The Crosstalk between N-Formyl Peptide Receptors and uPAR in Systemic Sclerosis: Molecular Mechanisms, Pathogenetic Role and Therapeutic Opportunities
Abstract
1. Introduction
2. Results
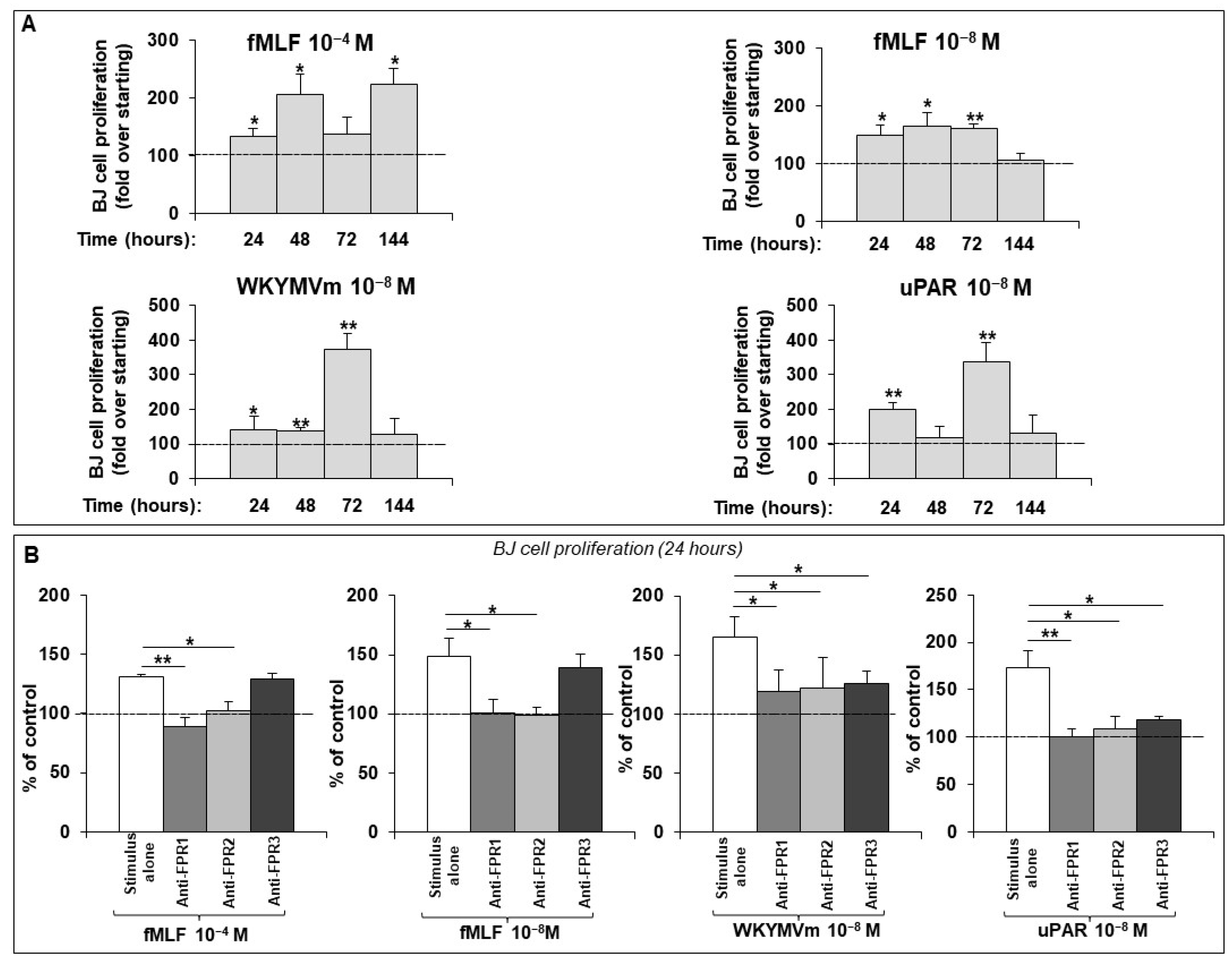
2.1. FPR Activation Promotes Proliferation in a Normal Human Dermal Fibroblast Cell Line
2.2. Rac1 and ERK1/2 Activation by FPRs in Dermal Fibroblast Proliferation
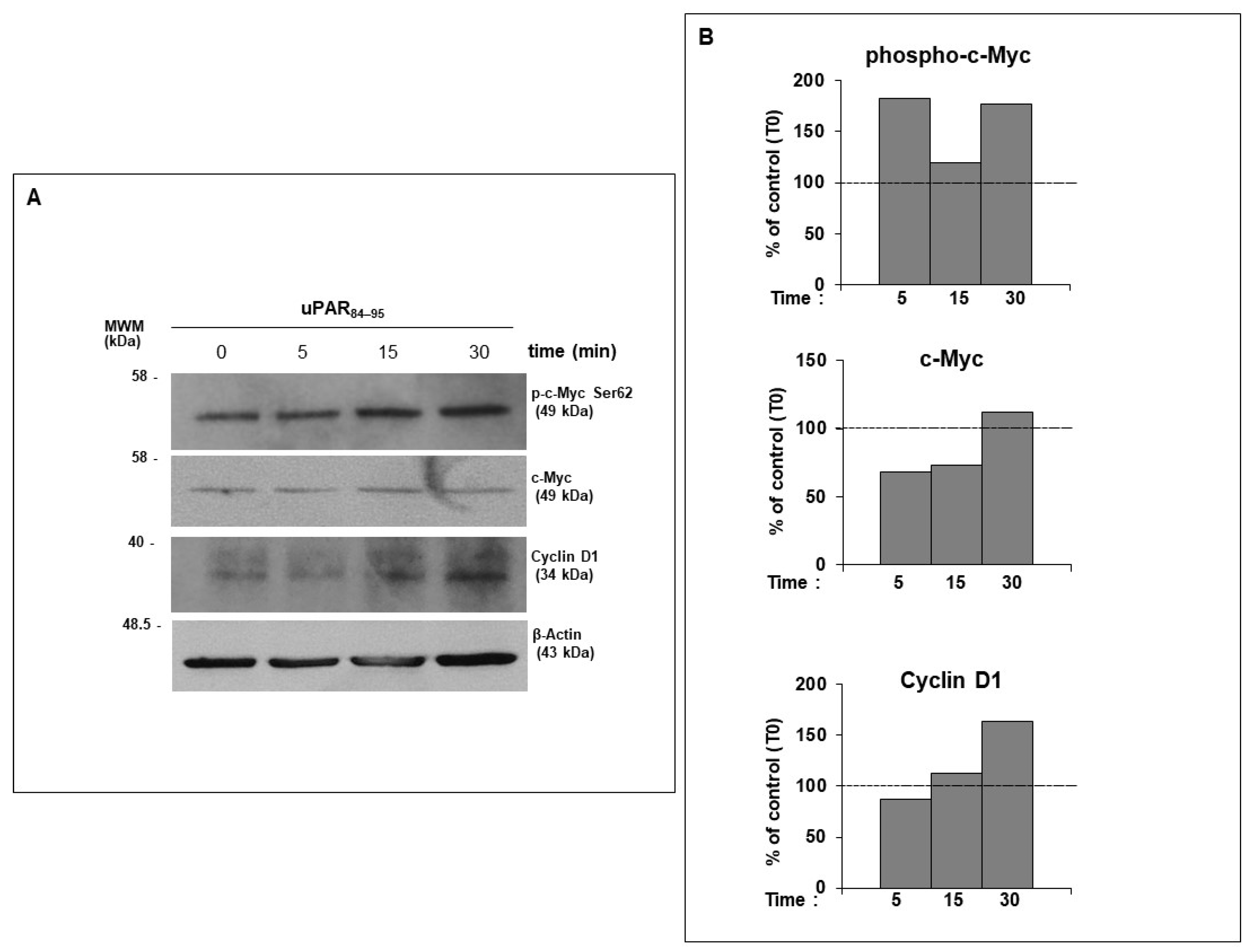
2.3. uPAR/FPRs Crosstalk-Dependent c-Myc Phosphorylation and Cyclin D1 Expression in Normal Human Dermal Fibroblasts
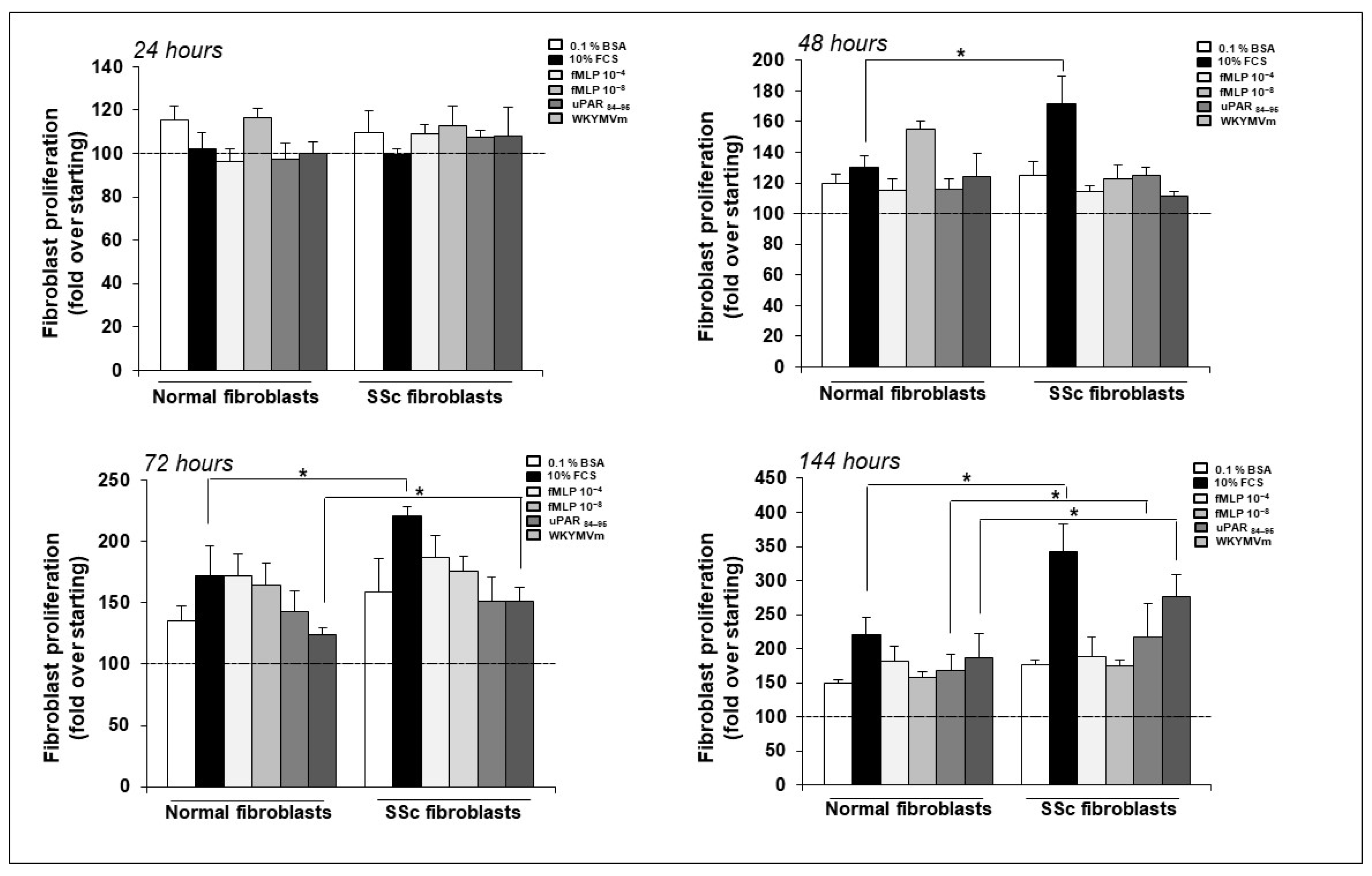
2.4. FPR/uPAR-Dependent Proliferation of Normal and SSc Primary Dermal Fibroblasts
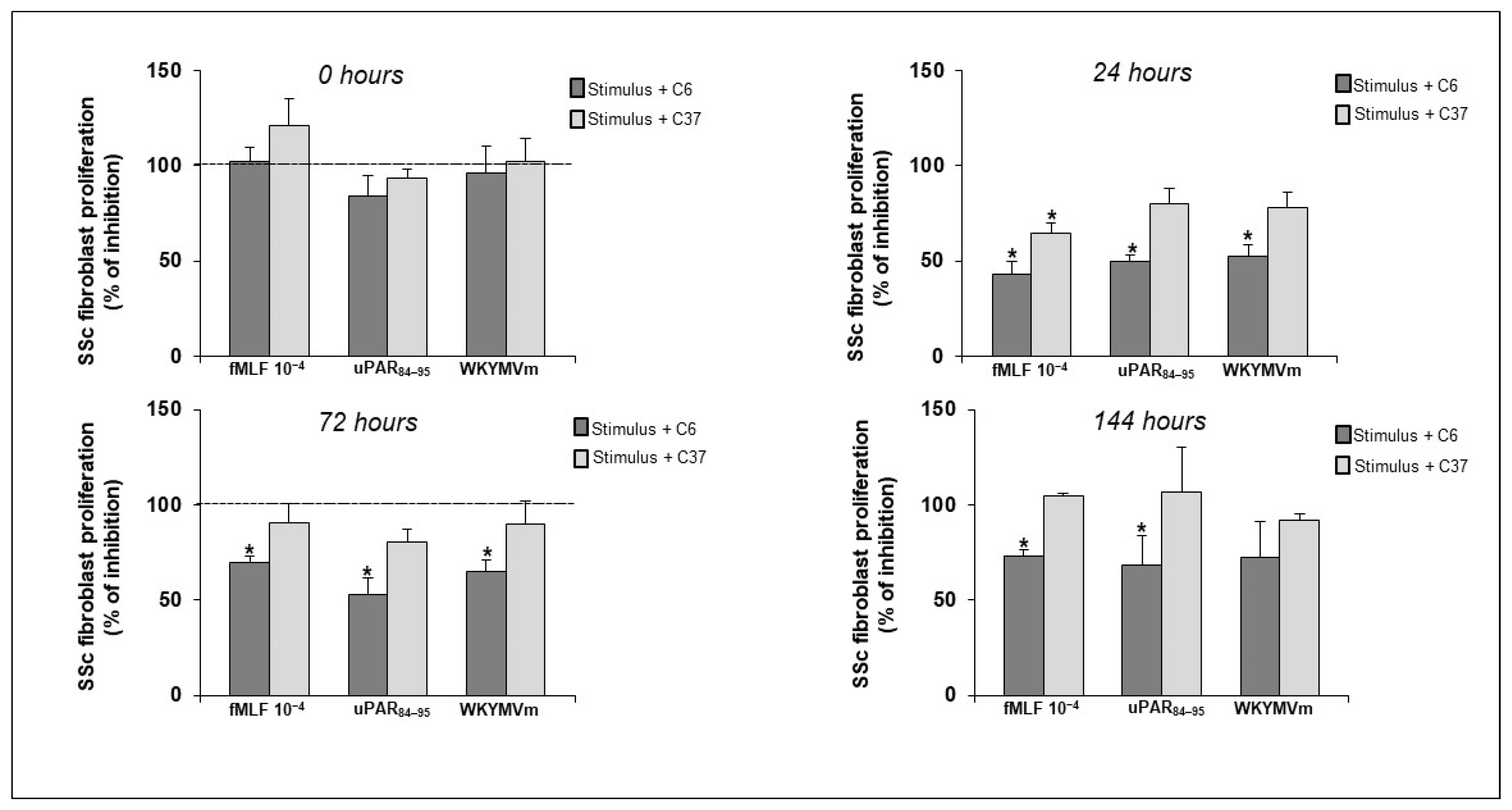
2.5. Effect of Selective Inhibitors of FPRs/uPAR Crosstalk on SSc Fibroblast Proliferation
3. Discussion
4. Materials and Methods
4.1. Peptides and Chemicals
4.2. Tissues and Patient Samples
4.3. Cell Cultures
4.4. Fibroblast Proliferation Assay
4.5. Western Blot
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kowalska-Kępczyńska, A. Systemic Scleroderma-Definition, Clinical Picture and Laboratory Diagnostics. J. Clin. Med. 2022, 11, 2299. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Gilbane, A.J.; Denton, C.P.; Holmes, A.M. Scleroderma pathogenesis: A pivotal role for fibroblasts as effector cells. Arthritis Res. Ther. 2013, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Pakshir, P.; Noskovicova, N.; Lodyga, M.; Son, D.O.; Schuster, R.; Goodwin, A.; Karvonen, H.; Hinz, B. The myofibroblast at a glance. J. Cell Sci. 2020, 133, jcs227900. [Google Scholar] [CrossRef]
