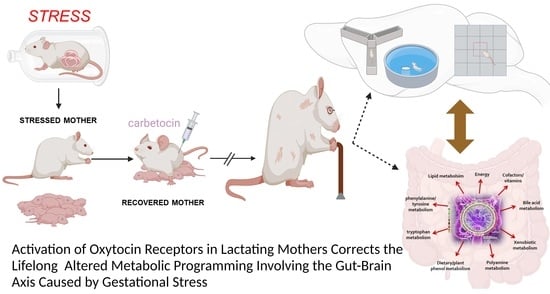
Postpartum Oxytocin Treatment via the Mother Reprograms Long-Term Behavioral Disorders Induced by Early Life Stress on the Plasma and Brain Metabolome in the Rat
Abstract
:1. Introduction
2. Results
2.1. Experimental Design
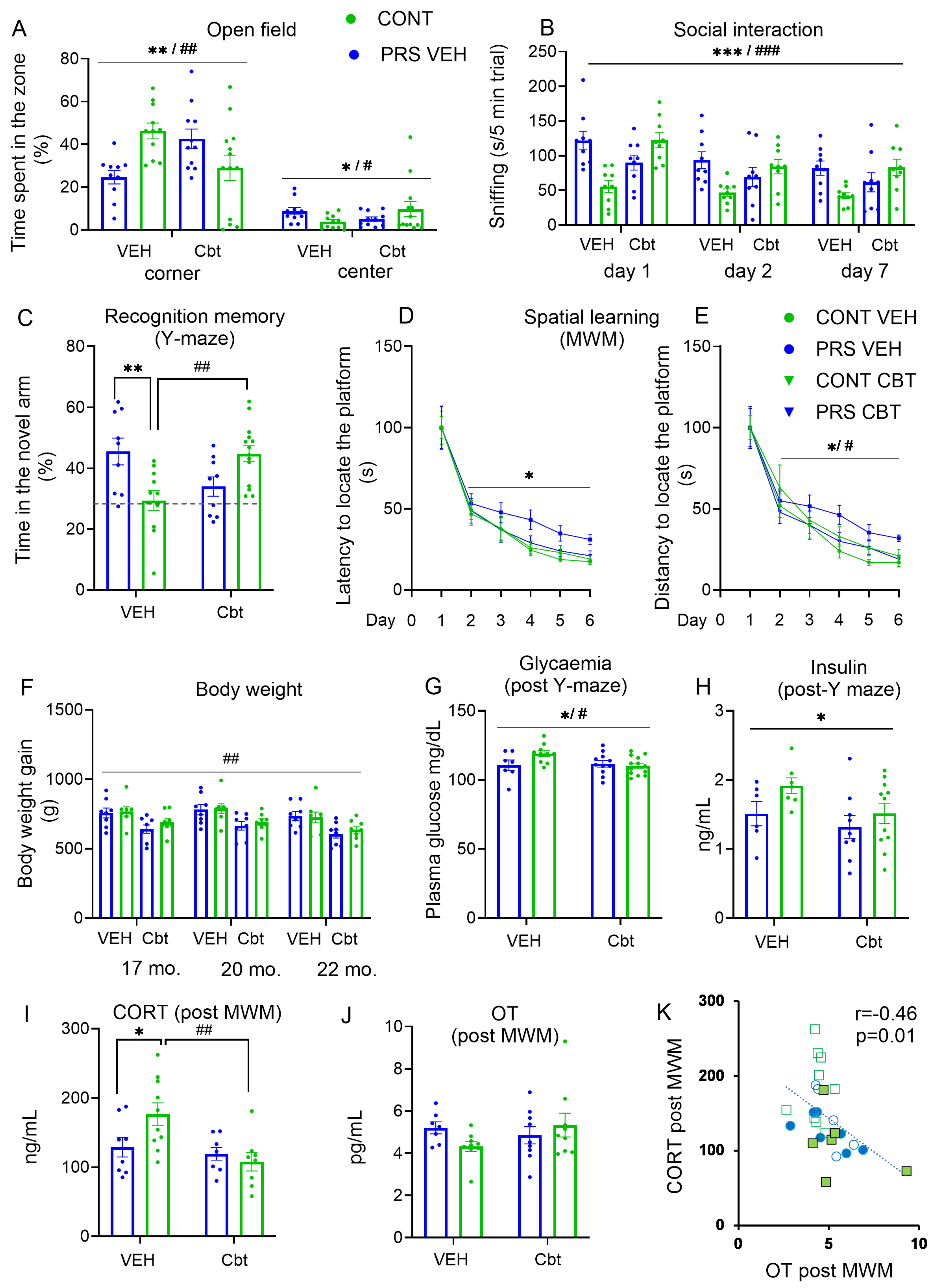
2.2. Behavioral Analysis
2.3. Physiological Analysis
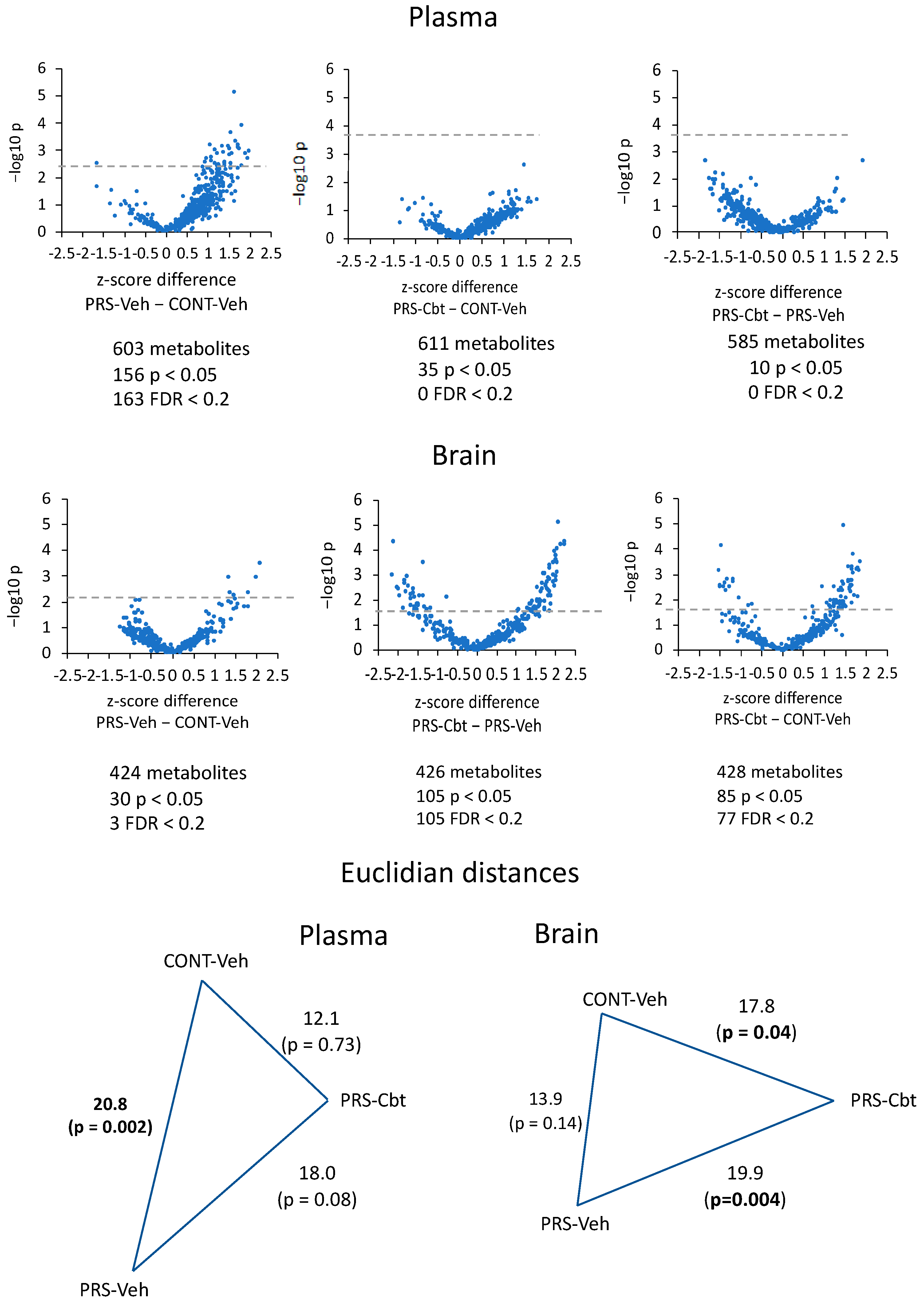
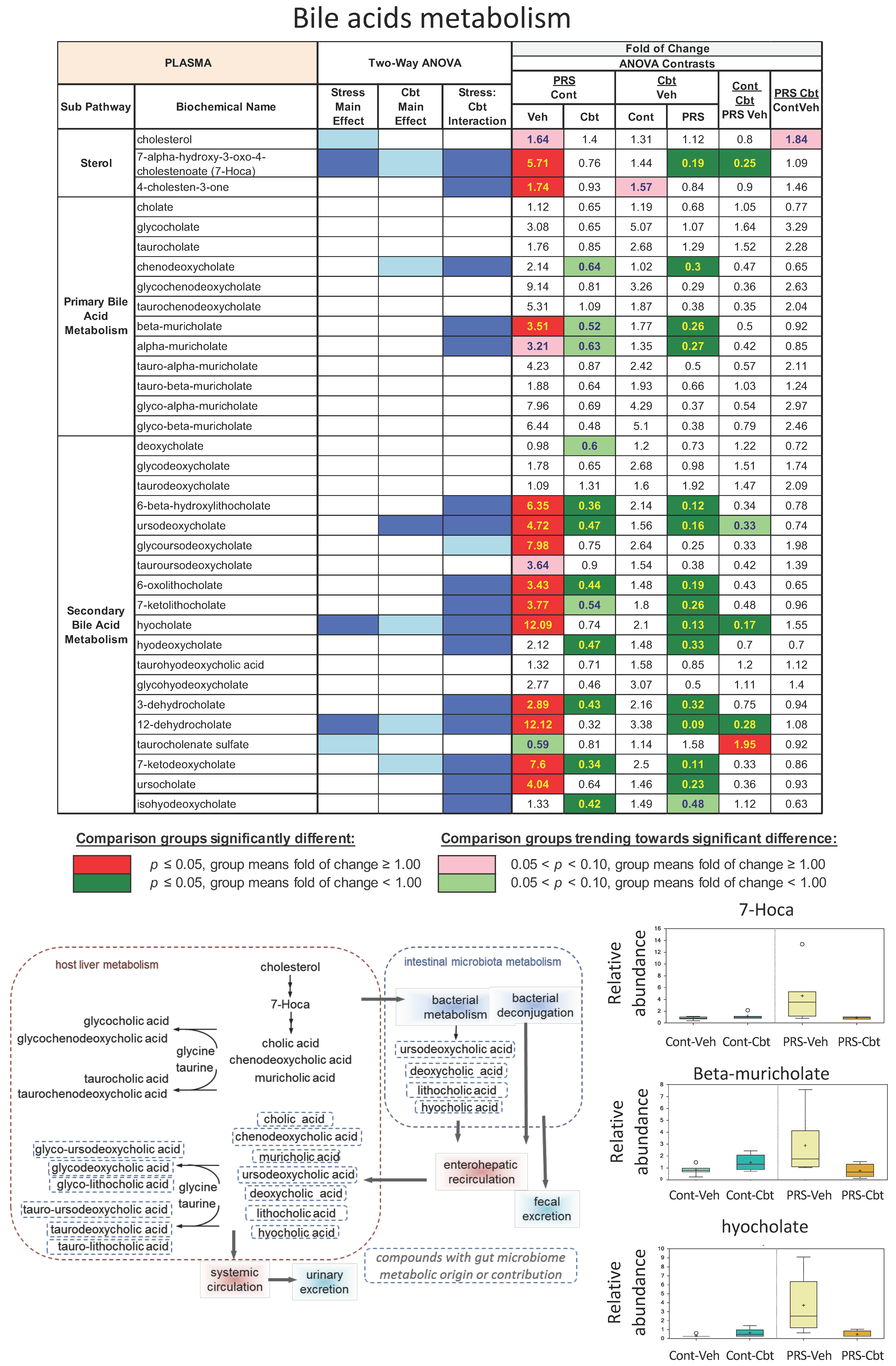
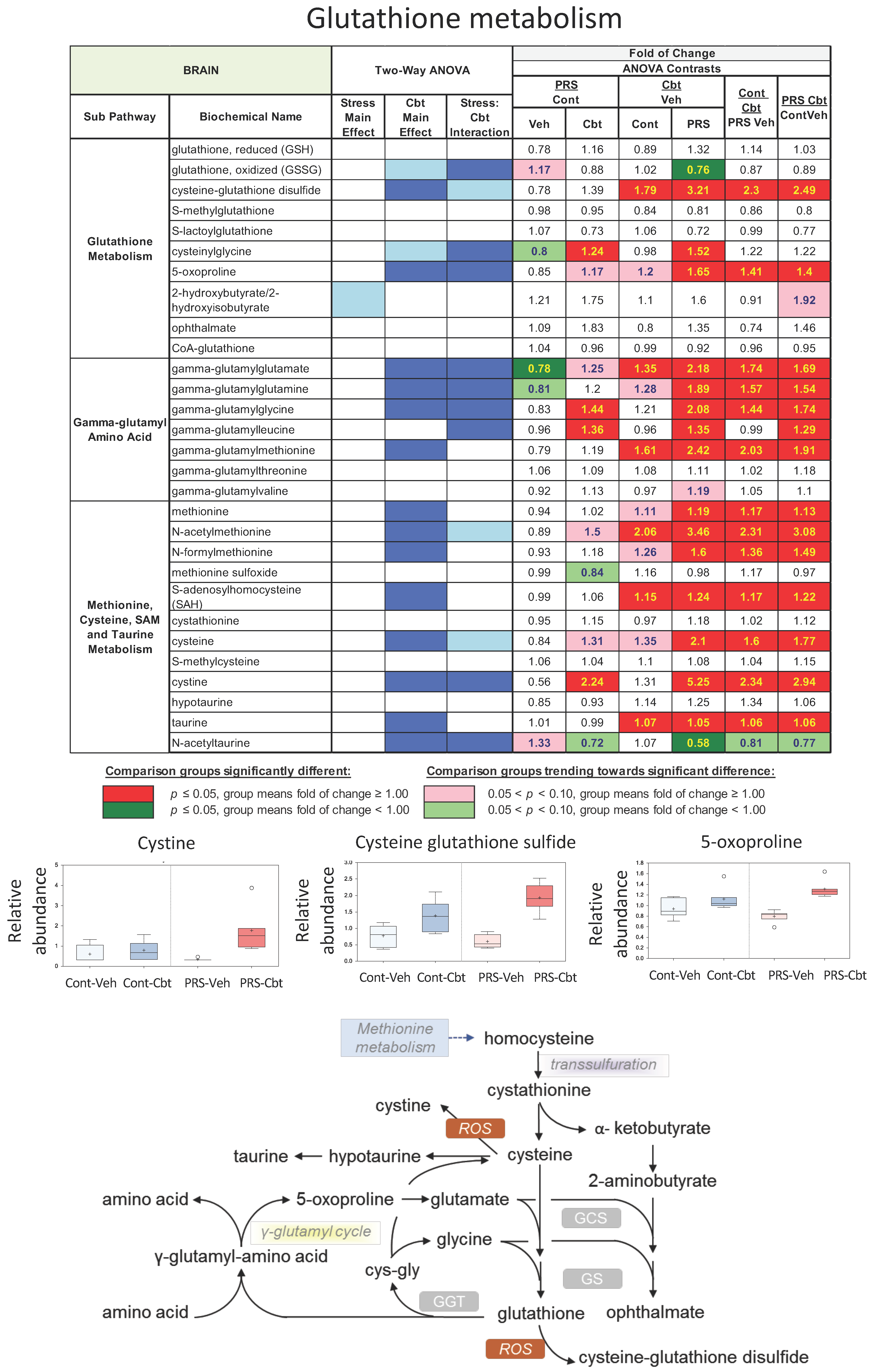
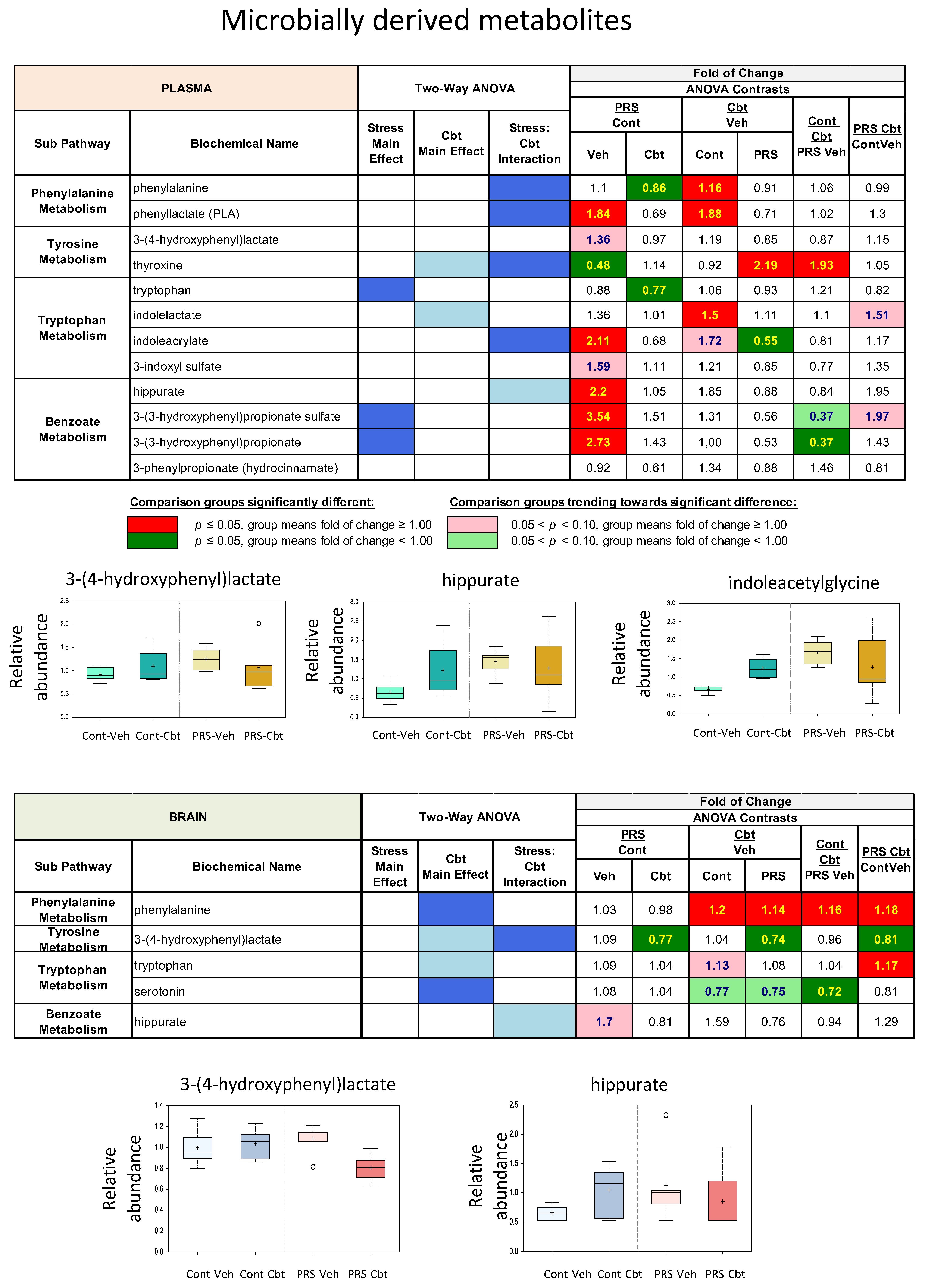
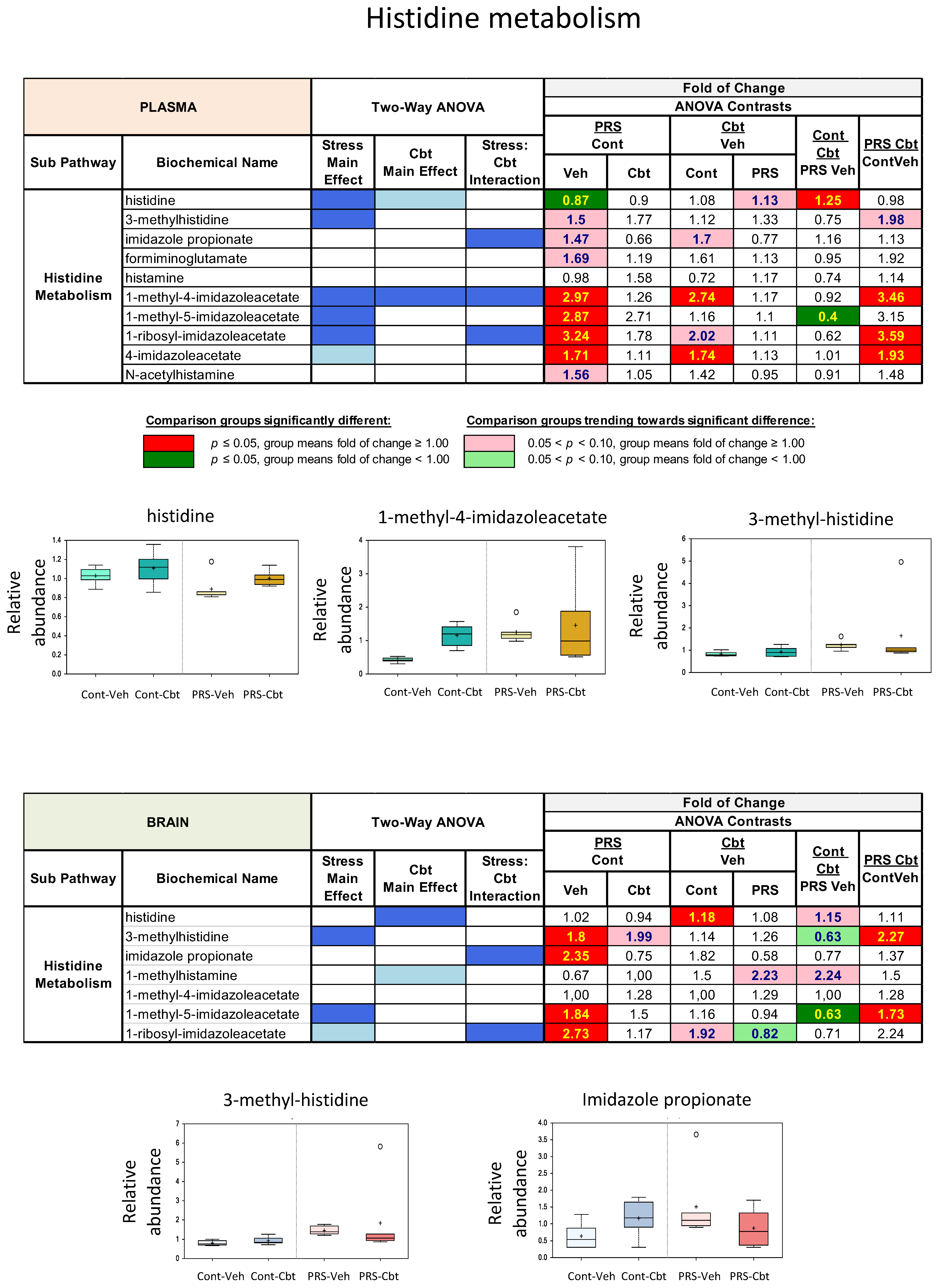
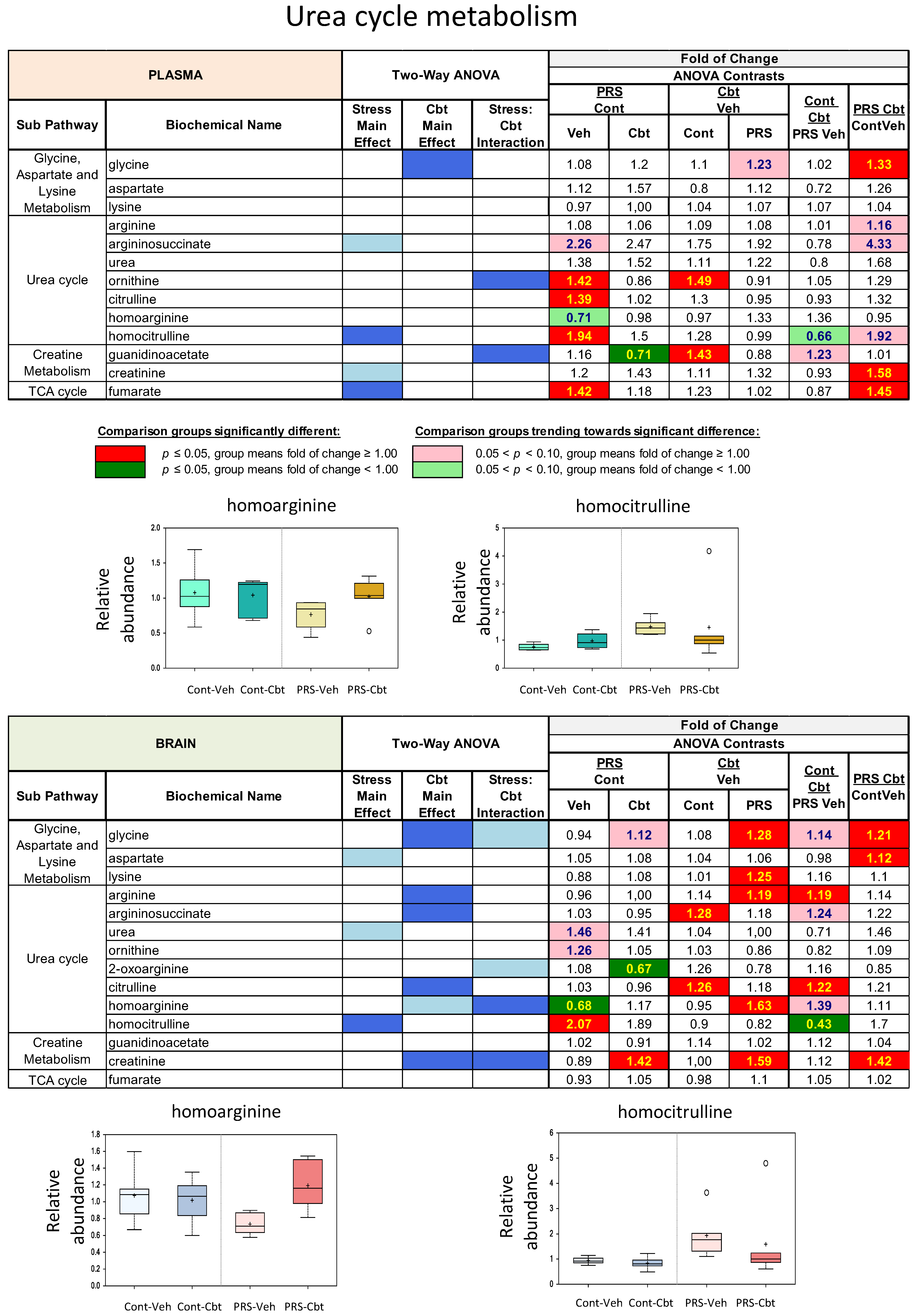
2.4. Untargeted Metabolomic Analysis
2.5. Enrichment Analysis of Functional Pathways in Plasma and Brain
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Perinatal Stress Procedure and Maternal Behavior
4.3. Carbetocin Treatment
4.4. Experimental Design
4.5. Behavioral Analysis
4.6. Physiological Analysis
4.7. Metabolome Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J. Fetal origins of coronary heart disease. Br. Heart J. 1993, 69, 195–196. [Google Scholar] [CrossRef]
- Lesseur, C.; Chen, J. Adverse Maternal Metabolic Intrauterine Environment and Placental Epigenetics: Implications for Fetal Metabolic Programming. Curr. Environ. Health Rep. 2018, 5, 531–543. [Google Scholar] [CrossRef]
- Fernandez-Capetillo, O. Intrauterine programming of ageing. EMBO Rep. 2010, 11, 32–36. [Google Scholar] [CrossRef]
- Langie, S.A.; Lara, J.; Mathers, J.C. Early determinants of the ageing trajectory. Best. Pr. Res. Clin. Endocrinol. Metab. 2012, 26, 613–626. [Google Scholar] [CrossRef]
