Animals in Respiratory Research
Abstract
1. Introduction
2. History of Animal Experimentation in Medical Research
3. Differences in the Respiratory Tract of Commonly Used Animals
4. Physiology and Anatomy
5. Histology
6. Use of Healthy Animals
7. Respiratory Diseases
Prevalence
8. Disease Models
8.1. Asthma
8.1.1. Rodents
8.1.2. Other Small Laboratory Animals
8.1.3. Larger Animals
8.2. Chronic Obstructive Pulmonary Disease (COPD)
8.2.1. Rodents
8.2.2. Other Small Laboratory Animals
8.2.3. Larger Animals
8.3. Cystic Fibrosis (CF)
8.3.1. Rodents
8.3.2. Other Small Laboratory Animals
8.3.3. Larger Animals
8.4. Pulmonary Hypertension (PH)
8.4.1. Rodents
8.4.2. Other Small Laboratory Animals
8.4.3. Larger Animals
8.5. Idiopathic Pulmonary Fibrosis (IPF)
8.5.1. Rodents
8.5.2. Other Small Laboratory Animals
8.5.3. Larger Animals
8.6. Tuberculosis (Tbc)
8.6.1. Rodents
8.6.2. Other Small Laboratory Animals
8.6.3. Larger Animals
8.7. Non-Mammalian Models
8.7.1. Zebrafish
8.7.2. Fruit Fly
9. General Aspects of Inhalation Testing in Animal Models
Induced Models versus Gene Editing Models
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- OECD. OECD Guidelines on the Testing of Chemicals; 28-Day (Subacute) Inhalation Toxicity Study. No. 412; OECD: Paris, France, 2018. [Google Scholar]
- Fitzgerald, D.; Creaner, G. Pharmaceutical Regulations, Organizations and Quality Standards You Must Know to Make Safe Medicines and Medical Devices. GetReskilled. 2024. Available online: https://www.getreskilled.com/pharmaceutical-companies/regulations/ (accessed on 11 November 2023).
- Kimmelman, J.; Federico, C. Consider drug efficacy before first-in-human trials. Nature 2017, 542, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.J.; Constantino, H.; Seidle, T. Phase-In to Phase-Out-Targeted, Inclusive Strategies Are Needed to Enable Full Replacement of Animal Use in the European Union. Animals 2022, 12, 863. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Animal testing and medicine. Heart Views 2011, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.S. Ibn Zuhr and experimental tracheostomy and tracheotomy. J. Am. Coll. Surg. 2004, 199, 665. [Google Scholar] [CrossRef] [PubMed]
- Franco, N.H. Animal Experiments in Biomedical Research: A Historical Perspective. Animals 2013, 3, 238–273. [Google Scholar] [CrossRef] [PubMed]
- Berkowitz, C. Disputed discovery: Vivisection and experiment in the 19th century. Endeavour 2006, 30, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Normandin, S. Claude Bernard and an introduction to the study of experimental medicine: “physical vitalism”, dialectic, and epistemology. J. Hist. Med. Allied Sci. 2007, 62, 495–528. [Google Scholar] [CrossRef]
- Ryan, A.H. History of the British Act of 1876: An act to amend the law relating to cruelty to animals. J. Med. Educ. 1963, 38, 182–194. [Google Scholar]
- Lam, C.; Patel, P. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Society, R.D.; Research, U.A.; Progress, C.f.M. Medical Advances and Animal Research: The Contribution of Animal Science to the Medical Revolution: Some Case Histories; Understanding Animal Research: London, UK, 2007. [Google Scholar]
- Hanahan, D.; Wagner, E.F.; Palmiter, R.D. The origins of oncomice: A history of the first transgenic mice genetically engineered to develop cancer. Genes Dev. 2007, 21, 2258–2270. [Google Scholar] [CrossRef]
- Bayne, K.; Morris, T.; France, M. Part 1: Implementing the Three Rs in Research Using Animals. In The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals; Hubrecht, R.C., Kirkwood, J., Eds.; Wiley: Hoboken, NJ, USA, 2010; pp. 107–123. [Google Scholar]
- Williams Lea Group. Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2022; Williams Lea Group: London, UK, 2023. Available online: https://www.gov.uk/government/statistics/statistics-of-scientific-procedures-on-living-animals-great-britain-2022/statistics-of-scientific-procedures-on-living-animals-great-britain-2022 (accessed on 1 February 2024).
- Rowan, A.N. The uncertain future of research chimpanzees. Science 2007, 315, 1493–1494. [Google Scholar]
- Understanding Animal Testing. Number of Animals Used. London. 2024. Available online: https://www.understandinganimalresearch.org.uk/what-is-animal-research/numbers-animals#GB (accessed on 3 February 2024).
- Understanding Animal Testing. Cat. London. 2024. Available online: https://www.understandinganimalresearch.org.uk/what-is-animal-research/a-z-animals/cat (accessed on 12 December 2023).
- Camphora, A.; Pucca, M. Horses used for large-scale production of immunoglobulins: An inter-species approach. Int. Anim. Health J. 2022, 8, 12–16. [Google Scholar]
- Li, H.; Li, H. Vaccines for Neglected Pathogens: Strategies, Achievements and Challenges: Focus on Leprosy, Leishmaniasis, Melioidosis and Tuberculosis; Christodoulides, M., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 139–170. [Google Scholar]
- Gehr, P.; Mwangi, D.K.; Ammann, A.; Maloiy, G.M.; Taylor, C.R.; Weibel, E.R. Design of the mammalian respiratory system. V. Scaling morphometric pulmonary diffusing capacity to body mass: Wild and domestic mammals. Respir. Physiol. 1981, 44, 61–86. [Google Scholar] [CrossRef] [PubMed]
- Bide, R.W.; Armour, S.J.; Yee, E. Allometric respiration/body mass data for animals to be used for estimates of inhalation toxicity to young adult humans. J. Appl. Toxicol. 2000, 20, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L. Learn How to Assess Normal Animal Vital Signs. 2023. Available online: https://www.yourvetonline.com/normal-animal-vital-signs/ (accessed on 14 November 2023).
- Ballard, S.T.; Inglis, S.K. Liquid secretion properties of airway submucosal glands. J. Physiol. 2004, 556, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.A.; Royer, C.M.; Pinkerton, K.E.; Schelegle, E.S. Nonhuman Primate Models of Respiratory Disease: Past, Present, and Future. ILAR J. 2017, 58, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Oakes, J.M.; Scadeng, M.; Breen, E.C.; Marsden, A.L.; Darquenne, C. Rat airway morphometry measured from in situ MRI-based geometric models. J. Appl. Physiol. 2012, 112, 1921–1931. [Google Scholar] [CrossRef] [PubMed]
- Schreider, J.P.; Hutchens, J.O. Morphology of the guinea pig respiratory tract. Anat. Rec. 1980, 196, 313–321. [Google Scholar] [CrossRef]
- Ramchandani, R.; Bates, J.H.; Shen, X.; Suki, B.; Tepper, R.S. Airway branching morphology of mature and immature rabbit lungs. J. Appl. Physiol. 2001, 90, 1584–1592. [Google Scholar] [CrossRef]
- Plopper, C.G.; Halsebo, J.E.; Berger, W.J.; Sonstegard, K.S.; Nettesheim, P. Distribution of nonciliated bronchiolar epithelial (Clara) cells in intra- and extrapulmonary airways of the rabbit. Exp. Lung Res. 1983, 5, 79–98. [Google Scholar] [CrossRef]
- Hajighasemi-Ossareh, M.; Borthwell, R.M.; Lachowicz-Scroggins, M.; Stevens, J.E.; Finkbeiner, W.E.; Widdicombe, J.H. Distribution and size of mucous glands in the ferret tracheobronchial tree. Anat. Rec. 2013, 296, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.P.; Venning, L.; Kyle, H.; Widdicombe, J.G. Quantitation of the secretory cells of the ferret tracheobronchial tree. J. Anat. 1986, 145, 173–188. [Google Scholar] [PubMed]
- Williams, A.; Orme, I.M. Animal Models of Tuberculosis: An Overview. Microbiol. Spectr. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C.R.; Jutkowitz, L.A.; Nelson, N.; Masseau, I.; Jennings, S.; Williams, K. Clinical features of canine pulmonary veno-occlusive disease and pulmonary capillary hemangiomatosis. J. Vet. Intern. Med. 2019, 33, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Phalen, R.F.; Oldham, M.J.; Wolff, R.K. The relevance of animal models for aerosol studies. J. Aerosol Med. Pulm. Drug Deliv. 2008, 21, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Meeusen, E.N.; Snibson, K.J.; Hirst, S.J.; Bischof, R.J. Sheep as a model species for the study and treatment of human asthma and other respiratory diseases. Drug Discov. Today Dis. Models 2009, 6, 101–106. [Google Scholar] [CrossRef]
- Kabilan, S.; Lin, C.L.; Hoffman, E.A. Characteristics of airflow in a CT-based ovine lung: A numerical study. J. Appl. Physiol. 2007, 102, 1469–1482. [Google Scholar] [CrossRef]