- Hinz, B.; David Lagares, B. Evasion of apoptosis by myofibroblasts: A hallmark of fibrotic diseases. Nat. Rev. Rheumatol. 2020, 16, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Myofibroblasts. Exp. Eye Res. 2016, 142, 56–70. [Google Scholar] [CrossRef]
- Leroy, E.C. Connective tissue synthesis by scleroderma skin fibroblasts in cell culture. J. Exp. Med. 1972, 135, 1351–1362. [Google Scholar] [CrossRef]
- Sappino, A.P.; Masouye, I.; Saurat, J.H.; Gabbiani, G. Smooth muscle differentiation in scleroderma fibroblastic cells. Am. J. Pathol. 1990, 137, 585–591. [Google Scholar]
- van Caam, A.; Vonk, M.; van den Hoogen, F.; van Lent, P.; van der Kraan, P. Unraveling SSc Pathophysiology; The Myofibroblast. Front. Immunol. 2018, 9, 2452. [Google Scholar]
- Santiago, B.; Galindo, M.; Rivero, M.; Pablos, J.L. Decreased susceptibility to Fas-induced apoptosis of systemic sclerosis dermal fibroblasts. Arthritis Rheum. 2001, 44, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Jelaska, A.; Korn, J.H. Role of apoptosis and transforming growth factor beta1 in fibroblast selection and activation in systemic sclerosis. Arthritis Rheum. 2000, 43, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.; Li, N.; Teng, W.; Wang, M.; Zhang, Y.; Xiao, Z. TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci. Rep. 2016, 6, 32231. [Google Scholar] [CrossRef] [PubMed]
- Henry, T.W.; Mendoza, F.A.; Jimenez, S.A. Role of microRNA in the pathogenesis of systemic sclerosis tissue fibrosis and vasculopathy. Autoimmun. Rev. 2019, 18, 102396. [Google Scholar] [CrossRef] [PubMed]
- Overed-Sayer, C.; Rapley, L.; Mustelin, T.; Clarke, D.L. Are mast cells instrumental for fibrotic diseases? Front. Pharmacol. 2013, 4, 174. [Google Scholar] [CrossRef] [PubMed]
