Canine Mammary Tumors: Classification, Biomarkers, Traditional and Personalized Therapies
Abstract
:1. Canine Mammary Tumors
1.1. General Information and Incidence
1.2. Classification and Immunohistochemical Markers
1.3. Grading System and Clinical Staging
1.4. Risk Factors
1.5. Biomarkers
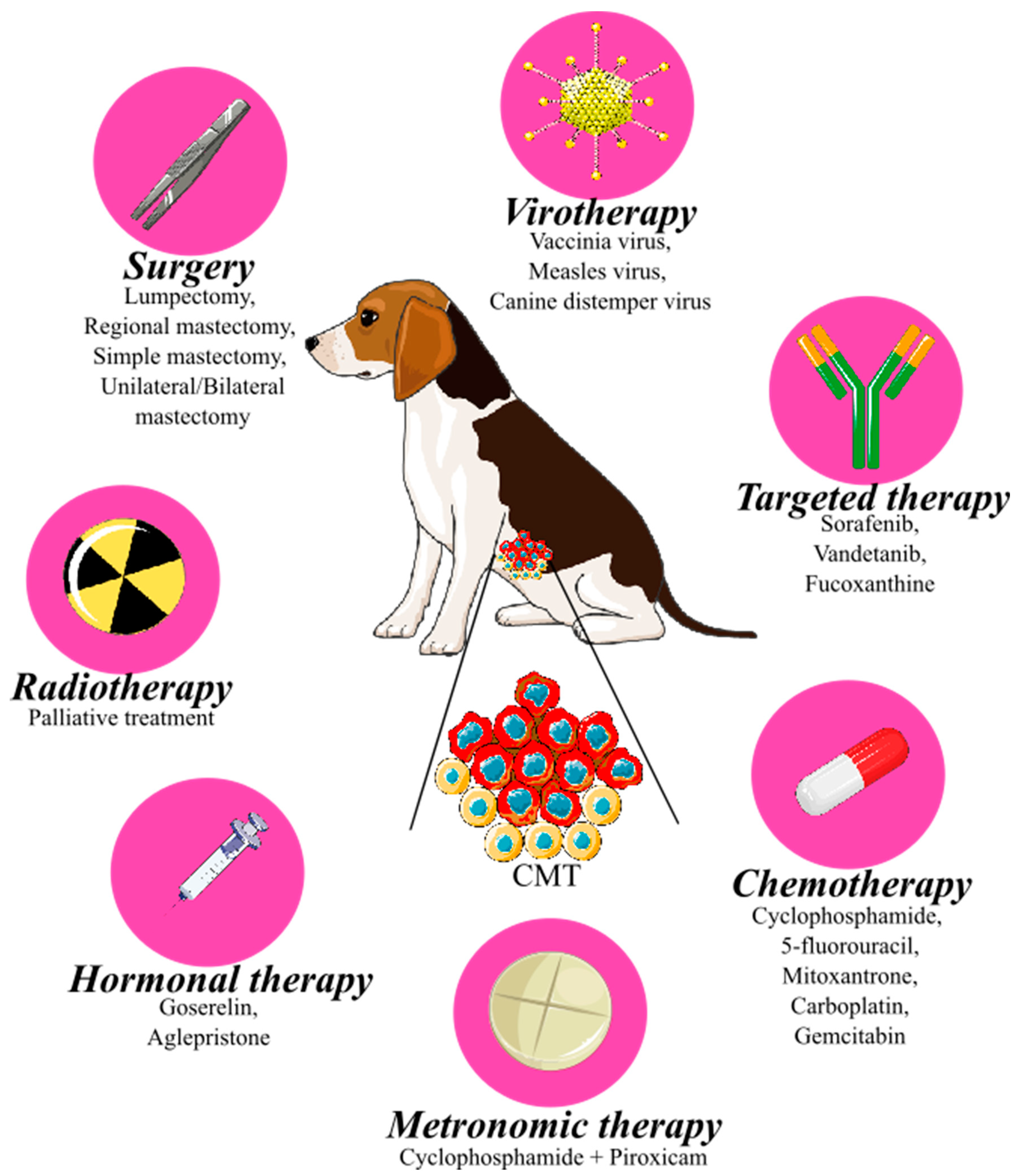
2. Treatment of Canine Mammary Tumors
3. Personalized—Precision Medicine in Cancer Therapy
Personalized Medicine in Veterinary Oncology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cassali, G.D.; Lavalle, G.E.; Nardi, A.B.D.; Ferreira, E.; Estrela-Lima, A.; Alessi, A.C.; Daleck, C.R.; Salgado, B.S.; Fernandes, C.G.; Sobral, R.A.; et al. Consensus for the Diagnosis, Prognosis and Treatment of Canine Mammary Tumors. Braz. J. Vet. Pathol. 2011, 4, 153–180. [Google Scholar]
- Schneider, R. Comparison of Age, Sex, and Incidence Rates in Human and Canine Breast Cancer. Cancer 1970, 26, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Varney, D.; O’Neill, D.; O’Neill, M.; Church, D.; Stell, A.; Beck, S.; Smalley, M.J.; Brodbelt, D. Epidemiology of Mammary Tumours in Bitches under Veterinary Care in the UK in 2016. Vet. Rec. 2023, 193, e3054. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-H.; Du, C.-T.; Yu, C.; Zhang, Y.-Z.; Huang, R.-L.; Tang, X.-Y.; Xie, G.-H. Epidemiological Investigation of Canine Mammary Tumors in Mainland China Between 2017 and 2021. Front. Vet. Sci. 2022, 9, 843390. [Google Scholar] [CrossRef]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of Mammary Tumors in the Canine Population Living in the Veneto Region (Northeastern Italy): Risk Factors and Similarities to Human Breast Cancer. Prev. Vet. Med. 2016, 126, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Sleeckx, N.; de Rooster, H.; Veldhuis Kroeze, E.J.B.; Van Ginneken, C.; Van Brantegem, L. Canine Mammary Tumours, an Overview. Reprod. Domest. Anim. Zuchthyg. 2011, 46, 1112–1131. [Google Scholar] [CrossRef] [PubMed]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological Study of Mammary Tumors in Female Dogs Diagnosed during the Period 2002–2012: A Growing Animal Health Problem. PLoS ONE 2015, 10, e0127381. [Google Scholar] [CrossRef]
- Gedon, J.; Wehrend, A.; Kessler, M. Ovariectomy Reduces the Risk of Tumour Development and Influences the Histologic Continuum in Canine Mammary Tumours. Vet. Comp. Oncol. 2022, 20, 476–483. [Google Scholar] [CrossRef]
- Misdorp, W. Tumors of the Mammary Gland. In Tumors in Domestic Animals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; pp. 575–606. ISBN 978-0-470-37692-8. [Google Scholar]
- Misdorp, W. Histological Classification of Mammary Tumors of the Dog and the Cat; International Histological Classification of Tumors of Domestic Animals Second Series; Armed Forces Institute of Pathology in Cooperation with the American Registry of Pathology and the World Health Organization Collaborating Center for Worldwide Reference on Comparative Oncology: Washington, DC, USA, 1999; ISBN 978-1-881041-66-5. [Google Scholar]
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and Grading of Canine Mammary Tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- Papparella, S.; Crescio, M.I.; Baldassarre, V.; Brunetti, B.; Burrai, G.P.; Cocumelli, C.; Grieco, V.; Iussich, S.; Maniscalco, L.; Mariotti, F.; et al. Reproducibility and Feasibility of Classification and National Guidelines for Histological Diagnosis of Canine Mammary Gland Tumours: A Multi-Institutional Ring Study. Vet. Sci. 2022, 9, 357. [Google Scholar] [CrossRef]
- Kiupel, M. Surgical Pathology of Tumors of Domestic Animals Volume 2, Mammary Tumors, 3rd ed.; Davis-Thompson Foundation: Gurnee, IL, USA, 2018. [Google Scholar]
- Kumar, P.; Pawaiya, R.; Ravindran, R. Histopathological Classification and Incidence of Canine Mammary Tumours. Indian J. Vet. Pathol. 2009, 33, 152–155. [Google Scholar]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current Biomarkers of Canine Mammary Tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef]
- Provenzano, E.; Ulaner, G.A.; Chin, S.-F. Molecular Classification of Breast Cancer. PET Clin. 2018, 13, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Varallo, G.R.; Gelaleti, G.B.; Maschio-Signorini, L.B.; Moschetta, M.G.; Lopes, J.R.; De Nardi, A.B.; Tinucci-Costa, M.; Rocha, R.M.; de Campos Zuccari, D.A.P. Prognostic Phenotypic Classification for Canine Mammary Tumors. Oncol. Lett. 2019, 18, 6545–6553. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Martin de las Mulas, J.; Ordás, J.; Millán, Y.; Fernández-Soria, V.; Ramón y Cajal, S. Oncogene HER-2 in Canine Mammary Gland Carcinomas: An Immunohistochemical and Chromogenic in Situ Hybridization Study. Breast Cancer Res. Treat. 2003, 80, 363–367. [Google Scholar] [CrossRef]
- Muscatello, L.V.; Gobbo, F.; Di Oto, E.; Sarli, G.; De Maria, R.; De Leo, A.; Tallini, G.; Brunetti, B. HER2 Overexpression and Cytogenetical Patterns in Canine Mammary Carcinomas. Vet. Sci. 2022, 9, 583. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Rasotto, R.; Zappulli, V.; Goldschmidt, M.H. Development, Anatomy, Histology, Lymphatic Drainage, Clinical Features, and Cell Differentiation Markers of Canine Mammary Gland Neoplasms. Vet. Pathol. 2011, 48, 85–97. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.T.; Pinho, S.S.; de Matos, A.J.; Hespanhol, V.; Reis, C.A.; Gärtner, F. MUC1 Expression in Canine Malignant Mammary Tumours and Relationship to Clinicopathological Features. Vet. J. 2009, 182, 491–493. [Google Scholar] [CrossRef]
- Arai, K.; Nakano, H.; Shibutani, M.; Naoi, M.; Matsuda, H. Expression of Class II β-Tubulin by Proliferative Myoepithelial Cells in Canine Mammary Mixed Tumors. Vet. Pathol. 2003, 40, 670–676. [Google Scholar] [CrossRef]
- Abd El-Rehim, D.M.; Pinder, S.E.; Paish, C.E.; Bell, J.; Blamey, R.W.; Robertson, J.F.R.; Nicholson, R.I.; Ellis, I.O. Expression of Luminal and Basal Cytokeratins in Human Breast Carcinoma. J. Pathol. 2004, 203, 661–671. [Google Scholar] [CrossRef]
- Böcker, W.; Moll, R.; Poremba, C.; Holland, R.; Van Diest, P.J.; Dervan, P.; Bürger, H.; Wai, D.; Ina Diallo, R.; Brandt, B.; et al. Common Adult Stem Cells in the Human Breast Give Rise to Glandular and Myoepithelial Cell Lineages: A New Cell Biological Concept. Lab. Investig. 2002, 82, 737–746. [Google Scholar] [CrossRef]
- Rasotto, R.; Goldschmidt, M.H.; Castagnaro, M.; Carnier, P.; Caliari, D.; Zappulli, V. The Dog as a Natural Animal Model for Study of the Mammary Myoepithelial Basal Cell Lineage and Its Role in Mammary Carcinogenesis. J. Comp. Pathol. 2014, 151, 166–180. [Google Scholar] [CrossRef]
- Gama, A.; Alves, A.; Schmitt, F. Expression and Prognostic Significance of CK19 in Canine Malignant Mammary Tumours. Vet. J. 2010, 184, 45–51. [Google Scholar] [CrossRef]
- Fhaikrue, I.; Srisawat, W.; Nambooppha, B.; Pringproa, K.; Thongtharb, A.; Prachasilchai, W.; Sthitmatee, N. Identification of Potential Canine Mammary Tumour Cell Biomarkers Using Proteomic Approach: Differences in Protein Profiles among Tumour and Normal Mammary Epithelial Cells by Two-Dimensional Electrophoresis-Based Mass Spectrometry. Vet. Comp. Oncol. 2020, 18, 787–795. [Google Scholar] [CrossRef]