- Judge, E.P.; Hughes, J.M.; Egan, J.J.; Maguire, M.; Molloy, E.L.; O’Dea, S. Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am. J. Respir. Cell Mol. Biol. 2014, 51, 334–343. [Google Scholar] [CrossRef]
- Hyde, D.M.; Hamid, Q.; Irvin, C.G. Anatomy, pathology, and physiology of the tracheobronchial tree: Emphasis on the distal airways. J. Allergy Clin. Immunol. 2009, 124, S72–S77. [Google Scholar] [CrossRef]
- Baum, G.; Schaff, B. The tracheal bronchus. J. Thorac. Surg. 1957, 33, 282–286. [Google Scholar]
- Schlesinger, R.B. Comparative deposition of inhaled aerosols in experimental animals and humans: A review. J. Toxicol. Environ. Health 1985, 15, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, S.; Shiosaki, J.; Al Alam, D. FGF Signaling in Lung Development and Disease: Human Versus Mouse. Front. Genet. 2019, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Albertine, K.H. Utility of large-animal models of BPD: Chronically ventilated preterm lambs. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2015, 308, L983–L1001. [Google Scholar] [CrossRef] [PubMed]
- Hayatdavoudi, G.; O’Neil, J.J.; Barry, B.E.; Freeman, B.A.; Crapo, J.D. Pulmonary injury in rats following continuous exposure to 60% O2 for 7 days. J. Appl. Physiol. 1981, 51, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- López, A.; Martinson, S.A. Respiratory System, Mediastinum, and Pleurae. Pathol. Basis Vet. Dis. 2017, 471–560.e1. [Google Scholar] [CrossRef]
- Felicetti, S.A.; Wolff, R.K.; Muggenburg, B.A. Comparison of tracheal mucous transport in rats, guinea pigs, rabbits, and dogs. J. Appl. Physiol. 1981, 51, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Donnelley, M.; Morgan, K.S.; Awadalla, M.; Farrow, N.R.; Hall, C.; Parsons, D.W. High-resolution mucociliary transport measurement in live excised large animal trachea using synchrotron X-ray imaging. Respir. Res. 2017, 18, 95. [Google Scholar] [CrossRef] [PubMed]
- Burn, A.; Schneiter, M.; Ryser, M.; Gehr, P.; Rička, J.; Frenz, M. A quantitative interspecies comparison of the respiratory mucociliary clearance mechanism. Eur. Biophys. J. 2022, 51, 51–65. [Google Scholar] [CrossRef]
- Hofmann, W.; Asgharian, B. Comparison of mucociliary clearance velocities in human and rat lungs for extrapolation modeling. Ann. Occup. Hyg. 2002, 46 (Suppl. 1), 323–325. [Google Scholar] [CrossRef]
- Basil, M.C.; Morrisey, E.E. Lung regeneration: A tale of mice and men. Semin. Cell Dev. Biol. 2020, 100, 88–100. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Food and Drug Administration. Guidance for Industry S6 Preclinical Safety Evaluation of Biotechnology-Derived Pharmaceuticals; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1997. [Google Scholar]
- Chambers, D.; Huang, C.; Matthews, G. Section 2 Respiratory Physiology. The Lower Airways; Cambridge University Press: Cambridge, UK, 2019; pp. 24–29. [Google Scholar]
- Pinkerton, K.; Plopper, C.; Hyde, D.; Harkema, J.; Tyler, W.; Morgan, K.; St. George, J.; Kay, M.; Mariassay, A. Handbook of Human Toxicology; Massaro, E.J., Ed.; CRC Press: Boca Raton, FL, USA, 1997; pp. 469–491. [Google Scholar]
- Parekh, K.R.; Nawroth, J.; Pai, A.; Busch, S.M.; Senger, C.N.; Ryan, A.L. Stem cells and lung regeneration. Am. J. Physiol. Cell Physiol. 2020, 319, C675–C693. [Google Scholar] [CrossRef] [PubMed]
- Pavelka, M.; Ronge, H.R.; Stockinger, G. Comparative study of tracheal epithelium of different mammals. Acta Anat. 1976, 94, 262–282. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, J.S.; Sheats, M.K.; Cooper, B.; Bayless, R. Asthma: The Use of Animal Models and Their Translational Utility. Cells 2023, 12, 1091. [Google Scholar] [CrossRef]
- Plopper, C.G.; Heidsiek, J.G.; Weir, A.J.; George, J.A.; Hyde, D.M. Tracheobronchial epithelium in the adult rhesus monkey: A quantitative histochemical and ultrastructural study. Am. J. Anat. 1989, 184, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Runft, S.; Färber, I.; Krüger, J.; Schöne, K.; Lehmbecker, A.; Baumgärtner, W. In Vitro Characteristics of Canine Primary Tracheal Epithelial Cells Maintained at an Air-Liquid Interface Compared to In Vivo Morphology. Int. J. Mol. Sci. 2023, 24, 4987. [Google Scholar] [CrossRef] [PubMed]
- Boers, J.E.; Ambergen, A.W.; Thunnissen, F.B. Number and proliferation of basal and parabasal cells in normal human airway epithelium. Am. J. Respir. Crit. Care Med. 1998, 157, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Plopper, C.G.; Hill, L.H.; Mariassy, A.T. Ultrastructure of the nonciliated bronchiolar epithelial (Clara) cell of mammalian lung. III. A study of man with comparison of 15 mammalian species. Exp. Lung Res. 1980, 1, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Konkimalla, A.; Tata, A.; Tata, P.R. Lung Regeneration: Cells, Models, and Mechanisms. Cold Spring Harb. Perspect. Biol. 2022, 14, a040873. [Google Scholar] [CrossRef]
- Jones-Freeman, B.; Starkey, M.R. Bronchioalveolar stem cells in lung repair, regeneration and disease. J. Pathol. 2020, 252, 219–226. [Google Scholar] [CrossRef]
- Crystal, R.G. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2014, 190, 1355–1362. [Google Scholar] [CrossRef]
- Serrano-Mollar, A.; Gay-Jordi, G.; Guillamat-Prats, R.; Closa, D.; Hernandez-Gonzalez, F.; Marin, P.; Burgos, F.; Martorell, J.; Sánchez, M.; Arguis, P.; et al. Safety and Tolerability of Alveolar Type II Cell Transplantation in Idiopathic Pulmonary Fibrosis. Chest 2016, 150, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Cottage, C.T.; Peterson, N.; Kearley, J.; Berlin, A.; Xiong, X.; Huntley, A.; Zhao, W.; Brown, C.; Migneault, A.; Zerrouki, K.; et al. Targeting p16-induced senescence prevents cigarette smoke-induced emphysema by promoting IGF1/Akt1 signaling in mice. Commun. Biol. 2019, 2, 307. [Google Scholar] [CrossRef]
- Balestrini, J.L.; Gard, A.L.; Liu, A.; Leiby, K.L.; Schwan, J.; Kunkemoeller, B.; Calle, E.A.; Sivarapatna, A.; Lin, T.; Dimitrievska, S.; et al. Production of decellularized porcine lung scaffolds for use in tissue engineering. Integr. Biol. 2015, 7, 1598–1610. [Google Scholar] [CrossRef] [PubMed]
- Oizumi, H.; Kato, H.; Endoh, M.; Suzuki, J.; Watarai, H.; Hamada, A.; Suzuki, K.; Nakahashi, K.; Sadahiro, M. Swine model for training surgeons in minimally invasive anatomic lung segmentectomy. J. Vis. Surg. 2017, 3, 72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Noda, M.; Shibasaki, I.; Okada, Y. Acquisition of surgical technique by surgical training using a Swine model—Evaluation of the suture technique using a WKS-2 simulator. AME Med. J. 2022, 7, 12. [Google Scholar] [CrossRef]
- Reczyńska, K.; Tharkar, P.; Kim, S.Y.; Wang, Y.; Pamuła, E.; Chan, H.K.; Chrzanowski, W. Animal models of smoke inhalation injury and related acute and chronic lung diseases. Adv. Drug Deliv. Rev. 2018, 123, 107–134. [Google Scholar] [CrossRef] [PubMed]
- OECD. Repeated Dose Inhalation Toxicity: 28-Day or 14-Day Study; No. 412; OECD: Paris, France, 1981. [Google Scholar]
- Navarro-Torné, A.; Vidal, M.; Trzaska, D.K.; Passante, L.; Crisafulli, A.; Laang, H.; van de Loo, J.W.; Berkouk, K.; Draghia-Akli, R. Chronic respiratory diseases and lung cancer research: A perspective from the European Union. Eur. Respir. J. 2015, 46, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M. Lung Diseases Overview. 2022. Available online: https://www.webmd.com/lung/lung-diseases-overview (accessed on 14 November 2023).
- Rittiphairoj, T.; Reilly, A.; Reddy, C.; Barrenho, E.; Colombo, F.; Atun, R. The State of Cardiovascular Disease in G20+ Countries; Health Systems Innovation Lab, Harvard University: Cambridge, MA, USA, 2022. [Google Scholar] [CrossRef]
- Mortimer, K.; Lesosky, M.; García-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; El Sony, A.; Ellwood, P.; Marks, G.B.; et al. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN Phase I study. Eur. Respir. J. 2022, 60, 2102865. [Google Scholar] [CrossRef]
- Landry, J. List of 85+ Rare Lung Diseases: Ultimate Guide. 2024. Available online: https://www.respiratorytherapyzone.com/rare-lung-diseases/ (accessed on 14 November 2023).
