Environmental Pollution and Risk of Childhood Cancer: A Scoping Review of Evidence from the Last Decade
Abstract
1. Introduction
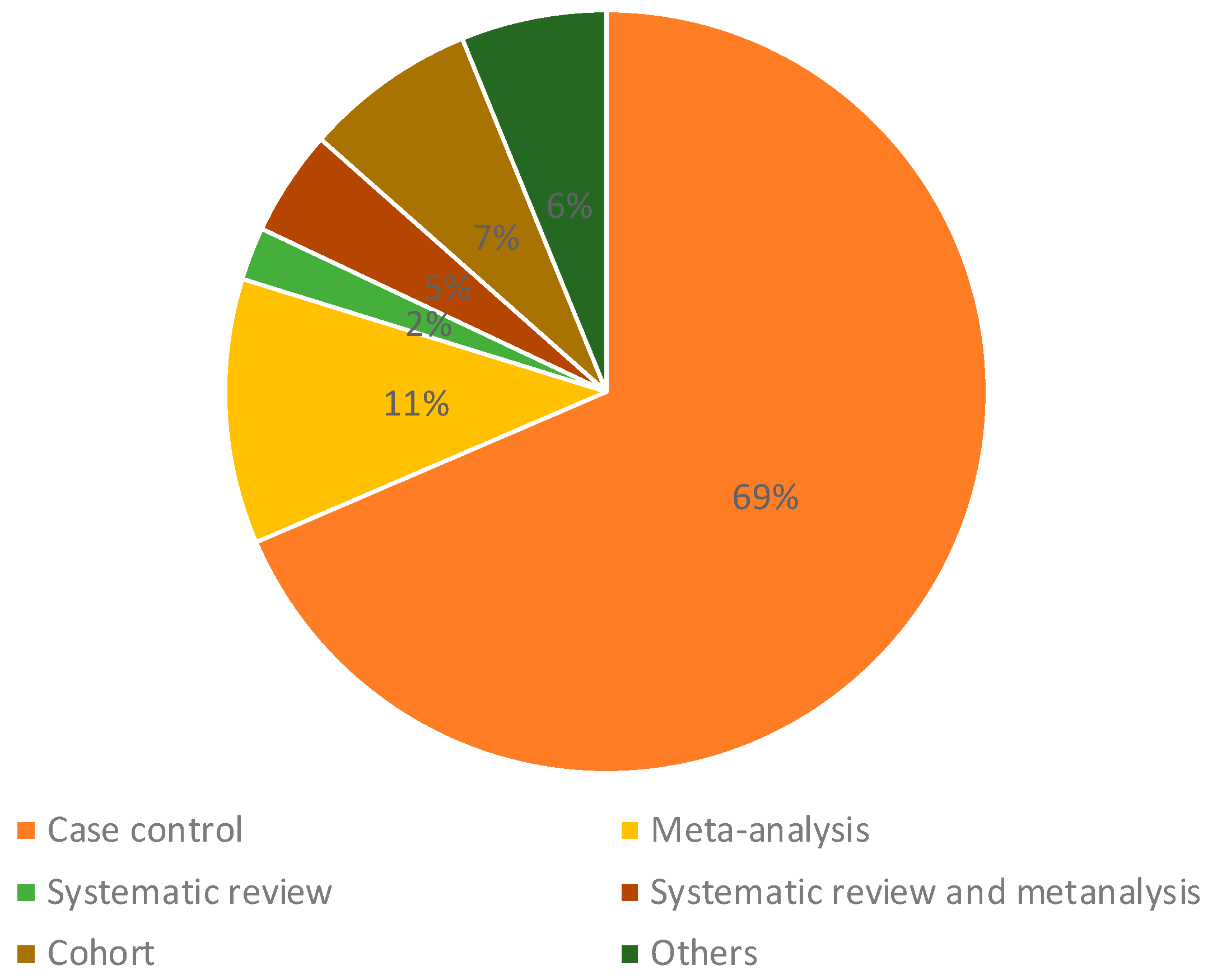
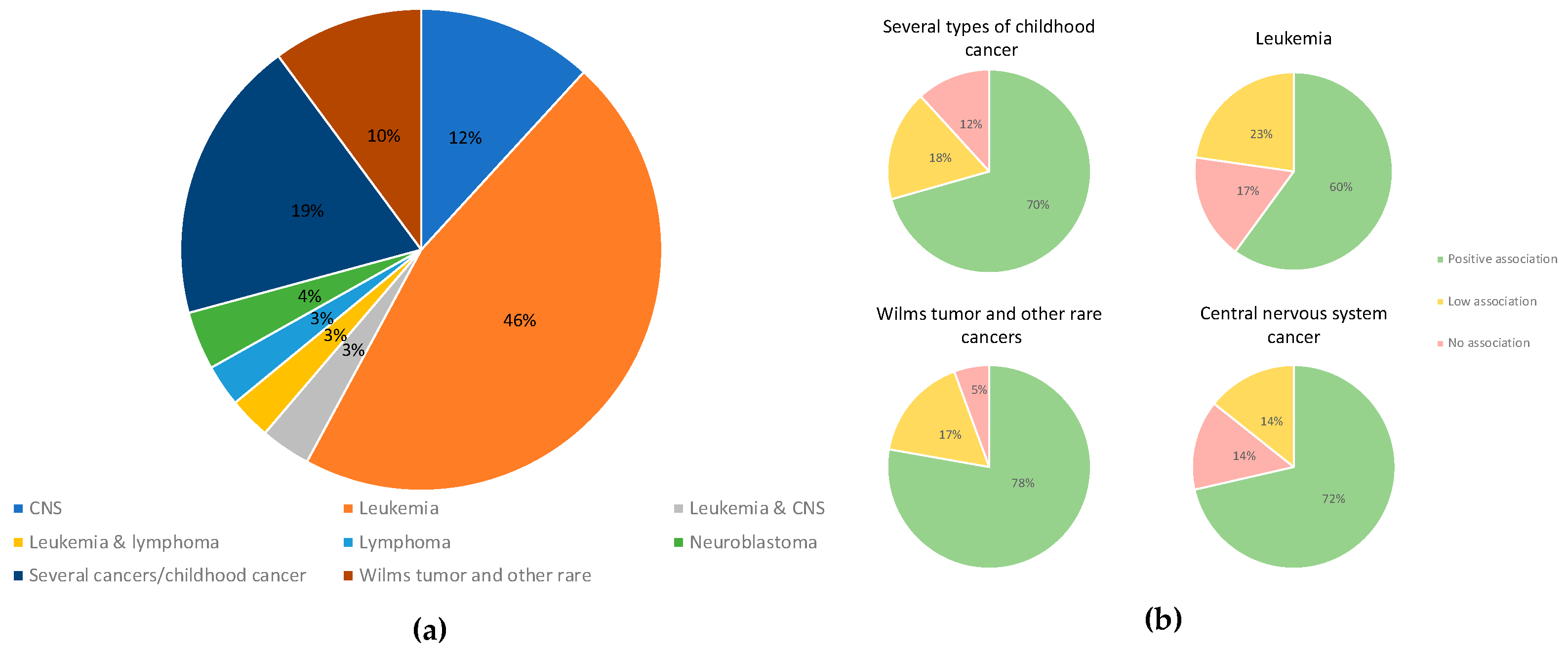
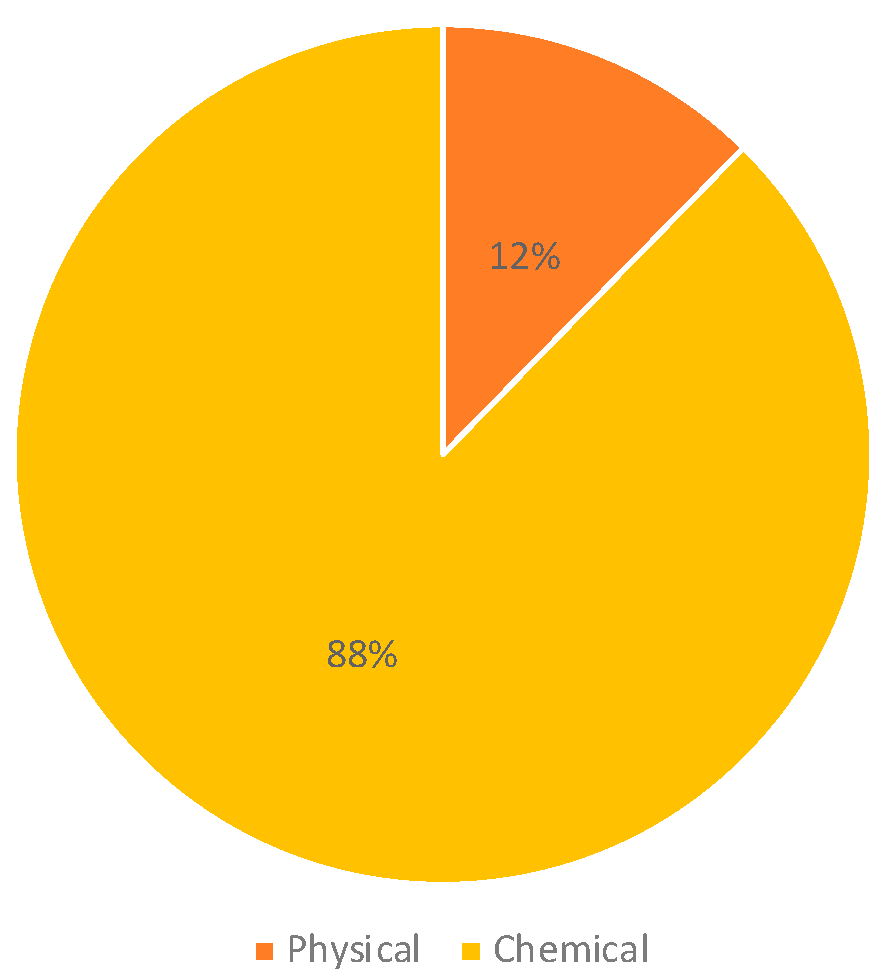
2. Results
3. Discussion
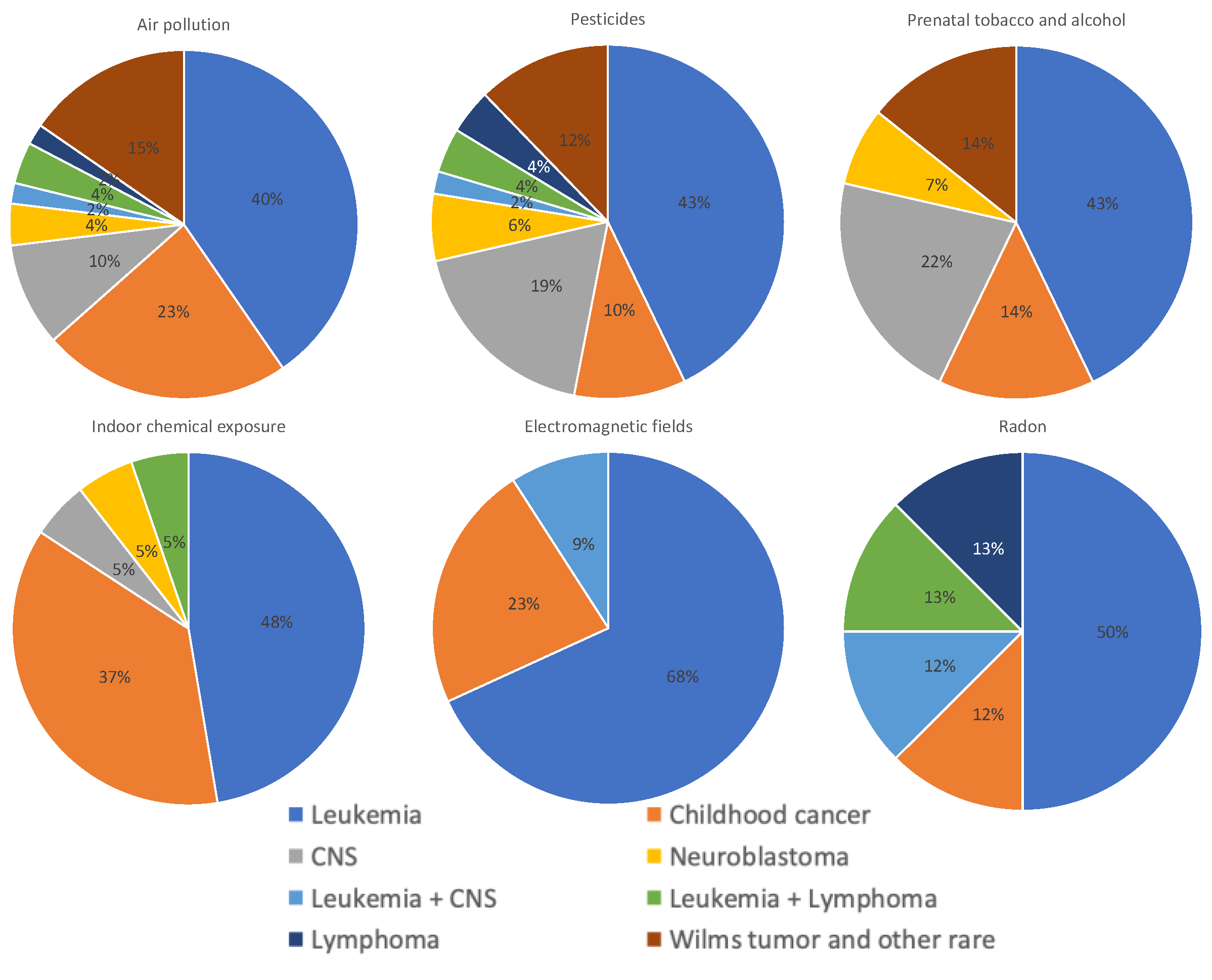
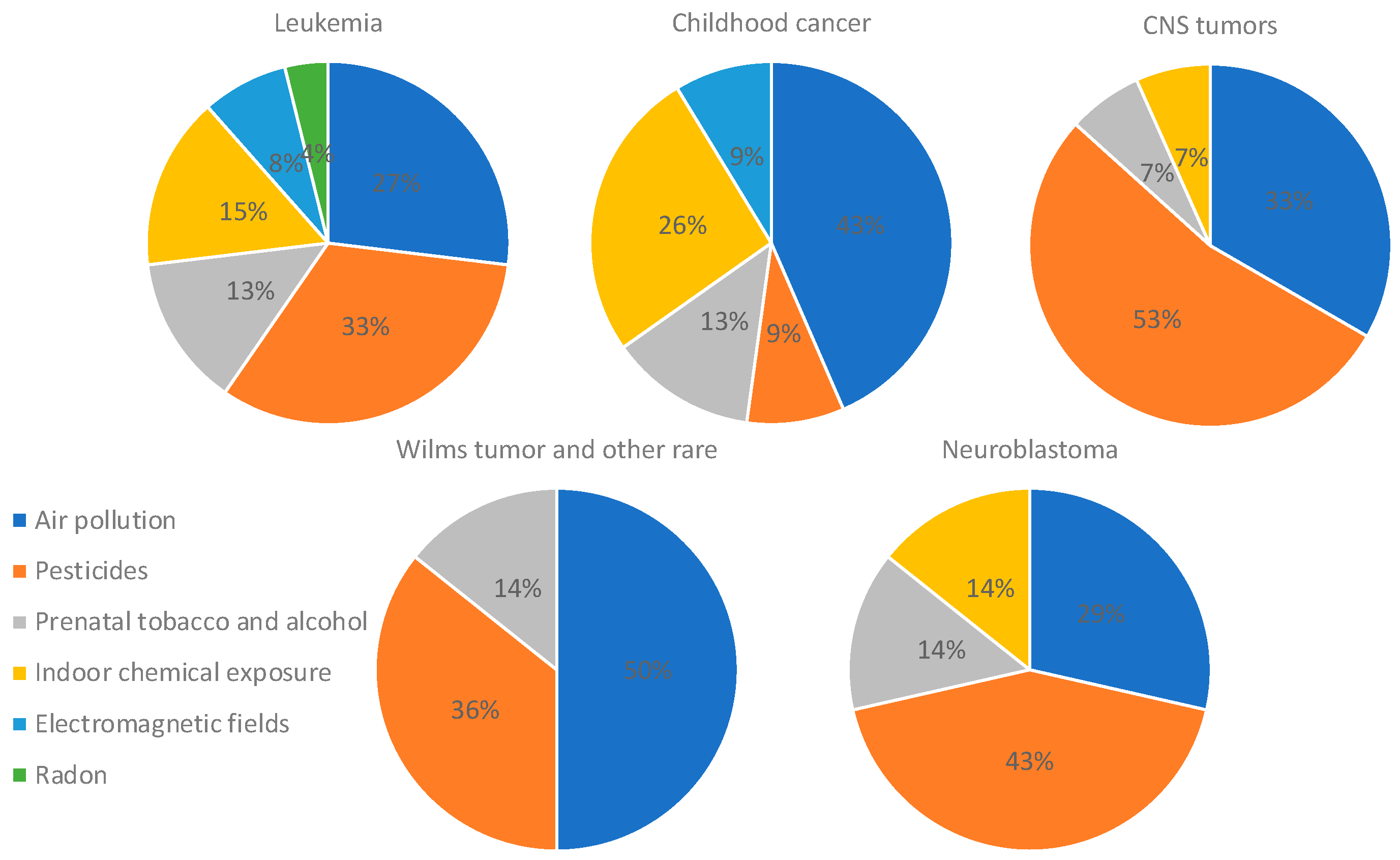
3.1. Air Pollution
3.2. Pesticides
3.3. Tobacco and Alcohol
3.4. Indoor Chemical Exposure
3.5. Electromagnetic Fields
3.6. Radon
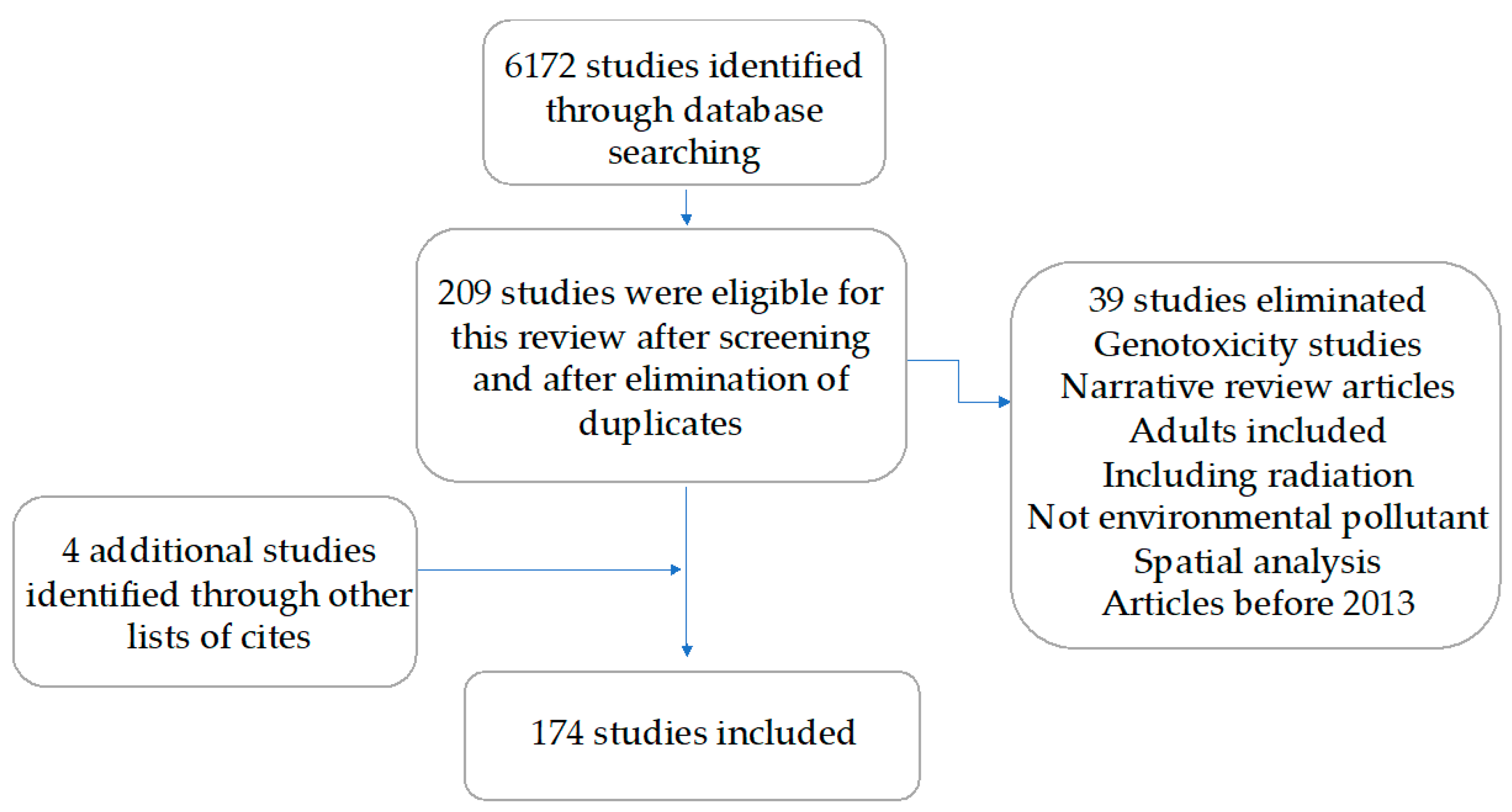
4. Materials and Methods
4.1. Data Source and Search Strategy
4.2. Study Selection
4.3. Data Extraction
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Buser, J.M.; Lake, K.; Ginier, E. Environmental Risk Factors for Childhood Cancer in an Era of Global Climate Change: A Scoping Review. J. Pediatr. Health Care Off. Publ. Natl. Assoc. Pediatr. Nurse Assoc. Pract. 2022, 36, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Cazzolla Gatti, R. Why We Will Continue to Lose Our Battle with Cancers If We Do Not Stop Their Triggers from Environmental Pollution. Int. J. Environ. Res. Public Health 2021, 18, 6107. [Google Scholar] [CrossRef] [PubMed]
- Childhood Cancer Inequalities in the WHO European Region. Available online: https://www.who.int/europe/publications/i/item/9789289057615 (accessed on 30 October 2023).
- PAHO WHO|Pan American Health Organization. Childhood and Adolescence Cancer. Available online: https://www.paho.org/en/topics/childhood-and-adolescence-cancer (accessed on 30 October 2023).
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Fucic, A.; Guszak, V.; Mantovani, A. Transplacental exposure to environmental carcinogens: Association with childhood cancer risks and the role of modulating factors. Reprod. Toxicol. 2017, 72, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.A.; Hornhardt, S.; Erdmann, F.; Sánchez-García, I.; Fischer, U.; Schüz, J.; Ziegelberger, G. Risk Factors for Childhood Leukemia: Radiation and Beyond. Front. Public Health 2021, 9, 805757. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Risk Factors and Causes of Childhood Cancer. Available online: https://www.cancer.org/cancer/cancer-in-children/risk-factors-and-causes.html (accessed on 30 October 2023).
- Iqbal, S.; Ali, S.; Ali, I. Maternal pesticide exposure and its relation to childhood cancer: An umbrella review of meta-analyses. Int. J. Environ. Health Res. 2022, 32, 1609–1627. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, R.; Morimoto, L.M.; Longcore, T.; Franklin, M.; Rogne, T.; Metayer, C.; Wiemels, J.L.; Ma, X. Outdoor artificial light at night, air pollution, and risk of childhood acute lymphoblastic leukemia in the California Linkage Study of Early-Onset Cancers. Sci. Rep. 2023, 13, 583. [Google Scholar] [CrossRef]
- Malavolti, M.; Malagoli, C.; Filippini, T.; Wise, L.A.; Bellelli, A.; Palazzi, G.; Cellini, M.; Costanzini, S.; Teggi, S.; Vinceti, M. Residential proximity to petrol stations and risk of childhood leukemia. Eur. J. Epidemiol. 2023, 38, 771–782. [Google Scholar] [CrossRef]
- Kreis, C.; Héritier, H.; Scheinemann, K.; Hengartner, H.; de Hoogh, K.; Röösli, M.; Spycher, B.D. Childhood cancer and traffic-related air pollution in Switzerland: A nationwide census-based cohort study. Environ. Int. 2022, 166, 107380. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lee, T.H.; Kim, S.; Song, M.; Bae, S. Association between long-term exposure to particulate matter and childhood cancer: A retrospective cohort study. Environ. Res. 2022, 205, 112418. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, A.; Konstantinoudis, G.; Kreis, C.; Diezi, M.; Ammann, R.A.; Zwahlen, M.; Kühni, C.; Spycher, B.D. Childhood cancer and residential proximity to petrol stations: A nationwide registry-based case-control study in Switzerland and an updated meta-analysis. Int. Arch. Occup. Environ. Health 2022, 95, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Asenjo, S.; Nuñez, O.; Segú-Tell, J.; Pardo Romaguera, E.; Cañete Nieto, A.; Martín-Méndez, I.; Bel-Lan, A.; García-Pérez, J.; Cárceles-Álvarez, A.; Ortega-García, J.A.; et al. Cadmium [Cd] and Lead [Pb] topsoil levels and incidence of childhood leukemias. Environ. Geochem. Health 2022, 44, 2341–2354. [Google Scholar] [CrossRef] [PubMed]
- Onyije, F.M.; Hosseini, B.; Togawa, K.; Schüz, J.; Olsson, A. Cancer Incidence and Mortality among Petroleum Industry Workers and Residents Living in Oil Producing Communities: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 4343. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.G.; Vermeulen, R.; Cardoso, M.R.A.; de Oliveira Latorre, M.d.R.D.; Hystad, P.; Downward, G.S.; Nardocci, A.C. Residential traffic exposure and lymphohematopoietic malignancies among children in the city of São Paulo, Brazil: An ecological study. Cancer Epidemiol. 2021, 70, 101859. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, E.; Lima, I.; Hatzopoulou, M.; Van Ryswyk, K.; van Donkelaar, A.; Martin, R.V.; Chen, H.; Stieb, D.M.; Crighton, E.; Burnett, R.T.; et al. Ambient ultrafine particle concentrations and incidence of childhood cancers. Environ. Int. 2020, 145, 106135. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Erdmann, F.; Urhoj, S.K.; Brandt, J.; Geels, C.; Ketzel, M.; Frohn, L.M.; Christensen, J.H.; Sørensen, M.; Raaschou-Nielsen, O. Residential Exposure to PM2.5 Components and Risk of Childhood Non-Hodgkin Lymphoma in Denmark: A Nationwide Register-Based Case-Control Study. Int. J. Environ. Res. Public Health 2020, 17, 8949. [Google Scholar] [CrossRef]
