Defective Biomechanics and Pharmacological Rescue of Human Cardiomyocytes with Filamin C Truncations
Abstract
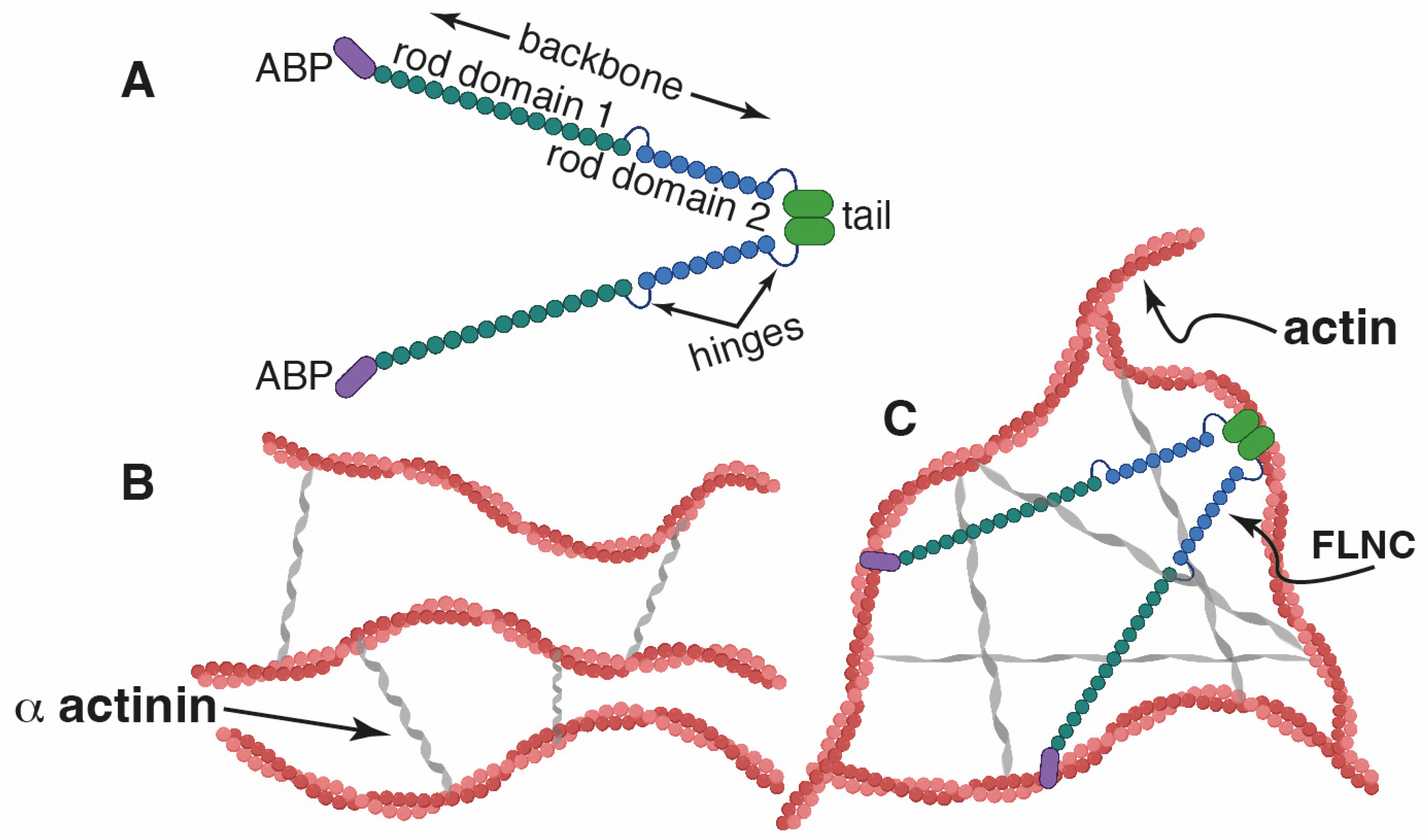
1. Introduction
2. Results and Discussion
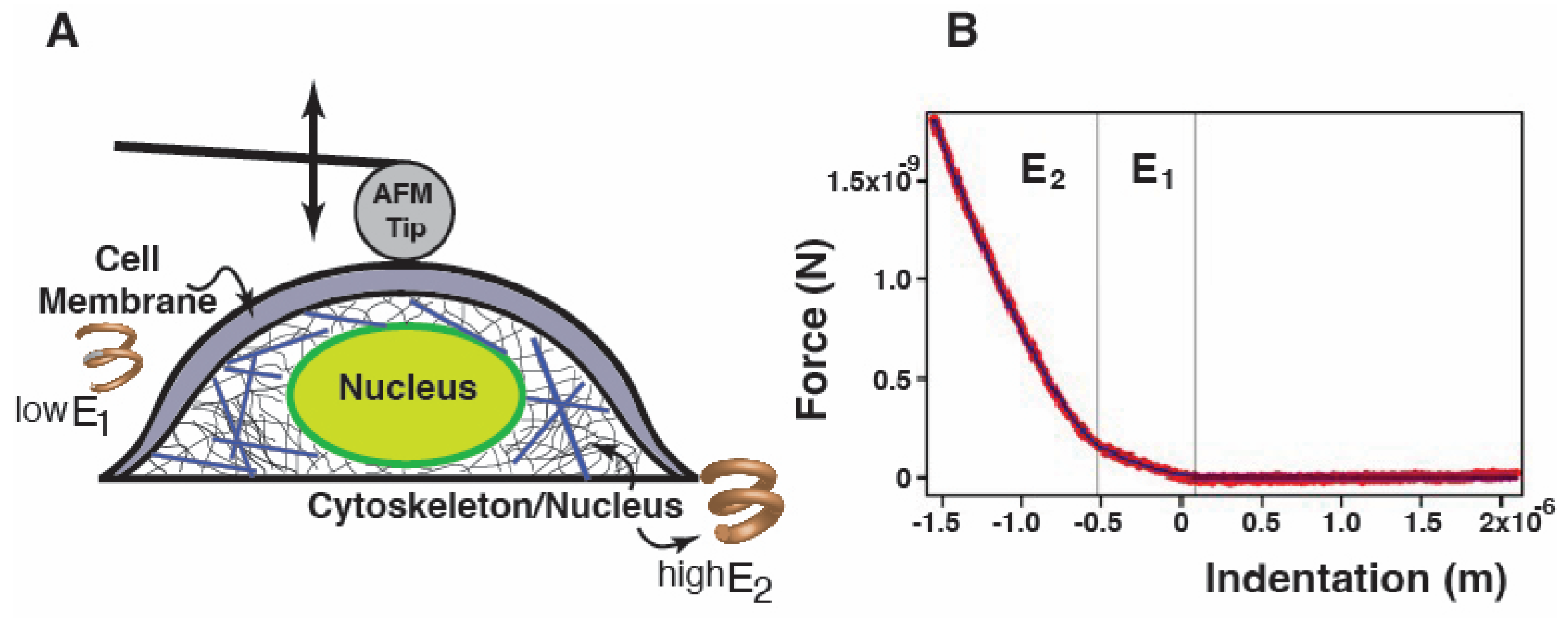
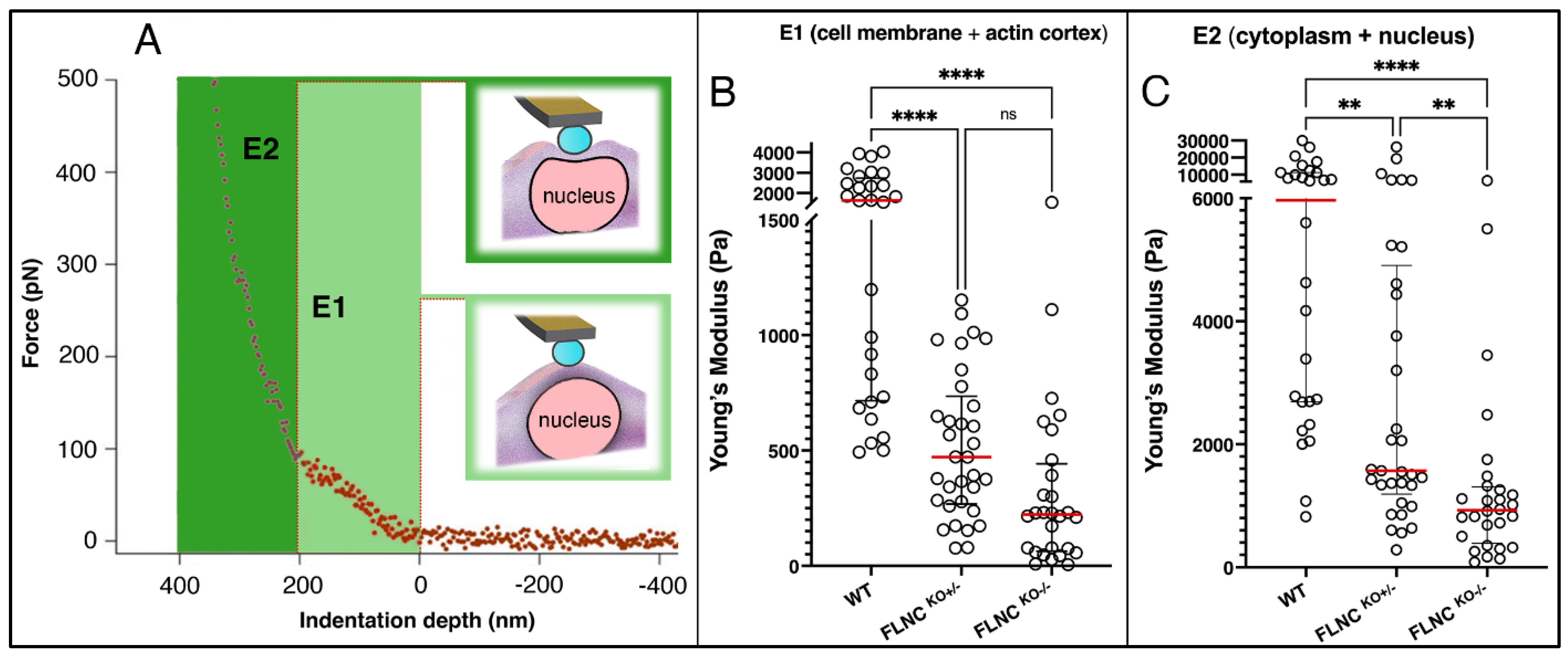
2.1. FLNC Deletion Disrupts the Mechanical Properties of hiPSC-CMs
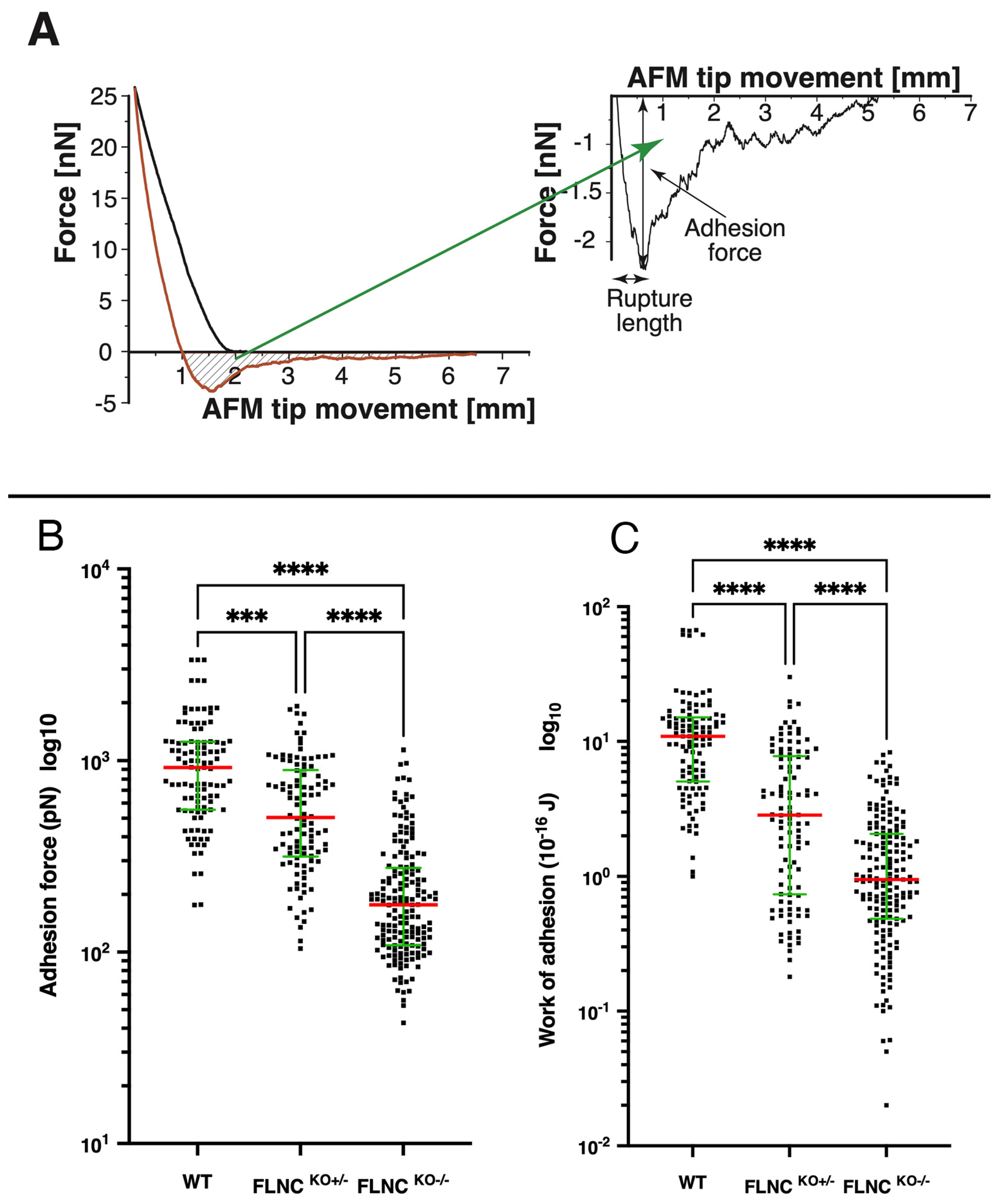
2.2. Loss of FLNC Also Reduces Adhesion Properties of hiPSC-CMs
2.3. Beating Frequency and Vertical Displacement in FLNC Models
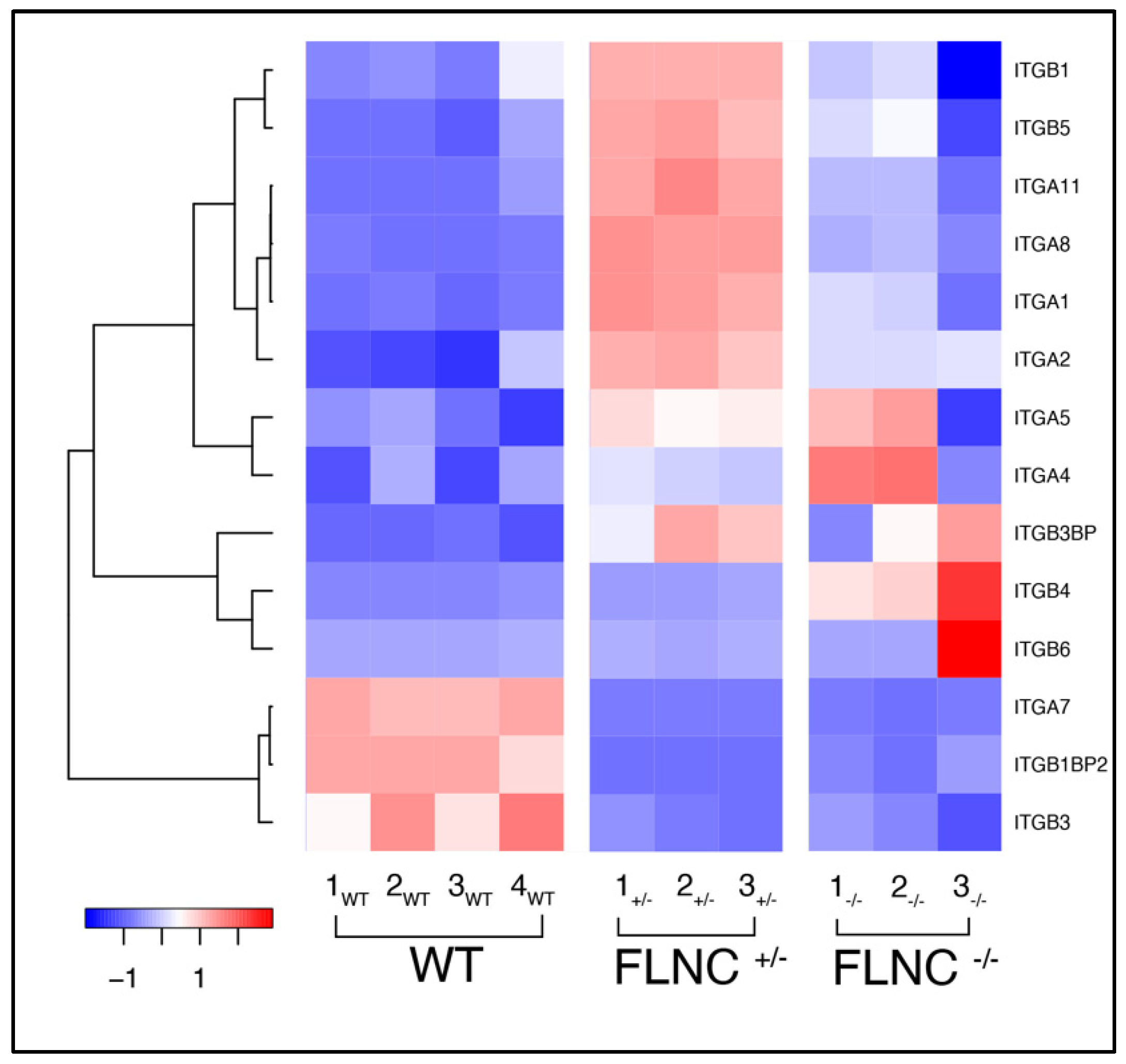
2.4. Defective Cell Adhesion in Mutant FLNC Is Associated with Altered Integrins Gene Expression
2.5. Recovering the Biomechanical Properties of FLNCtv Cardiomyocytes
2.6. Defective Cell–Cell Adhesion in FLNCtv Cardiomyocytes
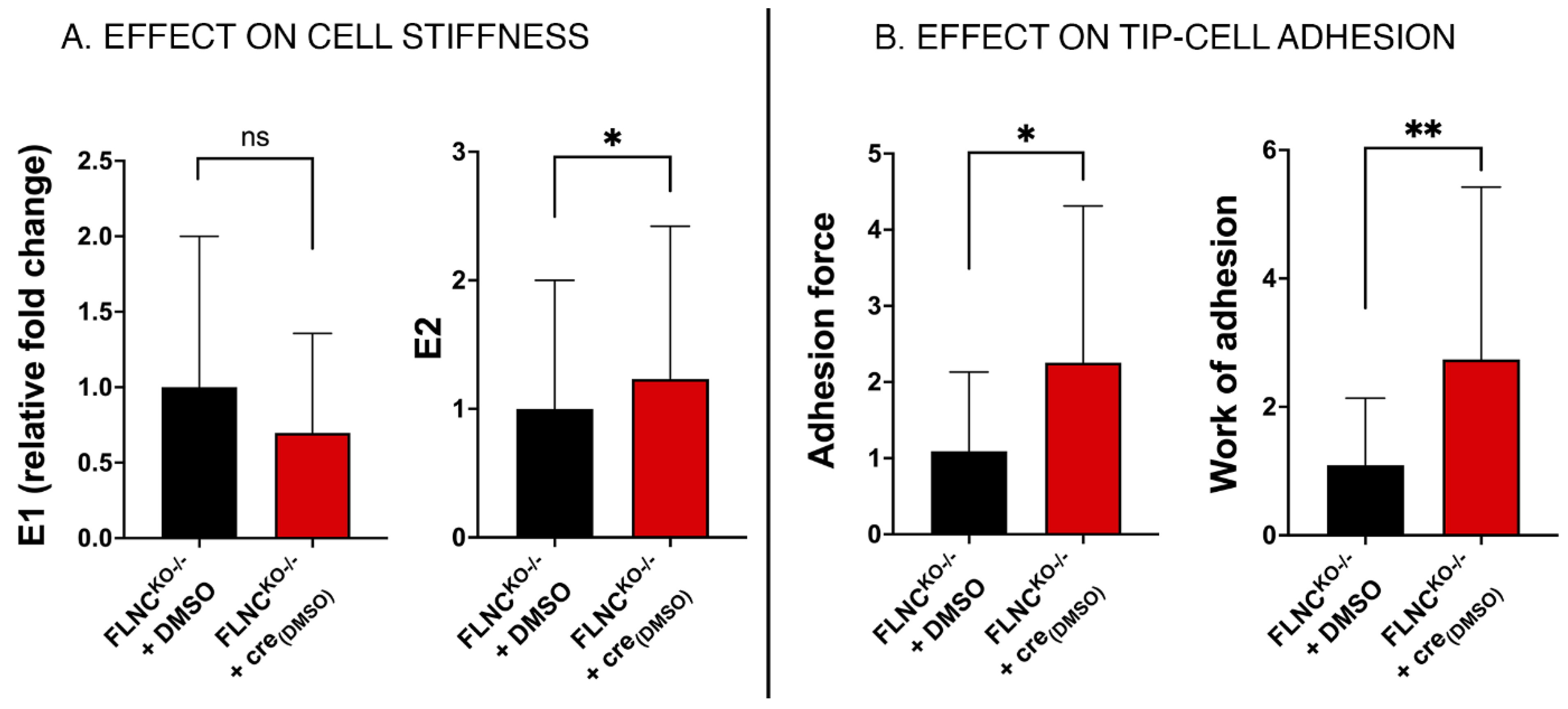
2.7. Inhibition of PDGRA Upregulation Improves Biomechanical Properties of FLNCtv Cardiomyocytes
3. Materials and Methods
3.1. FLNCKO hiPSC-CM Models
3.2. Atomic Force Microscopy—Single Cell Force Spectroscopy
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Lü, D.; Mao, D.; Long, M. Mechanomics: An emerging field between biology and biomechanics. Protein Cell 2014, 5, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Brodehl, A.; Gaertner-Rommel, A.; Milting, H. Molecular insights into cardiomyopathies associated with desmin (DES) mutations. Biophys. Rev. 2018, 10, 983–1006. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed]
- Stossel, T.P.; Condeelis, J.; Cooley, L.; Hartwig, J.H.; Noegel, A.; Schleicher, M.; Shapiro, S.S. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2001, 2, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Nakamura, F. Structure and Function of Filamin C in the Muscle Z-Disc. Int. J. Mol. Sci. 2020, 21, 2696. [Google Scholar] [CrossRef] [PubMed]
- Pecorari, I.; Mestroni, L.; Sbaizero, O. Current Understanding of the Role of Cytoskeletal Cross-Linkers in the Onset and Development of Cardiomyopathies. Int. J. Mol. Sci. 2020, 21, 5865. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, F.; Osborn, T.M.; Hartemink, C.A.; Hartwig, J.H.; Stossel, T.P. Structural basis of filamin A functions. J. Cell Biol. 2007, 179, 1011–1025. [Google Scholar] [CrossRef]
- Buehler, M.; Keten, S.; Ackbarow, T. Theoretical and computational hierarchical nanomechanics of protein materials: Deformation and fracture. Prog. Mater. Sci. 2008, 53, 1101–1241. [Google Scholar] [CrossRef]
- Keten, S.; Buehler, M.J. Strength limit of entropic elasticity in beta-sheet protein domains. Phys. Rev. E Stat. Nonlinear Biol. Soft Matter Phys. 2008, 78 Pt 1, 061913. [Google Scholar] [CrossRef]
- Mostaert, A.S.; Higgins, M.J.; Fukuma, T.; Rindi, F.; Jarvis, S.P. Nanoscale mechanical characterisation of amyloid fibrils discovered in a natural adhesive. J. Biol. Phys. 2006, 32, 393–401. [Google Scholar] [CrossRef]
