Real-Time Determination of Intracellular cAMP Reveals Functional Coupling of Gs Protein to the Melatonin MT1 Receptor
Abstract
1. Introduction
2. Results
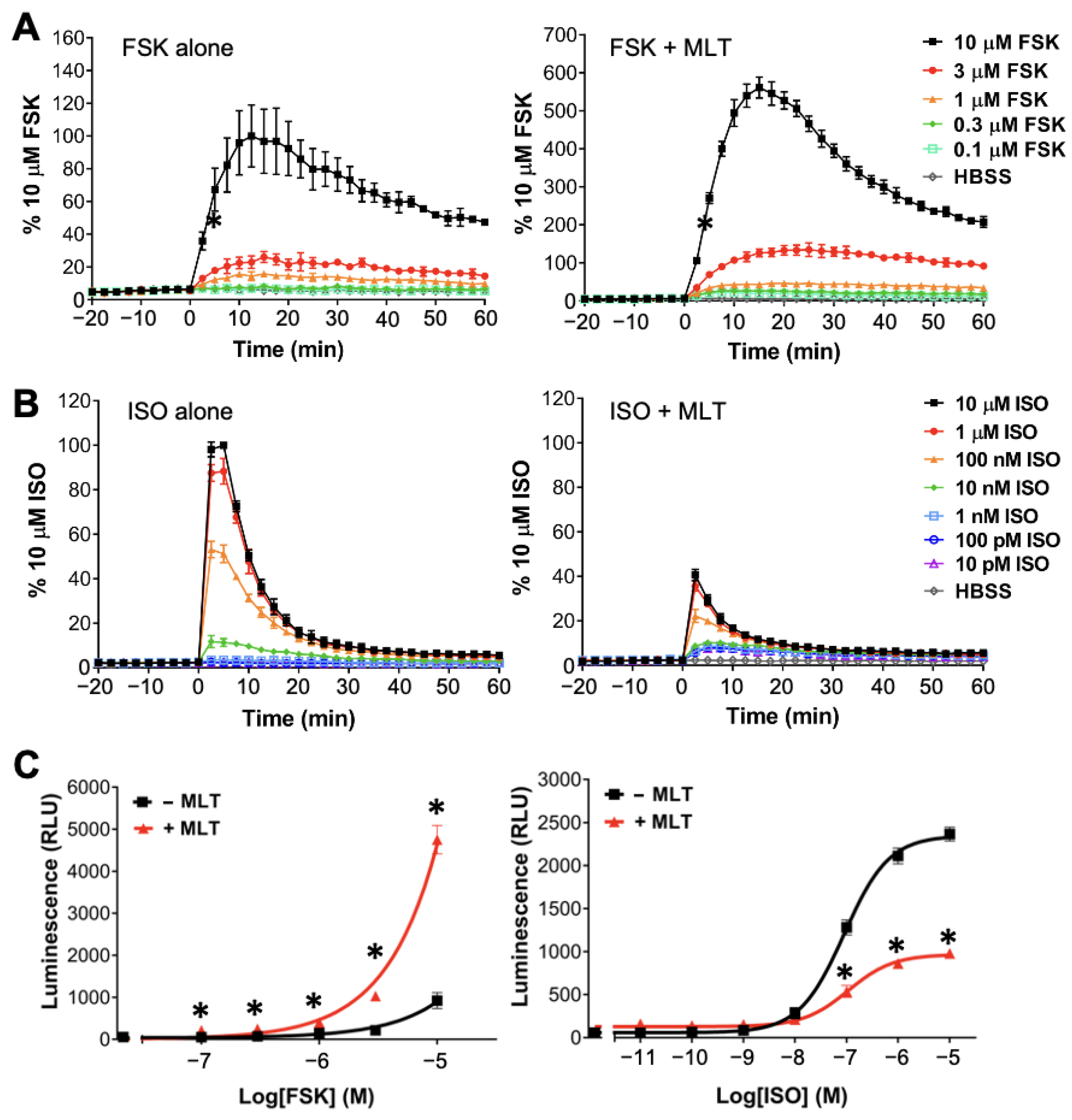
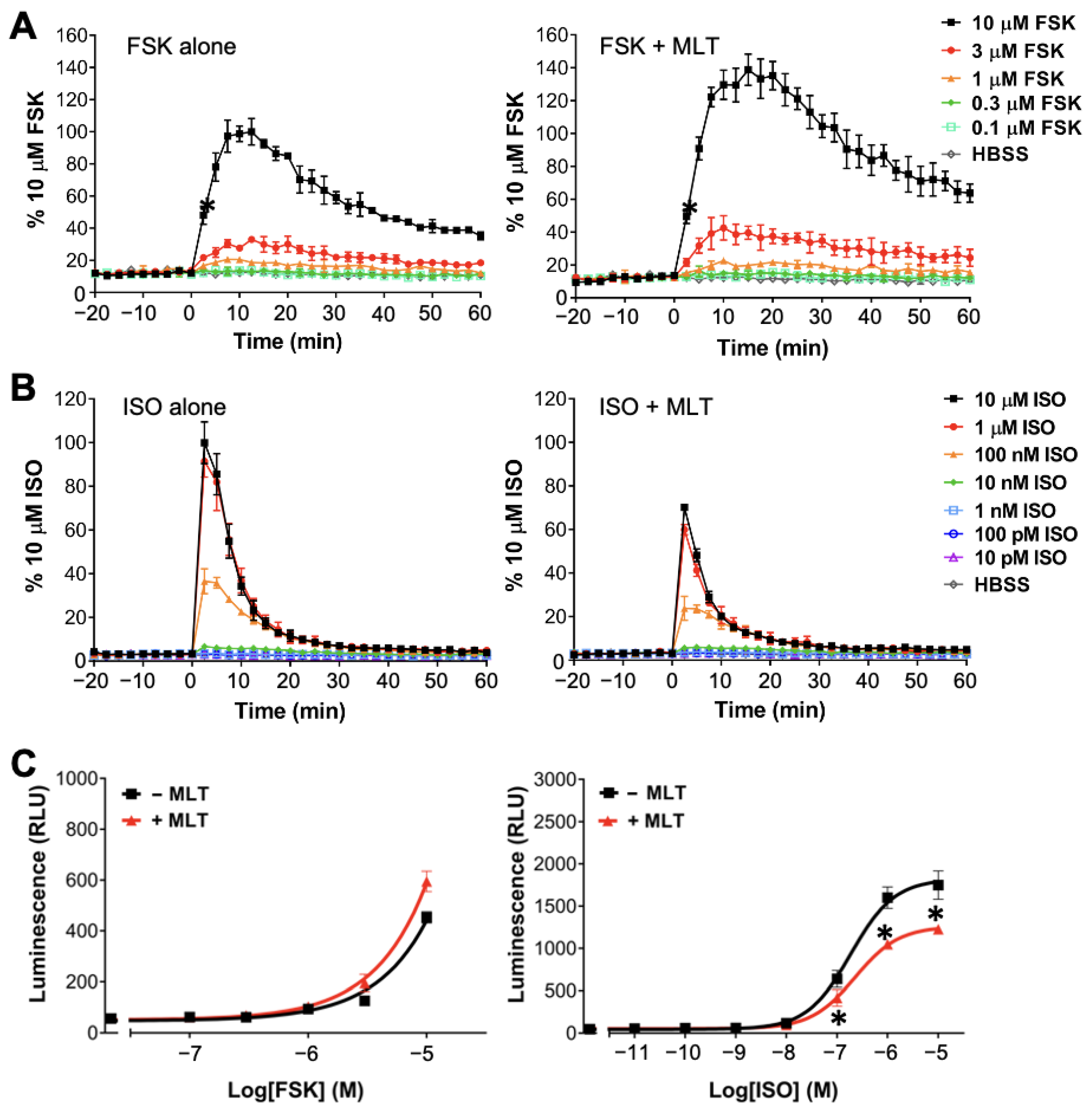
2.1. Realtime Detection of cAMP in Live HEK293 Cells
2.2. Atypical Regulations of cAMP by MT1
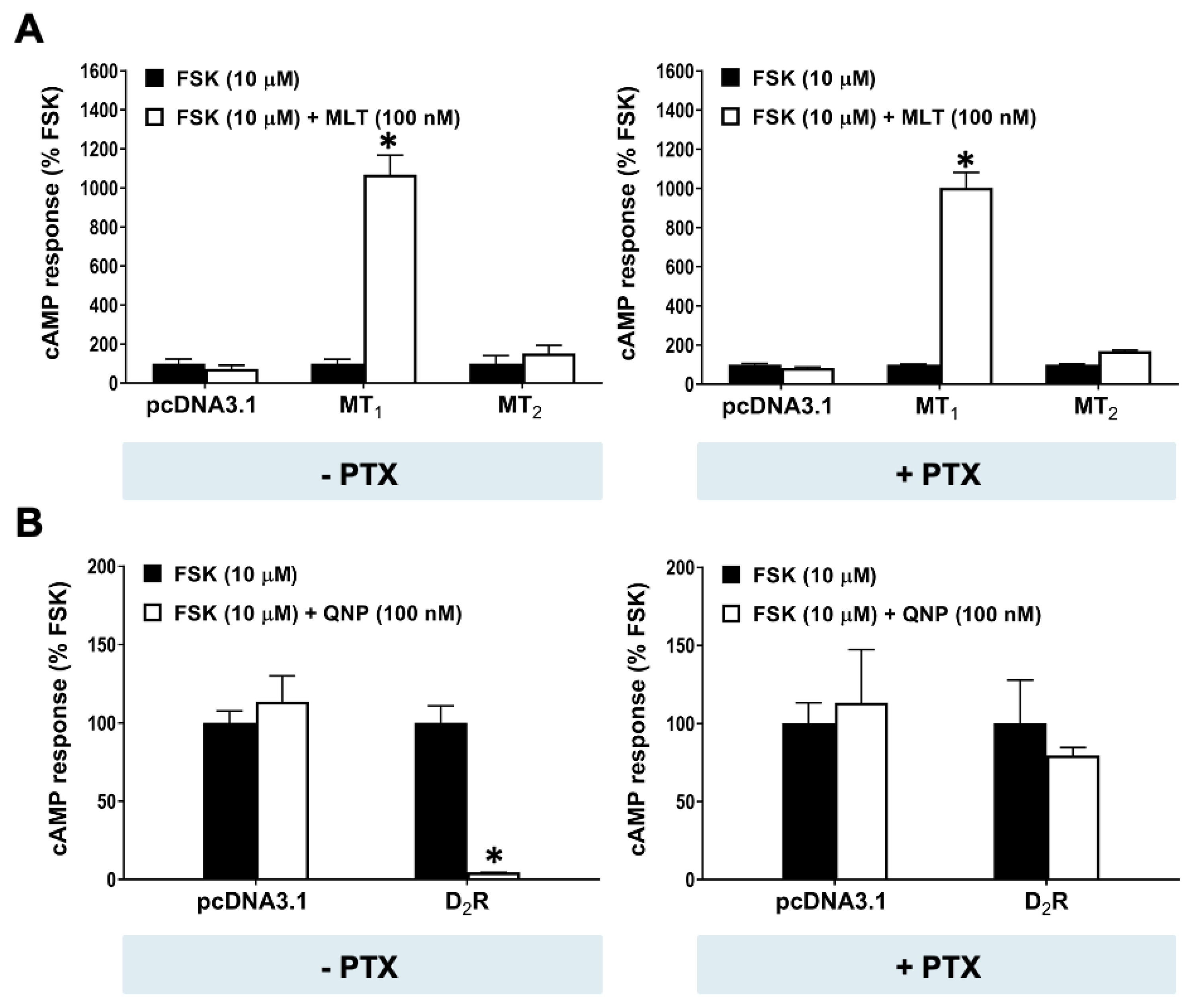
2.3. cAMP Stimulation by MT1 Is Independent of Gi Signaling
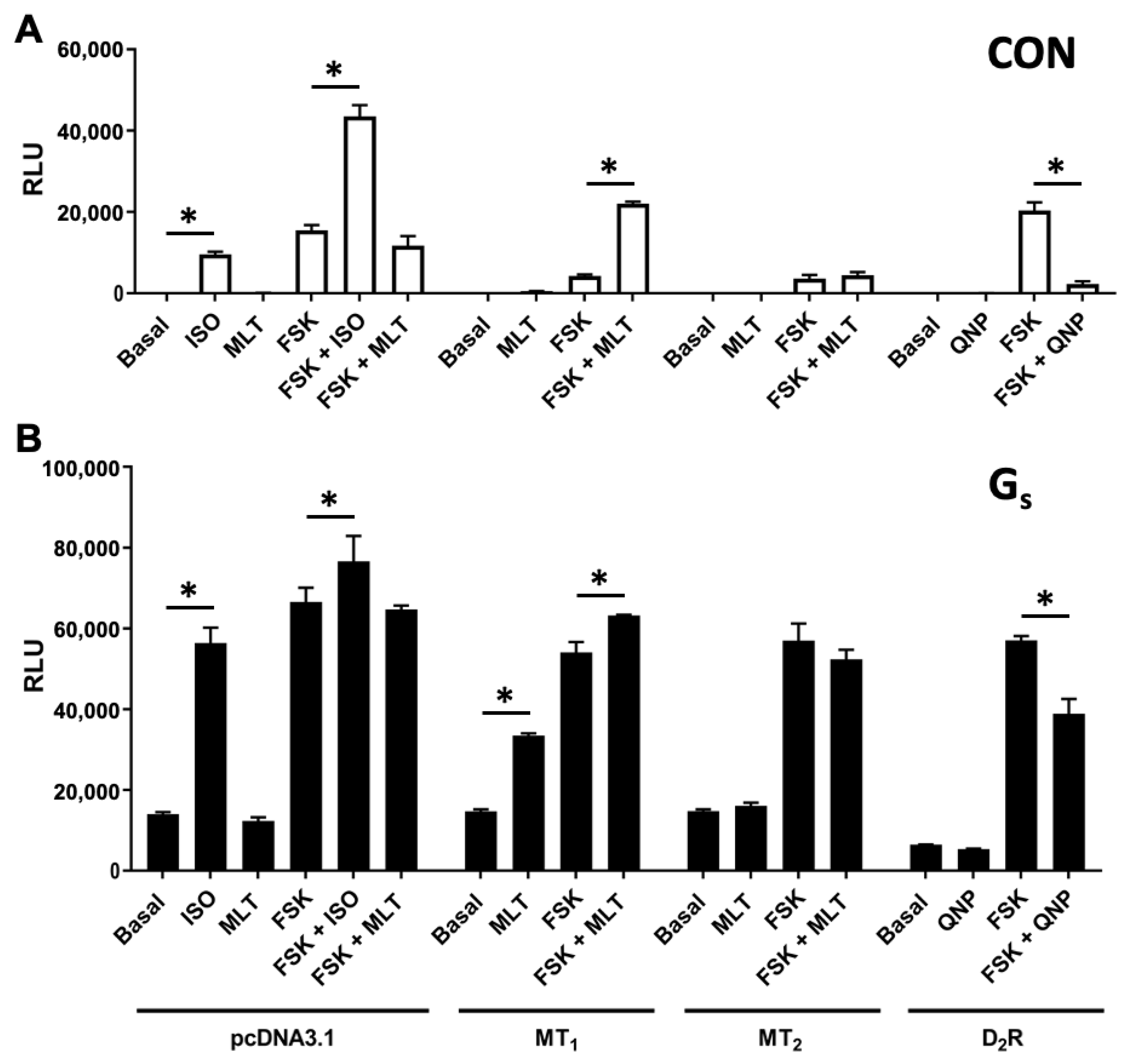
2.4. MT1 Stimulates cAMP Production through Gαs Protein
2.5. Molecular Modeling Predicts a Viable MT1-Gs Complex
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Transfection
4.3. GloSensor cAMP Assay
4.4. Loop Modeling and the Generation of Initial Structures
4.5. Determining Receptor-G Protein Interface via Local Docking
4.6. Scoring and Analysis of the Predicted Complex Models
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Reppert, S.M.; Weaver, D.R.; Ebisawa, T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 1994, 13, 1177–1185. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Godson, C.; Mahle, C.D.; Weaver, D.R.; Slaugenhaupt, S.A.; Gusella, J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA 1995, 92, 8734–8738. [Google Scholar] [CrossRef] [PubMed]
- Jockers, R.; Delagrange, P.; Dubocovich, M.L.; Markus, R.P.; Renault, N.; Tosini, G.; Cecon, E.; Zlotos, D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016, 173, 2702–2725. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Clough, S.J.; Hutchinson, A.J.; Adamah-Biassi, E.B.; Popovska-Gorevski, M.; Dubocovich, M.L. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 361–383. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Ayoub, M.A.; Ravid, R.; Angeloni, D.; Fraschini, F.; Meier, F.; Eckert, A.; Müller-Spahn, F.; Jockers, R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J. Pineal Res. 2005, 38, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Wong, K.Y.; Aquili, L.; Uddin, M.S.; Heng, B.C.; Tipoe, G.L.; Wong, K.H.; Fung, M.L.; Lim, L.W. Role of melatonin in Alzheimer’s disease: From preclinical studies to novel melatonin-based therapies. Front. Neuroendocrinol. 2022, 65, 100986. [Google Scholar] [CrossRef] [PubMed]