- Volk, J.; Heck, J.E.; Schmiegelow, K.; Hansen, J. Parental occupational exposure to diesel engine exhaust in relation to childhood leukaemia and central nervous system cancers: A register-based nested case-control study in Denmark 1968–2016. Occup. Environ. Med. 2019, 76, 809–817. [Google Scholar] [CrossRef]
- Peckham-Gregory, E.C.; Ton, M.; Rabin, K.R.; Danysh, H.E.; Scheurer, M.E.; Lupo, P.J. Maternal Residential Proximity to Major Roadways and the Risk of Childhood Acute Leukemia: A Population-Based Case-Control Study in Texas, 1995–2011. Int. J. Environ. Res. Public Health 2019, 16, 2029. [Google Scholar] [CrossRef]
- García-Pérez, J.; Gómez-Barroso, D.; Tamayo-Uria, I.; Ramis, R. Methodological approaches to the study of cancer risk in the vicinity of pollution sources: The experience of a population-based case-control study of childhood cancer. Int. J. Health Geogr. 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.H.; Li, J.; Wang, X.Y.; Yu, Y.; Ren, M.M.; Zhou, J. A Meta-analysis of Traffic-related Air Pollution and Risk of Childhood Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Hatch, E.E.; Rothman, K.J.; Heck, J.E.; Park, A.S.; Crippa, A.; Orsini, N.; Vinceti, M. Association between Outdoor Air Pollution and Childhood Leukemia: A Systematic Review and Dose-Response Meta-Analysis. Environ. Health Perspect. 2019, 127, 46002. [Google Scholar] [CrossRef] [PubMed]
- Seifi, M.; Niazi, S.; Johnson, G.; Nodehi, V.; Yunesian, M. Exposure to ambient air pollution and risk of childhood cancers: A population-based study in Tehran, Iran. Sci. Total Environ. 2019, 646, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Heck, J.E.; Ritz, B.; Cockburn, M.; Escobedo, L.A.; von Ehrenstein, O.S. Prenatal Exposure to Air Toxics and Malignant Germ Cell Tumors in Young Children. J. Occup. Environ. Med. 2019, 61, 529–534. [Google Scholar] [CrossRef]
- Iavarone, I.; Buzzoni, C.; Stoppa, G.; Steliarova-Foucher, E.; SENTIERI-AIRTUM Working Group. Cancer incidence in children and young adults living in industrially contaminated sites: From the Italian experience to the development of an international surveillance system. Epidemiol. Prev. 2018, 42, 76–85. [Google Scholar] [PubMed]
- Kirkeleit, J.; Riise, T.; Bjørge, T.; Christiani, D.C.; Bråtveit, M.; Baccarelli, A.; Mattioli, S.; Hollund, B.E.; Gjertsen, B.T. Maternal exposure to gasoline and exhaust increases the risk of childhood leukaemia in offspring—A prospective study in the Norwegian Mother and Child Cohort Study. Br. J. Cancer 2018, 119, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Lupo, P.J.; Pompeii, L.A.; Danysh, H.E. Maternal Residential Proximity to Major Roadways and Pediatric Embryonal Tumors in Offspring. Int. J. Environ. Res. Public Health 2018, 15, 505. [Google Scholar] [CrossRef]
- Ortega-García, J.A.; López-Hernández, F.A.; Cárceles-Álvarez, A.; Fuster-Soler, J.L.; Sotomayor, D.I.; Ramis, R. Childhood cancer in small geographical areas and proximity to air-polluting industries. Environ. Res. 2017, 156, 63–73. [Google Scholar] [CrossRef]
- Janitz, A.E.; Campbell, J.E.; Magzamen, S.; Pate, A.; Stoner, J.A.; Peck, J.D. Benzene and childhood acute leukemia in Oklahoma. Environ. Res. 2017, 158, 167–173. [Google Scholar] [CrossRef]
- Ramis, R.; Tamayo-Uria, I.; Gómez-Barroso, D.; López-Abente, G.; Morales-Piga, A.; Pardo Romaguera, E.; Aragones, N.; García-Pérez, J. Risk factors for central nervous system tumors in children: New findings from a case-control study. PLoS ONE 2017, 12, e0171881. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, É.; Bélair, M.A.; Do, M.T.; Stieb, D.M.; Hystad, P.; van Donkelaar, A.; Martin, R.V.; Crouse, D.L.; Crighton, E.; Chen, H.; et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada. Environ. Int. 2017, 100, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Spycher, B.D.; Lupatsch, J.E.; Huss, A.; Rischewski, J.; Schindera, C.; Spoerri, A.; Vermeulen, R.; Kuehni, C.E.; Swiss Paediatric Oncology Group; Swiss National Cohort Study Group. Parental occupational exposure to benzene and the risk of childhood cancer: A census-based cohort study. Environ. Int. 2017, 108, 84–91. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.; Morales-Piga, A.; Gómez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; López-Abente, G.; Ramis, R. Risk of bone tumors in children and residential proximity to industrial and urban areas: New findings from a case-control study. Sci. Total Environ. 2017, 579, 1333–1342. [Google Scholar] [CrossRef]
- Janitz, A.E.; Ramachandran, G.; Tomlinson, G.E.; Krailo, M.; Richardson, M.; Spector, L. Maternal and paternal occupational exposures and hepatoblastoma: Results from the HOPE study through the Children’s Oncology Group. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Janitz, A.E.; Campbell, J.E.; Magzamen, S.; Pate, A.; Stoner, J.A.; Peck, J.D. Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environ. Res. 2016, 148, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Danysh, H.E.; Zhang, K.; Mitchell, L.E.; Scheurer, M.E.; Lupo, P.J. Maternal residential proximity to major roadways at delivery and childhood central nervous system tumors. Environ. Res. 2016, 146, 315–322. [Google Scholar] [CrossRef]
