Overexpression of HbGRF4 or HbGRF4-HbGIF1 Chimera Improves the Efficiency of Somatic Embryogenesis in Hevea brasiliensis
Abstract
:1. Introduction
2. Results
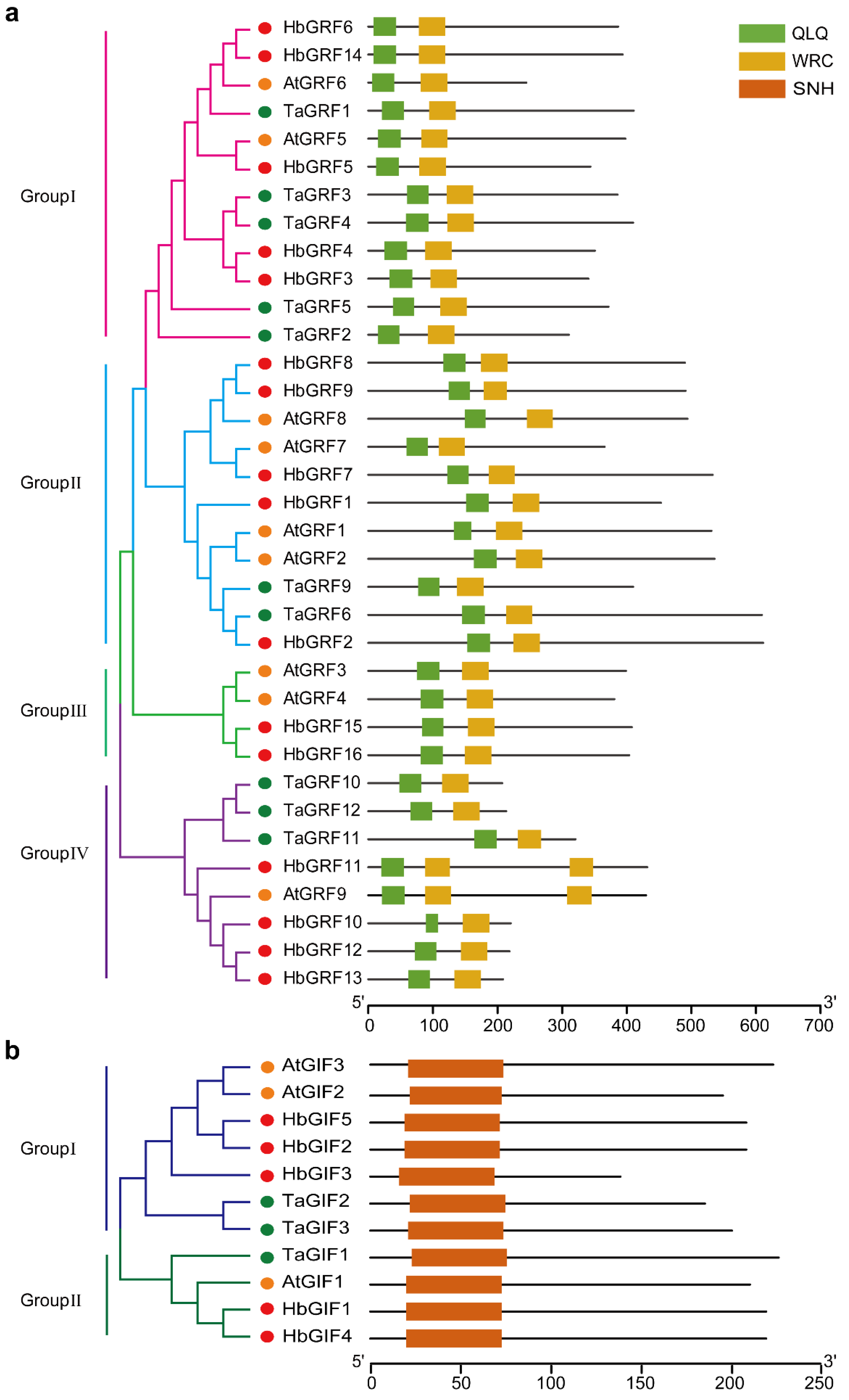
2.1. Identification of the GRF4 and GIF1 Homologues in H. brasiliensis
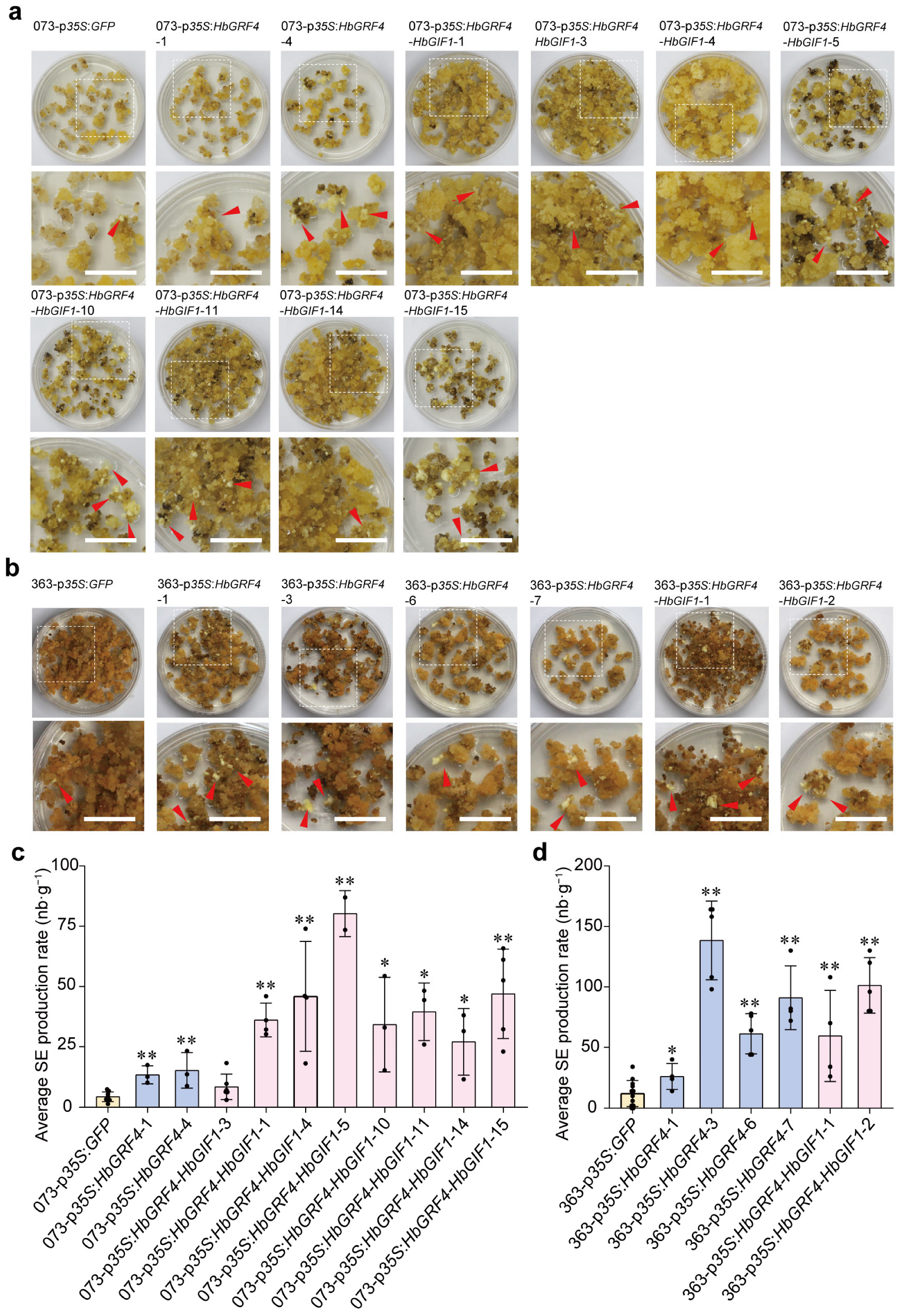
2.2. Overexpression of HbGRF4 or HbGRF4-HbGIF1 Promotes SE Production of Transgenic Callus Lines
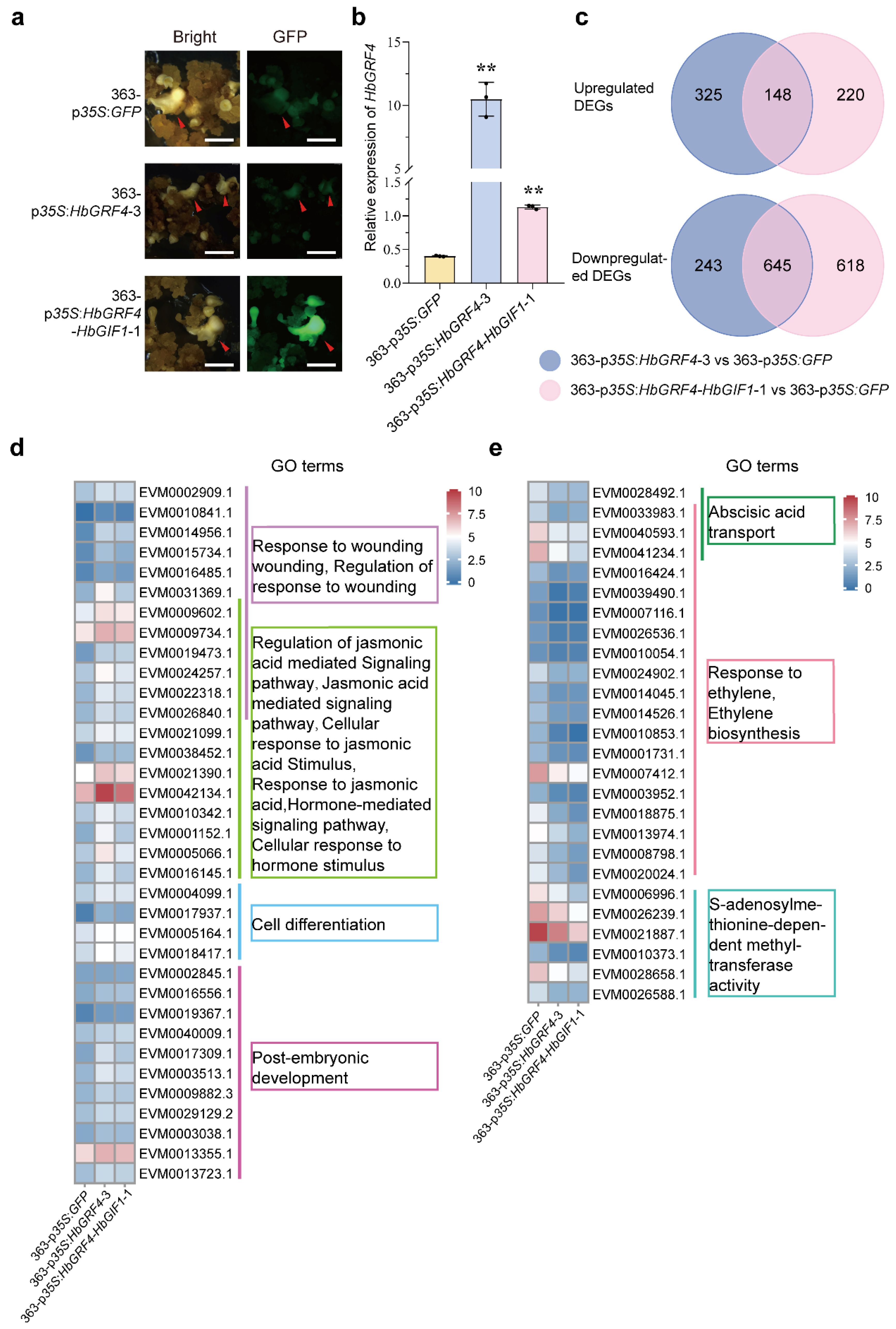
2.3. Transcriptome Analysis for the Mechanisms of HbGRF4-Promoting SE Production
3. Discussion
4. Materials and Methods
4.1. Identification of HbGRF and HbGIF Genes and Phylogenetic Analysis
4.2. Selection of Embryogenic Fragile Callus Lines
4.3. Preparation of Plasmids and Agrobacterium Cell Suspension
4.4. Inoculation, Cocultivation, Selection of Transgenic Callus Lines, and Somatic Embryo Production
4.5. RNA Extraction and Quantitative Real-Time PCR (RT PCR) Analysis
4.6. RNA Sequencing Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cherian, S.; Ryu, S.B.; Cornish, K. Natural rubber biosynthesis in plants, the rubber transferase complex, and metabolic engineering progress and prospects. Plant Biotechnol. J. 2019, 17, 2041–2061. [Google Scholar] [CrossRef] [PubMed]
- van Beilen, J.B.; Poirier, Y. Establishment of new crops for the production of natural rubber. Trends Biotechnol. 2007, 25, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.R. Distribution of latex in the plant kingdom. Econ. Bot. 1967, 21, 115–127. [Google Scholar] [CrossRef]
- Cornish, K. Alternative natural rubber crops: Why should we care? Technol. Innov. 2017, 18, 244–255. [Google Scholar] [CrossRef]
- Supriya, R.; Priyadarshan, P.M. Genomic technologies for Hevea breeding. Adv. Genet. 2019, 104, 1–73. [Google Scholar] [PubMed]
- Furtado, E.; Menten, J.; Passos, J. South American leaf blight intensity evaluated in six clones of young and adult rubber trees in the Vale do Ribeira region, Sao Paulo state, Brazil. Trop. Plant Pathol. 2008, 33, 130–137. [Google Scholar] [CrossRef]