- Barron, L.; Wynn, T.A. Fibrosis is regulated by Th2 and Th17 responses and by dynamic interactions between fibroblasts and macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G723–G728. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, T.; Yoshizaki-Ogawa, A.; Sato, S.; Yoshizaki, A. The role of B cells in systemic sclerosis. J. Dermatol. 2024, 00, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Weiß, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.D.; Boulay, F.; Wang, J.M.; Dahlgren, C.; Gerard, C.; Parmentier, M.; Serhan, C.N.; Murphy, P.M. International union of basic and clinical pharmacology. Lxxiii. Nomenclature for the formyl peptide receptor (fpr) family. Pharmacol. Rev. 2009, 61, 119–161. [Google Scholar] [CrossRef]
- Babbin, B.A.; Jesaitis, A.J.; Ivanov, A.I.; Kelly, D.; Laukoetter, M.; Nava, P.; Parkos, C.A.; Nusrat, A. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J. Immunol. 2007, 179, 8112–8121. [Google Scholar] [CrossRef]
- Zhang, X.G.; Hui, Y.N.; Huang, X.F.; Du, H.J.; Zhou, J.; Ma, J.X. Activation of formyl peptide receptor-1 enhances restitution of human retinal pigment epithelial cell monolayer under electric fields. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3160–3165. [Google Scholar] [CrossRef]
- Rossi, F.W.; Prevete, N.; Rivellese, F.; Napolitano, F.; Montuori, N.; Postiglione, L.; Selleri, C.; de Paulis, A. The Urokinase/Urokinase Receptor System in Mast Cells: Effects of its Functional Interaction with fMLF Receptors. Transl. Med. UniSa 2016, 15, 34–41. [Google Scholar]