- von Ehrenstein, O.S.; Heck, J.E.; Park, A.S.; Cockburn, M.; Escobedo, L.; Ritz, B. In Utero and Early-Life Exposure to Ambient Air Toxics and Childhood Brain Tumors: A Population-Based Case-Control Study in California, USA. Environ. Health Perspect. 2016, 124, 1093–1099. [Google Scholar] [CrossRef]
- Symanski, E.; Tee Lewis, P.G.; Chen, T.Y.; Chan, W.; Lai, D.; Ma, X. Air toxics and early childhood acute lymphocytic leukemia in Texas, a population based case control study. Environ. Health Glob. Access Sci. Source 2016, 15, 70. [Google Scholar] [CrossRef]
- Magnani, C.; Ranucci, A.; Badaloni, C.; Cesaroni, G.; Ferrante, D.; Miligi, L.; Mattioli, S.; Rondelli, R.; Bisanti, L.; Zambon, P.; et al. Road Traffic Pollution and Childhood Leukemia: A Nationwide Case-control Study in Italy. Arch. Med. Res. 2016, 47, 694–705. [Google Scholar] [CrossRef]
- García-Pérez, J.; Morales-Piga, A.; Gómez, J.; Gómez-Barroso, D.; Tamayo-Uria, I.; Romaguera, E.P.; Fernández-Navarro, P.; López-Abente, G.; Ramis, R. Association between residential proximity to environmental pollution sources and childhood renal tumors. Environ. Res. 2016, 147, 405–414. [Google Scholar] [CrossRef]
- García-Pérez, J.; Morales-Piga, A.; Gómez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; López-Abente, G.; Ramis, R. Residential proximity to environmental pollution sources and risk of rare tumors in children. Environ. Res. 2016, 151, 265–274. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.; Morales-Piga, A.; Gómez-Barroso, D.; Tamayo-Uria, I.; Pardo Romaguera, E.; Fernández-Navarro, P.; Lopez-Abente, G.; Ramis, R. Risk of neuroblastoma and residential proximity to industrial and urban sites: A case-control study. Environ. Int. 2016, 92–93, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Wallace, F.M.; Zhang, L.; Smith, M.T.; Rader, G.; Steinmaus, C. Parental, In Utero, and Early-Life Exposure to Benzene and the Risk of Childhood Leukemia: A Meta-Analysis. Am. J. Epidemiol. 2016, 183, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Filippini, T.; Heck, J.E.; Malagoli, C.; Del Giovane, C.; Vinceti, M. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2015, 33, 36–66. [Google Scholar] [CrossRef] [PubMed]
- Spycher, B.D.; Feller, M.; Röösli, M.; Ammann, R.A.; Diezi, M.; Egger, M.; Kuehni, C.E. Childhood cancer and residential exposure to highways: A nationwide cohort study. Eur. J. Epidemiol. 2015, 30, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, C.; Malavolti, M.; Costanzini, S.; Fabbri, S.; Tezzi, S.; Palazzi, G.; Arcolin, E.; Vinceti, M. Increased incidence of childhood leukemia in urban areas: A population-based case-control study. Epidemiol. Prev. 2015, 39 (Suppl. S1), 102–107. [Google Scholar]
- Heck, J.E.; Park, A.S.; Qiu, J.; Cockburn, M.; Ritz, B. Retinoblastoma and ambient exposure to air toxics in the perinatal period. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 182–186. [Google Scholar] [CrossRef]
- Houot, J.; Marquant, F.; Goujon, S.; Faure, L.; Honoré, C.; Roth, M.H.; Hémon, D.; Clavel, J. Residential Proximity to Heavy-Traffic Roads, Benzene Exposure, and Childhood Leukemia-The GEOCAP Study, 2002–2007. Am. J. Epidemiol. 2015, 182, 685–693. [Google Scholar] [CrossRef]
- Greenop, K.R.; Hinwood, A.L.; Fritschi, L.; Scott, R.J.; Attia, J.; Ashton, L.J.; Heath, J.A.; Armstrong, B.K.; Milne, E. Vehicle refuelling, use of domestic wood heaters and the risk of childhood brain tumours: Results from an Australian case-control study. Pediatr. Blood Cancer 2015, 62, 229–234. [Google Scholar] [CrossRef]
- García-Pérez, J.; López-Abente, G.; Gómez-Barroso, D.; Morales-Piga, A.; Romaguera, E.P.; Tamayo, I.; Fernández-Navarro, P.; Ramis, R. Childhood leukemia and residential proximity to industrial and urban sites. Environ. Res. 2015, 140, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, S.; Li, Z.; Zhu, J.; Bi, Y.; Bai, Y.; Wang, H. Maternal benzene exposure during pregnancy and risk of childhood acute lymphoblastic leukemia: A meta-analysis of epidemiologic studies. PLoS ONE 2014, 9, e110466. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Ritz, B.; Wilhelm, M.; Qiu, J.; Cockburn, M.; Heck, J.E. Prenatal exposure to air toxics and risk of Wilms tumor in 0- to 5-year-old children. J. Occup. Environ. Med. 2014, 56, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Boothe, V.L.; Boehmer, T.K.; Wendel, A.M.; Yip, F.Y. Residential traffic exposure and childhood leukemia: A systematic review and meta-analysis. Am. J. Prev. Med. 2014, 46, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Heck, J.E.; Park, A.S.; Qiu, J.; Cockburn, M.; Ritz, B. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int. J. Hyg. Environ. Health 2014, 217, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Badaloni, C.; Ranucci, A.; Cesaroni, G.; Zanini, G.; Vienneau, D.; Al-Aidrous, F.; De Hoogh, K.; Magnani, C.; Forastiere, F.; SETIL Study Group. Air pollution and childhood leukaemia: A nationwide case-control study in Italy. Occup. Environ. Med. 2013, 70, 876–883. [Google Scholar] [CrossRef]
- Heck, J.E.; Wu, J.; Lombardi, C.; Qiu, J.; Meyers, T.J.; Wilhelm, M.; Cockburn, M.; Ritz, B. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ. Health Perspect. 2013, 121, 1385–1391. [Google Scholar] [CrossRef]