- Hickie, I.B.; Rogers, N.L. Novel melatonin-based therapies: Potential advances in the treatment of major depression. Lancet 2011, 378, 621–631. [Google Scholar] [CrossRef]
- Bonnefond, A.; Clément, N.; Fawcett, K.; Yengo, L.; Vaillant, E.; Guillaume, J.-L.; Dechaume, A.; Payne, F.; Roussel, R.; Czernichow, S.; et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat. Genet. 2012, 44, 297. [Google Scholar] [CrossRef]
- Browning, C.; Beresford, I.; Fraser, N.; Giles, H. Pharmacological characterization of human recombinant melatonin mt1 and MT2 receptors. Br. J. Pharmacol. 2000, 129, 877–886. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.N.; Wong, Y.H.; Wong, J.T.Y.; Pang, S.F.; Shiu, S.Y.W. Molecular and cellular analyses of melatonin receptor-mediated cAMP signaling in rat corpus epididymis. J. Pineal Res. 1998, 25, 219–228. [Google Scholar] [CrossRef]
- Brydon, L.; Petit, L.; Delagrange, P.; Strosberg, A.D.; Jockers, R. Functional expression of MT2 (Mel1b) melatonin receptors in human PAZ6 adipocytes. Endocrinology 2001, 142, 4264–4271. [Google Scholar] [CrossRef]
- Kiefer, T.; Ram, P.T.; Yuan, L.; Hill, S.M. Melatonin inhibits estrogen receptor transactivation and cAMP levels in breast cancer cells. Breast Cancer Res. Treat. 2002, 71, 37–45. [Google Scholar] [CrossRef]
- Picinato, M.C.; Hirata, A.E.; Cipolla-Neto, J.; Curi, R.; Carvalho, C.R.O.; Anhê, G.F.; Carpinelli, A.R. Activation of insulin and IGF-1 signaling pathways by melatonin through MT1 receptor in isolated rat pancreatic islets. J. Pineal Res. 2008, 44, 88–94. [Google Scholar] [CrossRef]
- Carbajo-Pescador, S.; García-Palomo, A.; Martín-Renedo, J.; Piva, M.; González-Gallego, J.; Mauriz, J.L. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: Role of the MT1 receptor. J. Pineal Res. 2011, 51, 463–471. [Google Scholar] [CrossRef]
