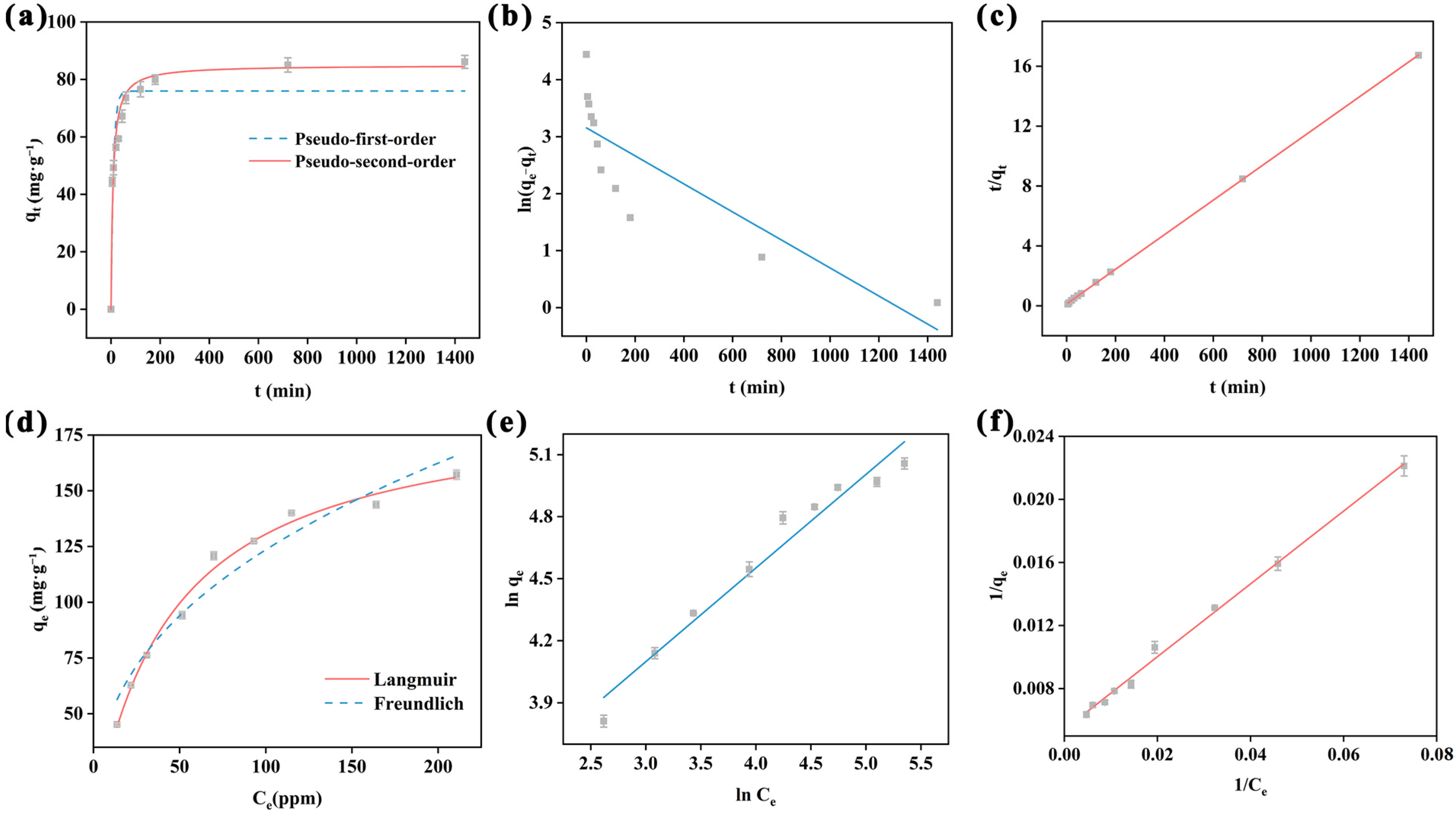
2.2.2. Adsorption Kinetics Study
2.2.3. Adsorption Isotherm Study
2.2.4. Adsorption Mechanism
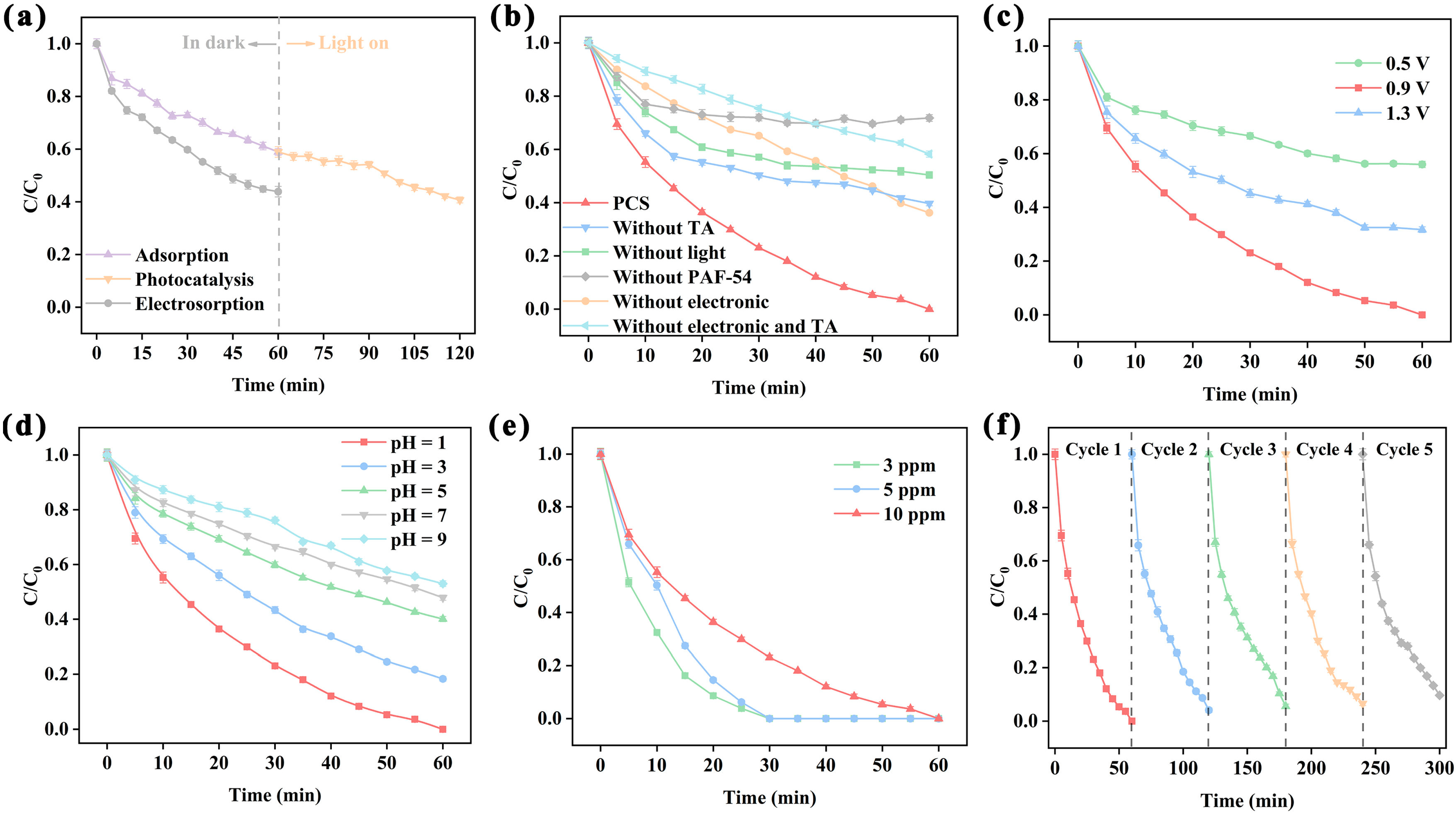
2.3. Combination of Photocatalysis and CDI
2.3.1. Characterization of PAF-54@CP for Cathode
2.3.2. Effect of Electric Field for PCS System
2.3.3. Effect of pH for PCS System
2.3.4. Catalytic Activity and Stability for PCS System
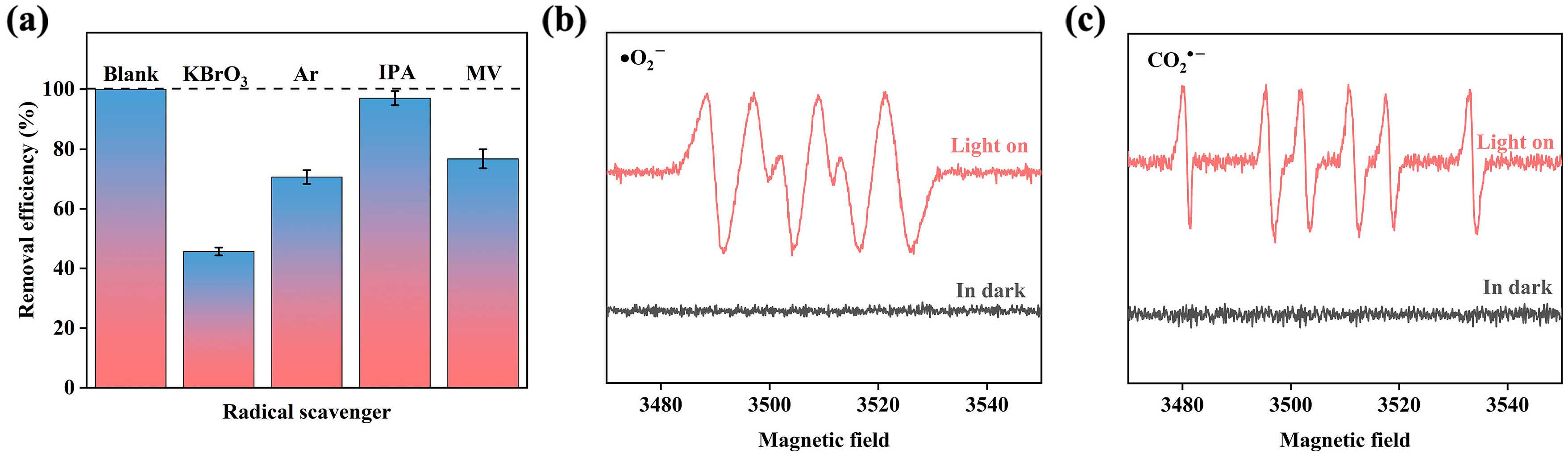
2.3.5. Possible Mechanism of PCS
3. Materials and Methods
3.1. Synthesis of PAF-54
3.2. Preparation of PCS Anode Electrode
3.3. Cr (VI) Adsorption Experiment
3.4. PCS System for Cr (VI) Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubilay, S.; Demirci, S.; Can, M.; Aktas, N.; Sahiner, N. Dichromate and arsenate anion removal by PEI microgel, cryogel, and bulkgel. J. Environ. Chem. Eng. 2021, 9, 104799. [Google Scholar] [CrossRef]
- Xiong, X.H.; Yu, Z.W.; Gong, L.L.; Tao, Y.; Gao, Z.; Wang, L.; Yin, W.H.; Yang, L.X.; Luo, F. Ammoniating Covalent Organic Framework (COF) for High-Performance and Selective Extraction of Toxic and Radioactive Uranium Ions. Adv. Sci. 2019, 6, 1900547. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Cheng, B.; Liang, J.; Guo, Y.; Wang, R. The aminated covalent organic polymers for reversible removal of concurrent perfluorooctane sulfonate and dichromate. Chem. Eng. J. 2022, 446, 137343. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, G.; He, Z.; Yang, W.; Li, H.; Wang, Y.; Pan, Q.; Shi, X. Luminescent detection of Cr(VI) and Mn(VII) based on a stable supramolecular organic framework. Cryst. Growth Des. 2020, 20, 6888–6895. [Google Scholar] [CrossRef]
- Zannotti, M.; Rossi, A.; Minicucci, M.; Ferraro, S.; Petetta, L.; Giovannetti, R. Water Decontamination from Cr(VI) by Transparent Silica Xerogel Monolith. Int. J. Mol. Sci. 2023, 24, 7430. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, V.; Kim, K.; Kwon, E.E.; Younis, S.A. Metal-organic frameworks for photocatalytic detoxification of chromium and uranium in water. Coord. Chem. Rev. 2021, 447, 214148. [Google Scholar] [CrossRef]