- Napolitano, F.; Rossi, F.W.; Pesapane, A.; Varricchio, S.; Ilardi, G.; Mascolo, M.; Staibano, S.; Lavecchia, A.; Ragno, P.; Selleri, C.; et al. N-Formyl Peptide Receptors Induce Radical Oxygen Production in Fibroblasts Derived From Systemic Sclerosis by Interacting With a Cleaved Form of Urokinase Receptor. Front. Immunol. 2018, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Ragone, C.; Minopoli, M.; Ingangi, V.; Botti, G.; Fratangelo, F.; Pessi, A.; Stoppelli, M.P.; Ascierto, P.A.; Ciliberto, G.; Motti, M.L.; et al. Targeting the cross-talk between Urokinase receptor and Formyl peptide receptor type 1 to prevent invasion and trans-endothelial migration of melanoma cells. J. Exp. Clin. Cancer Res. 2017, 36, 180. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Montuori, N. The Role of the Plasminogen Activation System in Angioedema: Novel Insights on the Pathogenesis. J. Clin. Med. 2021, 10, 518. [Google Scholar] [CrossRef]
- Napolitano, F.; Montuori, N. Role of Plasminogen Activation System in Platelet Pathophysiology: Emerging Concepts for Translational Applications. Int. J. Mol. Sci. 2022, 23, 6065. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, A.G.; Holloway, R.W.; Miller, V.A.; Waisman, D.M. Plasmin and Plasminogen System in the Tumor Microenvironment: Implications for Cancer Diagnosis, Prognosis, and Therapy. Cancers 2021, 13, 1838. [Google Scholar] [CrossRef]
- Kanno, Y. The uPA/uPAR System Orchestrates the Inflammatory Response, Vascular Homeostasis, and Immune System in Fibrosis Progression. Int. J. Mol. Sci. 2023, 24, 1796. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.J.H.; Petersen, J.E.V.; Eugen-Olsen, J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a Biomarker of Systemic Chronic Inflammation. Front. Immunol. 2021, 12, 780641. [Google Scholar] [CrossRef]
- Yuan, C.; Guo, Z.; Yu, S.; Jiang, L.; Huang, M. Development of inhibitors for uPAR: Blocking the interaction of uPAR with its partners. Drug Discov. Today 2021, 26, 1076–1085. [Google Scholar] [CrossRef]