- Esue, O.; Tseng, Y.; Wirtz, D. Alpha-actinin and filamin cooperatively enhance the stiffness of actin filament networks. PLoS ONE 2009, 4, e4411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geiger, B.; Bershadsky, A.; Pankov, R.; Yamada, K.M. Transmembrane crosstalk between the extracellular matrix—Cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2001, 2, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, L.S.; Morgan, T.; Bonafé, L.; Wessels, M.W.; Bialer, M.G.; Willems, P.J.; Cohn, D.H.; Krakow, D.; Robertson, S.P. Mutations in FLNB cause boomerang dysplasia. J. Med. Genet. 2005, 42, e43. [Google Scholar] [CrossRef] [PubMed]
- Vorgerd, M.; van der Ven, P.F.; Bruchertseifer, V.; Lowe, T.; Kley, R.A.; Schroder, R.; Lochmuller, H.; Himmel, M.; Koehler, K.; Furst, D.O.; et al. A mutation in the dimerization domain of filamin c causes a novel type of autosomal dominant myofibrillar myopathy. Am. J. Hum. Genet. 2005, 77, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Sheen, V.L.; Jansen, A.; Chen, M.H.; Parrini, E.; Morgan, T.; Ravenscroft, R.; Ganesh, V.; Underwood, T.; Wiley, J.; Leventer, R.; et al. Filamin A mutations cause periventricular heterotopia with Ehlers-Danlos syndrome. Neurology 2005, 64, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Kyndt, F.; Gueffet, J.P.; Probst, V.; Jaafar, P.; Legendre, A.; Le Bouffant, F.; Toquet, C.; Roy, E.; McGregor, L.; Lynch, S.A.; et al. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation 2007, 115, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Herring, J.A.; Swaney, S.S.; McClendon, T.B.; Gao, X.; Browne, R.H.; Rathjen, K.E.; Johnston, C.E.; Harris, S.; Cain, N.M.; et al. Mutations responsible for Larsen syndrome cluster in the FLNB protein. J. Med. Genet. 2006, 43, e24. [Google Scholar] [CrossRef]
- Feng, Y.; Walsh, C.A. The many faces of filamin: A versatile molecular scaffold for cell motility and signalling. Nat. Cell Biol. 2004, 6, 1034–1038. [Google Scholar] [CrossRef]
- Goetsch, S.C.; Martin, C.M.; Embree, L.J.; Garry, D.J. Myogenic progenitor cells express filamin C in developing and regenerating skeletal muscle. Stem Cells Dev. 2005, 14, 181–187. [Google Scholar] [CrossRef]
- Zhou, A.X.; Hartwig, J.H.; Akyurek, L.M. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010, 20, 113–123. [Google Scholar] [CrossRef]
- Leber, Y.; Ruparelia, A.A.; Kirfel, G.; van der Ven, P.F.; Hoffmann, B.; Merkel, R.; Bryson-Richardson, R.J.; Fürst, D.O. Filamin C is a highly dynamic protein associated with fast repair of myofibrillar microdamage. Hum. Mol. Genet. 2016, 25, 2776–2788. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Mitsuhashi, H.; Isogai, S.; Nakata, T.; Kawakami, A.; Nonaka, I.; Noguchi, S.; Hayashi, Y.K.; Nishino, I.; Kudo, A. Filamin C plays an essential role in the maintenance of the structural integrity of cardiac and skeletal muscles, revealed by the medaka mutant zacro. Dev. Biol. 2012, 361, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Tucker, N.R.; McLellan, M.A.; Hu, D.; Ye, J.; Parsons, V.A.; Mills, R.W.; Clauss, S.; Dolmatova, E.; Shea, M.A.; Milan, D.J.; et al. Novel Mutation in FLNC (Filamin C) Causes Familial Restrictive Cardiomyopathy. Circ. Cardiovasc. Genet. 