- Sen, A.; Dutta, S.; Dam, G.K.; Samanta, P.; Let, S.; Sharma, S.; Shirolkar, M.M.; Ghosh, S.K. Imidazolium-functionalized chemically robust ionic porous organic polymers (iPOPs) toward toxic oxo-pollutants capture from water. Chem. Eur. J. 2021, 27, 13442–13449. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Lin, L.; Shi, X.; Cheng, Y.; Luo, X.; Fang, C. Research progress of treatment technology and adsorption materials for removing chromate in the environment. Materials 2023, 16, 2797. [Google Scholar] [CrossRef]
- Deng, X.; Chen, Y.; Wen, J.; Xu, Y.; Zhu, J.; Bian, Z. Polyaniline-TiO2 composite photocatalysts for light-driven hexavalent chromium ions reduction. Sci. Bull. 2020, 65, 105–112. [Google Scholar] [CrossRef]
- Su, Y.; Shi, Y.; Jiang, M.; Chen, S. One-Step Synthesis of Nitrogen-Doped Porous Biochar Based on N-Doping Co-Activation Method and Its Application in Water Pollutants Control. Int. J. Mol. Sci. 2022, 23, 14618. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, X.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Noble-metal-free ultrathin Mxene coupled with In2S3 nanoflakes for ultrafast photocatalytic reduction of hexavalent chromium. Appl. Catal. B Environ. 2021, 284, 119754. [Google Scholar] [CrossRef]
- Trejo-Valdez, M.; Hernandez-Guzman, S.R.; Manriquez-Ramirez, M.E.; Sobral, H.; Martinez-Gutierrez, H.; Torres-Torres, C. Removal of aqueous chromium and environmental CO2 by using photocatalytic TiO2 doped with tungsten. J. Hazard. Mater. 2019, 370, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, toxicity, microbial remediation and phytoremediation of soil chromium contamination. Environ. Chem. Lett. 2021, 19, 1413–1431. [Google Scholar] [CrossRef]
- Bharath, G.; Hai, A.; Rambabu, K.; Savariraj, D.; Ibrahim, Y.; Banat, F. The fabrication of activated carbon and metalcarbide 2D framework-based asymmetric electrodes for the capacitive deionization of Cr(VI) ions toward industrial wastewater remediation. Environ. Sci. Water Res. Technol. 2020, 6, 351–361. [Google Scholar] [CrossRef]