- Manetti, M.; Rosa, I.; Milia, A.F.; Guiducci, S.; Carmeliet, P.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Inactivation of urokinase-type plasminogen activator receptor (uPAR) gene induces dermal and pulmonary fibrosis and peripheral microvasculopathy in mice: A new model of experimental scleroderma? Ann. Rheum. Dis. 2014, 73, 1700–1709. [Google Scholar] [CrossRef]
- Rossi, F.W.; Napolitano, F.; Pesapane, A.; Mascolo, M.; Staibano, S.; Matucci-Cerinic, M.; Guiducci, S.; Ragno, P.; di Spigna, G.; Postiglione, L.; et al. Upregulation of the N-formyl peptide receptors in scleroderma fibroblasts fosters the switch to myofibroblasts. J. Immunol. 2015, 194, 5161–5173. [Google Scholar] [CrossRef] [PubMed]
- Korman, B. Evolving insights into the cellular and molecular pathogenesis of fibrosis in systemic sclerosis. Transl. Res. 2019, 209, 77–89. [Google Scholar] [CrossRef]
- Burt, D.; Salvidio, G.; Tarabra, E.; Barutta, F.; Pinach, S.; Dentelli, P.; Camussi, G.; Cavallo Perin, P.; Gruden, G. The Monocyte Chemoattractant Protein-1/Cognate CC Chemokine Receptor 2 System Affects Cell Motility in Cultured Human Podocytes. Am. J. Pathol. 2007, 171, 1789–1799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salcedo, R.; Zhang, X.; Young, H.A.; Michael, N.; Wasserman, K.; Ma, W.H.; Martins-Green, M.; Murphy, W.J.; Oppenheim, J.J. Angiogenic effects of prostaglandin E2 are mediated by up-regulation of CXCR4 on human microvascular endothelial cells. Blood 2003, 102, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Park, W.S.; Ahn, S.Y.; Sung, D.K.; Sung, S.I.; Kim, J.H.; Chang, Y.S. WKYMVm hexapeptide, a strong formyl peptide receptor 2 agonist, attenuates hyperoxia-induced lung injuries in newborn mice. Sci. Rep. 2019, 9, 6815. [Google Scholar] [CrossRef]