- Borghesi, J.; Giancoli Kato Cano da Silva, M.; de Oliveira Pimenta Guimarães, K.; Mario, L.C.; de Almeida da Anunciação, A.R.; Silveira Rabelo, A.C.; Gonçalves Hayashi, R.; Lima, M.F.; Miglino, M.A.; Oliveira Favaron, P.; et al. Evaluation of Immunohistopathological Profile of Tubular and Solid Canine Mammary Carcinomas. Res. Vet. Sci. 2021, 136, 119–126. [Google Scholar] [CrossRef]
- Taneja, P.; Maglic, D.; Kai, F.; Zhu, S.; Kendig, R.D.; Fry Elizabeth, A.; Inoue, K. Classical and Novel Prognostic Markers for Breast Cancer and Their Clinical Significance. Clin. Med. Insights Oncol. 2010, 4, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Ludueña, R.F. Multiple Forms of Tubulin: Different Gene Products and Covalent Modifications. Int. Rev. Cytol. 1998, 178, 207–275. [Google Scholar] [CrossRef]
- Nami, B.; Wang, Z. Genetics and Expression Profile of the Tubulin Gene Superfamily in Breast Cancer Subtypes and Its Relation to Taxane Resistance. Cancers 2018, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Galmarini, C.M.; Treilleux, I.; Cardoso, F.; Bernard-Marty, C.; Durbecq, V.; Gancberg, D.; Bissery, M.-C.; Paesmans, M.; Larsimont, D.; Piccart, M.J.; et al. Class III Beta-Tubulin Isotype Predicts Response in Advanced Breast Cancer Patients Randomly Treated Either with Single-Agent Doxorubicin or Docetaxel. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 4511–4516. [Google Scholar] [CrossRef] [PubMed]
- Mozzetti, S.; Ferlini, C.; Concolino, P.; Filippetti, F.; Raspaglio, G.; Prislei, S.; Gallo, D.; Martinelli, E.; Ranelletti, F.O.; Ferrandina, G.; et al. Class III Beta-Tubulin Overexpression Is a Prominent Mechanism of Paclitaxel Resistance in Ovarian Cancer Patients. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 298–305. [Google Scholar] [CrossRef]
- Mariani, M.; Zannoni, G.F.; Sioletic, S.; Sieber, S.; Martino, C.; Martinelli, E.; Coco, C.; Scambia, G.; Shahabi, S.; Ferlini, C. Gender Influences the Class III and V β-Tubulin Ability to Predict Poor Outcome in Colorectal Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 2964–2975. [Google Scholar] [CrossRef]
- Gan, P.P.; Pasquier, E.; Kavallaris, M. Class III Beta-Tubulin Mediates Sensitivity to Chemotherapeutic Drugs in Non Small Cell Lung Cancer. Cancer Res. 2007, 67, 9356–9363. [Google Scholar] [CrossRef]
- Kanojia, D.; Morshed, R.A.; Zhang, L.; Miska, J.M.; Qiao, J.; Kim, J.W.; Pytel, P.; Balyasnikova, I.V.; Lesniak, M.S.; Ahmed, A.U. βIII-Tubulin Regulates Breast Cancer Metastases to the Brain. Mol. Cancer Ther. 2015, 14, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, M.C.; Manuali, E.; Pacifico, E.; Ferri, C.; Romagnoli, M.; Mangili, V.; Fruganti, G. Cancer Antigen 15/3: Possible Diagnostic Use in Veterinary Clinical Oncology. Preliminary Study. Vet. Res. Commun. 2010, 34 (Suppl. S1), S103–S106. [Google Scholar] [CrossRef] [PubMed]
- Rahn, J.J.; Dabbagh, L.; Pasdar, M.; Hugh, J.C. The Importance of MUC1 Cellular Localization in Patients with Breast Carcinoma: An Immunohistologic Study of 71 Patients and Review of the Literature. Cancer 2001, 91, 1973–1982. [Google Scholar] [CrossRef]
- Hollingsworth, M.A.; Swanson, B.J. Mucins in Cancer: Protection and Control of the Cell Surface. Nat. Rev. Cancer 2004, 4, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Agudelo, J.D.; Giraldo-Chalarca, L.; Cortes-Mera, D.M.; Ossa-López, P.A.; Morales-Álvarez, E.D.; Rivera-Páez, F.A. Detection of Single Nucleotide Polymorphisms (SNPs) in HER2, MUC1, ESR1, and BRCA1 Genes Associated with Canine Mammary Cancer. Vet. Stanica 2021, 52, 489–497. [Google Scholar] [CrossRef]
- Campos, L.C.; Silva, J.O.; Santos, F.S.; Araújo, M.R.; Lavalle, G.E.; Ferreira, E.; Cassali, G.D. Prognostic Significance of Tissue and Serum HER2 and MUC1 in Canine Mammary Cancer. J. Vet. Diagn. Investig. Off. Publ. Am. Assoc. Vet. Lab. Diagn. 2015, 27, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, Z.; Lin, Z.; Zhou, C.; Liu, G.; Lin, J.; Zhang, D.; Lin, D. Overexpression of Mucin 1 Suppresses the Therapeutical Efficacy of Disulfiram against Canine Mammary Tumor. Animals 2020, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Manuali, E.; De Giuseppe, A.; Feliziani, F.; Forti, K.; Casciari, C.; Marchesi, M.C.; Pacifico, E.; Pawłowski, K.M.; Majchrzak, K.; Król, M. CA 15-3 Cell Lines and Tissue Expression in Canine Mammary Cancer and the Correlation between Serum Levels and Tumour Histological Grade. BMC Vet. Res. 2012, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Baba, O.K.; Sood, N.K.; Gupta, K. Clinical Evaluation of Glycoproteins and Inflammatory Cytokines in the Serum of Dogs Affected with Canine Mammary Cancer. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 1465–1469. [Google Scholar] [CrossRef]
- Campos, L.C.; Lavalle, G.E.; Estrela-Lima, A.; Melgaço de Faria, J.C.; Guimarães, J.E.; Dutra, Á.P.; Ferreira, E.; de Sousa, L.P.; Rabelo, É.M.L.; Vieira da Costa, A.F.D.; et al. CA15.3, CEA and LDH in Dogs with Malignant Mammary Tumors. J. Vet. Intern. Med. 2012, 26, 1383–1388. [Google Scholar] [CrossRef]
- Apostolopoulos, V.; McKenzie, I.F.C. Cellular Mucins: Targets for Immunotherapy. Crit. Rev. Immunol. 2017, 37, 445–461. [Google Scholar] [CrossRef]
- Pathangey, L.B.; Lakshminarayanan, V.; Suman, V.J.; Pockaj, B.A.; Mukherjee, P.; Gendler, S.J. Aberrant Glycosylation of Anchor-Optimized MUC1 Peptides Can Enhance Antigen Binding Affinity and Reverse Tolerance to Cytotoxic T Lymphocytes. Biomolecules 2016, 6, 31. [Google Scholar] [CrossRef]
- Bloom, H.J.G.; Richardson, W.W. Histological Grading and Prognosis in Breast Cancer. Br. J. Cancer 1957, 11, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Elston, C.W.; Ellis, I.O. Pathological Prognostic Factors in Breast Cancer. I. The Value of Histological Grade in Breast Cancer: Experience from a Large Study with Long-Term Follow-Up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Canadas, A.; França, M.; Pereira, C.; Vilaça, R.; Vilhena, H.; Tinoco, F.; Silva, M.J.; Ribeiro, J.; Medeiros, R.; Oliveira, P.; et al. Canine Mammary Tumors: Comparison of Classification and Grading Methods in a Survival Study. Vet. Pathol. 2019, 56, 208–219. [Google Scholar] [CrossRef]
- Peña, L.; Andrés, P.J.D.; Clemente, M.; Cuesta, P.; Pérez-Alenza, M.D. Prognostic Value of Histological Grading in Noninflammatory Canine Mammary Carcinomas in a Prospective Study with Two-Year Follow-Up: Relationship with Clinical and Histological Characteristics. Vet. Pathol. 2013, 50, 94–105. [Google Scholar] [CrossRef]
- Yamagami, T.; Kobayashi, T.; Takahashi, K.; Sugiyama, M. Prognosis for Canine Malignant Mammary Tumors Based on TNM and Histologic Classification. J. Vet. Med. Sci. 1996, 58, 1079–1083. [Google Scholar] [CrossRef]
- Ferreira, E.; Bertagnolli, A.C.; Cavalcanti, M.F.; Schmitt, F.C.; Cassali, G.D. The Relationship between Tumour Size and Expression of Prognostic Markers in Benign and Malignant Canine Mammary Tumours. Vet. Comp. Oncol. 2009, 7, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Chang, C.-C.; Chang, T.-J.; Wong, M.-L. Prognostic Factors Associated with Survival Two Years after Surgery in Dogs with Malignant Mammary Tumors: 79 Cases (1998–2002). J. Am. Vet. Med. Assoc. 2005, 227, 1625–1629. [Google Scholar] [CrossRef]