- Yang, S.; Wei, K.; Ma, W.; Xie, K.; Wu, J.; Leic, Y. Kinetic mechanism of aluminum removal from diamond wire saw powder in HCl solution. J. Hazard. Mater. 2019, 308, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cervera Gabalda, L.; Gomez Polo, C. Magnetic Fe/Fe3C@C Nanoadsorbents for Efficient Cr (VI) Removal. Int. J. Mol. Sci. 2022, 23, 15135. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.; Subramanian, H.; Ramachandran, S.K.; Muthukumarasamy, A.; Ramadoss, D.; Mahalingam, A.; Rathinam, A.J.; Dahms, H.U.; Hwang, J.S. Synthesis of Bimetallic BiPO4/ZnO Nanocomposite: Enhanced Photocatalytic Dye Degradation and Antibacterial Applications. Int. J. Mol. Sci. 2023, 24, 1947. [Google Scholar] [CrossRef] [PubMed]
- Che, G.; Yang, W.; Luo, J.; Li, M.; Li, X.; Pan, Q. Efficient adsorption and photocatalysis over a photorenewable uranyl-organic framework for removal of diquat herbicide. Sep. Purif. Technol. 2024, 334, 126126. [Google Scholar] [CrossRef]
- Che, G.; Yang, W.; Wang, C.; Li, M.; Li, X.; Pan, Q. Efficient Photocatalytic Oxidative Coupling of Benzylamine over Uranyl-Organic Frameworks. Inorg. Chem. 2022, 61, 12301–12307. [Google Scholar] [CrossRef]
- Deng, M.; Guo, J.; Ma, X.; Fu, Y.; Du, H.; Hao, D.; Wang, Q. Enhanced photocatalytic Cr(VI) reduction performance by novel PDI/ COFs composite. Sep. Purif. Technol. 2023, 326, 124786. [Google Scholar] [CrossRef]
- Geng, W.; Chen, F.; Luo, Y.; Liu, Z.; Guo, S.; Zhang, Y.; Zhang, D.; Yu, X. Boosting photocatalytic Cr(VI) reduction activities of layered COF through regulating donor-acceptor units and the orientation of imine bonds. Microporous Mesoporous Mater. 2023, 351, 112479. [Google Scholar] [CrossRef]
- Liu, F.; Ma, Z.; Deng, Y.; Wang, M.; Zhou, P.; Liu, W.; Guo, S.; Tong, M.; Ma, D. Tunable Covalent Organic Frameworks with Different Heterocyclic Nitrogen Locations for Efficient Cr(VI) Reduction, Escherichia coli Disinfection, and Paracetamol Degradation under Visible-Light Irradiation. Environ. Sci. Technol. 2021, 55, 5371–5381. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, R.; Wang, J.; Yin, X.; Lu, Z.; Hou, L. Local Electron Donor Defects Induce Dipole Polarization Boosting on Covalent Organic Frameworks to Promote Photocatalysis. ACS Mater. Lett. 2023, 5, 2877–2886. [Google Scholar] [CrossRef]