- Ghosh, J.K.C.; Heck, J.E.; Cockburn, M.; Su, J.; Jerrett, M.; Ritz, B. Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am. J. Epidemiol. 2013, 178, 1233–1239. [Google Scholar] [CrossRef]
- Peters, S.; Glass, D.C.; Reid, A.; de Klerk, N.; Armstrong, B.K.; Kellie, S.; Ashton, L.J.; Milne, E.; Fritschi, L. Parental occupational exposure to engine exhausts and childhood brain tumors. Int. J. Cancer 2013, 132, 2975–2979. [Google Scholar] [CrossRef]
- Heck, J.E.; Park, A.S.; Qiu, J.; Cockburn, M.; Ritz, B. An exploratory study of ambient air toxics exposure in pregnancy and the risk of neuroblastoma in offspring. Environ. Res. 2013, 127, 1–6. [Google Scholar] [CrossRef]
- Ward, M.H.; Madrigal, J.M.; Jones, R.R.; Friesen, M.C.; Falk, R.T.; Koebel, D.; Metayer, C. Glyphosate in house dust and risk of childhood acute lymphoblastic leukemia in California. Environ. Int. 2023, 172, 107777. [Google Scholar] [CrossRef] [PubMed]
- Rafeeinia, A.; Asadikaram, G.; Moazed, V.; Darabi, M.K. Organochlorine pesticides may induce leukemia by methylation of CDKN2B and MGMT promoters and histone modifications. Gene 2023, 851, 146976. [Google Scholar] [CrossRef] [PubMed]
- Rossides, M.; Kampitsi, C.E.; Talbäck, M.; Mogensen, H.; Wiebert, P.; Tettamanti, G.; Feychting, M. Occupational exposure to pesticides in mothers and fathers and risk of cancer in the offspring: A register-based case-control study from Sweden [1960–2015]. Environ. Res. 2022, 214 Pt 1, 113820. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.; Ritz, B.; Cockburn, M.; Heck, J.E. Prenatal ambient pesticide exposure and childhood retinoblastoma. Int. J. Hyg. Environ. Health 2022, 245, 114025. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Feulefack, J.; Sergi, C.M. Pre-conceptional and prenatal exposure to pesticides and pediatric neuroblastoma. A meta-analysis of nine studies. Environ. Toxicol. Pharmacol. 2022, 90, 103790. [Google Scholar] [CrossRef]
- El-Helaly, S.; Khashaba, E.; El Domiaty, H.; Darwish, A. Parental occupational and environmental risk factors for childhood bone cancer in Mansoura oncology center: A case control study. Int. J. Environ. Health Res. 2022, 34, 248–256. [Google Scholar] [CrossRef]
- Khan, A.; Feulefack, J.; Sergi, C.M. Exposure to pesticides and pediatric Wilms’ tumor. A meta-analysis on pre-conception and pregnancy parental exposure with an IARC/WHO commentary. Hum. Exp. Toxicol. 2022, 41, 9603271221136211. [Google Scholar] [CrossRef] [PubMed]
- Bamouni, S.; Hémon, D.; Faure, L.; Clavel, J.; Goujon, S. Residential proximity to croplands at birth and childhood leukaemia. Environ. Health Glob. Access Sci. Source 2022, 21, 103. [Google Scholar] [CrossRef]
- Onyije, F.M.; Olsson, A.; Erdmann, F.; Magnani, C.; Petridou, E.; Clavel, J.; Miligi, L.; Bonaventure, A.; Ferrante, D.; Piro, S.; et al. Parental occupational exposure to combustion products, metals, silica and asbestos and risk of childhood leukaemia: Findings from the Childhood Cancer and Leukaemia International Consortium [CLIC]. Environ. Int. 2022, 167, 107409. [Google Scholar] [CrossRef]
- Feulefack, J.; Khan, A.; Forastiere, F.; Sergi, C.M. Parental Pesticide Exposure and Childhood Brain Cancer: A Systematic Review and Meta-Analysis Confirming the IARC/WHO Monographs on Some Organophosphate Insecticides and Herbicides. Children 2021, 8, 1096. [Google Scholar] [CrossRef]
- Nguyen, A.; Crespi, C.M.; Vergara, X.; Kheifets, L. Commercial outdoor plant nurseries as a confounder for electromagnetic fields and childhood leukemia risk. Environ. Res. 2022, 212, 113446. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, J.M.; Jones, R.R.; Gunier, R.B.; Whitehead, T.P.; Reynolds, P.; Metayer, C.; Ward, M.H. Residential exposure to carbamate, organophosphate, and pyrethroid insecticides in house dust and risk of childhood acute lymphoblastic leukemia. Environ. Res. 2021, 201, 111501. [Google Scholar] [CrossRef] [PubMed]
- Coste, A.; Bailey, H.D.; Kartal-Kaess, M.; Renella, R.; Berthet, A.; Spycher, B.D. Parental occupational exposure to pesticides and risk of childhood cancer in Switzerland: A census-based cohort study. BMC Cancer 2020, 20, 819. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.M.; Jones, R.R.; Booth, B.J.; Olsson, A.C.; Kromhout, H.; Straif, K.; Vermeulen, R.; Tikellis, G.; Paltiel, O.; Golding, J.; et al. Parental occupational exposure to pesticides, animals and organic dust and risk of childhood leukemia and central nervous system tumors: Findings from the International Childhood Cancer Cohort Consortium [I4C]. Int. J. Cancer 2020, 146, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Park, A.S.; Ritz, B.; Yu, F.; Cockburn, M.; Heck, J.E. Prenatal pesticide exposure and childhood leukemia—A California statewide case-control study. Int. J. Hyg. Environ. Health 2020, 226, 113486. [Google Scholar] [CrossRef]
- Mavoungou, S.; Rios, P.; Pacquement, H.; Nolla, M.; Rigaud, C.; Simonin, M.; Bertrand, Y.; Lambilliotte, A.; Faure, L.; Orsi, L.; et al. Maternal exposure to pesticides and risk of childhood lymphoma in France: A pooled analysis of the ESCALE and ESTELLE studies [SFCE]. Cancer Epidemiol. 2020, 68, 101797. [Google Scholar] [CrossRef] [PubMed]