- Hellmén, E.; Bergström, R.; Holmberg, L.; Spångberg, I.-B.; Hansson, K.; Lindgren, A. Prognostic Factors in Canine Mammary Tumors: A Multivariate Study of 202 Consecutive Cases. Vet. Pathol. 1993, 30, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Peña, L.; Pérez-Alenza, M.D.; Sánchez, M.A.; Flores, J.M.; Castaño, M. Immunohistologic Detection of Estrogen Receptor Alpha in Canine Mammary Tumors: Clinical and Pathologic Associations and Prognostic Significance. Vet. Pathol. 2000, 37, 239–247. [Google Scholar] [CrossRef]
- Salas-Araujo, Y.J.; Aburto, E.; Alonso, R.; Márquez-Alvarado, A.A.; Corona-Monjaras, H.; Romero-Romero, L. Association of Histological Features with Potential Risk Factors and Survival in Canine Mammary Tumors. Vet. México OA 2016, 3, 1. [Google Scholar] [CrossRef]
- Rivera, P.; Melin, M.; Biagi, T.; Fall, T.; Häggström, J.; Lindblad-Toh, K.; von Euler, H. Mammary Tumor Development in Dogs Is Associated with BRCA1 and BRCA2. Cancer Res. 2009, 69, 8770–8774. [Google Scholar] [CrossRef]
- Egenvall, A.; Bonnett, B.N.; Öhagen, P.; Olson, P.; Hedhammar, Å.; von Euler, H. Incidence of and Survival after Mammary Tumors in a Population of over 80,000 Insured Female Dogs in Sweden from 1995 to 2002. Prev. Vet. Med. 2005, 69, 109–127. [Google Scholar] [CrossRef]
- Sorenmo, K.U.; Kristiansen, V.M.; Cofone, M.A.; Shofer, F.S.; Breen, A.-M.; Langeland, M.; Mongil, C.M.; Grondahl, A.M.; Teige, J.; Goldschmidt, M.H. Canine Mammary Gland Tumours; a Histological Continuum from Benign to Malignant; Clinical and Histopathological Evidence. Vet. Comp. Oncol. 2009, 7, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, M.; Baioni, E.; Ru, G.; Carminato, A.; Mutinelli, F. Animal Tumour Registry of Two Provinces in Northern Italy: Incidence of Spontaneous Tumours in Dogs and Cats. BMC Vet. Res. 2009, 5, 39. [Google Scholar] [CrossRef]
- Burrai, G.P.; Gabrieli, A.; Moccia, V.; Zappulli, V.; Porcellato, I.; Brachelente, C.; Pirino, S.; Polinas, M.; Antuofermo, E. A Statistical Analysis of Risk Factors and Biological Behavior in Canine Mammary Tumors: A Multicenter Study. Animals 2020, 10, 1687. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Morimatsu, M.; Ochiai, K.; Ishiguro-Oonuma, T.; Wada, S.; Orino, K.; Watanabe, K. Reduced Canine BRCA2 Expression Levels in Mammary Gland Tumors. BMC Vet. Res. 2015, 11, 159. [Google Scholar] [CrossRef]
- Dutra, A.P.; Granja, N.V.M.; Schmitt, F.C.; Cassali, G.D. C-erbB-2 Expression and Nuclear Pleomorphism in Canine Mammary Tumors. Braz. J. Med. Biol. Res. 2004, 37, 1673–1681. [Google Scholar] [CrossRef]
- Rungsipipat, A.; Tateyama, S.; Yamaguchi, R.; Uchida, K.; Miyoshi, N.; Hayashi, T. Immunohistochemical Analysis of C-Yes and c-erbB-2 Oncogene Products and P53 Tumor Suppressor Protein in Canine Mammary Tumors. J. Vet. Med. Sci. 1999, 61, 27–32. [Google Scholar] [CrossRef]
- Haga, S.; Nakayama, M.; Tatsumi, K.; Maeda, M.; Imai, S.; Umesako, S.; Yamamoto, H.; Hilgers, J.; Sarkar, N.H. Overexpression of the P53 Gene Product in Canine Mammary Tumors. Oncol. Rep. 2001, 8, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.D.; Davidson, N.E. Estrogen Carcinogenesis in Breast Cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Dorn, C.R.; Taylor, D.O.N. Factors Influencing Canine Mammary Cancer Development and Postsurgical Survival2. JNCI J. Natl. Cancer Inst. 1969, 43, 1249–1261. [Google Scholar] [CrossRef]
- Lim, H.-Y.; Im, K.-S.; Kim, N.-H.; Kim, H.-W.; Shin, J.-I.; Yhee, J.-Y.; Sur, J.-H. Effects of Obesity and Obesity-Related Molecules on Canine Mammary Gland Tumors. Vet. Pathol. 2015, 52, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Trudeau, M.E.; Koo, J.; Madarnas, Y.; Hartwick, W.; Hoffman, B.; Hood, N. Fasting Insulin and Outcome in Early-Stage Breast Cancer: Results of a Prospective Cohort Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 42–51. [Google Scholar] [CrossRef]
- Yee, D.; Lee, A.V. Crosstalk between the Insulin-like Growth Factors and Estrogens in Breast Cancer. J. Mammary Gland Biol. Neoplasia 2000, 5, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, A.M.; Sukumar, S. Molecular Links between Obesity and Breast Cancer. Endocr. Relat. Cancer 2006, 13, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, L.; Gabai, G.; Wolfswinkel, J.; Mol, J.A. Mammary Steroid Metabolizing Enzymes in Relation to Hyperplasia and Tumorigenesis in the Dog. J. Steroid Biochem. Mol. Biol. 2004, 92, 167–173. [Google Scholar] [CrossRef]
- Singh-Ranger, G.; Mokbel, K. The Role of Cyclooxygenase-2 (COX-2) in Breast Cancer, and Implications of COX-2 Inhibition. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2002, 28, 729–737. [Google Scholar] [CrossRef]
- Hayes, A. Cancer, Cyclo-Oxygenase and Nonsteroidal Anti-Inflammatory Drugs—Can We Combine All Three? Vet. Comp. Oncol. 2007, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.N.M.; Stanfield, K.M.; Trajkovic, D.; Knapp, D.W. Expression of Cyclooxygenase-2 in Canine Renal Cell Carcinoma. Vet. Pathol. 2001, 38, 116–119. [Google Scholar] [CrossRef]
- Khan, K.N.M.; Knapp, D.W.; Denicola, D.B.; Harris, R.K. Expression of Cyclooxygenase-2 in Transitional Cell Carcinoma of the Urinary Bladder in Dogs. Am. J. Vet. Res. 2000, 61, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Pestili de Almeida, E.M.; Piché, C.; Sirois, J.; Doré, M. Expression of Cyclo-Oxygenase-2 in Naturally Occurring Squamous Cell Carcinomas in Dogs. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2001, 49, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Ristimäki, A.; Sivula, A.; Lundin, J.; Lundin, M.; Salminen, T.; Haglund, C.; Joensuu, H.; Isola, J. Prognostic Significance of Elevated Cyclooxygenase-2 Expression in Breast Cancer. Cancer Res. 2002, 62, 632–635. [Google Scholar] [PubMed]
- Queiroga, F.L.; Perez-Alenza, M.D.; Silvan, G.; Peña, L.; Lopes, C.; Illera, J.C. Cox-2 Levels in Canine Mammary Tumors, Including Inflammatory Mammary Carcinoma: Clinicopathological Features and Prognostic Significance. Anticancer Res. 2005, 25, 4269–4275. [Google Scholar]
- Millanta, F.; Citi, S.; Della Santa, D.; Porciani, M.; Poli, A. COX-2 Expression in Canine and Feline Invasive Mammary Carcinomas: Correlation with Clinicopathological Features and Prognostic Molecular Markers. Breast Cancer Res. Treat. 2006, 98, 115–120. [Google Scholar] [CrossRef]
- Dias Pereira, P.; Lopes, C.C.; Matos, A.J.F.; Santos, M.; Gärtner, F.; Medeiros, R.; Lopes, C. COX-2 Expression in Canine Normal and Neoplastic Mammary Gland. J. Comp. Pathol. 2009, 140, 247–253. [Google Scholar] [CrossRef]
- Lavalle, G.E.; Bertagnolli, A.C.; Tavares, W.L.F.; Cassali, G.D. Cox-2 Expression in Canine Mammary Carcinomas: Correlation with Angiogenesis and Overall Survival. Vet. Pathol. 2009, 46, 1275–1280. [Google Scholar] [CrossRef]
- Doré, M.; Lanthier, I.; Sirois, J. Cyclooxygenase-2 Expression in Canine Mammary Tumors. Vet. Pathol. 2003, 40, 207–212. [Google Scholar] [CrossRef]