- Mentis, A.F.; Kararizou, E. Does ageing originate in utero? Biogerontology 2010, 11, 725–729. [Google Scholar] [CrossRef]
- Vaiserman, A.M. Early-life nutritional programming of longevity. J. Dev. Orig. Health Dis. 2014, 5, 325–338. [Google Scholar] [CrossRef]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef]
- Maccari, S.; Polese, D.; Reynaert, M.L.; Amici, T.; Morley-Fletcher, S.; Fagioli, F. Early-life experiences and the development of adult diseases with a focus on mental illness: The Human Birth Theory. Neuroscience 2017, 342, 232–251. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Ozanne, S.E. Mechanisms of early life programming: Current knowledge and future directions. Am. J. Clin. Nutr. 2011, 94 (Suppl. S6), 1765S–1771S. [Google Scholar] [CrossRef]
- Duque-Guimarães, D.; Ozanne, S. Early nutrition and ageing: Can we intervene? Biogerontology 2017, 18, 893–900. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef]
- Gatta, E.; Mairesse, J.; Deruyter, L.; Marrocco, J.; Van Camp, G.; Bouwalerh, H.; Lo Guidice, J.M.; Morley-Fletcher, S.; Nicoletti, F.; Maccari, S. Reduced maternal behavior caused by gestational stress is predictive of life span changes in risk-taking behavior and gene expression due to altering of the stress/anti-stress balance. Neurotoxicology 2018, 66, 138–149. [Google Scholar] [CrossRef]
- Marrocco, J.; Verhaeghe, R.; Bucci, D.; Di Menna, L.; Traficante, A.; Bouwalerh, H.; Van Camp, G.; Ghiglieri, V.; Picconi, B.; Calabresi, P.; et al. Maternal stress programs accelerated aging of the basal ganglia motor system in offspring. Neurobiol. Stress 2020, 13, 100265. [Google Scholar] [CrossRef]