- Guyot, J.; Cilas, C.; Sache, I. Influence of host resistance and phenology on South American leaf blight of the rubber tree with special consideration of temporal dynamics. Eur. J. Plant Pathol. 2008, 120, 111–124. [Google Scholar] [CrossRef]
- Sterling, A.; Martínez-Viuche, E.J.; Pimentel-Parra, G.A.; Suárez-Córdoba, Y.D.; Fonseca-Restrepo, J.A.; Virguez-Díaz, Y.R. Dynamics of adaptive responses in growth and resistance of rubber tree clones under South American leaf blight non-escape conditions in the Colombian Amazon. Ind. Crops Prod. 2019, 141, 111811. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Azevedo, J.L. From forest to plantation: A brief history of the rubber tree. Indian J. Hist. Sci. 2023, 58, 74–78. [Google Scholar] [CrossRef]
- Arokiaraj, P.; Jones, H.; Cheong, K.F.; Coomber, S.; Charlwood, B.V. Gene insertion into Hevea brasiliensis. Plant Cell Rep. 1994, 13, 425–431. [Google Scholar] [CrossRef]
- Kalawong, S.; Srichuay, W.; Sirisom, Y.; Te-chato, S. The establishedment of Agrobacterium-mediated gene transformation in rubber tree through organized explants. J. Agric. Sci. Technol. 2004, 10, 493–503. [Google Scholar]
- Lardet, L.; Dessailly, F.; Carron, M.P.; Montoro, P.; Monteuuis, O. Influences of aging and cloning methods on the capacity for somatic embryogenesis of a mature Hevea brasiliensis genotype. Tree Physiol. 2009, 29, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Udayabhanu, J.; Huang, T.; Xin, S.; Cheng, J.; Hua, Y.; Huang, H. Optimization of the transformation protocol for increased efficiency of genetic transformation in Hevea brasiliensis. Plants 2022, 11, 1067. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Peng, S.; Wu, K.; Hong, L. Genetic transformation and regeration of Hevea brasiliensis transgenic plant with GAI gene by microparticle bombardment. Rom. Biotechnol. Lett. 2013, 18, 7910–7919. [Google Scholar]