- Heller, D.A.; Clifford, C.A.; Goldschmidt, M.H.; Holt, D.E.; Shofer, F.S.; Smith, A.; Sorenmo, K.U. Cyclooxygenase-2 Expression Is Associated with Histologic Tumor Type in Canine Mammary Carcinoma. Vet. Pathol. 2005, 42, 776–780. [Google Scholar] [CrossRef]
- Queiroga, F.L.; Pires, I.; Lobo, L.; Lopes, C.S. The Role of Cox-2 Expression in the Prognosis of Dogs with Malignant Mammary Tumours. Res. Vet. Sci. 2010, 88, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Queiroga, F.L.; Alves, A.; Pires, I.; Lopes, C. Expression of Cox-1 and Cox-2 in Canine Mammary Tumours. J. Comp. Pathol. 2007, 136, 177–185. [Google Scholar] [CrossRef]
- Henry, C.J. Biomarkers in Veterinary Cancer Screening: Applications, Limitations and Expectations. Vet. J. 2010, 185, 10–14. [Google Scholar] [CrossRef]
- Zuccari, D.A.P.C.; Santana, A.E.; Cury, P.M.; Cordeiro, J.A. Immunocytochemical Study of Ki-67 as a Prognostic Marker in Canine Mammary Neoplasia. Vet. Clin. Pathol. 2004, 33, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, E.; Horimoto, Y.; Arakawa, A.; Himuro, T.; Senuma, K.; Nakai, K.; Saito, M. Differences in Ki67 Expressions between Pre- and Post-Neoadjuvant Chemotherapy Specimens Might Predict Early Recurrence of Breast Cancer. Hum. Pathol. 2017, 63, 40–45. [Google Scholar] [CrossRef]
- Rodrigues, H.; Carvalho, M.I.; Pires, I.; Prada, J.; Queiroga, F.L. Clinicopathological Significance of Caspase-3 and Ki-67 Expression in Canine Mammary Gland Tumours. Acta Vet. Hung. 2016, 64, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Madej, J.A.; Pula, B.; Dziegiel, P.; Ciaputa, R. Expression of Matrix Metalloproteinase 2 (MMP-2), E-Cadherin and Ki-67 in Metastatic and Non-Metastatic Canine Mammary Carcinomas. Ir. Vet. J. 2015, 69, 9. [Google Scholar] [CrossRef]
- Nowak, M.; Madej, J.A.; Dziegiel, P. Expression of E-Cadherin, Beta-Catenin and Ki-67 Antigen and Their Reciprocal Relationships in Mammary Adenocarcinomas in Bitches. Folia Histochem. Cytobiol. 2007, 45, 233–238. [Google Scholar]
- Krishna, D.K.R.; Latha, D.M.J.; Lakshman, D.M.; Rani, D.M.U.; Kumar, D.Y.R.; Swathi, D.B. Expression Pattern of PCNA and Ki-67 Biomarkers in Canine Mammary Tumours. Pharma Innov. J. 2022, 11, 233–237. [Google Scholar]
- Prelich, G.; Tan, C.K.; Kostura, M.; Mathews, M.B.; So, A.G.; Downey, K.M.; Stillman, B. Functional Identity of Proliferating Cell Nuclear Antigen and a DNA Polymerase-Delta Auxiliary Protein. Nature 1987, 326, 517–520. [Google Scholar] [CrossRef]
- Maga, G.; Hubscher, U. Proliferating Cell Nuclear Antigen (PCNA): A Dancer with Many Partners. J. Cell Sci. 2003, 116, 3051–3060. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.I.; Pires, I.; Prada, J.; Lobo, L.; Queiroga, F.L. Ki-67 and PCNA Expression in Canine Mammary Tumors and Adjacent Nonneoplastic Mammary Glands: Prognostic Impact by a Multivariate Survival Analysis. Vet. Pathol. 2016, 53, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, A.; Ozmen, O.; Haligur, M.; Sipahi, C.; Ileri, D.; Haligur, A. Immunohistochemical Evaluation of Bcl-2, ER-Alpha, Caspase -3, -8, -9, PCNA and Ki-67 Expressions in Canine Mammary Carcinomas. Biotech. Histochem. 2018, 93, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Sorenmo, K. Canine Mammary Gland Tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, Y.; Liu, G.; Wei, Y. P53 and Ki-67 as Prognostic Markers in Triple-Negative Breast Cancer Patients. PLoS ONE 2017, 12, e0172324. [Google Scholar] [CrossRef]
- Lee, C.-H.; Kim, W.-H.; Lim, J.-H.; Kang, M.-S.; Kim, D.-Y.; Kweon, O.-K. Mutation and Overexpression of P53 as a Prognostic Factor in Canine Mammary Tumors. J. Vet. Sci. 2004, 5, 63–69. [Google Scholar] [CrossRef]
- Roberts, E.; Cossigny, D.A.F.; Quan, G.M.Y. The Role of Vascular Endothelial Growth Factor in Metastatic Prostate Cancer to the Skeleton. Prostate Cancer 2013, 2013, 418340. [Google Scholar] [CrossRef]
- Carmeliet, P. VEGF as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69 (Suppl. S3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of Epidermal Growth Factor Receptor in Breast Cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef]
- Millanta, F.; Silvestri, G.; Vaselli, C.; Citi, S.; Pisani, G.; Lorenzi, D.; Poli, A. The Role of Vascular Endothelial Growth Factor and Its Receptor Flk-1/KDR in Promoting Tumour Angiogenesis in Feline and Canine Mammary Carcinomas: A Preliminary Study of Autocrine and Paracrine Loops. Res. Vet. Sci. 2006, 81, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Asano, K.; Mogi, T.; Kutara, K.; Teshima, K.; Edamura, K.; Tsumagari, S.; Hasegawa, A.; Tanaka, S. Clinical Significance of Circulating Vascular Endothelial Growth Factor in Dogs with Mammary Gland Tumors. J. Vet. Med. Sci. 2007, 69, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Lin, D.; Wang, H.; Qiao, C.; Wang, J.; Zhang, T. Quantification of VEGF-C Expression in Canine Mammary Tumours. Aust. Vet. J. 2008, 86, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.W.; Lin, D.G.; Wang, J.Q.; Li, C.Y.; Deng, G.Z. Expression and Significance of PTEN and VEGF in Canine Mammary Gland Tumours. Vet. Res. Commun. 2008, 32, 463–472. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Guimarães, M.J.; Pires, I.; Prada, J.; Silva-Carvalho, R.; Lopes, C.; Queiroga, F.L. EGFR and Microvessel Density in Canine Malignant Mammary Tumours. Res. Vet. Sci. 2013, 95, 1094–1099. [Google Scholar] [CrossRef]
- Gama, A.; Gärtner, F.; Alves, A.; Schmitt, F. Immunohistochemical Expression of Epidermal Growth Factor Receptor (EGFR) in Canine Mammary Tissues. Res. Vet. Sci. 2009, 87, 432–437. [Google Scholar] [CrossRef]
- Kashiwagi, S.; Yashiro, M.; Takashima, T.; Nomura, S.; Noda, S.; Kawajiri, H.; Ishikawa, T.; Wakasa, K.; Hirakawa, K. Significance of E-Cadherin Expression in Triple-Negative Breast Cancer. Br. J. Cancer 2010, 103, 249–255. [Google Scholar] [CrossRef]
- Song, Y.; Ye, M.; Zhou, J.; Wang, Z.; Zhu, X. Targeting E-Cadherin Expression with Small Molecules for Digestive Cancer Treatment. Am. J. Transl. Res. 2019, 11, 3932–3944. [Google Scholar]
- López-Verdín, S.; de la Luz Martínez-Fierro, M.; Garza-Veloz, I.; Zamora-Perez, A.; Grajeda-Cruz, J.; González-González, R.; Molina-Frechero, N.; Arocena, M.; Bologna-Molina, R. E-Cadherin Gene Expression in Oral Cancer: Clinical and Prospective Data. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e444–e451. [Google Scholar] [CrossRef]
- Brunetti, B.; Sarli, G.; Preziosi, R.; Leprotti, S.; Benazzi, C. E-Cadherin Expression in Canine Mammary Carcinomas with Regional Lymph Node Metastases. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 496–500. [Google Scholar] [CrossRef]
- Matos, A.J.F.; Lopes, C.; Carvalheira, J.; Santos, M.; Rutteman, G.R.; Gärtner, F. E-Cadherin Expression in Canine Malignant Mammary Tumours: Relationship to Other Clinico-Pathological Variables. J. Comp. Pathol. 2006, 134, 182–189. [Google Scholar] [CrossRef]
- Gama, A.; Paredes, J.; Gärtner, F.; Alves, A.; Schmitt, F. Expression of E-Cadherin, P-Cadherin and Beta-Catenin in Canine Malignant Mammary Tumours in Relation to Clinicopathological Parameters, Proliferation and Survival. Vet. J. 2008, 177, 45–53. [Google Scholar] [CrossRef]