- Verhaeghe, R.; Gao, V.; Morley-Fletcher, S.; Bouwalerh, H.; Van Camp, G.; Cisani, F.; Nicoletti, F.; Maccari, S. Maternal stress programs a demasculinization of glutamatergic transmission in stress-related brain regions of aged rats. Geroscience 2022, 44, 1047–1069. [Google Scholar] [CrossRef]
- Van Camp, G.; Cigalotti, J.; Bouwalerh, H.; Mairesse, J.; Gatta, E.; Palanza, P.; Maccari, S.; Morley-Fletcher, S. Consequences of a double hit of stress during the perinatal period and midlife in female rats: Mismatch or cumulative effect? Psychoneuroendocrinology 2018, 93, 45–55. [Google Scholar] [CrossRef]
- Lawton, K.A.; Berger, A.; Mitchell, M.; Milgram, K.E.; Evans, A.M.; Guo, L.; Hanson, R.W.; Kalhan, S.C.; Ryals, J.A.; Milburn, M.V. Analysis of the adult human plasma metabolome. Pharmacogenomics 2008, 9, 383–397. [Google Scholar] [CrossRef]
- Houtkooper, R.H.; Argmann, C.; Houten, S.M.; Cantó, C.; Jeninga, E.H.; Andreux, P.A.; Thomas, C.; Doenlen, R.; Schoonjans, K.; Auwerx, J. The metabolic footprint of aging in mice. Sci. Rep. 2011, 1, 134. [Google Scholar] [CrossRef]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef]
- Johnson, L.C.; Martens, C.R.; Santos-Parker, J.R.; Bassett, C.J.; Strahler, T.R.; Cruickshank-Quinn, C.; Reisdorph, N.; McQueen, M.B.; Seals, D.R. Amino acid and lipid associated plasma metabolomic patterns are related to healthspan indicators with ageing. Clin. Sci. 2018, 132, 1765–1777. [Google Scholar] [CrossRef]
- Mellon, S.H.; Gautam, A.; Hammamieh, R.; Jett, M.; Wolkowitz, O.M. Metabolism, Metabolomics, and Inflammation in Posttraumatic Stress Disorder. Biol. Psychiatry 2018, 83, 866–875. [Google Scholar] [CrossRef]