- Wang, T.; Udayabhanu, J.; Gu, X.; Wu, R.; Xin, S.; Chen, Q.; Zhang, Y.; Yang, X.; Peng, S.; Chen, J.; et al. Induction of axillary bud swelling of Hevea brasiliensis to regenerate plants through Somatic embryogenesis and analysis of genetic stability. Plants 2023, 12, 1803. [Google Scholar] [CrossRef]
- Blanc, G.; Baptiste, C.; Oliver, G.; Martin, F.; Montoro, P. Efficient Agrobacterium tumefaciens-mediated transformation of embryogenic calli and regeneration of Hevea brasiliensis Mull Arg. plants. Plant Cell Rep. 2006, 24, 724–733. [Google Scholar] [CrossRef]
- Carron, M.P.; Dauzac, J.; Etienne, H.; Hadrami, I.E.; Housti, F.; Michaux, F.N.; Montoro, P. Biochemical and histological features of somatic embryogenesis in Hevea brasiliensis. Plant Cell Rep. 1992, 33, 331–338. [Google Scholar]
- Carron, M.P.; Etienne, H.; Michaux-Ferriere, N.; Montoro, P. Somatic embryogenesis in rubber tree (Hevea brasiliensis Mull. Arg). In Somatic Embryogenesis and Synthetic Seed I; Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1995; Volume 30. [Google Scholar]
- Piyatrakul, P.; Putranto, R.A.; Martin, F.; Rio, M.; Dessailly, F.; Leclercq, J.; Dufayard, J.F.; Lardet, L.; Montoro, P. Some ethylene biosynthesis and AP2/ERF genes reveal a specific pattern of expression during somatic embryogenesis in Hevea brasiliensis. BMC Plant Biol. 2012, 12, 244. [Google Scholar] [CrossRef] [PubMed]
- Auboiron Carron, M.P.; Michaux, F.N. Influence of atmospheric gases, particularly ethylene, on somatic embryogenesis of Hevea brasiliensis. Plant Cell Tissue Organ Cult. 1990, 21, 31–37. [Google Scholar] [CrossRef]
- Engelmann, F.; Lartaud, M.; Chabrillange, N.; Carron, M.P.; Etienne, H. Cryopreservation of embryogenic calluses of two commercial clones of Hevea brasiliensis. CryoLetters 1997, 18, 107–116. [Google Scholar]