- Rios, P.; Bauer, H.; Schleiermacher, G.; Pasqualini, C.; Boulanger, C.; Thebaud, E.; Gandemer, V.; Pellier, I.; Verschuur, A.; Sudour-Bonnange, H.; et al. Environmental exposures related to parental habits in the perinatal period and the risk of Wilms’ tumor in children. Cancer Epidemiol. 2020, 66, 101706. [Google Scholar] [CrossRef]
- Coste, A.; Goujon, S.; Faure, L.; Hémon, D.; Clavel, J. Agricultural crop density in the municipalities of France and incidence of childhood leukemia: An ecological study. Environ. Res. 2020, 187, 109517. [Google Scholar] [CrossRef]
- Patel, D.M.; Gyldenkærne, S.; Jones, R.R.; Olsen, S.F.; Tikellis, G.; Granström, C.; Dwyer, T.; Stayner, L.T.; Ward, M.H. Residential proximity to agriculture and risk of childhood leukemia and central nervous system tumors in the Danish national birth cohort. Environ. Int. 2020, 143, 105955. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Household exposure to pesticides and risk of leukemia in children and adolescents: Updated systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2019, 222, 49–67. [Google Scholar] [CrossRef]
- Bunch, K.J.; Kendall, G.M.; Stiller, C.A.; Vincent, T.J.; Murphy, M.F.G. Case-control study of paternal occupational exposures and childhood lymphoma in Great Britain, 1962–2010. Br. J. Cancer 2019, 120, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Dessypris, N.; Papadakis, V.; Tragiannidis, A.; Bouka, E.; Hatzipantelis, E.; Moschovi, M.; Papakonstantinou, E.; Polychronopoulou, S.; Sgouros, S.; et al. Perinatal and early life risk factors for childhood brain tumors: Is instrument-assisted delivery associated with higher risk? Cancer Epidemiol. 2019, 59, 178–184. [Google Scholar] [CrossRef]
- Hyland, C.; Gunier, R.B.; Metayer, C.; Bates, M.N.; Wesseling, C.; Mora, A.M. Maternal residential pesticide use and risk of childhood leukemia in Costa Rica. Int. J. Cancer 2018, 143, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Ferri, G.M.; Guastadisegno, C.M.; Intranuovo, G.; Cavone, D.; Birtolo, F.; Cecinati, V.; Pappalardi, B.; Corsi, P.; Vimercati, L.; Santoro, N. Maternal Exposure to Pesticides, Paternal Occupation in the Army/Police Force, and CYP2D6*4 Polymorphism in the Etiology of Childhood Acute Leukemia. J. Pediatr. Hematol. Oncol. 2018, 40, e207–e214. [Google Scholar] [CrossRef] [PubMed]
- Vidart d’Egurbide Bagazgoïtia, N.; Bailey, H.D.; Orsi, L.; Lacour, B.; Guerrini-Rousseau, L.; Bertozzi, A.I.; Leblond, P.; Faure-Conter, C.; Pellier, I.; Freycon, C.; et al. Maternal residential pesticide use during pregnancy and risk of malignant childhood brain tumors: A pooled analysis of the ESCALE and ESTELLE studies [SFCE]. Int. J. Cancer 2018, 142, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Desai, V. Exposure to permethrin and cancer risk: A systematic review. Crit. Rev. Toxicol. 2018, 48, 433–442. [Google Scholar] [CrossRef]
- Van Maele-Fabry, G.; Gamet-Payrastre, L.; Lison, D. Residential exposure to pesticides as risk factor for childhood and young adult brain tumors: A systematic review and meta-analysis. Environ. Int. 2017, 106, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Gunier, R.B.; Kang, A.; Hammond, S.K.; Reinier, K.; Lea, C.S.; Chang, J.S.; Does, M.; Scelo, G.; Kirsch, J.; Crouse, V.; et al. A task-based assessment of parental occupational exposure to pesticides and childhood acute lymphoblastic leukemia. Environ. Res. 2017, 156, 57–62. [Google Scholar] [CrossRef]
- Eerjaee, A.; Niknam, M.; Sadeghi, A.; Dehghani, M.; Safaei, Z.; Teshnizi, S.H.; Karimi, M. A Significant Breakthrough in the Incidence of Childhood Cancers and Evaluation of its Risk Factors in Southern Iran. Indian J. Med. Paediatr. Oncol. Off. J. Indian Soc. Med. Paediatr. Oncol. 2017, 38, 158–164. [Google Scholar]
- Rios, P.; Bailey, H.D.; Lacour, B.; Valteau-Couanet, D.; Michon, J.; Bergeron, C.; Boutroux, H.; Defachelles, A.S.; Gambart, M.; Sirvent, N.; et al. Maternal use of household pesticides during pregnancy and risk of neuroblastoma in offspring. A pooled analysis of the ESTELLE and ESCALE French studies [SFCE]. Cancer Causes Control CCC. 2017, 28, 1125–1132. [Google Scholar] [CrossRef]
- Omidakhsh, N.; Ganguly, A.; Bunin, G.R.; von Ehrenstein, O.S.; Ritz, B.; Heck, J.E. Residential Pesticide Exposures in Pregnancy and the Risk of Sporadic Retinoblastoma: A Report From the Children’s Oncology Group. Am. J. Ophthalmol. 2017, 176, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Febvey, O.; Schüz, J.; Bailey, H.D.; Clavel, J.; Lacour, B.; Orsi, L.; Lightfoot, T.; Roman, E.; Vermeulen, R.; Kromhout, H.; et al. Risk of Central Nervous System Tumors in Children Related to Parental Occupational Pesticide Exposures in three European Case-Control Studies. J. Occup. Environ. Med. 2016, 58, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Barroso, D.; García-Pérez, J.; López-Abente, G.; Tamayo-Uria, I.; Morales-Piga, A.; Pardo Romaguera, E.; Ramis, R. Agricultural crop exposure and risk of childhood cancer: New findings from a case-control study in Spain. Int. J. Health Geogr. 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, C.; Costanzini, S.; Heck, J.E.; Malavolti, M.; De Girolamo, G.; Oleari, P.; Palazzi, G.; Teggi, S.; Vinceti, M. Passive exposure to agricultural pesticides and risk of childhood leukemia in an Italian community. Int. J. Hyg. Environ. Health 2016, 219, 742–748. [Google Scholar] [CrossRef]