- Ghada Mohammed, A.; Katie, K.; Amy, M.; Hussain, A.A.-O. Criteria to define rare diseases and orphan drugs: A systematic review protocol. BMJ Open 2022, 12, e062126. [Google Scholar] [CrossRef]
- European Medicines Agency. Development of Medicines for Rare Diseases; European Medicines Agency: London, UK, 2018. [Google Scholar]
- Gammie, T.; Lu, C.Y.; Babar, Z.U. Access to Orphan Drugs: A Comprehensive Review of Legislations, Regulations and Policies in 35 Countries. PLoS ONE 2015, 10, e0140002. [Google Scholar] [CrossRef]
- Lu, Y.; Han, J. The definition of rare disease in China and its prospects. Intractable Rare Dis. Res. 2022, 11, 29–30. [Google Scholar] [CrossRef]
- Doyle, A.; McGarry, M.P.; Lee, N.A.; Lee, J.J. The construction of transgenic and gene knockout/knockin mouse models of human disease. Transgenic Res. 2012, 21, 327–349. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.; Cho, A.; Limaye, A.; Cho, K.; Khillan, J.; Kulkarni, A.B. Genome Editing in Mice Using CRISPR/Cas9 Technology. Curr. Protoc. Cell Biol. 2018, 81, e57. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Rubio, G.A.; Limper, A.H.; Williams, K.; Elliot, S.J.; Ninou, I.; Aidinis, V.; Tzouvelekis, A.; Glassberg, M.K. Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis. Front. Med. 2017, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Walkin, L.; Herrick, S.E.; Summers, A.; Brenchley, P.E.; Hoff, C.M.; Korstanje, R.; Margetts, P.J. The role of mouse strain differences in the susceptibility to fibrosis: A systematic review. Fibrogenesis Tissue Repair 2013, 6, 18. [Google Scholar] [CrossRef]
- Gul, A.; Yang, F.; Xie, C.; Du, W.; Mohammadtursun, N.; Wang, B.; Le, J.; Dong, J. Pulmonary fibrosis model of mice induced by different administration methods of bleomycin. BMC Pulm. Med. 2023, 23, 91. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G. Harnessing opportunities in non-animal asthma research for a 21st-century science. Drug Discov. Today 2011, 16, 914–927. [Google Scholar] [CrossRef][Green Version]
- Malm-Erjefält, M.; Persson, C.G.; Erjefält, J.S. Degranulation status of airway tissue eosinophils in mouse models of allergic airway inflammation. Am. J. Respir. Cell Mol. Biol. 2001, 24, 352–359. [Google Scholar] [CrossRef]
- Szelenyi, I. Animal models of bronchial asthma. Inflamm. Res. 2000, 49, 639–654. [Google Scholar] [CrossRef]
- D’Erchia, A.M.; Gissi, C.; Pesole, G.; Saccone, C.; Arnason, U. The guinea-pig is not a rodent. Nature 1996, 381, 597–600. [Google Scholar] [CrossRef]
- Sullivan, J.; Swofford, D. Are Guinea Pigs Rodents? The Importance of Adequate Models in Molecular Phylogenetics. J. Mamm. Evol. 1997, 4, 77–86. [Google Scholar] [CrossRef]
- Busse, W.W.; Lemanske, R.F., Jr. Asthma. N. Engl. J. Med. 2001, 344, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Harker, J.A.; Lloyd, C.M. T helper 2 cells in asthma. J. Exp. Med. 2023, 220, e20221094. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kumar, R.K.; Foster, P.S. Pathogenesis of steroid-resistant airway hyperresponsiveness: Interaction between IFN-gamma and TLR4/MyD88 pathways. J. Immunol. 2009, 182, 5107–5115. [Google Scholar] [CrossRef]
- Mercer, P.F.; Abbott-Banner, K.; Adcock, I.M.; Knowles, R.G. Translational models of lung disease. Clin. Sci. 2015, 128, 235–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adner, M.; Canning, B.J.; Meurs, H.; Ford, W.; Ramos Ramírez, P.; van den Berg, M.P.M.; Birrell, M.A.; Stoffels, E.; Lundblad, L.K.A.; Nilsson, G.P.; et al. Back to the future: Re-establishing guinea pig in vivo asthma models. Clin. Sci. 2020, 134, 1219–1242. [Google Scholar] [CrossRef] [PubMed]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef]
- Lloyd, C. Building Better Mouse Models of Asthma. Curr. Allergy Asthma Rep. 2007, 7, 231–236. [Google Scholar] [CrossRef]
- Baron, R.M.; Choi, A.J.; Owen, C.A.; Choi, A.M. Genetically manipulated mouse models of lung disease: Potential and pitfalls. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L485–L497. [Google Scholar] [CrossRef]
- Carroll, O.R.; Pillar, A.L.; Brown, A.C.; Feng, M.; Chen, H.; Donovan, C. Advances in respiratory physiology in mouse models of experimental asthma. Front. Physiol. 2023, 14, 1099719. [Google Scholar] [CrossRef]
- Holmes, A.M.; Solari, R.; Holgate, S.T. Animal models of asthma: Value, limitations and opportunities for alternative approaches. Drug Discov. Today 2011, 16, 659–670. [Google Scholar] [CrossRef]
- Mullane, K.; Williams, M. Animal models of asthma: Reprise or reboot? Biochem. Pharmacol. 2014, 87, 131–139. [Google Scholar] [CrossRef]
- Périz, M.; Pérez-Cano, F.J.; Rodríguez-Lagunas, M.J.; Cambras, T.; Pastor-Soplin, S.; Best, I.; Castell, M.; Massot-Cladera, M. Development and Characterization of an Allergic Asthma Rat Model for Interventional Studies. Int. J. Mol. Sci. 2020, 21, 3841. [Google Scholar] [CrossRef] [PubMed]
- Brewer, N.R.; Cruise, L.J. The Respiratory System of the Guinea Pig: Emphasis on Species Differences. Contemp. Top. Lab. Anim. Sci. 1997, 36, 100–108. [Google Scholar] [PubMed]
- Regal, J.F. Immunoglobulin G- and immunoglobulin E-mediated airway smooth muscle contraction in the guinea pig. J. Pharmacol. Exp. Ther. 1984, 228, 116–120. [Google Scholar] [PubMed]
- Skappak, C.; Ilarraza, R.; Wu, Y.Q.; Drake, M.G.; Adamko, D.J. Virus-induced asthma attack: The importance of allergic inflammation in response to viral antigen in an animal model of asthma. PLoS ONE 2017, 12, e0181425. [Google Scholar] [CrossRef] [PubMed]
- Keir, S.; Page, C. The rabbit as a model to study asthma and other lung diseases. Pulm. Pharmacol. Ther. 2008, 21, 721–730. [Google Scholar] [CrossRef]
- Tanner, L.; Single, A.B. Animal Models Reflecting Chronic Obstructive Pulmonary Disease and Related Respiratory Disorders: Translating Pre-Clinical Data into Clinical Relevance. J. Innate Immun. 2020, 12, 203–225. [Google Scholar] [CrossRef]
- Kurucz, I.; Szelenyi, I. Current animal models of bronchial asthma. Curr. Pharm. Des. 2006, 12, 3175–3194. [Google Scholar] [CrossRef]