- Burillo, J.; Marqués, P.; Jiménez, B.; González-Blanco, C.; Benito, M.; Guillén, C. Insulin Resistance and Diabetes Mellitus in Alzheimer’s Disease. Cells 2021, 10, 1236. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Marogianni, C.; Sokratous, M.; Dardiotis, E.; Hadjigeorgiou, G.M.; Bogdanos, D.; Xiromerisiou, G. Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 8421. [Google Scholar] [CrossRef]
- Ebner, N.C.; Maura, G.M.; Macdonald, K.; Westberg, L.; Fischer, H. Oxytocin and socioemotional aging: Current knowledge and future trends. Front. Hum. Neurosci. 2013, 7, 487. [Google Scholar] [CrossRef]
- Hofmann, A.F. The continuing importance of bile acids in liver and intestinal disease. Arch. Intern. Med. 1999, 159, 2647–2658. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Cardoso, V.F.; Corlianò, M.; Singaraja, R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. Neuromolecular Med. 2021, 23, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Yeo, X.Y.; Tan, L.Y.; Chae, W.R.; Lee, D.Y.; Lee, Y.A.; Wuestefeld, T.; Jung, S. Liver’s influence on the brain through the action of bile acids. Front. Neurosci. 2023, 17, 1123967. [Google Scholar] [CrossRef]
- Qu, Y.; Su, C.; Zhao, Q.; Shi, A.; Zhao, F.; Tang, L.; Xu, D.; Xiang, Z.; Wang, Y.; Wang, Y.; et al. Gut Microbiota-Mediated Elevated Production of Secondary Bile Acids in Chronic Unpredictable Mild Stress. Front. Pharmacol. 