- He, H.Q.; Ye, R.D. The Formyl Peptide Receptors: Diversity of Ligands and Mechanism for Recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef]
- Alfano, D.; Franco, P.; Stoppelli, M.P. Modulation of Cellular Function by the Urokinase Receptor Signalling: A Mechanistic View. Front. Cell Dev. Biol. 2022, 10, 818616. [Google Scholar] [CrossRef]
- Smith, H.W.; Marshall, C.J. Regulation of cell signalling by uPAR. Nat. Rev. Mol. Cell Biol. 2010, 11, 23–36. [Google Scholar] [CrossRef]
- Panaro, M.A.; Acquafredda, A.; Sisto, M.; Lisi, S.; Maffione, A.B.; Mitolo, V. Biological role of the N-formyl peptide receptors. Immunopharmacol. Immunotoxicol. 2006, 28, 103–127. [Google Scholar] [CrossRef]
- Fu, H.; Björkman, L.; Janmey, P.; Karlsson, A.; Karlsson, J.; Movitz, C.; Dahlgren, C. The two neutrophil members of the formylpeptide receptor family activate the NADPH-oxidase through signals that differ in sensitivity to a gelsolin derived phosphoinositide-binding peptide. BMC Cell Biol. 2004, 5, 50. [Google Scholar] [CrossRef]
- Miller, D.M.; Thomas, S.D.; Islam, A.; Muench, D.; Sedoris, K. c-Myc and Cancer Metabolism. Clin. Cancer Res. 2012, 18, 5546–5553. [Google Scholar] [CrossRef] [PubMed]
- Devaiah, B.N.; Mu, J.; Akman, B.; Uppal, S.; Weissman, J.D.; Cheng, D.; Baranello, L.; Nie, Z.; Levens, D.; Singer, D.S. MYC protein stability is negatively regulated by BRD4. Proc. Natl. Acad. Sci. USA 2020, 117, 13457–13467. [Google Scholar] [CrossRef] [PubMed]
- Montalto, F.I.; De Amicis, F. Cyclin D1 in Cancer: A Molecular Connection for Cell Cycle Control, Adhesion and Invasion in Tumor and Stroma. Cells 2020, 9, 2648. [Google Scholar] [CrossRef] [PubMed]