- Yu, R.M.C.; Cheah, Y.K. The Roles of miRNAs in Human Breast Cancer and Canine Mammary Tumor. Appl. Cancer Res. 2017, 37, 37. [Google Scholar] [CrossRef]
- O’Day, E.; Lal, A. MicroRNAs and Their Target Gene Networks in Breast Cancer. Breast Cancer Res. 2010, 12, 201. [Google Scholar] [CrossRef]
- Bertoli, G.; Cava, C.; Castiglioni, I. MicroRNAs: New Biomarkers for Diagnosis, Prognosis, Therapy Prediction and Therapeutic Tools for Breast Cancer. Theranostics 2015, 5, 1122–1143. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Srivastava, D. A Developmental View of microRNA Function. Trends Biochem. Sci. 2007, 32, 189–197. [Google Scholar] [CrossRef]
- Calin, G.A.; Liu, C.-G.; Sevignani, C.; Ferracin, M.; Felli, N.; Dumitru, C.D.; Shimizu, M.; Cimmino, A.; Zupo, S.; Dono, M.; et al. MicroRNA Profiling Reveals Distinct Signatures in B Cell Chronic Lymphocytic Leukemias. Proc. Natl. Acad. Sci. USA 2004, 101, 11755–11760. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.; Jewell, D.A.; Liang, Y.; Ridzon, D.; Moore, J.H.; Chen, C.; Ambros, V.R.; Israel, M.A. Characterization of microRNA Expression Levels and Their Biological Correlates in Human Cancer Cell Lines. Cancer Res. 2007, 67, 2456–2468. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Yasuda, T.; Saigo, K.; Urashima, T.; Toyoda, H.; Okanoue, T.; Shimotohno, K. Comprehensive Analysis of microRNA Expression Patterns in Hepatocellular Carcinoma and Non-Tumorous Tissues. Oncogene 2006, 25, 2537–2545. [Google Scholar] [CrossRef]
- Heishima, K.; Ichikawa, Y.; Yoshida, K.; Iwasaki, R.; Sakai, H.; Nakagawa, T.; Tanaka, Y.; Hoshino, Y.; Okamura, Y.; Murakami, M.; et al. Circulating microRNA-214 and -126 as Potential Biomarkers for Canine Neoplastic Disease. Sci. Rep. 2017, 7, 2301. [Google Scholar] [CrossRef]
- Osaki, T.; Sunden, Y.; Sugiyama, A.; Azuma, K.; Murahata, Y.; Tsuka, T.; Ito, N.; Imagawa, T.; Okamoto, Y. Establishment of a Canine Mammary Gland Tumor Cell Line and Characterization of Its miRNA Expression. J. Vet. Sci. 2016, 17, 385–390. [Google Scholar] [CrossRef]
- Abbate, J.M.; Arfuso, F.; Riolo, K.; Capparucci, F.; Brunetti, B.; Lanteri, G. Epigenetics in Canine Mammary Tumors: Upregulation of miR-18a and miR-18b Oncogenes Is Associated with Decreased ERS1 Target mRNA Expression and ERα Immunoexpression in Highly Proliferating Carcinomas. Animals 2023, 13, 1086. [Google Scholar] [CrossRef]
- Boggs, R.M.; Wright, Z.M.; Stickney, M.J.; Porter, W.W.; Murphy, K.E. MicroRNA Expression in Canine Mammary Cancer. Mamm. Genome 2008, 19, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Bulkowska, M.; Rybicka, A.; Senses, K.M.; Ulewicz, K.; Witt, K.; Szymanska, J.; Taciak, B.; Klopfleisch, R.; Hellmén, E.; Dolka, I.; et al. MicroRNA Expression Patterns in Canine Mammary Cancer Show Significant Differences between Metastatic and Non-Metastatic Tumours. BMC Cancer 2017, 17, 728. [Google Scholar] [CrossRef] [PubMed]
- Fish, E.J.; Irizarry, K.J.; DeInnocentes, P.; Ellis, C.J.; Prasad, N.; Moss, A.G.; Curt Bird, R. Malignant Canine Mammary Epithelial Cells Shed Exosomes Containing Differentially Expressed microRNA That Regulate Oncogenic Networks. BMC Cancer 2018, 18, 832. [Google Scholar] [CrossRef]
- Cai, W.-Y.; Wei, T.-Z.; Luo, Q.-C.; Wu, Q.-W.; Liu, Q.-F.; Yang, M.; Ye, G.-D.; Wu, J.-F.; Chen, Y.-Y.; Sun, G.-B.; et al. The Wnt-β-Catenin Pathway Represses Let-7 microRNA Expression through Transactivation of Lin28 to Augment Breast Cancer Stem Cell Expansion. J. Cell Sci. 2013, 126, 2877–2889. [Google Scholar] [CrossRef]
- Chhabra, R.; Saini, N. MicroRNAs in Cancer Stem Cells: Current Status and Future Directions. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 8395–8405. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, J.D.; Barr, M.; Fennell, D.; Richard, D.; Reynolds, J.; O’Leary, J.; O’Byrne, K. The Cancer Stem-Cell Hypothesis: Its Emerging Role in Lung Cancer Biology and Its Relevance for Future Therapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2012, 7, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Rybicka, A.; Król, M. Identification and Characterization of Cancer Stem Cells in Canine Mammary Tumors. Acta Vet. Scand. 2016, 58, 86. [Google Scholar] [CrossRef]
- Rybicka, A.; Mucha, J.; Majchrzak, K.; Taciak, B.; Hellmen, E.; Motyl, T.; Krol, M. Analysis of microRNA Expression in Canine Mammary Cancer Stem-like Cells Indicates Epigenetic Regulation of Transforming Growth Factor-Beta Signaling. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2015, 66, 29–37. [Google Scholar]
- Im, K.S.; Jang, Y.G.; Shin, J.I.; Kim, N.H.; Lim, H.Y.; Lee, S.M.; Kim, J.H.; Sur, J.H. CD44+/CD24- Cancer Stem Cells Are Associated with Higher Grade of Canine Mammary Carcinomas. Vet. Pathol. 2015, 52, 1041–1044. [Google Scholar] [CrossRef]
- Papazoglou, L. Current Surgical Options for Mammary Tumor Removal in Dogs. J. Vet. Sci. Med. 2012, 1, 6. [Google Scholar] [CrossRef]
- Bartels, K.E.; Ferguson, H.R.; Gillette, E.L.; Ferguson, H.L. Simultaneous Bilateral Mastectomy in the Dog. Vet. Surg. 1978, 7, 97–102. [Google Scholar] [CrossRef]
- Novosad, C.A. Principles of Treatment for Mammary Gland Tumors. Clin. Tech. Small Anim. Pract. 2003, 18, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Coomer, A.; Farese, J.; Milner, R.; Liptak, J.; Bacon, N.; Lurie, D. Radiation Therapy for Canine Appendicular Osteosarcoma. Vet. Comp. Oncol. 2009, 7, 15–27. [Google Scholar] [CrossRef]
- Elliot, K.M.; Mayer, M.N. Radiation Therapy for Tumors of the Nasal Cavity and Paranasal Sinuses in Dogs. Can. Vet. J. 2009, 50, 309–312. [Google Scholar]
- Buchholz, J.; Hagen, R.; Leo, C.; Ebling, A.; Roos, M.; Kaser-Hotz, B.; Bley, C.R. 3D Conformal Radiation Therapy for Palliative Treatment of Canine Nasal Tumors. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2009, 50, 679–683. [Google Scholar] [CrossRef]
- Lombardi, P.; Florio, S.; Pagnini, U.; Crispino, A.; Avallone, L. Ovarian Function Suppression with a GnRH Analogue: D-Ser(But[t])[6]-Arzgly[10]-LHRH (Goserelin) in Hormone Dependent Canine Mammary Cancer. J. Vet. Pharmacol. Ther. 1999, 22, 56–61. [Google Scholar] [CrossRef]
- Stuart-Harris, R.; Davis, A. Optimal Adjuvant Endocrine Therapy for Early Breast Cancer. Women’s Health Lond. Engl. 2010, 6, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.S.; Dobson, J.M.; Bostock, D.E. Use of Tamoxifen in the Control of Canine Mammary Neoplasia. Vet. Rec. 1993, 133, 539–542. [Google Scholar] [CrossRef]
- Guil-Luna, S.; Sánchez-Céspedes, R.; Millán, Y.; De Andrés, F.J.; Rollón, E.; Domingo, V.; Guscetti, F.; Martín de Las Mulas, J. Aglepristone Decreases Proliferation in Progesterone Receptor-Positive Canine Mammary Carcinomas. J. Vet. Intern. Med. 2011, 25, 518–523. [Google Scholar] [CrossRef]
- Karayannopoulou, M.; Kaldrymidou, E.; Constantinidis, T.C.; Dessiris, A. Adjuvant Post-Operative Chemotherapy in Bitches with Mammary Cancer. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2001, 48, 85–96. [Google Scholar] [CrossRef]
- Clemente, M.; De Andrés, P.J.; Peña, L.; Pérez-Alenza, M.D. Survival Time of Dogs with Inflammatory Mammary Cancer Treated with Palliative Therapy Alone or Palliative Therapy plus Chemotherapy. Vet. Rec. 2009, 165, 78–81. [Google Scholar] [CrossRef]