2017, 10, e001780. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Mas, R.; Gutierrez-Fernandez, A.; Gomez, J.; Coto, E.; Astudillo, A.; Puente, D.A.; Reguero, J.R.; Alvarez, V.; Moris, C.; Leon, D.; et al. Mutations in filamin C cause a new form of familial hypertrophic cardiomyopathy. Nat. Commun. 2014, 5, 5326. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Genga, M.F.; Cuenca, S.; Dal Ferro, M.; Zorio, E.; Salgado-Aranda, R.; Climent, V.; Padron-Barthe, L.; Duro-Aguado, I.; Jimenez-Jaimez, J.; Hidalgo-Olivares, V.M.; et al. Truncating FLNC Mutations Are Associated with High-Risk Dilated and Arrhythmogenic Cardiomyopathies. J. Am. Coll. Cardiol. 2016, 68, 2440–2451. [Google Scholar] [CrossRef] [PubMed]
- Gigli, M.; Merlo, M.; Graw, S.L.; Barbati, G.; Rowland, T.J.; Slavov, D.B.; Stolfo, D.; Haywood, M.E.; Dal Ferro, M.; Altinier, A.; et al. Genetic Risk of Arrhythmic Phenotypes in Patients with Dilated Cardiomyopathy. J. Am. Coll. Cardiol. 2019, 74, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Furst, D.O.; Goldfarb, L.G.; Kley, R.A.; Vorgerd, M.; Olive, M.; van der Ven, P.F. Filamin C-related myopathies: Pathology and mechanisms. Acta Neuropathol. 2013, 125, 33–46. [Google Scholar] [CrossRef]
- Verdonschot, J.A.J.; Vanhoutte, E.K.; Claes, G.R.F.; Helderman-van den Enden, A.; Hoeijmakers, J.G.J.; Hellebrekers, D.; de Haan, A.; Christiaans, I.; Lekanne Deprez, R.H.; Boen, H.M.; et al. A mutation update for the FLNC gene in myopathies and cardiomyopathies. Hum. Mutat. 2020, 41, 1091–1111. [Google Scholar] [CrossRef]
- Brodehl, A.; Ferrier, R.A.; Hamilton, S.J.; Greenway, S.C.; Brundler, M.A.; Yu, W.; Gibson, W.T.; McKinnon, M.L.; McGillivray, B.; Alvarez, N.; et al. Mutations in FLNC are Associated with Familial Restrictive Cardiomyopathy. Hum. Mutat. 2016, 37, 269–279. [Google Scholar] [CrossRef]
- Brun, F.; Gigli, M.; Graw, S.L.; Judge, D.P.; Merlo, M.; Murray, B.; Calkins, H.; Sinagra, G.; Taylor, M.R.; Mestroni, L.; et al. FLNC truncations cause arrhythmogenic right ventricular cardiomyopathy. J. Med. Genet. 2020, 57, 254–257. [Google Scholar] [CrossRef]
- Begay, R.L.; Graw, S.L.; Sinagra, G.; Asimaki, A.; Rowland, T.J.; Slavov, D.B.; Gowan, K.; Jones, K.L.; Brun, F.; Merlo, M.; et al. Filamin C Truncation Mutations Are Associated with Arrhythmogenic Dilated Cardiomyopathy and Changes in the Cell-Cell Adhesion Structures. JACC Clin. Electrophysiol. 2018, 4, 504–514. [Google Scholar] [CrossRef]
- Begay, R.L.; Tharp, C.A.; Martin, A.; Graw, S.L.; Sinagra, G.; Miani, D.; Sweet, M.E.; Slavov, D.B.; Stafford, N.; Zeller, M.J.; et al. FLNC Gene Splice Mutations Cause Dilated Cardiomyopathy. JACC Basic Transl. Sci. 2016, 1, 344–359. [Google Scholar] [CrossRef]