- Guo, J.; Ma, D.; Sun, F.; Zhuang, G.; Wang, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Substituent engineering in g-C3N4/COF heterojunctions for rapid charge separation and high photo-redox activity. Sci. China Chem. 2022, 65, 1704–1709. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, Y.; Li, S.; Wang, S.; Que, X.; Sheng, L.; Peng, J.; Zhao, L.; Yuan, L.; Zhai, M. Enhanced photo-reduction of chromium(VI) from aqueous solution by nanosheet hybrids of covalent organic framework and graphene-phase carbon nitride. Sep. Purif. Technol. 2022, 294, 121204. [Google Scholar] [CrossRef]
- Samajdar, S.; Golda, A.S.; Lakhera, S.K.; Ghosh, S. Recent progress in chromium removal from wastewater using covalent organic frameworks—A review. Chemosphere 2023, 350, 141028. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Duan, P.; Yang, W.; Huang, X.; Zhao, Y.; Wang, C.; Pan, Q. Facile fabrication of melamine sponge@covalent organic framework composite for enhanced degradation of tetracycline under visible light. Chem. Eng. J. 2022, 430, 132817. [Google Scholar] [CrossRef]
- Hou, S.; Xu, X.; Wang, M.; Lu, T.; Sun, C.Q.; Pan, L. Synergistic conversion and removal of total Cr from aqueous solution by photocatalysis and capacitive deionization. Chem. Eng. J. 2018, 337, 398–404. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Wen, B.; Li, Y.; Zhou, B.; Wang, Z.; Yang, S.; Zhao, R. Nitrogen-rich covalent triazine frameworks for high-efficient removal of anion dyes and the synergistic adsorption of cationic dye. Chemosphere 2021, 272, 129622. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Y.; Sun, F.; Zhu, G. Targeted synthesis of novel porous aromatic frameworks with selective separation of CO2/CH4 and CO2/N2. Chin. Chem. Lett. 2014, 25, 1407–1410. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, X.; Liu, S.; Li, X.; Wang, S.; Feng, Z.; Wu, Z.; Wang, L.; Jiang, Z.; Zhang, Y. One-step preparation of Fe/N co-doped porous biochar for chromium(VI) and bisphenol a decontamination in water: Insights to co-activation and adsorption mechanisms. Bioresour. Technol. 2022, 361, 127718. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cao, S.; Luo, D.; Zhang, N.; Lei, Y.Z.; Wang, Y. Polydopamine-assisted polyethylenimine grafting melamine foam and the application in wastewater purification. Chemosphere 2022, 287, 132054. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ren, G.; Ma, Y.; Wang, C.; Li, M.; Pang, X.; Pan, Q.; Li, J. Adsorption and reutilization of Pb(II) based on acid-resistant metal-organic gel. Sep. Purif. Technol. 2022, 295, 121253. [Google Scholar] [CrossRef]
- Duan, P.; Lin, D.; Yang, W.; Huang, X.; Sun, A.; Pan, Q. Facile preparation of covalent organic frameworks@alginate composite beads for enhanced uranium(VI) adsorption. Rare Met. 2022, 41, 1323–1331. [Google Scholar] [CrossRef]
- Zhua, D.; Zhou, S.; Zhou, Z.; Li, R.; Jia, Y.; Xu, Z.; Lan, S.; Zhang, Y.; Miao, S.; Wang, W. Highly efficient and selective removal of Cr(VI) by covalent organic frameworks: Structure, performance and mechanism. Colloids Surf. A 2020, 600, 124910. [Google Scholar] [CrossRef]
- Nayak, S.; Sahoo, A.; Naidu, G.S.; Giri, A.; Patra, A. Pyridinium-functionalized ionic porous organic polymer for rapid scavenging of oxoanions from water. Macromol. Rapid Commun. 2023, 44, 2300138. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhang, Y.; Kong, X.; Yu, J.; Lv, X.; Wu, Y.; Guo, Z.; Li, J. Zr(IV)-based metal-organic framework with T-shaped ligand: Unique Structure, high stability, selective detection, and rapid adsorption of Cr2O72− in water. ACS Appl. Mater. Interfaces 2018, 10, 16650–16659. [Google Scholar] [CrossRef]
- Deng, S.; Mo, X.; Zheng, S.; Jin, X.; Gao, Y.; Cai, S.; Fan, J.; Zhang, W. Hydrolytically stable nanotubular cationic metal−organic framework for rapid and efficient removal of toxic oxo-anions and dyes from water. Inorg. Chem. 2019, 58, 2899–2909. [Google Scholar] [CrossRef]
- Daliran, S.; Oveisi, A.R.; Khajeh, M.; Barkhordar, A.; Dhakshinamoorthy, A. Zirconium-based cyclodextrin porous coordination polymer for highly efficient uptake of Cr(VI) species. Polyhedron 2023, 237, 116392. [Google Scholar] [CrossRef]