- Becker, A.B.; Hershkovich, J.; Simons, F.E.; Simons, K.J.; Lilley, M.K.; Kepron, M.W. Development of chronic airway hyperresponsiveness in ragweed-sensitized dogs. J. Appl. Physiol. 1989, 66, 2691–2697. [Google Scholar] [CrossRef]
- Sasaki, H.; Yanai, M.; Shimura, S.; Okayama, H.; Aikawa, T.; Sasaki, T.; Takishima, T. Late asthmatic response to Ascaris antigen challenge in dogs treated with metyrapone. Am. Rev. Respir. Dis. 1987, 136, 1459–1465. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.F.; Becker, A.B.; Lazarus, S.C.; Frick, O.L.; Nadel, J.A.; Gold, W.M. Antigen-induced airway hyperresponsiveness and pulmonary inflammation in allergic dogs. J. Appl. Physiol. 1985, 58, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Bice, D.E.; Weissman, D.N.; Muggenburg, B.A. Long-term maintenance of localized antibody responses in the lung. Immunology 1991, 74, 215–222. [Google Scholar] [PubMed]
- Schelegle, E.S.; Gershwin, L.J.; Miller, L.A.; Fanucchi, M.V.; Van Winkle, L.S.; Gerriets, J.P.; Walby, W.F.; Omlor, A.M.; Buckpitt, A.R.; Tarkington, B.K.; et al. Allergic asthma induced in rhesus monkeys by house dust mite (Dermatophagoides farinae). Am. J. Pathol. 2001, 158, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.; Harris, K.; Pruzansky, J. Induction of IgE-mediated cutaneous, cellular, and airway reactivity in rhesus monkeys by Ascaris suum infection. Transl. Res. 1983, 101, 864–872. [Google Scholar] [CrossRef]
- Ferreira, F.D.; Mayer, P.; Sperr, W.R.; Valent, P.; Seiberler, S.; Ebner, C.; Liehl, E.; Scheiner, O.; Kraft, D.; Valenta, R. Induction of IgE antibodies with predefined specificity in rhesus monkeys with recombinant birch pollen allergens, Bet v 1 and Bet v 2. J. Allergy Clin. Immunol. 1996, 97, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Bertho, N.; Meurens, F. The pig as a medical model for acquired respiratory diseases and dysfunctions: An immunological perspective. Mol. Immunol. 2021, 135, 254–267. [Google Scholar] [CrossRef]
- Senior, R.; Shapiro, S. Fishman’s Pulmonary Diseases and Disorders; Fishman, A.P., Elias, J.A., Fishman, J.A., Grippi, M.A., Kaiser, L.R., Senoir, R.M., Eds.; McGraw-Hill: New York, NY, USA, 1998; pp. 659–681. [Google Scholar]
- Churg, A.; Sin, D.D.; Wright, J.L. Everything prevents emphysema: Are animal models of cigarette smoke-induced chronic obstructive pulmonary disease any use? Am. J. Respir. Cell Mol. Biol. 2011, 45, 1111–1115. [Google Scholar] [CrossRef]
- Rydell-Törmänen, K.; Johnson, J.R. The Applicability of Mouse Models to the Study of Human Disease. Methods Mol. Biol. 2019, 1940, 3–22. [Google Scholar] [CrossRef]
- Shapiro, S.D. Transgenic and gene-targeted mice as models for chronic obstructive pulmonary disease. Eur. Respir. J. 2007, 29, 375–378. [Google Scholar] [CrossRef]
- De Oliveira, M.; Silva, P.; Rocco, P. Animal Models of Chronic Obstructive Pulmonary Disease Exacerbations: A Review of the Current Status. J. Biomed. Sci. 2016, 5, 1. [Google Scholar] [CrossRef]
- Yoshida, M.; Sakiyama, S.; Kenzaki, K.; Toba, H.; Uyama, K.; Takehisa, M.; Kondo, K.; Tangoku, A. Functional evaluation of pallid mice with genetic emphysema. Lab. Investig. 2009, 89, 760–768. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimbori, C.; Takechi, M.; Shiota, N.; Niibayashi, T.; Tanaka, T.; Okunishi, H. A Novel Mouse Model of Spontaneous Pulmonary Emphysema: Mayumi-Emphysema Mouse. Shimane J. Med. Sci. 2015, 32, 19–21. [Google Scholar]
- Hubeau, C.; Kubera, J.E.; Masek-Hammerman, K.; Williams, C.M. Interleukin-6 neutralization alleviates pulmonary inflammation in mice exposed to cigarette smoke and poly(I:C). Clin. Sci. 2013, 125, 483–493. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, S.; Chen, Z.; Liu, W.; Zhang, L.; Li, J.; Zhu, Z.; Zhou, L. Animal models: An essential tool to dissect the heterogeneity of chronic obstructive pulmonary disease. J. Transl. Int. Med. 2023, 11, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Nikula, K.J.; Green, F.H. Animal models of chronic bronchitis and their relevance to studies of particle-induced disease. Inhal. Toxicol. 2000, 12 (Suppl. 4), 123–153. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.W.; Hoffman, A.M.; Mazan, M.R.; Ingenito, E.P. Bronchoscopic measurement of collateral ventilation in a sheep model of emphysema. Respiration 2007, 74, 565–571. [Google Scholar] [CrossRef]
- Boucher, R.C. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu. Rev. Med. 2007, 58, 157–170. [Google Scholar] [CrossRef]
- Knowles, M.R.; Boucher, R.C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Investig. 2002, 109, 571–577. [Google Scholar] [CrossRef]
- Mall, M.A. ENaC inhibition in cystic fibrosis: Potential role in the new era of CFTR modulator therapies. Eur. Respir. J. 2020, 56, 2000946. [Google Scholar] [CrossRef]
- Wilke, M.; Buijs-Offerman, R.M.; Aarbiou, J.; Colledge, W.H.; Sheppard, D.N.; Touqui, L.; Bot, A.; Jorna, H.; de Jonge, H.R.; Scholte, B.J. Mouse models of cystic fibrosis: Phenotypic analysis and research applications. J. Cyst. Fibros. 2011, 10 (Suppl. 2), S152–S171. [Google Scholar] [CrossRef] [PubMed]
- Hodges, C. Case Western Reserve University—Cystic Fibrosis Mouse Models Core. 2024. Available online: https://case.edu/medicine/genetics/core-facilities/cystic-fibrosis-mouse-models-core (accessed on 1 February 2024).
- McCarron, A.; Parsons, D.; Donnelley, M. Animal and Cell Culture Models for Cystic Fibrosis: Which Model Is Right for Your Application? Am. J. Pathol. 2021, 191, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Semaniakou, A.; Croll, R.P.; Chappe, V. Animal Models in the Pathophysiology of Cystic Fibrosis. Front. Pharmacol. 2018, 9, 1475. [Google Scholar] [CrossRef] [PubMed]