- Gigli, M.; Stolfo, D.; Graw, S.L.; Merlo, M.; Gregorio, C.; Nee Chen, S.; Dal Ferro, M.; Paldino, M.A.; De Angelis, G.; Brun, F.; et al. Phenotypic Expression, Natural History, and Risk Stratification of Cardiomyopathy Caused by Filamin C Truncating Variants. Circulation 2021, 144, 1600–1611. [Google Scholar] [CrossRef]
- Bueno-Beti, C.; Tafuni, A.; Chelko, S.P.; Sheppard, M.N.; Field, E.; Tollit, J.; Heenan, I.K.; Barnes, A.; Taylor, M.R.; Mestroni, L.; et al. Innate immune signaling in hearts and buccal mucosa cells of patients with arrhythmogenic cardiomyopathy. Heart Rhythm O2 2023, 4, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Lam, C.K.; Wan, Y.W.; Gao, S.; Malak, O.A.; Zhao, S.R.; Lombardi, R.; Ambardekar, A.V.; Bristow, M.R.; Cleveland, J.; et al. Activation of PDGFRA signaling contributes to filamin C-related arrhythmogenic cardiomyopathy. Sci. Adv. 2022, 8, eabk0052. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; He, L.; Lam, C.K.; Taylor, M.R.G.; Mestroni, L.; Lombardi, R.; Chen, S.N. Filamin C Deficiency Impairs Sarcomere Stability and Activates Focal Adhesion Kinase through PDGFRA Signaling in Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Cells 2024, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef]
- Burridge, P.W.; Holmstrom, A.; Wu, J.C. Chemically Defined Culture and Cardiomyocyte Differentiation of Human Pluripotent Stem Cells. Curr. Protoc. Hum. Genet. 2015, 87, 21–23. [Google Scholar] [CrossRef]
- Burridge, P.W.; Diecke, S.; Matsa, E.; Sharma, A.; Wu, H.; Wu, J.C. Modeling Cardiovascular Diseases with Patient-Specific Human Pluripotent Stem Cell-Derived Cardiomyocytes. Methods Mol. Biol. 2016, 1353, 119–130. [Google Scholar] [CrossRef]
- Prabhune, M.; Belge, G.; Dotzauer, A.; Bullerdiek, J.; Radmacher, M. Comparison of mechanical properties of normal and malignant thyroid cells. Micron 2012, 43, 1267–1272. [Google Scholar] [CrossRef]
- Rico, F.; Roca-Cusachs, P.; Gavara, N.; Farré, R.; Rotger, M.; Navajas, D. Probing mechanical properties of living cells by atomic force microscopy with blunted pyramidal cantilever tips. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005, 72 Pt 1, 021914. [Google Scholar] [CrossRef]
- Guz, N.; Dokukin, M.; Kalaparthi, V.; Sokolov, I. If cell mechanics can be described by elastic modulus: Study of different models and probes used in indentation experiments. Biophys. J. 2014, 107, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Weisenhorn, A.L.; Khorsandi, M.; Kasas, S.; Gotzos, V.; Butt, H.J. Deformation and height anomaly of soft surfaces studied with an AFM. Nanotechnology 1993, 4, 106–113. [Google Scholar] [CrossRef]
- Radmacher, M.; Fritz, M.; Hansma, P.K. Imaging soft samples with the atomic force microscope: Gelatin in water and propanol. Biophys. J. 1995, 69, 264–270. [Google Scholar] [CrossRef]