- Chen, S.; Gu, S.; Wang, Y.; Yao, Y.; Wang, G.; Jin, Y.; Wu, Y. Exposure to pyrethroid pesticides and the risk of childhood brain tumors in East China. Environ. Pollut. 2016, 218, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chang, C.H.; Tao, L.; Lu, C. Residential Exposure to Pesticide During Childhood and Childhood Cancers: A Meta-Analysis. Pediatrics 2015, 136, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Maryam, Z.; Sajad, A.; Maral, N.; Zahra, L.; Sima, P.; Zeinab, A.; Zahra, M.; Fariba, E.; Sezaneh, H.; Davood, M. Relationship between exposure to pesticides and occurrence of acute leukemia in Iran. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 239–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Shi, R.; Chen, D.; Wang, X.; Kamijima, M.; Sakai, K.; Nakajima, T.; Khalequzzaman, M.; Zhou, Y.; et al. Household pesticide exposure and the risk of childhood acute leukemia in Shanghai, China. Environ. Sci. Pollut. Res. Int. 2015, 22, 11755–11763. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Tian, Y.; Shi, R.; Wang, X.; Hu, Y.; Ji, X.; Han, K.; Hu, S.; Mao, S.; et al. Relationship between risk of childhood acute leukemia and children’s and parents’ lifestyles and household environment exposure. Chin. J. Prev. Med. 2015, 49, 792–799. [Google Scholar]
- Bailey, H.D.; Infante-Rivard, C.; Metayer, C.; Clavel, J.; Lightfoot, T.; Kaatsch, P.; Roman, E.; Magnani, C.; Spector, L.G.; Th Petridou, E.T.; et al. Home pesticide exposures and risk of childhood leukemia: Findings from the childhood leukemia international consortium. Int. J. Cancer 2015, 137, 2644–2663. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, Q.; Zhang, Q.; Yang, L.; Zhang, Z.; Huang, F. Occupational exposure to pentachlorophenol causing lymphoma and hematopoietic malignancy for two generations. Toxicol. Ind. Health 2015, 31, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.; Bae, S.; Singh, K.P.; Roy, D. Increased risk of childhood brain tumors among children whose parents had farm-related pesticide exposures during pregnancy. JP J. Biostat. 2014, 11, 89–101. [Google Scholar] [PubMed]
- Kumar, A.; Vashist, M.; Rathee, R. Maternal factors and risk of childhood leukemia. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 781–784. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bailey, H.D.; Fritschi, L.; Infante-Rivard, C.; Glass, D.C.; Miligi, L.; Dockerty, J.D.; Lightfoot, T.; Clavel, J.; Roman, E.; Spector, L.G.; et al. Parental occupational pesticide exposure and the risk of childhood leukemia in the offspring: Findings from the childhood leukemia international consortium. Int. J. Cancer 2014, 135, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Hoet, P.; Lison, D. Parental occupational exposure to pesticides as risk factor for brain tumors in children and young adults: A systematic review and meta-analysis. Environ. Int. 2013, 56, 19–31. [Google Scholar] [CrossRef]
- Ferreira, J.D.; Couto, A.C.; Pombo-de-Oliveira, M.S.; Koifman, S.; Brazilian Collaborative Study Group of Infant Acute Leukemia. In utero pesticide exposure and leukemia in Brazilian children < 2 years of age. Environ. Health Perspect. 2013, 121, 269–275. [Google Scholar] [PubMed]
- Greenop, K.R.; Peters, S.; Bailey, H.D.; Fritschi, L.; Attia, J.; Scott, R.J.; Glass, D.C.; De Klerk, N.H.; Alvaro, F.; Armstrong, B.K.; et al. Exposure to pesticides and the risk of childhood brain tumors. Cancer Causes Control CCC 2013, 24, 1269–1278. [Google Scholar] [CrossRef]
- Metayer, C.; Colt, J.S.; Buffler, P.A.; Reed, H.D.; Selvin, S.; Crouse, V.; Ward, M.H. Exposure to herbicides in house dust and risk of childhood acute lymphoblastic leukemia. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 363–370. [Google Scholar] [CrossRef]
- Abdolahi, A.; van Wijngaarden, E.; McClean, M.D.; Herrick, R.F.; Allen, J.G.; Ganguly, A.; Bunin, G.R. A case-control study of paternal occupational exposures and the risk of childhood sporadic bilateral retinoblastoma. Occup. Environ. Med. 2013, 70, 372–379. [Google Scholar] [CrossRef]
- Wimberly, C.E.; Gulrajani, N.B.; Russ, J.B.; Landi, D.; Wiemels, J.L.; Towry, L.; Wiencke, J.K.; Walsh, K.M. Maternal prenatal use of alcohol, tobacco, and illicit drugs and associations with childhood cancer subtypes. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2024, 33, 347–354. [Google Scholar] [CrossRef]
- Xu, K.; Li, S.; Whitehead, T.P.; Pandey, P.; Kang, A.Y.; Morimoto, L.M.; Kogan, S.C.; Metayer, C.; Wiemels, J.L.; de Smith, A.J. Epigenetic biomarkers of prenatal tobacco smoke exposure are associated with gene deletions in childhood acute lymphoblastic leukemia. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2021, 30, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Alyahya, M.S.; Al-Sheyab, N.A.; Amro, B. Parental Smoking Behavior and Childhood Cancer: A Case-control Study. Am. J. Health Behav. 2020, 44, 572–590. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, L.E.; Erdmann, F.; Wesseling, C.; Winther, J.F.; Mora, A.M. Parental tobacco smoking and risk of childhood leukemia in Costa Rica: A population-based case-control study. Environ. Res. 2020, 180, 108827. [Google Scholar] [CrossRef]