- Dominguez, P.A.; Dervisis, N.G.; Cadile, C.D.; Sarbu, L.; Kitchell, B.E. Combined Gemcitabine and Carboplatin Therapy for Carcinomas in Dogs. J. Vet. Intern. Med. 2009, 23, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Lorenzo, R.M.; Abramo, F.; Ratto, A.; Zini, E. Adjuvant Gemcitabine after Surgical Removal of Aggressive Malignant Mammary Tumours in Dogs. Vet. Comp. Oncol. 2008, 6, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Schoenrock, D.; Baumgärtner, W.; Nolte, I. Postoperative Adjuvant Treatment of Invasive Malignant Mammary Gland Tumors in Dogs with Doxorubicin and Docetaxel. J. Vet. Intern. Med. 2006, 20, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- de M. Souza, C.H.; Toledo-Piza, E.; Amorin, R.; Barboza, A.; Tobias, K.M. Inflammatory Mammary Carcinoma in 12 Dogs: Clinical Features, Cyclooxygenase-2 Expression, and Response to Piroxicam Treatment. Can. Vet. J. 2009, 50, 506–510. [Google Scholar]
- Knapp, D.W.; Glickman, N.W.; Mohammed, S.I.; DeNicola, D.B.; Widmer, W.R.; Bonney, P.L.; DeGortari, A.E. Antitumor Effects of Piroxicam in Spontaneous Canine Invasive Urinary Bladder Cancer, a Relevant Model of Human Invasive Bladder Cancer. In Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury, 5; Honn, K.V., Marnett, L.J., Nigam, S., Dennis, E., Serhan, C., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2002; Volume 507, pp. 377–380. [Google Scholar] [CrossRef]
- Knapp, D.W.; Glickman, N.W.; Widmer, W.R.; DeNicola, D.B.; Adams, L.G.; Kuczek, T.; Bonney, P.L.; DeGortari, A.E.; Han, C.; Glickman, L.T. Cisplatin versus Cisplatin Combined with Piroxicam in a Canine Model of Human Invasive Urinary Bladder Cancer. Cancer Chemother. Pharmacol. 2000, 46, 221–226. [Google Scholar] [CrossRef]
- Valdivia, G.; Alonso-Diez, Á.; Pérez-Alenza, D.; Peña, L. From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Vet. Sci. 2021, 8, 623800. [Google Scholar] [CrossRef]
- Derry, S.; Loke, Y.K. Risk of Gastrointestinal Haemorrhage with Long Term Use of Aspirin: Meta-Analysis. BMJ 2000, 321, 1183–1187. [Google Scholar] [CrossRef]
- Arun, B.; Goss, P. The Role of COX-2 Inhibition in Breast Cancer Treatment and Prevention. Semin. Oncol. 2004, 31, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gurpinar, E.; Grizzle, W.E.; Piazza, G.A. NSAIDs Inhibit Tumorigenesis, but How? Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 1104–1113. [Google Scholar] [CrossRef]
- Iturriaga, M.P.; Paredes, R.; Arias, J.I.; Torres, C.G. Meloxicam Decreases the Migration and Invasion of CF41.Mg Canine Mammary Carcinoma Cells. Oncol. Lett. 2017, 14, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Ustün Alkan, F.; Ustüner, O.; Bakırel, T.; Cınar, S.; Erten, G.; Deniz, G. The Effects of Piroxicam and Deracoxib on Canine Mammary Tumour Cell Line. Sci. World J. 2012, 2012, 976740. [Google Scholar] [CrossRef]
- Sonzogni-Desautels, K.; Knapp, D.W.; Sartin, E.; Doré, M. Effect of Cyclooxygenase Inhibitors in a Xenograft Model of Canine Mammary Tumours. Vet. Comp. Oncol. 2011, 9, 161–171. [Google Scholar] [CrossRef]
- Tamura, D.; Saito, T.; Murata, K.; Kawashima, M.; Asano, R. Celecoxib Exerts Antitumor Effects in Canine Mammary Tumor Cells via COX-2-independent Mechanisms. Int. J. Oncol. 2015, 46, 1393–1404. [Google Scholar] [CrossRef]
- Hurst, E.A.; Pang, L.Y.; Argyle, D.J. The Selective Cyclooxygenase-2 Inhibitor Mavacoxib (Trocoxil) Exerts Anti-Tumour Effects in Vitro Independent of Cyclooxygenase-2 Expression Levels. Vet. Comp. Oncol. 2019, 17, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Arenas, C.; Peña, L.; Granados-Soler, J.L.; Pérez-Alenza, M.D. Adjuvant Therapy for Highly Malignant Canine Mammary Tumours: Cox-2 Inhibitor versus Chemotherapy: A Case-Control Prospective Study. Vet. Rec. 2016, 179, 125. [Google Scholar] [CrossRef] [PubMed]
- Brandi, A.; de Faria Lainetti, P.; Elias, F.; Rodrigues, M.M.P.; Fagundes Moraes, L.; Laufer-Amorim, R.; de Camargo, L.S.; Salles Gomes, C.d.O.M.; Fonseca-Alves, C.E. Firocoxib as a Potential Neoadjuvant Treatment in Canine Patients with Triple-Negative Mammary Gland Tumors. Animals 2023, 13, 60. [Google Scholar] [CrossRef]
- Mpekris, F.; Voutouri, C.; Panagi, M.; Baish, J.W.; Jain, R.K.; Stylianopoulos, T. Normalizing Tumor Microenvironment with Nanomedicine and Metronomic Therapy to Improve Immunotherapy. J. Control. Release 2022, 345, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Elmslie, R.E.; Glawe, P.; Dow, S.W. Metronomic Therapy with Cyclophosphamide and Piroxicam Effectively Delays Tumor Recurrence in Dogs with Incompletely Resected Soft Tissue Sarcomas. J. Vet. Intern. Med. 2008, 22, 1373–1379. [Google Scholar] [CrossRef]
- Setyo, L.; Ma, M.; Bunn, T.; Wyatt, K.; Wang, P. Furosemide for Prevention of Cyclophosphamide-Associated Sterile Haemorrhagic Cystitis in Dogs Receiving Metronomic Low-Dose Oral Cyclophosphamide. Vet. Comp. Oncol. 2017, 15, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Ranieri, G.; Patruno, R.; Ruggieri, E.; Montemurro, S.; Valerio, P.; Ribatti, D. Vascular Endothelial Growth Factor (VEGF) as a Target of Bevacizumab in Cancer: From the Biology to the Clinic. Curr. Med. Chem. 2006, 13, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yan, H.; Zhao, P.; Yang, Y.; Cao, B. Efficacy and Safety of Bevacizumab Combined with Chemotherapy for Managing Metastatic Breast Cancer: A Meta-Analysis of Randomized Controlled Trials. Sci. Rep. 2015, 5, 15746. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yao, H.; Hu, H. Disrupting Tumor Angiogenesis and “the Hunger Games” for Breast Cancer. In Translational Research in Breast Cancer: Biomarker Diagnosis, Targeted Therapies and Approaches to Precision Medicine; Song, E., Hu, H., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2017; pp. 171–195. ISBN 978-981-10-6020-5. [Google Scholar]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef]
- Prado, M.C.M.; Macedo, S.d.A.L.; Guiraldelli, G.G.; de Faria Lainetti, P.; Leis-Filho, A.F.; Kobayashi, P.E.; Laufer-Amorim, R.; Fonseca-Alves, C.E. Investigation of the Prognostic Significance of Vasculogenic Mimicry and Its Inhibition by Sorafenib in Canine Mammary Gland Tumors. Front. Oncol. 2019, 9, 1445. [Google Scholar] [CrossRef]
- Kennedy, K.C.; Qurollo, B.A.; Rose, B.J.; Thamm, D.H. Epidermal Growth Factor Enhances the Malignant Phenotype in Canine Mammary Carcinoma Cell Lines. Vet. Comp. Oncol. 2011, 9, 196–206. [Google Scholar] [CrossRef]
- Jang, H.; Choi, J.; Park, J.-K.; Won, G.; Seol, J.-W. Fucoxanthin Exerts Anti-Tumor Activity on Canine Mammary Tumor Cells via Tumor Cell Apoptosis Induction and Angiogenesis Inhibition. Animals 2021, 11, 1512. [Google Scholar] [CrossRef]
- Sánchez, D.; Cesarman-Maus, G.; Amador-Molina, A.; Lizano, M. Oncolytic Viruses for Canine Cancer Treatment. Cancers 2018, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Al-Shammari, A.M.; Al-Mudhafr, M.A.; Chalap Al- Grawi, E.D.; Al-Hili, Z.A.; Yaseen, N. Newcastle Disease Virus Suppresses Angiogenesis in Mammary Adenocarcinoma Models. Bulg. J. Vet. Med. 2022, 25, 33–45. [Google Scholar] [CrossRef]