- Birket, S.E.; Davis, J.M.; Fernandez-Petty, C.M.; Henderson, A.G.; Oden, A.M.; Tang, L.; Wen, H.; Hong, J.; Fu, L.; Chambers, A.; et al. Ivacaftor Reverses Airway Mucus Abnormalities in a Rat Model Harboring a Humanized G551D-CFTR. Am. J. Respir. Crit. Care Med. 2020, 202, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Livraghi-Butrico, A.; Hou, X.; Rajagopalan, C.; Zhang, J.; Song, J.; Jiang, H.; Wei, H.G.; Wang, H.; Bouhamdan, M.; et al. Phenotypes of CF rabbits generated by CRISPR/Cas9-mediated disruption of the CFTR gene. JCI Insight 2021, 6, e139813. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yan, Z.; Yi, Y.; Li, Z.; Lei, D.; Rogers, C.S.; Chen, J.; Zhang, Y.; Welsh, M.J.; Leno, G.H.; et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J. Clin. Investig. 2008, 118, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Keiser, N.W.; Birket, S.E.; Evans, I.A.; Tyler, S.R.; Crooke, A.K.; Sun, X.; Zhou, W.; Nellis, J.R.; Stroebele, E.K.; Chu, K.K.; et al. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am. J. Respir. Cell Mol. Biol. 2015, 52, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Perisse, I.V.; Fan, Z.; Singina, G.N.; White, K.L.; Polejaeva, I.A. Improvements in Gene Editing Technology Boost Its Applications in Livestock. Front. Genet. 2020, 11, 614688. [Google Scholar] [CrossRef]
- Rogers, C.S.; Hao, Y.; Rokhlina, T.; Samuel, M.; Stoltz, D.A.; Li, Y.; Petroff, E.; Vermeer, D.W.; Kabel, A.C.; Yan, Z.; et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J. Clin. Investig. 2008, 118, 1571–1577. [Google Scholar] [CrossRef]
- Paemka, L.; McCullagh, B.N.; Abou Alaiwa, M.H.; Stoltz, D.A.; Dong, Q.; Randak, C.O.; Gray, R.D.; McCray, P.B., Jr. Monocyte derived macrophages from CF pigs exhibit increased inflammatory responses at birth. J. Cyst. Fibros. 2017, 16, 471–474. [Google Scholar] [CrossRef]
- Hoegger, M.J.; Fischer, A.J.; McMenimen, J.D.; Ostedgaard, L.S.; Tucker, A.J.; Awadalla, M.A.; Moninger, T.O.; Michalski, A.S.; Hoffman, E.A.; Zabner, J.; et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 2014, 345, 818–822. [Google Scholar] [CrossRef]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, D.T.; Lajkosz, K.; Brogly, S.B.; Lougheed, M.D.; Jiang, L.; Housin, A.; Barber, D.; Johnson, A.; Doliszny, K.M.; Archer, S.L. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e003973. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmüller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: State of the art and research perspectives. Eur. Respir. J. 2019, 53, 1801887. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.H.; Ma, J.L.; Ding, D.; Ma, Y.J.; Wei, Y.P.; Jing, Z.C. Experimental animal models of pulmonary hypertension: Development and challenges. Anim. Model Exp. Med. 2022, 5, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Webb, S.; Tucker, A.; Rabinovitch, M.; O’Brien, R.F.; McMurtry, I.F.; Stelzner, T.J. Factors influencing the idiopathic development of pulmonary hypertension in the fawn hooded rat. Am. Rev. Respir. Dis. 1992, 145, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Morrell, N.W.; Aldred, M.A.; Chung, W.K.; Elliott, C.G.; Nichols, W.C.; Soubrier, F.; Trembath, R.C.; Loyd, J.E. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 2019, 53, 1801899. [Google Scholar] [CrossRef]
- Hansmann, G.; de Jesus Perez, V.A.; Alastalo, T.P.; Alvira, C.M.; Guignabert, C.; Bekker, J.M.; Schellong, S.; Urashima, T.; Wang, L.; Morrell, N.W.; et al. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J. Clin. Investig. 2008, 118, 1846–1857. [Google Scholar] [CrossRef]
- Paulin, R.; Dromparis, P.; Sutendra, G.; Gurtu, V.; Zervopoulos, S.; Bowers, L.; Haromy, A.; Webster, L.; Provencher, S.; Bonnet, S.; et al. Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metab. 2014, 20, 827–839. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.; Saleem, S.J.; Mizuno, S.; Syed, A.A.; Bogaard, H.J.; Abbate, A.; Taraseviciene-Stewart, L.; Sung, Y.; Kraskauskas, D.; Farkas, D.; et al. A brief overview of mouse models of pulmonary arterial hypertension: Problems and prospects. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2012, 302, L977–L991. [Google Scholar] [CrossRef]
- Sztuka, K.; Jasińska-Stroschein, M. Animal models of pulmonary arterial hypertension: A systematic review and meta-analysis of data from 6126 animals. Pharmacol. Res. 2017, 125, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Boucherat, O.; Agrawal, V.; Lawrie, A.; Bonnet, S. The Latest in Animal Models of Pulmonary Hypertension and Right Ventricular Failure. Circ. Res. 2022, 130, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.; Gillespie, M.; McMurtry, I. Fifty Years of Monocrotaline-Induced Pulmonary Hypertension. What Has It Meant to the Field? Chest 2017, 152, 1106–1108. [Google Scholar] [CrossRef] [PubMed]
- Dignam, J.P.; Scott, T.E.; Kemp-Harper, B.K.; Hobbs, A.J. Animal models of pulmonary hypertension: Getting to the heart of the problem. Br. J. Pharmacol. 2022, 179, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Suen, C.M.; Chaudhary, K.R.; Deng, Y.; Jiang, B.; Stewart, D.J. Fischer rats exhibit maladaptive structural and molecular right ventricular remodelling in severe pulmonary hypertension: A genetically prone model for right heart failure. Cardiovasc. Res. 2019, 115, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Stenmark, K.R.; Meyrick, B.; Galie, N.; Mooi, W.J.; McMurtry, I.F. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 297, L1013–L1032. [Google Scholar] [CrossRef] [PubMed]
- Taraseviciene-Stewart, L.; Kasahara, Y.; Alger, L.; Hirth, P.; Mc Mahon, G.; Waltenberger, J.; Voelkel, N.F.; Tuder, R.M. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001, 15, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Steigman, D.M.; Spence, C.L.; Janssens, S.P.; Hales, C.A. Chronic hypoxic pulmonary hypertension in the guinea pig: Effect of three levels of hypoxia. J. Appl. Physiol. 1993, 74, 916–921. [Google Scholar] [CrossRef]
- Quarck, R.; Wagenaar, A.; Tielemans, B.; Hautefort, A.; Belge, C.; Montani, D.; Delcroix, M.; Anitigny, F.; Perros, F. Administration of mitomycin results in pulmonary hypertension and vascular remodeling in rabbits. Eur. Respir. J. 2017, 50, PA2376. [Google Scholar] [CrossRef]
- Ohar, J.A.; Pyle, J.A.; Waller, K.S.; Hyers, T.M.; Webster, R.O.; Lagunoff, D. A rabbit model of pulmonary hypertension induced by the synthetic platelet-activating factor acetylglyceryl ether phosphorylcholine. Am. Rev. Respir. Dis. 1990, 141, 104–110. [Google Scholar] [CrossRef]