- Masucci, M.T.; Minopoli, M.; Di Carluccio, G.; Motti, M.L.; Carriero, M.V. Therapeutic Strategies Targeting Urokinase and Its Receptor in Cancer. Cancers 2022, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, C.; Pesapane, A.; Formisano, L.; Rosa, R.; D’Amato, V.; Ciciola, P.; Servetto, A.; Marciano, R.; Clara Orsini, R.; Monteleone, F.; et al. Urokinase-type plasminogen activator receptor (uPAR) expression enhances invasion and metastasis in RAS mutated tumors. Sci. Rep. 2017, 7, 9388. [Google Scholar] [CrossRef]
- Lee, H.Y.; Lee, M.; Bae, Y.S. Formyl Peptide Receptors in Cellular Differentiation and Inflammatory Diseases. J. Cell Biochem. 2017, 118, 1300–1307. [Google Scholar] [CrossRef]
- Garrett, S.M.; Baker Frost, D.; Feghali-Bostwick, C. The Mighty Fibroblast and Its Utility in Scleroderma Research. J. Scleroderma Relat. Disord. 2017, 2, 100–107. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Izzo, A.; Mollo, N.; Napolitano, F.; Limone, A.; Margheri, F.; Mocali, A.; Minopoli, G.; Lo Bianco, A.; Di Maggio, F.; et al. Inhibition of 37/67kDa Laminin-1 Receptor Restores APP Maturation and Reduces Amyloid-β in Human Skin Fibroblasts from Familial Alzheimer’s Disease. J. Pers. Med. 2020, 10, 232. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Ames, I.A., Ed.; Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]

 ); overexpressed form of uPAR (
); overexpressed form of uPAR ( ); inhibition (
); inhibition ( ); structural and functional interaction (
); structural and functional interaction ( ); intracellular signaling cascade elicited by FPRs/uPAR crosstalk (
); intracellular signaling cascade elicited by FPRs/uPAR crosstalk ( ).