- Hwang, C.C.; Umeki, S.; Kubo, M.; Hayashi, T.; Shimoda, H.; Mochizuki, M.; Maeda, K.; Baba, K.; Hiraoka, H.; Coffey, M.; et al. Oncolytic Reovirus in Canine Mast Cell Tumor. PLoS ONE 2013, 8, e73555. [Google Scholar] [CrossRef]
- Gentschev, I.; Adelfinger, M.; Josupeit, R.; Rudolph, S.; Ehrig, K.; Donat, U.; Weibel, S.; Chen, N.G.; Yu, Y.A.; Zhang, Q.; et al. Preclinical Evaluation of Oncolytic Vaccinia Virus for Therapy of Canine Soft Tissue Sarcoma. PLoS ONE 2012, 7, e37239. [Google Scholar] [CrossRef] [PubMed]
- Laborda, E.; Puig-Saus, C.; Rodriguez-García, A.; Moreno, R.; Cascalló, M.; Pastor, J.; Alemany, R. A pRb-Responsive, RGD-Modified, and Hyaluronidase-Armed Canine Oncolytic Adenovirus for Application in Veterinary Oncology. Mol. Ther. J. Am. Soc. Gene Ther. 2014, 22, 986–998. [Google Scholar] [CrossRef]
- Shoji, K.; Yoneda, M.; Fujiyuki, T.; Amagai, Y.; Tanaka, A.; Matsuda, A.; Ogihara, K.; Naya, Y.; Ikeda, F.; Matsuda, H.; et al. Development of New Therapy for Canine Mammary Cancer with Recombinant Measles Virus. Mol. Ther.-Oncolytics 2016, 3, 15022. [Google Scholar] [CrossRef]
- Suter, S.E.; Chein, M.B.; von Messling, V.; Yip, B.; Cattaneo, R.; Vernau, W.; Madewell, B.R.; London, C.A. In Vitro Canine Distemper Virus Infection of Canine Lymphoid Cells: A Prelude to Oncolytic Therapy for Lymphoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 1579–1587. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Chen, G.; Zhang, X.; Lin, D.; Zhou, Y.; Yu, Y.; Liu, W.; Zhang, D. Oncolytic Activity of Canine Distemper Virus in Canine Mammary Tubular Adenocarcinoma Cells. Vet. Comp. Oncol. 2019, 17, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The Growing Role of Precision and Personalized Medicine for Cancer Treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2011; ISBN 978-0-309-22222-8. [Google Scholar]
- Verma, M. Personalized Medicine and Cancer. J. Pers. Med. 2012, 2, 1–14. [Google Scholar] [CrossRef]
- Cheng, Y.Q.; Wang, S.B.; Liu, J.H.; Jin, L.; Liu, Y.; Li, C.Y.; Su, Y.R.; Liu, Y.R.; Sang, X.; Wan, Q.; et al. Modifying the Tumour Microenvironment and Reverting Tumour Cells: New Strategies for Treating Malignant Tumours. Cell Prolif. 2020, 53, e12865. [Google Scholar] [CrossRef]
- Gray, M.; Meehan, J.; Turnbull, A.K.; Martínez-Pérez, C.; Kay, C.; Pang, L.Y.; Argyle, D.J. The Importance of the Tumor Microenvironment and Hypoxia in Delivering a Precision Medicine Approach to Veterinary Oncology. Front. Vet. Sci. 2020, 7, 598338. [Google Scholar] [CrossRef] [PubMed]
- Supplitt, S.; Karpinski, P.; Sasiadek, M.; Laczmanska, I. Current Achievements and Applications of Transcriptomics in Personalized Cancer Medicine. Int. J. Mol. Sci. 2021, 22, 1422. [Google Scholar] [CrossRef]
- Buzdin, A.; Sorokin, M.; Garazha, A.; Glusker, A.; Aleshin, A.; Poddubskaya, E.; Sekacheva, M.; Kim, E.; Gaifullin, N.; Giese, A.; et al. RNA Sequencing for Research and Diagnostics in Clinical Oncology. Semin. Cancer Biol. 2020, 60, 311–323. [Google Scholar] [CrossRef]
- Jaeger, P.A.; Doherty, C.; Ideker, T. Modeling Transcriptome Dynamics in a Complex World. Cell 2012, 151, 1161–1162. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Lönnberg, T. Global and Targeted Approaches to Single-Cell Transcriptome Characterization. Brief. Funct. Genom. 2018, 17, 209–219. [Google Scholar] [CrossRef]
- Larance, M.; Lamond, A.I. Multidimensional Proteomics for Cell Biology. Nat. Rev. Mol. Cell Biol. 2015, 16, 269–280. [Google Scholar] [CrossRef]
- Monteiro, M.S.; Carvalho, M.; Bastos, M.L.; Guedes de Pinho, P. Metabolomics Analysis for Biomarker Discovery: Advances and Challenges. Curr. Med. Chem. 2013, 20, 257–271. [Google Scholar] [CrossRef]
- Esteva, F.J. Monoclonal Antibodies, Small Molecules, and Vaccines in the Treatment of Breast Cancer. Oncologist 2004, 9 (Suppl. S3), 4–9. [Google Scholar] [CrossRef] [PubMed]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer Immunotherapy Comes of Age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards Personalized, Tumour-Specific, Therapeutic Vaccines for Cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]
- Ye, B.; Stary, C.M.; Li, X.; Gao, Q.; Kang, C.; Xiong, X. Engineering Chimeric Antigen Receptor-T Cells for Cancer Treatment. Mol. Cancer 2018, 17, 32. [Google Scholar] [CrossRef]
- Morris, E.C.; Stauss, H.J. Optimizing T-Cell Receptor Gene Therapy for Hematologic Malignancies. Blood 2016, 127, 3305–3311. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cardona, X.E.; Luo, W.; Mohammed, S.I. Advances and Challenges of CAR T Therapy and Suitability of Animal Models (Review). Mol. Clin. Oncol. 2022, 17, 134. [Google Scholar] [CrossRef]
- Klopfleisch, R. Personalised Medicine in Veterinary Oncology: One to Cure Just One. Vet. J. 2015, 205, 128–135. [Google Scholar] [CrossRef]
- London, C.A. Tyrosine Kinase Inhibitors in Veterinary Medicine. Top. Companion Anim. Med. 2009, 24, 106–112. [Google Scholar] [CrossRef] [PubMed]
- London, C.; Mathie, T.; Stingle, N.; Clifford, C.; Haney, S.; Klein, M.K.; Beaver, L.; Vickery, K.; Vail, D.M.; Hershey, B.; et al. Preliminary Evidence for Biologic Activity of Toceranib Phosphate (Palladia®) in Solid Tumors. Vet. Comp. Oncol. 2012, 10, 194–205. [Google Scholar] [CrossRef]
- London, C.A.; Hannah, A.L.; Zadovoskaya, R.; Chien, M.B.; Kollias-Baker, C.; Rosenberg, M.; Downing, S.; Post, G.; Boucher, J.; Shenoy, N.; et al. Phase I Dose-Escalating Study of SU11654, a Small Molecule Receptor Tyrosine Kinase Inhibitor, in Dogs with Spontaneous Malignancies. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2003, 9, 2755–2768. [Google Scholar]
- Gattino, F.; Maniscalco, L.; Iussich, S.; Biasato, I.; Martano, M.; Morello, E.; Gola, C.; Ruiz, Y.M.; Saeki, N.; Buracco, P.; et al. PDGFR-α, PDGFR-β, VEGFR-2 and CD117 Expression in Canine Mammary Tumours and Evaluation of the in Vitro Effects of Toceranib Phosphate in Neoplastic Mammary Cell Lines. Vet. Rec. 2018, 183, 221. [Google Scholar] [CrossRef]
- Hahn, K.A.; Oglivie, G.; Rusk, T.; Devauchelle, P.; Leblanc, A.; Legendre, A.; Powers, B.; Leventhal, P.S.; Kinet, J.-P.; Palmerini, F.; et al. Masitinib Is Safe and Effective for the Treatment of Canine Mast Cell Tumors. J. Vet. Intern. Med. 2008, 22, 1301–1309. [Google Scholar] [CrossRef]
- Hernandez, B.; Adissu, H.A.; Wei, B.-R.; Michael, H.T.; Merlino, G.; Simpson, R.M. Naturally Occurring Canine Melanoma as a Predictive Comparative Oncology Model for Human Mucosal and Other Triple Wild-Type Melanomas. Int. J. Mol. Sci. 2018, 19, 394. [Google Scholar] [CrossRef]
- Wang, G.; Wu, M.; Maloneyhuss, M.A.; Wojcik, J.; Durham, A.C.; Mason, N.J.; Roth, D.B. Actionable Mutations in Canine Hemangiosarcoma. PLoS ONE 2017, 12, e0188667. [Google Scholar] [CrossRef] [PubMed]
- Monks, N.R.; Cherba, D.M.; Kamerling, S.G.; Simpson, H.; Rusk, A.W.; Carter, D.; Eugster, E.; Mooney, M.; Sigler, R.; Steensma, M.; et al. A Multi-Site Feasibility Study for Personalized Medicine in Canines with Osteosarcoma. J. Transl. Med. 2013, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Mealey, K.L.; Martinez, S.E.; Villarino, N.F.; Court, M.H. Personalized Medicine: Going to the Dogs? Hum. Genet. 2019, 138, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Klopfleisch, R.; Klose, P.; Gruber, A.D. The Combined Expression Pattern of BMP2, LTBP4, and DERL1 Discriminates Malignant from Benign Canine Mammary Tumors. Vet. Pathol. 2010, 47, 446–454. [Google Scholar] [CrossRef]