- Doganis, D.; Katsimpris, A.; Panagopoulou, P.; Bouka, P.; Bouka, E.; Moschovi, M.; Polychronopoulou, S.; Papakonstantinou, E.; Tragiannidis, A.; Katzilakis, N.; et al. Maternal lifestyle characteristics and Wilms tumor risk in the offspring: A systematic review and meta-analysis. Cancer Epidemiol. 2020, 67, 101769. [Google Scholar] [CrossRef] [PubMed]
- Medina-Sanson, A.; Núñez-Enríquez, J.C.; Hurtado-Cordova, E.; Pérez-Saldivar, M.L.; Martínez-García, A.; Jiménez-Hernández, E.; Fernández-López, J.C.; Martín-Trejo, J.A.; Pérez-Lorenzana, H.; Flores-Lujano, J.; et al. Genotype-Environment Interaction Analysis of NQO1, CYP2E1, and NAT2 Polymorphisms and the Risk of Childhood Acute Lymphoblastic Leukemia: A Report From the Mexican Interinstitutional Group for the Identification of the Causes of Childhood Leukemia. Front. Oncol. 2020, 10, 571869. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, J.; Lu, J. Paternal Smoking Before Conception and During Pregnancy Is Associated With an Increased Risk of Childhood Acute Lymphoblastic Leukemia: A Systematic Review and Meta-Analysis of 17 Case-Control Studies. J. Pediatr. Hematol. Oncol. 2020, 42, 32–40. [Google Scholar] [CrossRef]
- Dong, C.; Wang, M.; Zhang, J.; Zhang, R.; Liu, X.; Zheng, Z.; Yang, L. Tobacco smoke exposure and the risk of childhood acute lymphoblastic leukemia and acute myeloid leukemia: A meta-analysis. Medicine 2019, 98, e16454. [Google Scholar]
- Rios, P.; Bailey, H.D.; Poulalhon, C.; Valteau-Couanet, D.; Schleiermacher, G.; Bergeron, C.; Petit, A.; Defachelles, A.-S.; Marion, G.; Sirvent, N.; et al. Parental smoking, maternal alcohol consumption during pregnancy and the risk of neuroblastoma in children. A pooled analysis of the ESCALE and ESTELLE French studies. Int. J. Cancer 2019, 145, 2907–2916. [Google Scholar] [CrossRef]
- Kessous, R.; Wainstock, T.; Sheiner, E. Smoking during pregnancy as a possible risk factor for pediatric neoplasms in the offspring: A population-based cohort study. Addict. Behav. 2019, 90, 349–353. [Google Scholar] [CrossRef]
- Milne, E.; Greenop, K.R.; Petridou, E.; Bailey, H.D.; Orsi, L.; Kang, A.Y.; Baka, M.; Bonaventure, A.; Kourti, M.; Metayer, C.; et al. Maternal consumption of coffee and tea during pregnancy and risk of childhood ALL: A pooled analysis from the childhood Leukemia International Consortium. Cancer Causes Control CCC 2018, 29, 539–550. [Google Scholar] [CrossRef]
- de Smith, A.J.; Kaur, M.; Gonseth, S.; Endicott, A.; Selvin, S.; Zhang, L.; Roy, R.; Shao, X.; Hansen, H.M.; Kang, A.Y.; et al. Correlates of Prenatal and Early-Life Tobacco Smoke Exposure and Frequency of Common Gene Deletions in Childhood Acute Lymphoblastic Leukemia. Cancer Res. 2017, 77, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, G.; Ljung, R.; Mathiesen, T.; Schwartzbaum, J.; Feychting, M. Maternal smoking during pregnancy and the risk of childhood brain tumors: Results from a Swedish cohort study. Cancer Epidemiol. 2016, 40, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Vienneau, D.; Infanger, D.; Feychting, M.; Schüz, J.; Schmidt, L.S.; Poulsen, A.H.; Tettamanti, G.; Klæboe, L.; Kuehni, C.E.; Tynes, T.; et al. A multinational case-control study on childhood brain tumours, anthropogenic factors, birth characteristics and prenatal exposures: A validation of interview data. Cancer Epidemiol. 2016, 40, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Azary, S.; Ganguly, A.; Bunin, G.R.; Lombardi, C.; Park, A.S.; Ritz, B.; Heck, J.E. Sporadic Retinoblastoma and Parental Smoking and Alcohol Consumption before and after Conception: A Report from the Children’s Oncology Group. PLoS ONE 2016, 11, e0151728. [Google Scholar] [CrossRef] [PubMed]
- Metayer, C.; Petridou, E.; Aranguré, J.M.M.; Roman, E.; Schüz, J.; Magnani, C.; Mora, A.M.; Mueller, B.A.; de Oliveira, M.S.P.; Dockerty, J.D.; et al. Parental Tobacco Smoking and Acute Myeloid Leukemia: The Childhood Leukemia International Consortium. Am. J. Epidemiol. 2016, 184, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Wang, H.; Han, S.; Jin, Y.; Lu, J.; Han, W.; Shi, J.; Guo, Y.; Ni, X. Maternal smoking during pregnancy and risk of childhood neuroblastoma: Systematic review and meta-analysis. J. Cancer Res. Ther. 2016, 12, 999–1005. [Google Scholar] [PubMed]
- Orsi, L.; Rudant, J.; Ajrouche, R.; Leverger, G.; Baruchel, A.; Nelken, B.; Pasquet, M.; Michel, G.; Bertrand, Y.; Ducassou, S.; et al. Parental smoking, maternal alcohol, coffee and tea consumption during pregnancy, and childhood acute leukemia: The ESTELLE study. Cancer Causes Control CCC 2015, 26, 1003–1017. [Google Scholar] [CrossRef]
- Momen, N.C.; Olsen, J.; Gissler, M.; Li, J. Exposure to maternal smoking during pregnancy and risk of childhood cancer: A study using the Danish national registers. Cancer Causes Control CCC 2016, 27, 341–349. [Google Scholar] [CrossRef]
- Mattioli, S.; Farioli, A.; Legittimo, P.; Miligi, L.; Benvenuti, A.; Ranucci, A.; Salvan, A.; Rondelli, R.; Magnani, C.; on behalf of the SETIL Study Group. Tobacco smoke and risk of childhood acute non-lymphocytic leukemia: Findings from the SETIL study. PLoS ONE 2014, 9, e111028. [Google Scholar] [CrossRef][Green Version]
- Grufferman, S.; Lupo, P.J.; Vogel, R.I.; Danysh, H.E.; Erhardt, E.B.; Ognjanovic, S. Parental military service, agent orange exposure, and the risk of rhabdomyosarcoma in offspring. J. Pediatr. 2014, 165, 1216–1221. [Google Scholar] [CrossRef]