- Lardet, L.; Martin, F.; Dessailly, F.; Carron, M.P.; Montoro, P. Effect of exogenous calcium on post-thaw growth recovery and subsequent plant regeneration of cryopreserved embryogenic calli of Hevea brasiliensis (Mull. Arg.). Plant Cell Rep. 2007, 26, 559–569. [Google Scholar] [CrossRef]
- Montoro, P.; Etienne, H.; Ferriere, N.M.; Carron, M.P. Callus friability and somatic embryogenesis in Hevea brasiliensis. Plant Cell Tissue Organ Cult. 1993, 33, 331–338. [Google Scholar] [CrossRef]
- Lardet, L.; Dessailly, F.; Carron, M.P.; Rio, M.A.; Ferrière, N.; Montoro, P. Secondary somatic embryogenesis in Hevea brasiliensis (Müll. Arg.) an alternative process for long-term somatic embryogenesis. J. Rubb. Res. 2009, 12, 215–228. [Google Scholar]
- Li, Z.; Sun, A.H.; Hung, T.D.; Zhou, Q.N.; Dai, X.M.; Hung, H.S.; Lin, W.F.; Lardet, L.; Montoro, P. Friable and embryogenic callus induction and plant regeneration from rubber tree clone Reyan88-13 (Hevea brasiliensis). Chin. J. Trop. Crops 2010, 31, 2166–2173. [Google Scholar]
- Lu, L.; Holt, A.; Chen, X.; Liu, Y.; Knauer, S.; Tucker, E.J.; Sarkar, A.K.; Hao, Z.; Roodbarkelari, F.; Shi, J.; et al. MiR394 enhances WUSCHEL-induced somatic embryogenesis in Arabidopsis thaliana. New Phytol. 2023, 238, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Palmgren, M. GRF-GIF chimeras boost plant regeneration. Trends Plant Sci. 2021, 26, 201–204. [Google Scholar] [CrossRef]
- Park, J.S.; Park, K.H.; Park, S.J.; Ko, S.R.; Moon, K.B.; Koo, H.; Cho, H.S.; Park, S.U.; Jeon, J.H.; Kim, H.S.; et al. WUSCHEL controls genotype-dependent shoot regeneration capacity in potato. Plant Physiol. 2023, 193, 661–676. [Google Scholar] [CrossRef]
- Wang, N.; Ryan, L.; Sardesai, N.; Wu, E.; Lenderts, B.; Lowe, K.; Che, P.; Anand, A.; Worden, A.; van Dyk, D.; et al. Leaf transformation for efficient random integration and targeted genome modification in maize and sorghum. Nat. Plants 2023, 9, 255–270. [Google Scholar] [CrossRef]