- Ohashi, T.; Ishii, Y.; Ishikawa, Y.; Matsumoto, T.; Sato, M. Experimental and numerical analyses of local mechanical properties measured by atomic force microscopy for sheared endothelial cells. Biomed. Mater. Eng. 2002, 12, 319–327. [Google Scholar]
- Kuznetsova, T.G.; Starodubtseva, M.N.; Yegorenkov, N.I.; Chizhik, S.A.; Zhdanov, R.I. Atomic force microscopy probing of cell elasticity. Micron 2007, 38, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Andolfi, L.; Frattini, F.; Mayer, F.; Lazzarino, M.; Hu, J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nat. Commun. 2015, 6, 8512. [Google Scholar] [CrossRef]
- Andolfi, L.; Masiero, E.; Giolo, E.; Martinelli, M.; Luppi, S.; Dal Zilio, S.; Delfino, I.; Bortul, R.; Zweyer, M.; Ricci, G.; et al. Investigating the mechanical properties of zona pellucida of whole human oocytes by atomic force spectroscopy. Integr. Biol. 2016, 8, 886–893. [Google Scholar] [CrossRef]
- Bugiardini, E.; Nunes, A.M.; Oliveira-Santos, A.; Dagda, M.; Fontelonga, T.M.; Barraza-Flores, P.; Pittman, A.M.; Morrow, J.M.; Parton, M.; Houlden, H.; et al. Integrin α7 Mutations Are Associated with Adult-Onset Cardiac Dysfunction in Humans and Mice. J. Am. Heart Assoc. 2022, 11, e026494. [Google Scholar] [CrossRef]
- Haroon, M.; Boers, H.E.; Bakker, A.D.; Bloks, N.G.C.; Hoogaars, W.M.H.; Giordani, L.; Musters, R.J.P.; Deldicque, L.; Koppo, K.; Le Grand, F.; et al. Reduced growth rate of aged muscle stem cells is associated with impaired mechanosensitivity. Aging 2022, 14, 28–53. [Google Scholar] [CrossRef]
- Powers, J.D.; Kirkland, N.J.; Liu, C.; Razu, S.S.; Fang, X.; Engler, A.J.; Chen, J.; McCulloch, A.D. Subcellular Remodeling in Filamin C Deficient Mouse Hearts Impairs Myocyte Tension Development during Progression of Dilated Cardiomyopathy. Int. J. Mol. Sci. 2022, 23, 871. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Zhang, L.; Zhu, M.; Tan, C.; Zhou, X.; Evans, S.M.; Fang, X.; Feng, W.; Chen, J. Loss of Filamin C Is Catastrophic for Heart Function. Circulation 2020, 141, 869–871. [Google Scholar] [CrossRef]
- Wagner, B.; Tharmann, R.; Haase, I.; Fischer, M.; Bausch, A.R. Cytoskeletal polymer networks: The molecular structure of cross-linkers determines macroscopic properties. Proc. Natl. Acad. Sci. USA 2006, 103, 13974–13978. [Google Scholar] [CrossRef]
- Yang, F.; Gao, Y.; Zhang, Y.; Chen, J.; Long, M. Developing a microfluidic-based system to quantify cell capture efficiency. Sci. China Ser. C Life Sci. 2009, 52, 173–181. [Google Scholar] [CrossRef]
- Berro, J.; Michelot, A.; Blanchoin, L.; Kovar, D.R.; Martiel, J.L. Attachment conditions control actin filament buckling and the production of forces. Biophys. J. 2007, 92, 2546–2558. [Google Scholar] [CrossRef]