- Travnickova-Bendova, Z.; Cermakian, N.; Reppert, S.M.; Sassone-Corsi, P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc. Natl. Acad. Sci. USA 2002, 99, 7728–7733. [Google Scholar] [CrossRef]
- Peschke, E.; Bach, A.G.; Mühlbauer, E. Parallel signaling pathways of melatonin in the pancreatic β-cell. J. Pineal Res. 2006, 40, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Schuster, C.; Williams, L.M.; Morris, A.; Morgan, P.J.; Barrett, P. The human MT1 melatonin receptor stimulates cAMP production in the human neuroblastoma cell line SH-SY5Y cells via a calcium-calmodulin signal transduction Pathway. J. Neuroendocrinol. 2005, 17, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.Y.W.; Pang, B.; Tam, C.W.; Yao, K.-M. Signal transduction of receptor-mediated antiproliferative action of melatonin on human prostate epithelial cells involves dual activation of Gαs and Gαq proteins. J. Pineal Res. 2010, 49, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, X.; Zhang, Y.; Chen, X.; Lai, X.; Shao, J.; Shi, Y.; Zhou, N. Melatonin receptor type 1 signals to extracellular signal-regulated kinase 1 and 2 via Gi and Gs dually coupled pathways in HEK-293 cells. Biochemistry 2014, 53, 2827–2839. [Google Scholar] [CrossRef] [PubMed]
- Huete-Toral, F.; Crooke, A.; Martínez-Águila, A.; Pintor, J. Melatonin receptors trigger cAMP production and inhibit chloride movements in nonpigmented ciliary epithelial cells. J. Pharmacol. Exp. Ther. 2015, 352, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Wang, Z.; Dong, Y.; Chen, Y.; Cao, J. Mel1b and Mel1c melatonin receptors mediate green light-induced secretion of growth hormone in chick adenohypophysis cells via the AC/PKA and ERK1/2 signalling pathways. J. Photochem. Photobiol. B Biol. 2021, 225, 112322. [Google Scholar] [CrossRef]
- MacKenzie, R.S.; Melan, M.A.; Passey, D.K.; Witt-Enderby, P.A. Dual coupling of MT1 and MT2 melatonin receptors to cyclic AMP and phosphoinositide signal transduction cascades and their regulation following melatonin exposure. Biochem. Pharmacol. 2002, 63, 587–595. [Google Scholar] [CrossRef]