- Lowe, K.; Wu, E.; Wang, N.; Hoerster, G.; Hastings, C.; Cho, M.J.; Scelonge, C.; Lenderts, B.; Chamberlin, M.; Cushatt, J.; et al. Morphogenic regulators baby boom and wuschel improve monocot transformation. Plant Cell 2016, 28, 1998–2015. [Google Scholar] [CrossRef]
- Ercoli, M.F.; Ferela, A.; Debernardi, J.M.; Perrone, A.P.; Rodriguez, R.E.; Palatnik, J.F. GIF transcriptional coregulators control root meristem homeostasis. Plant Cell 2018, 30, 347–359. [Google Scholar] [CrossRef]
- Kim, J.H.; Kende, H. A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 13374–13379. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, P.; Wang, J.; Xu, Z.Y. Growth-regulating factors: Conserved and divergent roles in plant growth and development and potential value for crop improvement. Plant J. 2023, 113, 1122–1145. [Google Scholar] [CrossRef]
- Omidbakhshfard, M.A.; Proost, S.; Fujikura, U.; Mueller-Roeber, B. Growth-regulating factors (GRFs): A small transcription factor family with important functions in plant biology. Mol. Plant 2015, 8, 998–1010. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF-GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef]
- Feng, Q.; Xiao, L.; He, Y.; Liu, M.; Wang, J.; Tian, S.; Zhang, X.; Yuan, L. Highly efficient, genotype-independent transformation and gene editing in watermelon (Citrullus lanatus) using a chimeric ClGRF4-GIF1 gene. J. Integr. Plant Biol. 2021, 63, 2038–2042. [Google Scholar] [CrossRef]
- Qiu, F.; Xing, S.; Xue, C.; Liu, J.; Chen, K.; Chai, T.; Gao, C. Transient expression of a TaGRF4-TaGIF1 complex stimulates wheat regeneration and improves genome editing. Sci. China Life Sci. 2022, 65, 731–738. [Google Scholar] [CrossRef]