2022, 13, 837543. [Google Scholar] [CrossRef] [PubMed]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease-An emerging role for gut microbiome. Alzheimers Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Baloni, P.; Funk, C.C.; Yan, J.; Yurkovich, J.T.; Kueider-Paisley, A.; Nho, K.; Heinken, A.; Jia, W.; Mahmoudiandehkordi, S.; Louie, G.; et al. Metabolic Network Analysis Reveals Altered Bile Acid Synthesis and Metabolism in Alzheimer’s Disease. Cell Rep. Med. 2020, 1, 100138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Tang, C.; Ma, Q.; Wang, W.; Shi, M.; Zhou, X.; Chen, F.; Ma, C.; Li, X.; Chen, G.; et al. Comprehensive serum metabolic and proteomic characterization on cognitive dysfunction in Parkinson’s disease. Ann. Transl. Med. 2021, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wei, J.; Yang, X.; Dou, Y.; Zhao, L.; Qi, X.; Yu, X.; Guo, W.; Wang, Q.; Deng, W.; et al. Plasma metabolites were associated with spatial working memory in major depressive disorder. Medicine 2021, 100, e24581. [Google Scholar] [CrossRef]
- Wu, L.; Han, Y.; Zheng, Z.; Peng, G.; Liu, P.; Yue, S.; Zhu, S.; Chen, J.; Lv, H.; Shao, L.; et al. Altered Gut Microbial Metabolites in Amnestic Mild Cognitive Impairment and Alzheimer’s Disease: Signals in Host-Microbe Interplay. Nutrients 2021, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Li, S.; Li, S.; Wang, X.; Wang, Y.; Xie, Z.; Zhao, Y.; Zhang, J.; Luo, A. Gut Microbiome and Plasma Metabolome Signatures in Middle-Aged Mice With Cognitive Dysfunction Induced by Chronic Neuropathic Pain. Front. Mol. Neurosci. 2022, 14, 806700. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.; Bel Lassen, P.; Henricsson, M.; Wu, H.; Adriouch, S.; Belda, E.; Chakaroun, R.; Nielsen, T.; Bergh, P.O.; Rouault, C.; et al. Imidazole propionate is increased in diabetes and associated with dietary patterns and altered microbial ecology. Nat. Commun. 2020, 11, 5881. [Google Scholar] [CrossRef]