- Tsu, R.C.; Wong, Y.H. Gi-mediated stimulation of type II adenylyl cyclase is augmented by Gq-coupled receptor activation and phorbol esters treatment. J. Neurosci. 1996, 16, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.L.; Lai, F.P.L.; Lo, R.K.H.; Voyno-Yasenetskaya, T.A.; Stanbridge, E.J.; Wong, Y.H. Melatonin mt1 and MT2 receptors stimulate c-Jun N-terminal kinase via pertussis toxin-sensitive and -insensitive G proteins. Cell. Signal. 2002, 14, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Yung, L.Y.; Tsim, S.T.; Wong, Y.H. Stimulation of cAMP accumulation by the cloned Xenopus melatonin receptor through Gi and Gz proteins. FEBS Lett. 1995, 372, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Katada, T.; Gilman, A.G.; Ross, E.M. Activation of the inhibitory GTP-binding protein of adenylate cyclase, Gi, by β-adrenergic receptors in reconstituted phospholipid vesicles. J. Biol. Chem. 1984, 259, 9351–9354. [Google Scholar] [CrossRef] [PubMed]
- Daaka, Y.; Luttrell, L.M.; Lefkowitz, R.J. Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature 1997, 390, 88–91. [Google Scholar] [CrossRef]
- Ciani, E.; Fontaine, R.; Maugars, G.; Mizrahi, N.; Mayer, I.; Levavi-Sivan, B.; Weltzien, F.A. Melatonin receptors in Atlantic salmon stimulate cAMP levels in heterologous cell lines and show season-dependent daily variations in pituitary expression levels. J. Pineal Res. 2019, 67, e12590. [Google Scholar] [CrossRef]
- Nelson, C.S.; Marino, J.L.; Allen, C.N. Melatonin receptor potentiation of cyclic AMP and the cystic fibrosis transmembrane conductance regulator ion channel. J. Pineal Res. 1999, 26, 113–121. [Google Scholar] [CrossRef]
- Dessauer, C.W.; Scully, T.T.; Gilman, A.G. Interactions of forskolin and ATP with the cytosolic domains of mammalian adenylyl cyclase. J. Biol. Chem. 1997, 272, 22272–22277. [Google Scholar] [CrossRef]