- Richards, K.L.; Motsinger-Reif, A.A.; Chen, H.-W.; Fedoriw, Y.; Fan, C.; Nielsen, D.M.; Small, G.W.; Thomas, R.; Smith, C.; Dave, S.S.; et al. Gene Profiling of Canine B-Cell Lymphoma Reveals Germinal Center and Postgerminal Center Subtypes with Different Survival Times, Modeling Human DLBCL. Cancer Res. 2013, 73, 5029–5039. [Google Scholar] [CrossRef] [PubMed]
- Frantz, A.M.; Sarver, A.L.; Ito, D.; Phang, T.L.; Karimpour-Fard, A.; Scott, M.C.; Valli, V.E.O.; Lindblad-Toh, K.; Burgess, K.E.; Husbands, B.D.; et al. Molecular Profiling Reveals Prognostically Significant Subtypes of Canine Lymphoma. Vet. Pathol. 2013, 50, 693–703. [Google Scholar] [CrossRef]
- Gaines, P.J.; Powell, T.D.; Walmsley, S.J.; Estredge, K.L.; Wisnewski, N.; Stinchcomb, D.T.; Withrow, S.J.; Lana, S.E. Identification of Serum Biomarkers for Canine B-Cell Lymphoma by Use of Surface-Enhanced Laser Desorption-Ionization Time-of-Flight Mass Spectrometry. Am. J. Vet. Res. 2007, 68, 405–410. [Google Scholar] [CrossRef]
- Zamani-Ahmadmahmudi, M.; Nassiri, S.M.; Rahbarghazi, R. Serological Proteome Analysis of Dogs with Breast Cancer Unveils Common Serum Biomarkers with Human Counterparts. Electrophoresis 2014, 35, 901–910. [Google Scholar] [CrossRef]
- da Costa, A.; Kohn, B.; Gruber, A.D.; Klopfleisch, R. Multiple RT-PCR Markers for the Detection of Circulating Tumour Cells of Metastatic Canine Mammary Tumours. Vet. J. 2013, 196, 34–39. [Google Scholar] [CrossRef]
- Abadie, J.; Nguyen, F.; Loussouarn, D.; Peña, L.; Gama, A.; Rieder, N.; Belousov, A.; Bemelmans, I.; Jaillardon, L.; Ibisch, C.; et al. Canine Invasive Mammary Carcinomas as Models of Human Breast Cancer. Part 2: Immunophenotypes and Prognostic Significance. Breast Cancer Res. Treat. 2018, 167, 459–468. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Moskwa, N.; Kang, W.; Fan, T.M.; Lee, C. Canine as a Comparative and Translational Model for Human Mammary Tumor. J. Breast Cancer 2023, 26, 1–13. [Google Scholar] [CrossRef]
- Razazan, A.; Behravan, J.; Arab, A.; Barati, N.; Arabi, L.; Gholizadeh, Z.; Hatamipour, M.; Reza Nikpoor, A.; Momtazi-Borojeni, A.A.; Mosaffa, F.; et al. Conjugated Nanoliposome with the HER2/Neu-Derived Peptide GP2 as an Effective Vaccine against Breast Cancer in Mice Xenograft Model. PLoS ONE 2017, 12, e0185099. [Google Scholar] [CrossRef]
- Farzad, N.; Barati, N.; Momtazi-Borojeni, A.A.; Yazdani, M.; Arab, A.; Razazan, A.; Shariat, S.; Mansourian, M.; Abbasi, A.; Saberi, Z.; et al. P435 HER2/Neu-Derived Peptide Conjugated to Liposomes Containing DOPE as an Effective Prophylactic Vaccine Formulation for Breast Cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 664–672. [Google Scholar] [CrossRef]
- Muhammadnejad, A.; Keyhani, E.; Mortazavi, P.; Behjati, F.; Haghdoost, I.S. Overexpression of Her-2/Neu in Malignant Mammary Tumors; Translation of Clinicopathological Features from Dog to Human. Asian Pac. J. Cancer Prev. 2012, 13, 6415–6421. [Google Scholar] [CrossRef]
- Gao, T.; Cen, Q.; Lei, H. A Review on Development of MUC1-Based Cancer Vaccine. Biomed. Pharmacother. 2020, 132, 110888. [Google Scholar] [CrossRef]
- Gheybi, E.; Salmanian, A.H.; Fooladi, A.A.I.; Salimian, J.; Hosseini, H.M.; Halabian, R.; Amani, J. Immunogenicity of Chimeric MUC1-HER2 Vaccine against Breast Cancer in Mice. Iran. J. Basic Med. Sci. 2018, 21, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Klein Wolterink, R.G.J.; Gulaia, V.; Cloosen, S.; Ehlers, F.A.I.; Wieten, L.; Graus, Y.F.; Bos, G.M.J.; Germeraad, W.T.V. Defucosylation of Tumor-Specific Humanized Anti-MUC1 Monoclonal Antibody Enhances NK Cell-Mediated Anti-Tumor Cell Cytotoxicity. Cancers 2021, 13, 2579. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yazdanifar, M.; Das Roy, L.; Whilding, L.M.; Gavrill, A.; Maher, J.; Mukherjee, P. CAR T Cells Targeting the Tumor MUC1 Glycoprotein Reduce Triple-Negative Breast Cancer Growth. Front. Immunol. 2019, 10, 1149. [Google Scholar] [CrossRef] [PubMed]
- Fürdös, I.; Fazekas, J.; Singer, J.; Jensen-Jarolim, E. Translating Clinical Trials from Human to Veterinary Oncology and Back. J. Transl. Med. 2015, 13, 265. [Google Scholar] [CrossRef]
- Vafaei, R.; Samadi, M.; Hosseinzadeh, A.; Barzaman, K.; Esmailinejad, M.; Khaki, Z.; Farahmand, L. Comparison of Mucin-1 in Human Breast Cancer and Canine Mammary Gland Tumor: A Review Study. Cancer Cell Int. 2022, 22, 14. [Google Scholar] [CrossRef] [PubMed]




 | Duct ectasia |
| Lobular hyperplasia (adenosis) | |
| Epitheliosis | |
| Papillomatosis | |
 | Simple benign tumors |
| Non-simple benign tumors | |
| Ductal-associated benign tumors | |
 | Simple carcinomas |
| Non-simple carcinoma | |
| Ductal-associated carcinoma | |
 | Squamous cell carcinoma |
| Adenosquamous carcinoma | |
| Mucinous carcinoma | |
| Lipid-rich (secretory) carcinoma | |
| Spindle cell carcinoma | |
| Malignant myoepithelioma | |
 | Osteosarcoma |
| Chondrosarcoma | |
| Fibrosarcoma | |
| Hemangiosarcoma | |
| Other sarcomas | |
 | |
 | Melanosis of the skin of the teat |
| Hyperplasia of the teat | |
 | Benign ductal-associated neoplasms |
| Malignant ductal-associated neoplasms | |
| Carcinoma with epidermal infiltration (Paget-like disease) |
| Grade of Malignancy | Tumor Differentiation | Total Score |
|---|---|---|
| I. | well-differentiated | 3–5 |
| II. | moderately differentiated | 6–7 |
| III. | poorly differentiated | 8–9 |
 | Toceranib |
| Masitinib | |
| Imatinib | |
 | Nanoliposomes conjugated with HER-2-derived peptide |
| MUC-1 vaccine | |
| Chimeric MUC-1/HER-2 vaccine | |
 | Anti-MUC-1 specific CAR T-cells |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosalova, N.; Huniadi, M.; Horňáková, Ľ.; Valenčáková, A.; Horňák, S.; Nagoos, K.; Vozar, J.; Cizkova, D. Canine Mammary Tumors: Classification, Biomarkers, Traditional and Personalized Therapies. Int. J. Mol. Sci. 2024, 25, 2891. https://doi.org/10.3390/ijms25052891
Nosalova N, Huniadi M, Horňáková Ľ, Valenčáková A, Horňák S, Nagoos K, Vozar J, Cizkova D. Canine Mammary Tumors: Classification, Biomarkers, Traditional and Personalized Therapies. International Journal of Molecular Sciences. 2024; 25(5):2891. https://doi.org/10.3390/ijms25052891
Chicago/Turabian StyleNosalova, Natalia, Mykhailo Huniadi, Ľubica Horňáková, Alexandra Valenčáková, Slavomir Horňák, Kamil Nagoos, Juraj Vozar, and Dasa Cizkova. 2024. "Canine Mammary Tumors: Classification, Biomarkers, Traditional and Personalized Therapies" International Journal of Molecular Sciences 25, no. 5: 2891. https://doi.org/10.3390/ijms25052891
APA StyleNosalova, N., Huniadi, M., Horňáková, Ľ., Valenčáková, A., Horňák, S., Nagoos, K., Vozar, J., & Cizkova, D. (2024). Canine Mammary Tumors: Classification, Biomarkers, Traditional and Personalized Therapies. International Journal of Molecular Sciences, 25(5), 2891. https://doi.org/10.3390/ijms25052891