- Peng, X.; Yan, Z.; Hu, L.; Zhang, R.; Liu, S.; Wang, A.; Yu, X.; Chen, L. Adsorption behavior of hexavalent chromium in aqueous solution by polyvinylimidazole modified cellulose. Int. J. Biol. Macromol. 2020, 155, 1184–1193. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, J.; Liang, J.; Li, M.; Fu, Y.; Wang, H.; Tu, S.; Li, J. Hypercrosslinked mesoporous poly(ionic liquid)s with high density of ion pairs: Efficient adsorbents for Cr(VI) removal via ion-exchange. Chem. Eng. J. 2019, 378, 122107. [Google Scholar] [CrossRef]
- Li, X.; Jin, L.; Huang, L.; Ge, X.; Deng, H.; Wang, H.; Li, Y.; Chai, L.; Ma, S. Imidazolium-based cationic polymeric nanotraps for efficient removal of Cr2O72−. J. Environ. Chem. Eng. 2021, 9, 106357. [Google Scholar] [CrossRef]
- Jin, X.; Wang, G.; Ma, D.; Deng, S.; Cai, S.; Fan, J.; Zhang, W.; Zheng, S. Cationic amorphous metal−organic cage-based materials for the removal of oxo-anions from water. ACS Appl. Energy Mater. 2019, 2, 5824–5832. [Google Scholar] [CrossRef]
- Li, Z.; Xue, H.; Zhang, Y.; Hua, H.; Zheng, X. Construction of a cationic organic network for highly efficient removal of anionic contaminants from water. New J. Chem. 2019, 43, 11604–11609. [Google Scholar] [CrossRef]
- Fei, W.; Gao, J.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. A visible-light active p-n heterojunction NiFe-LDH/Co3O4 supported on Ni foam as photoanode for photoelectrocatalytic removal of contaminants. J. Hazard. Mater. 2021, 402, 123515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Shi, Z.; Lu, T.; Chen, D.; Wang, Q.; Zhan, Z. A direct Z-scheme BiOBr/TzDa COF heterojunction photocatalyst with enhanced performance on visible-light driven removal of organic dye and Cr(VI). Sep. Purif. Technol. 2021, 275, 119216. [Google Scholar] [CrossRef]
- Xu, M.; Wu, J.; Wang, J.; Mao, Y.; Liu, M.; Yang, Y.; Yang, C.; Sun, L.; Du, Y.; Li, Y.; et al. Regulating energy band structures of triazine covalent organic frameworks with electron-donating/withdrawing substituents for visible-light-responsive photocatalytic tetracycline degradation and Cr (VI) reduction. J. Hazard. Mater. 2023, 446, 130756. [Google Scholar] [CrossRef]