- Jin, J.-X.; Sun, J.-T.; Jiang, C.-Q.; Cui, H.-D.; Bian, Y.; Lee, S.; Zhang, L.; Lee, B.C.; Liu, Z.-H. Melatonin regulates lipid metabolism in porcine cumulus–oocyte complexes via the melatonin receptor 2. Antioxidants 2022, 11, 687. [Google Scholar] [CrossRef]
- Binkowski, B.F.; Butler, B.L.; Stecha, P.F.; Eggers, C.T.; Otto, P.; Zimmerman, K.; Vidugiris, G.; Wood, M.G.; Encell, L.P.; Fan, F.; et al. A luminescent biosensor with increased dynamic range for intracellular cAMP. ACS Chem. Biol. 2011, 6, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- DiRaddo, J.O.; Miller, E.J.; Hathaway, H.A.; Grajkowska, E.; Wroblewska, B.; Wolfe, B.B.; Liotta, D.C.; Wroblewski, J.T. A real-time method for measuring cAMP production modulated by Gαi/o-coupled metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 2014, 349, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Goulding, J.; Maya, L.T.; Hill, S.J. Characterisation of endogenous A2A and A2B receptor-mediated cyclic AMP responses in HEK 293 cells using the GloSensorTM biosensor: Evidence for an allosteric mechanism of action for the A2B-selective antagonist PSB 603. Biochem. Pharmacol. 2018, 147, 55–66. [Google Scholar] [CrossRef]
- Insel, P.A.; Ostrom, R.S. Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell. Mol. Neurobiol. 2003, 23, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.M.; Stork, P.J.S. β2-adrenergic receptor activates extracellular signal-regulated kinases (ERKs) via the small G protein Rap1 and the serine/threonine kinase B-Raf. J. Biol. Chem. 2000, 275, 25342–25350. [Google Scholar] [CrossRef]
- Violin, J.D.; DiPilato, L.M.; Yildirim, N.; Elston, T.C.; Zhang, J.; Lefkowitz, R.J. β2-adrenergic receptor signaling and desensitization elucidated by quantitative modeling of real time cAMP dynamics. J. Biol. Chem. 2008, 283, 2949–2961. [Google Scholar] [CrossRef]
- Tang, W.J.; Gilman, A.G. Type-specific regulation of adenylyl cyclase by G protein beta gamma subunits. Science 1991, 254, 1500–1503. [Google Scholar] [CrossRef]
- Kurose, H.; Katada, T.; Amano, T.; Ui, M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. J. Biol. Chem. 1983, 258, 4870–4875. [Google Scholar] [CrossRef]