- Yarra, R.; Krysan, P.J. GRF-GIF duo and GRF-GIF-BBM: Novel transformation methodologies for enhancing regeneration efficiency of genome-edited recalcitrant crops. Planta 2023, 257, 60. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Martin-Ortigosa, S.; Finer, J.; Orchard, N.; Gunadi, A.; Batts, L.A.; Thakare, D.; Rush, B.; Schmitz, O.; Stuiver, M.; et al. Overexpression of the transcription factor GROWTH-REGULATING FACTOR 5 improves transformation of dicot and monocot species. Front. Plant Sci. 2020, 11, 572319. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Cheng, Z.; Han, Z.; Yang, H.; Zhang, W.; Zhang, H. Efficient genetic transformation and CRISPR/Cas9-mediated genome editing of watermelon assisted by genes encoding developmental regulators. J. Zhejiang Univ.-Sci. B 2022, 23, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Karumamkandathil, R.; Jayasree, P.K.; Radha, J.; Uthup, T.K.; Mathew, S.A.; Sathik, M.B.M. Genetics and Genomics of Abiotic Stress in Rubber Tree (Hevea Brasiliensis). In Genomic Designing for Abiotic Stress Resistant Technical Crops; Kole, C., Ed.; Springer: Cham, Switzerland, 2022; pp. 245–298. [Google Scholar]
- Mira, M.M.; Wally, O.S.; Elhiti, M.; El-Shanshory, A.; Reddy, D.S.; Hill, R.D.; Stasolla, C. Jasmonic acid is a downstream component in the modulation of somatic embryogenesis by Arabidopsis Class 2 phytoglobin. J. Exp. Bot. 2016, 67, 2231–2246. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K.; Manners, J.M. MYC2: The master in action. Mol. Plant 2013, 6, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Gao, Y.Q.; Zhang, L.; Chen, D.R.; Gan, J.H.; Murchie, A.I.H. The identification and characterization of a selected SAM-dependent methyltransferase ribozyme that is present in natural sequences. Nat. Catal. 2021, 4, 872–881. [Google Scholar] [CrossRef]