).
 ); overexpressed form of uPAR (
); overexpressed form of uPAR ( ); inhibition (
); inhibition ( ); structural and functional interaction (
); structural and functional interaction ( ); intracellular signaling cascade elicited by FPRs/uPAR crosstalk (
); intracellular signaling cascade elicited by FPRs/uPAR crosstalk ( ).
).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Napolitano, F.; Rossi, F.W.; de Paulis, A.; Lavecchia, A.; Montuori, N. The Crosstalk between N-Formyl Peptide Receptors and uPAR in Systemic Sclerosis: Molecular Mechanisms, Pathogenetic Role and Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 3156. https://doi.org/10.3390/ijms25063156
Napolitano F, Rossi FW, de Paulis A, Lavecchia A, Montuori N. The Crosstalk between N-Formyl Peptide Receptors and uPAR in Systemic Sclerosis: Molecular Mechanisms, Pathogenetic Role and Therapeutic Opportunities. International Journal of Molecular Sciences. 2024; 25(6):3156. https://doi.org/10.3390/ijms25063156
Chicago/Turabian StyleNapolitano, Filomena, Francesca Wanda Rossi, Amato de Paulis, Antonio Lavecchia, and Nunzia Montuori. 2024. "The Crosstalk between N-Formyl Peptide Receptors and uPAR in Systemic Sclerosis: Molecular Mechanisms, Pathogenetic Role and Therapeutic Opportunities" International Journal of Molecular Sciences 25, no. 6: 3156. https://doi.org/10.3390/ijms25063156
APA StyleNapolitano, F., Rossi, F. W., de Paulis, A., Lavecchia, A., & Montuori, N. (2024). The Crosstalk between N-Formyl Peptide Receptors and uPAR in Systemic Sclerosis: Molecular Mechanisms, Pathogenetic Role and Therapeutic Opportunities. International Journal of Molecular Sciences, 25(6), 3156. https://doi.org/10.3390/ijms25063156