- Sutkowski, E.M.; Tang, W.-J.; Broome, C.W.; Robbins, J.D.; Seamon, K.B. Regulation of forskolin interactions with type I, II, V, and VI adenylyl cyclases by Gs alpha. Biochemistry 1994, 33, 12852–12859. [Google Scholar] [CrossRef]
- Xiao, R.P. β-adrenergic signaling in the heart: Dual coupling of the β2- adrenergic receptor to Gs and Gi proteins. Sci. STKE 2001, 2001, re15. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, Q.; Guo, Q.; Teng, M.; Gong, Q.; Li, X.; Du, Y.; Liu, Z.; Tao, Y. Structural basis of the ligand binding and signaling mechanism of melatonin receptors. Nat. Commun. 2022, 13, 454. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Z.; Dong, Y.; Cao, J.; Chen, Y. Physiological crosstalk between the AC/PKA and PLC/PKC pathways modulates melatonin-mediated, monochromatic-light-induced proliferation of T-lymphocytes in chickens. Cell Tissue Res. 2017, 369, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Dubocovich, M.L.; Markowska, M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine 2005, 27, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Alewijnse, A.E.; Smit, M.J.; Pena, M.S.R.; Verzijl, D.; Timmerman, H.; Leurs, R. Modulation of forskolin-mediated adenylyl cyclase activation by constitutively active Gs-coupled receptors. FEBS Lett. 1997, 419, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Lavriha, P.; Mehta, V.; Khanppnavar, B.; Mohammed, I.; Li, Y.; Lazaratos, M.; Schaefer, J.V.; Dreier, B.; Plückthun, A.; et al. Structural basis of adenylyl cyclase 9 activation. Nat. Commun. 2022, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Roka, F.; Brydon, L.; Waldhoer, M.; Strosberg, A.D.; Freissmuth, M.; Jockers, R.; Nanoff, C. Tight association of the human Mel1a-melatonin receptor and Gi: Precoupling and constitutive activity. Mol. Pharmacol. 1999, 56, 1014–1024. [Google Scholar] [CrossRef]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.L.; Pagliara, A.S.; Chase, L.R.; Kipnis, D.M. Radioimmunoassay for cyclic nucleotides: II. adenosine 3′,5′-monophosphate and guanosine 3′,5′-monophosphate in mammalian tissues and body fluids. J. Biol. Chem. 1972, 247, 1114–1120. [Google Scholar] [CrossRef]