- Monnet, E.; Chachques, J.C. Animal models of heart failure: What is new? Ann. Thorac. Surg. 2005, 79, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.Q.; Liu, R.; Liao, H.X.; Zhang, X.F.; Qian, Y.X.; Liu, B.H.; Wu, Q.H.; Zhao, J.; Gu, W.W.; Li, H.T. Single intraperitoneal injection of monocrotaline as a novel large animal model of chronic pulmonary hypertension in Tibet minipigs. PLoS ONE 2013, 8, e78965. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Hall, C.M.; Griffith, G.W.; Johnson, K.F.; McGillicuddy, J.W.; Bartlett, R.H.; Cook, K.E. Large animal model of chronic pulmonary hypertension. ASAIO J. 2008, 54, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Degryse, A.L.; Lawson, W.E. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am. J. Med. Sci. 2011, 341, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Ramirez, A.M.; Ritzenthaler, J.D.; Torres-Gonzalez, E.; Roser-Page, S.; Mora, A.L.; Brigham, K.L.; Jones, D.P.; Roman, J.; Rojas, M. Oxidation of extracellular cysteine/cystine redox state in bleomycin-induced lung fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L37–L45. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.; Lawson, W.E.; Oury, T.D.; Sisson, T.H.; Raghavendran, K.; Hogaboam, C.M. Animal models of fibrotic lung disease. Am. J. Respir. Cell Mol. Biol. 2013, 49, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.E.; Ahmad, S.A.; Fitch, P.M.; Lamb, J.R.; Howie, S.E. FITC-induced murine pulmonary inflammation: CC10 up-regulation and concurrent Shh expression. Cell Biol. Int. 2005, 29, 868–876. [Google Scholar] [CrossRef]
- Hams, E.; Armstrong, M.E.; Barlow, J.L.; Saunders, S.P.; Schwartz, C.; Cooke, G.; Fahy, R.J.; Crotty, T.B.; Hirani, N.; Flynn, R.J.; et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl. Acad. Sci. USA 2014, 111, 367–372. [Google Scholar] [CrossRef]
- Miles, T.; Hoyne, G.F.; Knight, D.A.; Fear, M.W.; Mutsaers, S.E.; Prêle, C.M. The contribution of animal models to understanding the role of the immune system in human idiopathic pulmonary fibrosis. Clin. Transl. Immunol. 2020, 9, e1153. [Google Scholar] [CrossRef]
- Sime, P.J.; Xing, Z.; Graham, F.L.; Csaky, K.G.; Gauldie, J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J. Clin. Investig. 1997, 100, 768–776. [Google Scholar] [CrossRef]
- Tepper, J.S.; Kuehl, P.J.; Cracknell, S.; Nikula, K.J.; Pei, L.; Blanchard, J.D. Symposium Summary: “Breathe In, Breathe Out, Its Easy: What You Need to Know About Developing Inhaled Drugs”. Int. J. Toxicol. 2016, 35, 376–392. [Google Scholar] [CrossRef]
- Carrington, R.; Jordan, S.; Pitchford, S.C.; Page, C.P. Use of animal models in IPF research. Pulm. Pharmacol. Ther. 2018, 51, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Last, J.A.; Gelzleichter, T.; Harkema, J.; Parks, W.C.; Mellick, P. Effects of 20 months of ozone exposure on lung collagen in Fischer 344 rats. Toxicology 1993, 84, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Blanco, J.A.; Aguilera, M.; Domènech, A.; Tarrasón, G.; Prats, N.; Miralpeix, M.; De Alba, J. Enhanced cough reflex in a model of bleomycin-induced lung fibrosis in guinea pigs. Clin. Sci. 2015, 129, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Che, P.; Wang, M.; Larson-Casey, J.L.; Hu, R.H.; Cheng, Y.; El Hamdaoui, M.; Zhao, X.K.; Grytz, R.; Brent Carter, A.; Ding, Q. A novel tree shrew model of pulmonary fibrosis. Lab. Investig. 2021, 101, 116–124. [Google Scholar] [CrossRef]
- Fleischman, R.W.; Baker, J.R.; Thompson, G.R.; Schaeppi, U.H.; Illievski, V.R.; Cooney, D.A.; Davis, R.D. Bleomycin-induced interstitial pneumonia in dogs. Thorax 1971, 26, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Organ, L.; Bacci, B.; Koumoundouros, E.; Barcham, G.; Kimpton, W.; Nowell, C.J.; Samuel, C.; Snibson, K. A novel segmental challenge model for bleomycin-induced pulmonary fibrosis in sheep. Exp. Lung Res. 2015, 41, 115–134. [Google Scholar] [CrossRef]
- Im, J.G.; Itoh, H.; Lee, K.S.; Han, M.C. CT-pathology correlation of pulmonary tuberculosis. Crit. Rev. Diagn. Imaging 1995, 36, 227–285. [Google Scholar]
- Sholeye, A.R.; Williams, A.A.; Loots, D.T.; Tutu van Furth, A.M.; van der Kuip, M.; Mason, S. Tuberculous Granuloma: Emerging Insights From Proteomics and Metabolomics. Front. Neurol. 2022, 13, 804838. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Tang, W.; Wang, F.; Wang, Y. Research progress of tuberculosis infection and immune response mechanism. Chin. Med. J. 2019, 47, 3. [Google Scholar]
- Dharmadhikari, A.S.; Nardell, E.A. What animal models teach humans about tuberculosis. Am. J. Respir. Cell Mol. Biol. 2008, 39, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H. How can immunology contribute to the control of tuberculosis? Nat. Rev. Immunol. 2001, 1, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, U.D.; Katoch, V.M. Animal models of tuberculosis. Tuberculosis 2005, 85, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Lenaerts, A.J.; Gruppo, V.; Brooks, J.V.; Orme, I.M. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 2003, 47, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Scanga, C.A.; Mohan, V.P.; Joseph, H.; Yu, K.; Chan, J.; Flynn, J.L. Reactivation of latent tuberculosis: Variations on the Cornell murine model. Infect. Immun. 1999, 67, 4531–4538. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gupta, U. Animal models of tuberculosis: Lesson learnt. Indian J. Med. Res. 2018, 147, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.C.; Kesavan, A.K.; Lopez-Molina, J.; Hatem, C.L.; Brooks, M.; Fujiwara, R.; Hochstein, K.; Pitt, M.L.; Tufariello, J.; Chan, J.; et al. The aerosol rabbit model of TB latency, reactivation and immune reconstitution inflammatory syndrome. Tuberculosis 2008, 88, 187–196. [Google Scholar] [CrossRef]
- Peña, J.C.; Ho, W.Z. Monkey models of tuberculosis: Lessons learned. Infect. Immun. 2015, 83, 852–862. [Google Scholar] [CrossRef]
- Szaluś-Jordanow, O.; Augustynowicz-Kopeć, E.; Czopowicz, M.; Olkowski, A.; Łobaczewski, A.; Rzewuska, M.; Sapierzyński, R.; Wiatr, E.; Garncarz, M.; Frymus, T. Intracardiac tuberculomas caused by Mycobacterium tuberculosis in a dog. BMC Vet. Res. 2016, 12, 109. [Google Scholar] [CrossRef]