- Ercan-Sencicek, A.G.; Stillman, A.A.; Ghosh, A.K.; Bilguvar, K.; O’Roak, B.J.; Mason, C.E.; Abbott, T.; Gupta, A.; King, R.A.; Pauls, D.L.; et al. L-histidine decarboxylase and Tourette’s syndrome. N. Engl. J. Med. 2010, 362, 1901–1908. [Google Scholar] [CrossRef]
- Kanbayashi, T.; Kodama, T.; Kondo, H.; Satoh, S.; Inoue, Y.; Chiba, S.; Shimizu, T.; Nishino, S. CSF histamine contents in narcolepsy, idiopathic hypersomnia and obstructive sleep apnea syndrome. Sleep 2009, 32, 181–187. [Google Scholar] [CrossRef]
- Nishino, S.; Sakurai, E.; Nevsimalova, S.; Yoshida, Y.; Watanabe, T.; Yanai, K.; Mignot, E. Decreased CSF histamine in narcolepsy with and without low CSF hypocretin-1 in comparison to healthy controls. Sleep 2009, 32, 175–180. [Google Scholar] [CrossRef]
- Rodionov, R.N.; Oppici, E.; Martens-Lobenhoffer, J.; Jarzebska, N.; Brilloff, S.; Burdin, D.; Demyanov, A.; Kolouschek, A.; Leiper, J.; Maas, R.; et al. A Novel Pathway for Metabolism of the Cardiovascular Risk Factor Homoarginine by alanine:glyoxylate aminotransferase 2. Sci. Rep. 2016, 6, 35277. [Google Scholar] [CrossRef]
- Bernstein, H.G.; Jäger, K.; Dobrowolny, H.; Steiner, J.; Keilhoff, G.; Bogerts, B.; Laube, G. Possible sources and functions of L-homoarginine in the brain: Review of the literature and own findings. Amino Acids 2015, 47, 1729–1740. [Google Scholar] [CrossRef] [PubMed]
- Gropman, A.L.; Batshaw, M.L. Cognitive outcome in urea cycle disorders. Mol. Genet. Metab. 2004, 81 (Suppl. S1), S58–S62. [Google Scholar] [CrossRef] [PubMed]