- Skomedal, T.; Grynne, B.; Osnes, J.B.; Sjetnan, A.E.; Øye, I. A radioimmunoassay for cyclic AMP (cAMP) obtained by acetylation of both unlabeled and labeled (3H-cAMP) ligand, or of unlabeled ligand only. Acta Pharmacol. Toxicol. 1980, 46, 200–204. [Google Scholar] [CrossRef]
- Witt-Enderby, P.A.; Dubocovich, M.L. Characterization and regulation of the human ML1A melatonin receptor stably expressed in Chinese hamster ovary cells. Mol. Pharmacol. 1996, 50, 166–174. [Google Scholar] [PubMed]
- Wang, J.; Miao, Y. Recent advances in computational studies of GPCR-G protein interactions. Adv. Protein Chem. Struct. Biol. 2019, 116, 397–419. [Google Scholar] [PubMed]
- Clark, J.J.; Benson, M.L.; Smith, R.D.; Carlson, H.A. Inherent versus induced protein flexibility: Comparisons within and between Apo and holo structures. PLoS Comput. Biol. 2019, 15, e1006705. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Katiyar, A.; Lenka, S.K.; Lakshmi, K.; Chinnusamy, V.; Bansal, K.C. In silico characterization and homology modeling of thylakoid bound ascorbate peroxidase from a drought tolerant wheat cultivar. Genom. Proteom. Bioinform. 2009, 7, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Paknejad, N.; Zhu, L.; Wang, J.; Do, H.N.; Miao, Y.; Liu, W.; Hite, R.K.; Huang, X.-Y. Structures of β1-adrenergic receptor in complex with Gs and ligands of different efficacies. Nat. Commun. 2022, 13, 4095. [Google Scholar] [CrossRef]
- Lyskov, S.; Gray, J.J. The Rosettadock server for local protein-protein docking. Nucleic Acids Res. 2008, 36, W233–W238. [Google Scholar] [CrossRef]
- Chaudhury, S.; Berrondo, M.; Weitzner, B.D.; Muthu, P.; Bergman, H.; Gray, J.J. Benchmarking and analysis of protein docking performance in Rosetta v3.2. PLoS ONE 2011, 6, e22477. [Google Scholar] [CrossRef]