- Käs, J.; Strey, H.; Sackmann, E. Direct imaging of reptation for semiflexible actin filaments. Nature 1994, 368, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Gurmessa, B.; Ricketts, S.; Robertson-Anderson, R.M. Nonlinear Actin Deformations Lead to Network Stiffening, Yielding, and Nonuniform Stress Propagation. Biophys. J. 2017, 113, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Broedersz, C.P.; Depken, M.; Yao, N.Y.; Pollak, M.R.; Weitz, D.A.; MacKintosh, F.C. Cross-link-governed dynamics of biopolymer networks. Phys. Rev. Lett. 2010, 105, 238101. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, D.; Tardin, C.; Schmidt, C.F.; Mackintosh, F.C. Nonequilibrium mechanics of active cytoskeletal networks. Science 2007, 315, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, D.; Duggan, C.; Saha, D.; Smith, D.; Käs, J. Active fluidization of polymer networks through molecular motors. Nature 2002, 416, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Wachsstock, D.H.; Schwarz, W.H.; Pollard, T.D. Cross-linker dynamics determine the mechanical properties of actin gels. Biophys. J. 1994, 66 Pt 1, 801–809. [Google Scholar] [CrossRef]
- Xu, J.; Wirtz, D.; Pollard, T.D. Dynamic cross-linking by alpha-actinin determines the mechanical properties of actin filament networks. J. Biol. Chem. 1998, 273, 9570–9576. [Google Scholar] [CrossRef]
- Gardel, M.L.; Nakamura, F.; Hartwig, J.H.; Crocker, J.C.; Stossel, T.P.; Weitz, D.A. Prestressed F-actin networks cross-linked by hinged filamins replicate mechanical properties of cells. Proc. Natl. Acad. Sci. USA 2006, 103, 1762–1767. [Google Scholar] [CrossRef]
- Wu, T.; Xu, Y.; Zhang, L.; Liang, Z.; Zhou, X.; Evans, S.M.; Chen, J. Filamin C is Essential for mammalian myocardial integrity. PLoS Genet. 2023, 19, e1010630. [Google Scholar] [CrossRef]
- Puzzi, L.; Borin, D.; Gurha, P.; Lombardi, R.; Martinelli, V.; Weiss, M.; Andolfi, L.; Lazzarino, M.; Mestroni, L.; Marian, A.J.; et al. Knock Down of Plakophillin 2 Dysregulates Adhesion Pathway through Upregulation of miR200b and Alters the Mechanical Properties in Cardiac Cells. Cells 2019, 8, 1639. [Google Scholar] [CrossRef] [PubMed]
- Gontier, Y.; Taivainen, A.; Fontao, L.; Sonnenberg, A.; van der Flier, A.; Carpen, O.; Faulkner, G.; Borradori, L. The Z-disc proteins myotilin and FATZ-1 interact with each other and are connected to the sarcolemma via muscle-specific filamins. J. Cell Sci. 2005, 118 Pt 16, 3739–3749. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.G.; Chan, Y.M.; Hack, A.A.; Brosius, M.; Rajala, M.; Lidov, H.G.; McNally, E.M.; Watkins, S.; Kunkel, L.M. Filamin 2 (FLN2): A muscle-specific sarcoglycan interacting protein. J. Cell Biol. 2000, 148, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Glogauer, M.; Arora, P.; Chou, D.; Janmey, P.A.; Downey, G.P.; McCulloch, C.A. The role of actin-binding protein 280 in integrin-dependent mechanoprotection. J. Biol. Chem. 1998, 273, 1689–1698. [Google Scholar] [CrossRef] [PubMed]