- Chen, W.; Yang, Z.; Xie, Z.; Li, Y.; Yu, X.; Lu, F.; Chen, L. Benzothiadiazole functionalized D–A type covalent organic frameworks for effective photocatalytic reduction of aqueous chromium(VI). J. Mater. Chem. A 2019, 7, 997. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, Z.; Zhang, Y.; Wang, X.; Lu, T.; Wang, Q.; Zhan, Z.; Zhang, P. COF TzDa/Ag/AgBr Z-scheme heterojunction photocatalyst for efficient visible light driven elimination of antibiotics tetracycline and heavy metal ion Cr(VI). Sep. Purif. Technol. 2022, 288, 120717. [Google Scholar] [CrossRef]
- Geng, W.; Lu, X.; Zhang, H.; Luo, Y.; Wang, Z.; Guo, S.; Zhou, Z.; Zhang, D. Effective design and synthesis of donor-acceptor covalent triazine polymers with boosted photocatalytic performance for Cr(VI) reduction. Sep. Purif. Technol. 2022, 290, 120829. [Google Scholar] [CrossRef]
- Yi, X.; Gao, Y.; Wang, C.; Li, Y.; Chu, H.; Wang, P. Photocatalytic Cr(VI) reduction over MIL-88A(Fe) on polyurethane sponge: From batch to continuous-flow operation. Chin. Chem. Lett. 2023, 34, 108029. [Google Scholar] [CrossRef]
- Tang, P.; Ji, B.; Sun, G. Wearable super-adsorptive fibrous equipment in situ grafted with porous organic polymers for carcinogenic fumigant defense and detoxification. J. Mater. Chem. A 2020, 8, 24128–24136. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Lin, Y.; Gan, L.; Zhao, K.; Zhao, X.; Pan, Q.; Fu, G. Enhancement of Mass Transfer Process for Photocatalytic Reduction in Cr(VI) by Electric Field Assistance. Int. J. Mol. Sci. 2024, 25, 2832. https://doi.org/10.3390/ijms25052832
Feng X, Lin Y, Gan L, Zhao K, Zhao X, Pan Q, Fu G. Enhancement of Mass Transfer Process for Photocatalytic Reduction in Cr(VI) by Electric Field Assistance. International Journal of Molecular Sciences. 2024; 25(5):2832. https://doi.org/10.3390/ijms25052832
Chicago/Turabian StyleFeng, Xi, Yonghui Lin, Letian Gan, Kaiyuan Zhao, Xiaojun Zhao, Qinhe Pan, and Guohua Fu. 2024. "Enhancement of Mass Transfer Process for Photocatalytic Reduction in Cr(VI) by Electric Field Assistance" International Journal of Molecular Sciences 25, no. 5: 2832. https://doi.org/10.3390/ijms25052832
APA StyleFeng, X., Lin, Y., Gan, L., Zhao, K., Zhao, X., Pan, Q., & Fu, G. (2024). Enhancement of Mass Transfer Process for Photocatalytic Reduction in Cr(VI) by Electric Field Assistance. International Journal of Molecular Sciences, 25(5), 2832. https://doi.org/10.3390/ijms25052832