- Gil, O.; Díaz, I.; Vilaplana, C.; Tapia, G.; Díaz, J.; Fort, M.; Cáceres, N.; Pinto, S.; Caylà, J.; Corner, L.; et al. Granuloma encapsulation is a key factor for containing tuberculosis infection in minipigs. PLoS ONE 2010, 5, e10030. [Google Scholar] [CrossRef]
- Wilson, J.M.; Laurent, P. Fish gill morphology: Inside out. J. Exp. Zool. 2002, 293, 192–213. [Google Scholar] [CrossRef] [PubMed]
- Macirella, R.; Brunelli, E. Morphofunctional Alterations in Zebrafish (Danio rerio) Gills after Exposure to Mercury Chloride. Int. J. Mol. Sci. 2017, 18, 824. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Nakamura, K.; Kudo, H.; Tran, Y.H.; Yamamoto, Y.; Doi, H.; Hirose, S. Characterization of the column and autocellular junctions that define the vasculature of gill lamellae. J. Histochem. Cytochem. 2007, 55, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Progatzky, F.; Cook, H.T.; Lamb, J.R.; Bugeon, L.; Dallman, M.J. Mucosal inflammation at the respiratory interface: A zebrafish model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L551–L561. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Kim, C.-Y.; Ryu, B.; Kim, U.; Kim, J.; Lee, J.-M.; Lee, B.-H.; Moon, J.; Jung, C.-R.; Park, J.-H. Respiratory Toxicity of Polyhexamethylene Guanidine Phosphate Exposure in Zebrafish. Zebrafish 2018, 15, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Epperly, M.W.; Bahary, N.; Quader, M.; Dewald, V.; Greenberger, J.S. The zebrafish-Danio rerio—Is a useful model for measuring the effects of small-molecule mitigators of late effects of ionizing irradiation. In Vivo 2012, 26, 889–897. [Google Scholar]
- Hwang, P.P.; Chou, M.Y. Zebrafish as an animal model to study ion homeostasis. Pflug. Arch. 2013, 465, 1233–1247. [Google Scholar] [CrossRef]
- Plasschaert, L.W.; Žilionis, R.; Choo-Wing, R.; Savova, V.; Knehr, J.; Roma, G.; Klein, A.M.; Jaffe, A.B. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 2018, 560, 377–381. [Google Scholar] [CrossRef]
- Tobin, D.M.; Ramakrishnan, L. Comparative pathogenesis of Mycobacterium marinum and Mycobacterium tuberculosis. Cell. Microbiol. 2008, 10, 1027–1039. [Google Scholar] [CrossRef]
- Myllymäki, H.; Bäuerlein, C.A.; Rämet, M. The Zebrafish Breathes New Life into the Study of Tuberculosis. Front. Immunol. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Takaki, K.; Cosma, C.L.; Troll, M.A.; Ramakrishnan, L. An in vivo platform for rapid high-throughput antitubercular drug discovery. Cell Rep. 2012, 2, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.; Lechartier, B.; Zhang, M.; Neres, J.; van der Sar, A.M.; Raadsen, S.A.; Hartkoorn, R.C.; Ryabova, O.B.; Vocat, A.; Decosterd, L.A.; et al. Towards a new combination therapy for tuberculosis with next generation benzothiazinones. EMBO Mol. Med. 2014, 6, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J. Tracheal System; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1011–1015. [Google Scholar]
- Scholl, A.; Ndoja, I.; Jiang, L. Drosophila Trachea as a Novel Model of COPD. Int. J. Mol. Sci. 2021, 22, 12730. [Google Scholar] [CrossRef] [PubMed]
- Webster, M.R.; Socha, J.J.; Teresi, L.; Nardinocchi, P.; De Vita, R. Structure of tracheae and the functional implications for collapse in the American cockroach. Bioinspir. Biomim. 2015, 10, 066011. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, B.; El-Merhie, N.; Kovacevic, D.; Schramm, J.; Bossen, J.; Roeder, T.; Krauss-Etschmann, S. Airway remodeling: The Drosophila model permits a purely epithelial perspective. Front. Allergy 2022, 3, 876673. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.K.; Triplett, A.A.; Christensen, A.C. A peritrophin-like protein expressed in the embryonic tracheae of Drosophila melanogaster. Insect Biochem. Mol. Biol. 1999, 29, 319–327. [Google Scholar] [CrossRef]
- Roeder, T.; Isermann, K.; Kallsen, K.; Uliczka, K.; Wagner, C. Recent Advances on Model Hosts; Mylonakis, E., Ausubel, F., Gilmore, M., Casadevall, A., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2012; Volume 710, pp. 37–47. [Google Scholar]
- Kim, K.; Lane, E.A.; Saftien, A.; Wang, H.; Xu, Y.; Wirtz-Peitz, F.; Perrimon, N. Drosophila as a model for studying cystic fibrosis pathophysiology of the gastrointestinal system. Proc. Natl. Acad. Sci. USA 2020, 117, 10357–10367. [Google Scholar] [CrossRef]
- Dionne, M.S.; Ghori, N.; Schneider, D.S. Drosophila melanogaster is a genetically tractable model host for Mycobacterium marinum. Infect. Immun. 2003, 71, 3540–3550. [Google Scholar] [CrossRef]
- Sécher, T.; Bodier-Montagutelli, E.; Guillon, A.; Heuzé-Vourc’h, N. Correlation and clinical relevance of animal models for inhaled pharmaceuticals and biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 167, 148–169. [Google Scholar] [CrossRef]
- Van der Velden, J.; Snibson, K.J. Airway disease: The use of large animal models for drug discovery. Pulm. Pharmacol. Ther. 2011, 24, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.D.; Saadeh, C.; Ross, D. Clinical applications of forced oscillation to assess peripheral airway function. Respir. Physiol. Neurobiol. 2005, 148, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Castile, R.G.; Davis, S.D. Kendig & Chernick’s Disorders of the Respiratory Tract in Children, 8th ed.; Wilmott, R.W., Boat, T.F., Bush, A., Chernick, V., Deterding, R.R., Ratjen, F., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2012; pp. 211–233. [Google Scholar]
- Jones, B.; Donovan, C.; Li, G.; Gomez, H.; Chimankar, V.; Harrison, C.; Wiegman, C.; Adcock, I.; Knight, D.; Hirota, J.; et al. Animal models of COPD: What do they tell us? Respirology 2017, 22, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Seeger, W.; Adir, Y.; Barberà, J.A.; Champion, H.; Coghlan, J.G.; Cottin, V.; De Marco, T.; Galiè, N.; Ghio, S.; Gibbs, S.; et al. Pulmonary hypertension in chronic lung diseases. J. Am. Coll. Cardiol. 2013, 62, D109–D116. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. BMC Vet. Res. 2020, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Serghini, A.; Portelli, S.; Ascher, D.B. AI-Driven Enhancements in Drug Screening and Optimization. Methods Mol. Biol. 2024, 2714, 269–294. [Google Scholar] [CrossRef]