- Kiema, T.; Lad, Y.; Jiang, P.; Oxley, C.L.; Baldassarre, M.; Wegener, K.L.; Campbell, I.D.; Ylänne, J.; Calderwood, D.A. The molecular basis of filamin binding to integrins and competition with talin. Mol. Cell 2006, 21, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.P.; Ezzell, R.M.; Arnaout, M.A. Direct interaction of filamin (ABP-280) with the beta 2-integrin subunit CD18. J. Immunol. 1995, 154, 3461–3470. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, M.; Liu, S.; Erle, D.J.; Ginsberg, M.H. Integrin beta cytoplasmic domains differentially bind to cytoskeletal proteins. J. Biol. Chem. 1998, 273, 6104–6109. [Google Scholar] [CrossRef]
- Yan, B.; Calderwood, D.A.; Yaspan, B.; Ginsberg, M.H. Calpain cleavage promotes talin binding to the beta 3 integrin cytoplasmic domain. J. Biol. Chem. 2001, 276, 28164–28170. [Google Scholar] [CrossRef]
- Travis, M.A.; van der Flier, A.; Kammerer, R.A.; Mould, A.P.; Sonnenberg, A.; Humphries, M.J. Interaction of filamin A with the integrin beta 7 cytoplasmic domain: Role of alternative splicing and phosphorylation. FEBS Lett. 2004, 569, 185–190. [Google Scholar] [CrossRef]
- Calderwood, D.A. Integrin activation. J. Cell Sci. 2004, 117 Pt 5, 657–666. [Google Scholar] [CrossRef]
- Kim, H.; Nakamura, F.; Lee, W.; Shifrin, Y.; Arora, P.; McCulloch, C.A. Filamin A is required for vimentin-mediated cell adhesion and spreading. Am. J. Physiol. Cell Physiol. 2010, 298, C221–C236. [Google Scholar] [CrossRef]
- Kim, H.; Nakamura, F.; Lee, W.; Hong, C.; Pérez-Sala, D.; McCulloch, C.A. Regulation of cell adhesion to collagen via beta1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp. Cell Res. 2010, 316, 1829–1844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cao, G.; Collier, M.P.; Qiu, X.; Broadway-Stringer, S.; Šaman, D.; Ng, J.Z.Y.; Sen, N.; Azad, A.J.; Hooper, C.; et al. Cardiac stress leads to regulation of Filamin C dimerisation via an ancient phosphorylation-modulated interaction with HSPB7. bioRxiv 2024, 2024.01.05.574393. [Google Scholar] [CrossRef]
- Yang, X.; Pabon, L.; Murry, C.E. Engineering adolescence: Maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2014, 114, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Lanzicher, T.; Martinelli, V.; Long, C.S.; Del Favero, G.; Puzzi, L.; Borelli, M.; Mestroni, L.; Taylor, M.R.; Sbaizero, O. AFM single-cell force spectroscopy links altered nuclear and cytoskeletal mechanics to defective cell adhesion in cardiac myocytes with a nuclear lamin mutation. Nucleus 2015, 6, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Lanzicher, T.; Martinelli, V.; Puzzi, L.; Del Favero, G.; Codan, B.; Long, C.S.; Mestroni, L.; Taylor, M.R.; Sbaizero, O. The Cardiomyopathy Lamin A/C D192G Mutation Disrupts Whole-Cell Biomechanics in Cardiomyocytes as Measured by Atomic Force Microscopy Loading-Unloading Curve Analysis. Sci. Rep. 2015, 5, 13388. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, N.; Bruce, M.A.; Wu, J.C.; Butte, M.J. Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PLoS ONE 2012, 7, e37559. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.B.; Collinsworth, A.M.; Reichert, W.M.; Kraus, W.E.; Truskey, G.A. Endothelial, cardiac muscle and skeletal muscle exhibit different viscous and elastic properties as determined by atomic force microscopy. J. Biomech. 2001, 34, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: http://www.R-project.org/ (accessed on 28 October 2023).

| MEAN ± SD | ||||
|---|---|---|---|---|
| Cells | E1 (Pa) | E2 (Pa) | Max Adh Force (pN) | Work of Adhesion (10−16 J) |
| FLNCWT, N = 28 | 1804 ± 1139 | 8219 ± 7619 | 1036 ± 659 | 13.4 ± 13.9 |
| FLNCKO+/−, N = 33 | 518 ± 314 | 3933 ± 5484 | 233 ± 194 | 4.77 ± 5.17 |
| FLNCKO−/−, N = 28 | 318 ± 356 | 1324 ± 1509 | 630 ± 411 | 1.57 ± 1.64 |
| MEDIAN/IQR | ||||
| Cells | E1 (Pa) | E2 (Pa) | Max Adh Force | Work of Adhesion |
| WT N = 28 | 1637/2020 | 5966/8536 | 921/699 | 10.9/10.0 |
| FLNC +/− N = 33 | 471/467 | 1570/3720 | 505/577 | 2.85/7.07 |
| FLNC −/− N = 28 | 222/379 | 927/916 | 177/167 | 0.949/1.58 |
| MEAN ± SD | |||
|---|---|---|---|
| Cells | E2 | Max Adh Force (pN) | Work of Adhesion (0.1 fJ) |
| CTRL N = 22 | 1048 ± 894 | 95 ± 56 | 0.244 ± 0.259 |
| CRE N = 22 | 1257 ± 1066 | 165 ± 116 | 0.696 ± 0696 |
| MEDIAN/IQR | |||
| Cells | E2 | Max Adh Force | Work of Adhesion |
| CTRL N = 22 | 755/409 | 77/60 | 0.140/0.259 |
| CRE N = 22 | 984/427 | 110/187 | 0.260/1.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazzarino, M.; Zanetti, M.; Chen, S.N.; Gao, S.; Peña, B.; Lam, C.K.; Wu, J.C.; Taylor, M.R.G.; Mestroni, L.; Sbaizero, O. Defective Biomechanics and Pharmacological Rescue of Human Cardiomyocytes with Filamin C Truncations. Int. J. Mol. Sci. 2024, 25, 2942. https://doi.org/10.3390/ijms25052942
Lazzarino M, Zanetti M, Chen SN, Gao S, Peña B, Lam CK, Wu JC, Taylor MRG, Mestroni L, Sbaizero O. Defective Biomechanics and Pharmacological Rescue of Human Cardiomyocytes with Filamin C Truncations. International Journal of Molecular Sciences. 2024; 25(5):2942. https://doi.org/10.3390/ijms25052942
Chicago/Turabian StyleLazzarino, Marco, Michele Zanetti, Suet Nee Chen, Shanshan Gao, Brisa Peña, Chi Keung Lam, Joseph C. Wu, Matthew R. G. Taylor, Luisa Mestroni, and Orfeo Sbaizero. 2024. "Defective Biomechanics and Pharmacological Rescue of Human Cardiomyocytes with Filamin C Truncations" International Journal of Molecular Sciences 25, no. 5: 2942. https://doi.org/10.3390/ijms25052942
APA StyleLazzarino, M., Zanetti, M., Chen, S. N., Gao, S., Peña, B., Lam, C. K., Wu, J. C., Taylor, M. R. G., Mestroni, L., & Sbaizero, O. (2024). Defective Biomechanics and Pharmacological Rescue of Human Cardiomyocytes with Filamin C Truncations. International Journal of Molecular Sciences, 25(5), 2942. https://doi.org/10.3390/ijms25052942