- Posset, R.; Gropman, A.L.; Nagamani SC, S.; Burrage, L.C.; Bedoyan, J.K.; Wong, D.; Berry, G.T.; Baumgartner, M.R.; Yudkoff, M.; Zielonka, M.; et al. Impact of Diagnosis and Therapy on Cognitive Function in Urea Cycle Disor-ders. Ann. Neurol. 2019, 86, 116–128. [Google Scholar] [CrossRef]
- Imbard, A.; Bouchereau, J.; Arnoux, J.B.; Brassier, A.; Schiff, M.; Bérat, C.M.; Pontoizeau, C.; Benoist, J.F.; Josse, C.; Montestruc, F.; et al. Citrullina nella gestione dei pazienti con disturbi del ciclo dell’urea. Orphanet J. Rare Dis. 2023, 18, 207. [Google Scholar] [CrossRef]
- Angelidou, A.; Asadi, S.; Alysandratos, K.D.; Karagkouni, A.; Kourembanas, S.; Theoharides, T.C. Perinatal stress, brain inflammation and risk of autism-review and proposal. BMC Pediatr. 2012, 12, 89. [Google Scholar] [CrossRef]
- Suhre, K.; Zaghlool, S. Connecting the epigenome, metabolome and proteome for a deeper understanding of disease. J. Intern. Med. 2021, 290, 527–548. [Google Scholar] [CrossRef] [PubMed]
- Maccari, S.; Krugers, H.J.; Morley-Fletcher, S.; Szyf, M.; Brunton, P.J. The consequences of early-life adversity: Neurobiological, behavioural and epigenetic adaptations. J. Neuroendocrinol. 2014, 26, 707–723. [Google Scholar] [CrossRef]
- Dellu, F.; Fauchey, V.; Le Moal, M.; Simon, H. Extension of a new two-trial memory task in the rat: Influence of environmental context on recognition processes. Neurobiol. Learn. Mem. 1997, 67, 112–120. [Google Scholar] [CrossRef]
- Engelmann, M.; Wotjak, C.T.; Landgraf, R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol. Behav. 1995, 58, 315–321. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef] [PubMed]