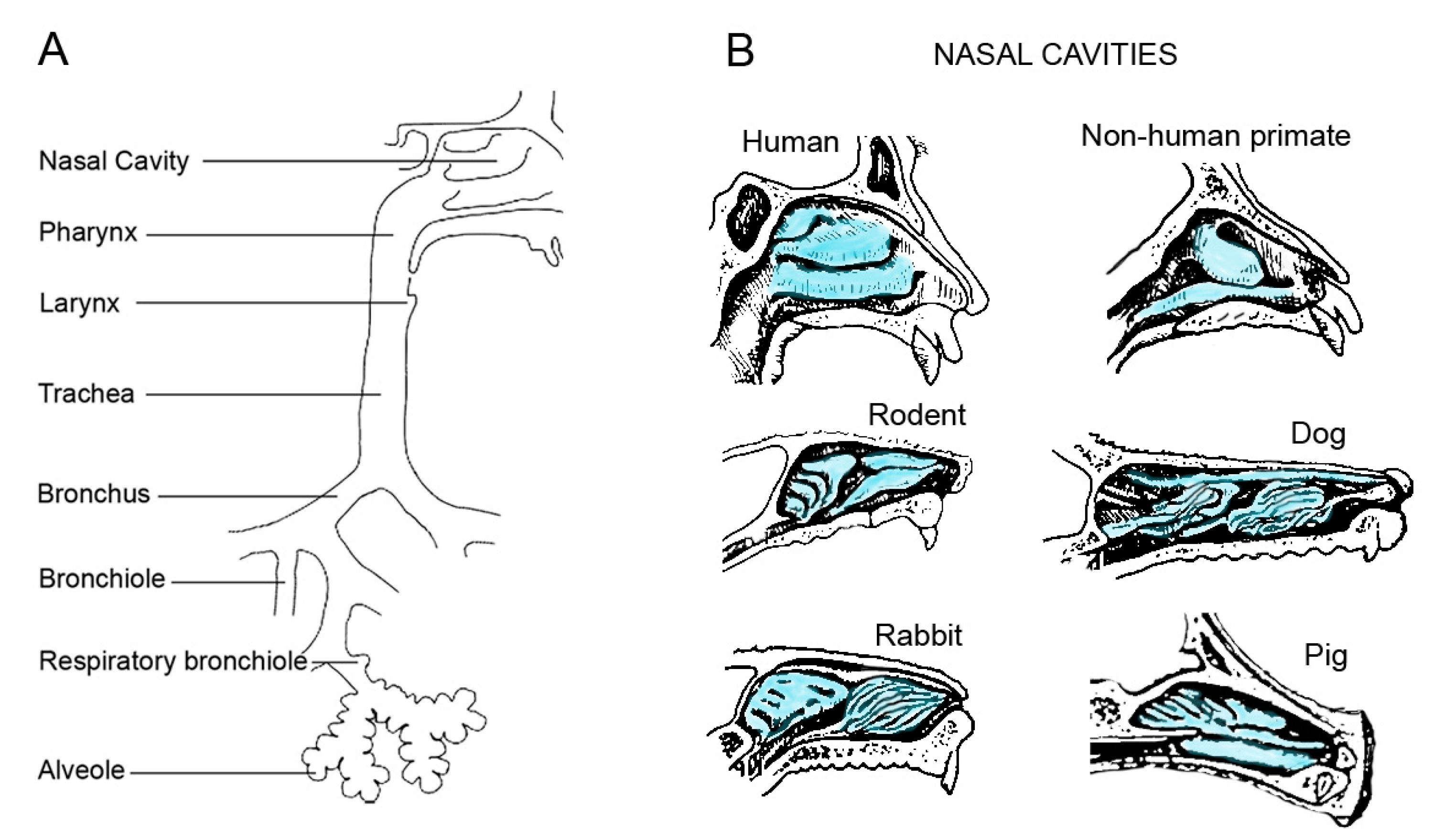
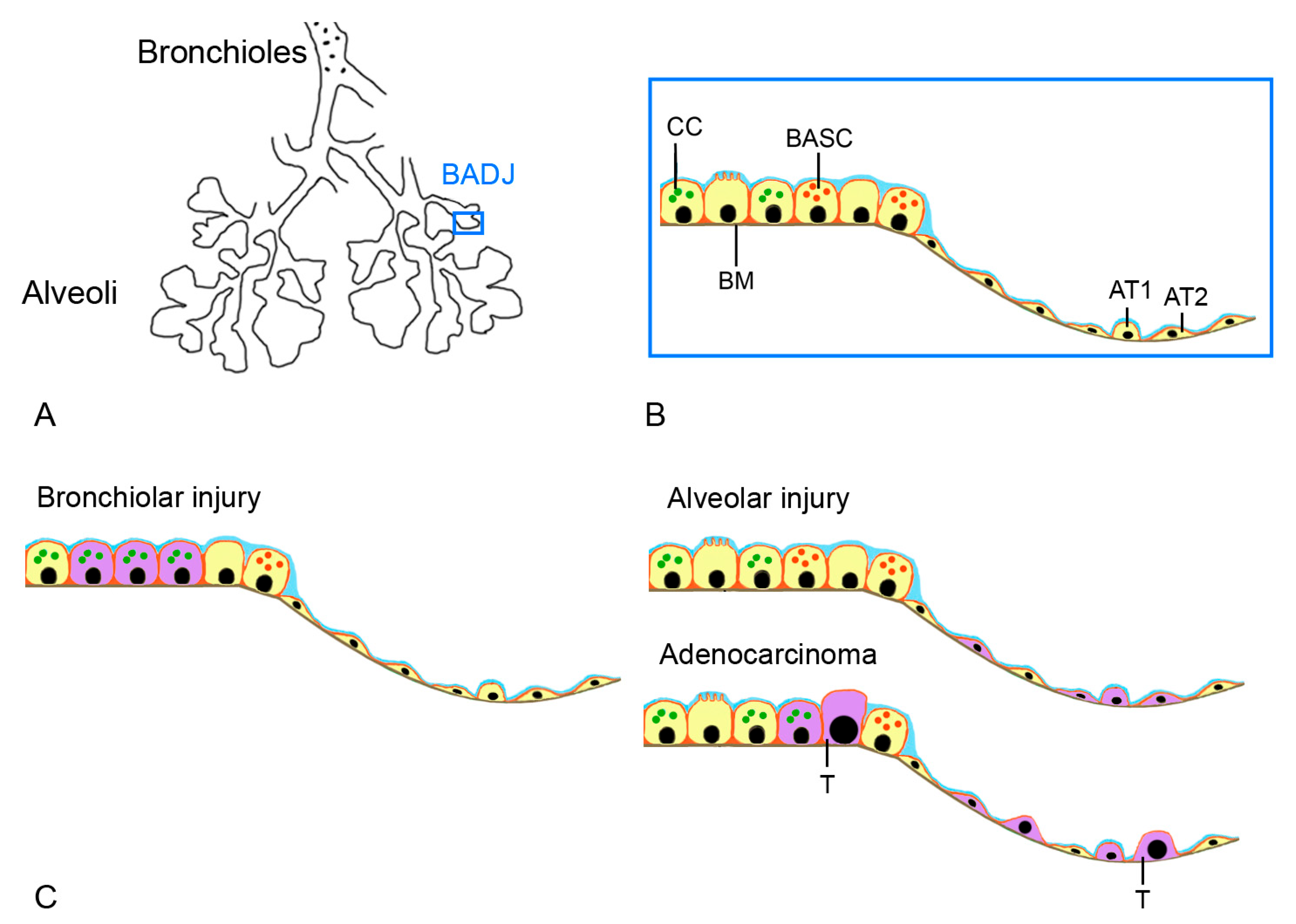

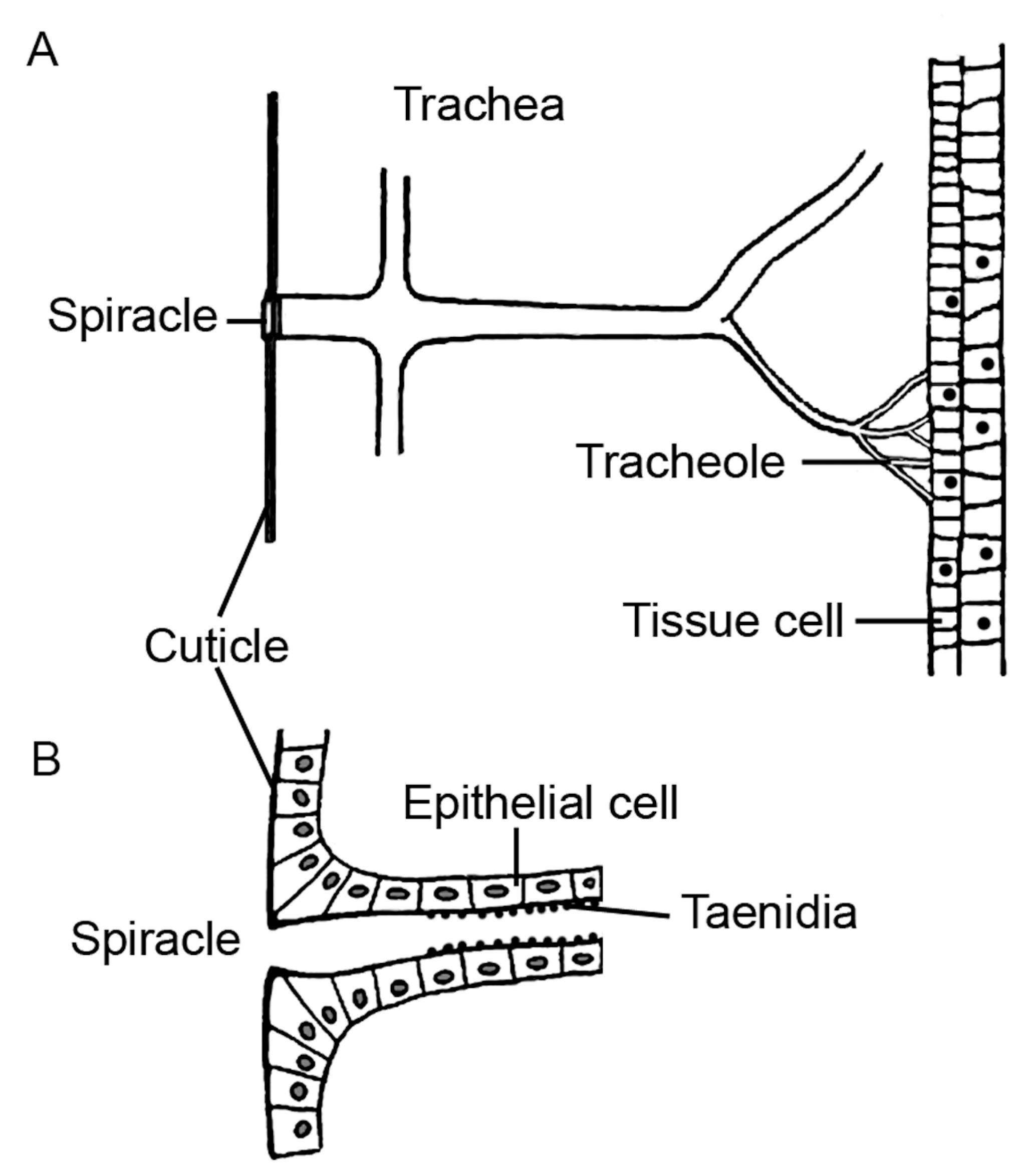
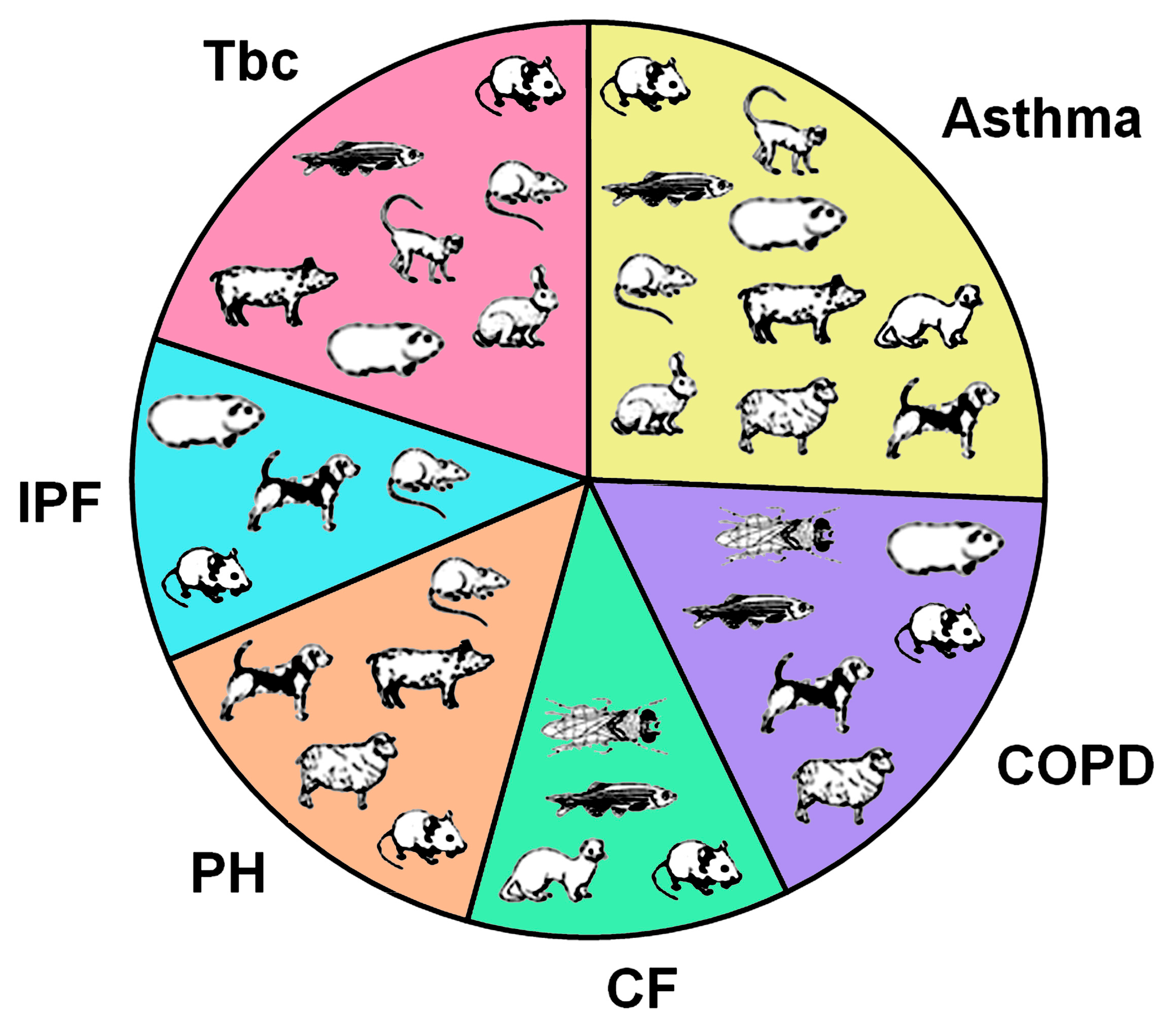
| Parameter | Human | Mouse | Rat | Guinea Pig | Rabbit | Ferret | Monkey | Dog | Sheep | Pig |
|---|---|---|---|---|---|---|---|---|---|---|
| Breathing | Mouth and nose | Obligatory nose | Obligatory nose | Obligatory nose | Obligatory nose | Mouth and nose | Mouth and nose | Mouth and nose | Mouth breathing possible | Mouth and nose |
| Cough reflex | present | absent | absent | present | present | absent | present | present | present | present |
| Lung architecture | 5 lobes, 2 left, 3 right | 5 lobes, 1 left, 4 right | 5 lobes, 1 left, 4 right | 7 lobes, 3 left, 4 right | 7 lobes, 3 left, 4 right | 6 lobes: 2 left, 4 right | 6 lobes: 2 left, 4 right | 6 lobes: 2 left, 4 right | 6 lobes: 2 left, 4 right | 6 lobes: 2 left, 4 right |
| Branching | Dichotomous | Monopodial | Monopodial | Monopodial | Monopodial | Monopodial | Monopodial | Monopodial; dichotomous | Dichotomous | Monopodial |
| Branchings to alveolarized bronchiole | 17–21 | 13–17 | 8–25 or 13–32, depending on lobe | 14 | 32–36 | >6 | 13–17 | 15–22 | 7–13 | 21–23 |
| Submucosal glands | Trachea, bronchi | Trachea (1/3) | Trachea (1/3) | Trachea (1/3) | Absent | Trachea, bronchi | Trachea, bronchi | Trachea, bronchi | Trachea, bronchi | Trachea, bronchi |
| Other | Chest wall less stiff | Chest wall less stiff | Chest wall less stiff | Chest wall less stiff | No interlobular septa | No interlobular septa | Prominent interlobar septa | Prominent interlobar septa | ||
| References | [25,26] | [25,26] | [25,26,27] | [25,26,28] | [25,26,29,30] | [31,32] | [25,26,33] | [25,34,35] | [25,36,37] | [25,36,38] |
| Parameter | Human | Mouse | Rat | Guinea Pig | Rabbit | Ferret | Monkey | Dog | Sheep | Pig |
|---|---|---|---|---|---|---|---|---|---|---|
| Thickness of epithelial layer (μm) | 100–50 | 14–11 | 24–13 | 11 | 21–29 | 17–20 | 30–20 | 33 | 59–32 | 50–30 |
| Cells/mm BM | 303 ± 20 | 215 | 126–116 | 307 | 194–114 | n.a. | 181 ± 51 | n.a. | 285–284 | 303 |
| Ciliated cells (%) | 49 | 39 | 35–53 | 32 | 43–49 | Cells/mm: 80–20 | 33 | n.a. | 48–39 | 43 |
| Mucous goblet cells (%) | 9 | <1 | <1 | 5 | 1 | Cells/mm: 20–60 | 17 | 9.6 | 4–8 | 3 |
| Serous cells (%) | n.a. | 21 | 0 | 0 | 0 | n.a. | <1 | n.a. | 0 | 0 |
| Club (Clara) cells (%) | n.a. | 49 | 0 | n.a. | 22–41 | n.a. | <1 | n.a. | n.a. | n.a. |
| Basal cells (%) | 33 | 10 | 27–14 | 34 | 27–49 | n.a. | 42 | n.a. | 18–19 | 31 |
| Ref. | [53] | [54] | [53] | [55] | [53,56] | [32] | [57] | [58] | [53] | [55] |
| Species | Advantages | Disadvantages |
|---|---|---|
| Mouse | Low costs, many providers, short breeding time, easy handling, comparably low ethical considerations, most test reagents available, transgenic animals, vast literature data, numerous inbred strains, good for mechanistic studies | Small size (aerosol delivery difficult, sample volumes small), short life span, different lung structure, obligatory nose breathers, low mucus production, limited airway musculature, no chronic models, strain-specific responses |
| Rat | Larger lung surface than other rodents, good for pharmacodynamic and toxicological testing | Strain-specific responses, obligatory nose breathers, higher mucociliary clearance, different airway macrostructure and epithelial composition |
| Guinea pig | Best models for inflammation and AHR (asthma, Tbc) | Shortage of inbred strains, axon reflex, difficult blood collection because of thick skin and lack of tail, few reagents available. |
| Rabbit | Procedures (tracheotomy) like for large animals, good availability, easy handling, longer observation times | Difficult intubation, differences in airway architecture and epithelial composition |
| Ferret | Comparable respiratory tract, ideal models for COPD, genetically modified animals available | Limited availability, complex husbandry, not fully annotated genome, few inbred strains, handling difficult (biting), reagents not easily available |
| Non-human primates | Genetic and morphological similarity to humans, reagents available due to cross-reactivity with human | Ethical problems, high costs, skilled handling and specialized equipment necessary |
| Dog | Easy intubation due to large mouth opening, greater number of alveoli than rodents, broad spectrum of breeds, use of human inhalers possible | Ethical problems, larger airways make identification of constriction difficult, difference due to outbred strains |
| Sheep | Similar mucus composition to humans, use of human devices possible, lungs can be treated separately, serve as surrogate models for surfactant dysfunction | High costs, intense labor, vomiting possible upon intubation |
| Pig | Genetic homology, body weight, metabolism, organ structure similar to humans, human devices can be used, long observation time, genetically modified animals available | Differences in pharyngeal anatomy, handling more difficult, higher costs, laryngospasm possible upon intubation |
| Zebrafish | Costs low, large number of eggs, transgenic animals | No lung |
| Fruit fly | High-throughput testing possible, simple protocols | Low conserved homology with human genome, no lung |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröhlich, E. Animals in Respiratory Research. Int. J. Mol. Sci. 2024, 25, 2903. https://doi.org/10.3390/ijms25052903
Fröhlich E. Animals in Respiratory Research. International Journal of Molecular Sciences. 2024; 25(5):2903. https://doi.org/10.3390/ijms25052903
Chicago/Turabian StyleFröhlich, Eleonore. 2024. "Animals in Respiratory Research" International Journal of Molecular Sciences 25, no. 5: 2903. https://doi.org/10.3390/ijms25052903
APA StyleFröhlich, E. (2024). Animals in Respiratory Research. International Journal of Molecular Sciences, 25(5), 2903. https://doi.org/10.3390/ijms25052903