- Luo, H.; Hansen, A.S.L.; Yang, L.; Schneider, K.; Kristensen, M.; Christensen, U.; Christensen, H.B.; Du, B.; Ozdemir, E.; Feist, A.M.; et al. Coupling S-adenosylmethionine-dependent methylation to growth: Design and uses. PLoS Biol. 2019, 17, e2007050. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, R.; Kumar, N.; Gautam, A.; Kumar Dubey, A.; Pandey, S.N.; Mallick, S. Chlorella sp. modulates the glutathione mediated detoxification and S-adenosylmethionine dependent methyltransferase to counter arsenic toxicity in Oryza sativa L. Ecotoxicol. Environ. Saf. 2021, 208, 111418. [Google Scholar] [CrossRef]
- Leclercq, J.; Lardet, L.; Martin, F.; Chapuset, T.; Oliver, G.; Montoro, P. The green fluorescent protein as an efficient selection marker for Agrobacterium tumefaciens-mediated transformation in Hevea brasiliensis (Mull. Arg). Plant Cell Rep. 2010, 29, 513–522. [Google Scholar] [CrossRef]
- Lestari, R.; Rio, M.; Martin, F.; Leclercq, J.; Woraathasin, N.; Roques, S.; Dessailly, F.; Clement-Vidal, A.; Sanier, C.; Fabre, D.; et al. Overexpression of Hevea brasiliensis ethylene response factor HbERF-IXc5 enhances growth and tolerance to abiotic stress and affects laticifer differentiation. Plant Biotechnol. J. 2018, 16, 322–336. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, X.; Zhang, Y.; Zhou, M.; Liu, K.; Zhang, S.; Ye, D.; Tang, C.; Cao, J. Overexpression of HbGRF4 or HbGRF4-HbGIF1 Chimera Improves the Efficiency of Somatic Embryogenesis in Hevea brasiliensis. Int. J. Mol. Sci. 2024, 25, 2921. https://doi.org/10.3390/ijms25052921
Luo X, Zhang Y, Zhou M, Liu K, Zhang S, Ye D, Tang C, Cao J. Overexpression of HbGRF4 or HbGRF4-HbGIF1 Chimera Improves the Efficiency of Somatic Embryogenesis in Hevea brasiliensis. International Journal of Molecular Sciences. 2024; 25(5):2921. https://doi.org/10.3390/ijms25052921
Chicago/Turabian StyleLuo, Xiaomei, Yi Zhang, Miaomiao Zhou, Kaiye Liu, Shengmin Zhang, De Ye, Chaorong Tang, and Jie Cao. 2024. "Overexpression of HbGRF4 or HbGRF4-HbGIF1 Chimera Improves the Efficiency of Somatic Embryogenesis in Hevea brasiliensis" International Journal of Molecular Sciences 25, no. 5: 2921. https://doi.org/10.3390/ijms25052921