| ELISA Kit | Manufacture | Sensitivity | Intra CV (%) | Inter CV (%) |
|---|---|---|---|---|
| Insulin-rat | Demeditec (DEV 8811) Kiel, Germany | 0.1 ng/mL | <6% | <9.5% |
| Corticosterone rat/mouse | Demeditec (DEV 9922), Kiel, Germany | 6.1 ng/mL | <9% | <8.5 |
| Oxytocin rat | CUSABIO (CSB-E14197r), Houston, TX, USA | 9.4 pg/mL | <15% | <15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morley-Fletcher, S.; Gaetano, A.; Gao, V.; Gatta, E.; Van Camp, G.; Bouwalerh, H.; Thomas, P.; Nicoletti, F.; Maccari, S. Postpartum Oxytocin Treatment via the Mother Reprograms Long-Term Behavioral Disorders Induced by Early Life Stress on the Plasma and Brain Metabolome in the Rat. Int. J. Mol. Sci. 2024, 25, 3014. https://doi.org/10.3390/ijms25053014
Morley-Fletcher S, Gaetano A, Gao V, Gatta E, Van Camp G, Bouwalerh H, Thomas P, Nicoletti F, Maccari S. Postpartum Oxytocin Treatment via the Mother Reprograms Long-Term Behavioral Disorders Induced by Early Life Stress on the Plasma and Brain Metabolome in the Rat. International Journal of Molecular Sciences. 2024; 25(5):3014. https://doi.org/10.3390/ijms25053014
Chicago/Turabian StyleMorley-Fletcher, Sara, Alessandra Gaetano, Vance Gao, Eleonora Gatta, Gilles Van Camp, Hammou Bouwalerh, Pierre Thomas, Ferdinando Nicoletti, and Stefania Maccari. 2024. "Postpartum Oxytocin Treatment via the Mother Reprograms Long-Term Behavioral Disorders Induced by Early Life Stress on the Plasma and Brain Metabolome in the Rat" International Journal of Molecular Sciences 25, no. 5: 3014. https://doi.org/10.3390/ijms25053014