- Dijkman, P.M.; Castell, O.K.; Goddard, A.D.; Munoz-Garcia, J.C.; de Graaf, C.; Wallace, M.I.; Watts, A. Dynamic tuneable G protein-coupled receptor monomer-dimer populations. Nat. Commun. 2018, 9, 1710. [Google Scholar] [CrossRef]
- Kaczor, A.A.; Guixà-González, R.; Carriõ, P.; Poso, A.; Dove, S.; Pastor, M.; Selent, J. Multi-component protein-protein docking based protocol with external scoring for modeling dimers of G protein-coupled receptors. Mol. Inform. 2015, 34, 246–255. [Google Scholar] [CrossRef]
- Xue, L.C.; Rodrigues, J.P.; Kastritis, P.L.; Bonvin, A.M.; Vangone, A. PRODIGY: A web server for predicting the binding affinity of protein-protein complexes. Bioinformatics 2016, 32, 3676–3678. [Google Scholar] [CrossRef] [PubMed]
- Goddard, T.D.; Huang, C.C.; Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Morris, J.H.; Ferrin, T.E. UCSF Chimerax: Meeting modern challenges in visualization and analysis. Protein Sci. 2017, 27, 14–25. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, L.H.; Cheung, S.T.; Lee, S.; Wong, Y.H. Real-Time Determination of Intracellular cAMP Reveals Functional Coupling of Gs Protein to the Melatonin MT1 Receptor. Int. J. Mol. Sci. 2024, 25, 2919. https://doi.org/10.3390/ijms25052919
Tse LH, Cheung ST, Lee S, Wong YH. Real-Time Determination of Intracellular cAMP Reveals Functional Coupling of Gs Protein to the Melatonin MT1 Receptor. International Journal of Molecular Sciences. 2024; 25(5):2919. https://doi.org/10.3390/ijms25052919
Chicago/Turabian StyleTse, Lap Hang, Suet Ting Cheung, Seayoung Lee, and Yung Hou Wong. 2024. "Real-Time Determination of Intracellular cAMP Reveals Functional Coupling of Gs Protein to the Melatonin MT1 Receptor" International Journal of Molecular Sciences 25, no. 5: 2919. https://doi.org/10.3390/ijms25052919
APA StyleTse, L. H., Cheung, S. T., Lee, S., & Wong, Y. H. (2024). Real-Time Determination of Intracellular cAMP Reveals Functional Coupling of Gs Protein to the Melatonin MT1 Receptor. International Journal of Molecular Sciences, 25(5), 2919. https://doi.org/10.3390/ijms25052919