- Farioli, A.; Legittimo, P.; Mattioli, S.; Miligi, L.; Benvenuti, A.; Ranucci, A.; Salvan, A.; Rondelli, R.; Conter, V.; Magnani, C. Tobacco smoke and risk of childhood acute lymphoblastic leukemia: Findings from the SETIL case-control study. Cancer Causes Control CCC 2014, 25, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Greenop, K.R.; Miller, M.; Attia, J.; Ashton, L.J.; Cohn, R.; Armstrong, B.K.; Milne, E. Maternal consumption of coffee and tea during pregnancy and risk of childhood brain tumors: Results from an Australian case-control study. Cancer Causes Control CCC 2014, 25, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Huang, J.; Lan, H.; Zhao, G.; Huang, C. A meta-analysis of parental smoking and the risk of childhood brain tumors. PLoS ONE 2014, 9, e102910. [Google Scholar] [CrossRef] [PubMed]
- Barrington-Trimis, J.L.; Searles Nielsen, S.; Preston-Martin, S.; Gauderman, W.J.; Holly, E.A.; Farin, F.M.; Mueller, B.A.; McKean-Cowdin, R. Parental smoking and risk of childhood brain tumors by functional polymorphisms in polycyclic aromatic hydrocarbon metabolism genes. PLoS ONE 2013, 8, e79110. [Google Scholar] [CrossRef] [PubMed]
- Metayer, C.; Zhang, L.; Wiemels, J.L.; Bartley, K.; Schiffman, J.; Ma, X.; Aldrich, M.C.; Chang, J.S.; Selvin, S.; Fu, C.H.; et al. Tobacco smoke exposure and the risk of childhood acute lymphoblastic and myeloid leukemias by cytogenetic subtype. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2013, 22, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Milne, E.; Greenop, K.R.; Scott, R.J.; Ashton, L.J.; Cohn, R.J.; de Klerk, N.H.; Lyon, J.L.; Swanson, G.M.; Weiss, N.S.; West, D.; et al. Parental smoking and risk of childhood brain tumors. Int. J. Cancer 2013, 133, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Rossides, M.; Kampitsi, C.E.; Talbäck, M.; Mogensen, H.; Wiebert, P.; Feychting, M.; Tettamanti, G. Risk of Cancer in Children of Parents Occupationally Exposed to Hydrocarbon Solvents and Engine Exhaust Fumes: A Register-Based Nested Case-Control Study from Sweden [1960–2015]. Environ. Health Perspect. 2022, 130, 77002. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, D.; Shi, R.; Kamijima, M.; Sakai, K.; Tian, Y.; Gao, Y. Indoor volatile organic compounds exposures and risk of childhood acute leukemia: A case-control study in shanghai. J. Environ. Sci. Health Part A Tox Hazard. Subst. Environ. Eng. 2021, 56, 190–198. [Google Scholar] [CrossRef]
- Stayner, L.T.; Schullehner, J.; Semark, B.D.; Jensen, A.S.; Trabjerg, B.B.; Pedersen, M.; Olsen, J.; Hansen, B.; Ward, M.H.; Jones, R.R.; et al. Exposure to nitrate from drinking water and the risk of childhood cancer in Denmark. Environ. Int. 2021, 155, 106613. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Erdmann, F.; Urhøj, S.K.; Brandt, J.; Geels, C.; Ketzel, M.; Frohn, L.M.; Christensen, J.H.; Sørensen, M.; Raaschou-Nielsen, O. Air pollution exposure at the residence and risk of childhood cancers in Denmark: A nationwide register-based case-control study. eClinicalMedicine 2020, 28, 100569. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, P.; Liang, G.; Zhang, N.; Wang, C.; Wang, Y.; Nie, L.; Lv, X.; Li, W.; Guo, Q.; et al. Maternal prenatal exposure to environmental factors and risk of childhood acute lymphocytic leukemia: A hospital-based case-control study in China. Cancer Epidemiol. 2019, 58, 146–152. [Google Scholar] [CrossRef]
- Raaschou-Nielsen, O.; Hvidtfeldt, U.A.; Roswall, N.; Hertel, O.; Poulsen, A.H.; Sørensen, M. Ambient benzene at the residence and risk for subtypes of childhood leukemia, lymphoma and CNS tumor. Int. J. Cancer 2018, 143, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Loffredo, C.A.; Mitra, P.S.; Trnovec, T.; Palkovicova Murinova, L.; Sovcikova, E.; Hoffman, E.P.; Makambi, K.H.; Dutta, S.K. PCB exposure and potential future cancer incidence in Slovak children: An assessment from molecular finger printing by Ingenuity Pathway Analysis [IPA®] derived from experimental and epidemiological investigations. Environ. Sci. Pollut. Res. Int. 2018, 25, 16493–16507. [Google Scholar] [CrossRef] [PubMed]
- Park, A.S.; Ritz, B.; Ling, C.; Cockburn, M.; Heck, J.E. Exposure to ambient dichloromethane in pregnancy and infancy from industrial sources and childhood cancers in California. Int. J. Hyg. Environ. Health 2017, 220, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, T.P.; Adhatamsoontra, P.; Wang, Y.; Arcolin, E.; Sender, L.; Selvin, S.; Metayer, C. Home remodeling and risk of childhood leukemia. Ann. Epidemiol. 2017, 27, 140–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Metayer, C.; Scelo, G.; Kang, A.Y.; Gunier, R.B.; Reinier, K.; Lea, S.; Chang, J.S.; Selvin, S.; Kirsch, J.; Crouse, V.; et al. A task-based assessment of parental occupational exposure to organic solvents and other compounds and the risk of childhood leukemia in California. Environ. Res. 2016, 151, 174–183. [Google Scholar] [CrossRef]
- Jiang, W.C.; Wu, S.Y.; Ke, Y.B. Association of exposure to environmental chemicals with risk of childhood acute lymphocytic leukemia. Chin. J. Prev. Med. 2016, 50, 893–899. [Google Scholar]
- Bailey, H.D.; Metayer, C.; Milne, E.; Petridou, E.T.; Infante-Rivard, C.; Spector, L.G.; Clavel, J.; Dockerty, J.D.; Zhang, L.; Armstrong, B.K.; et al. Home paint exposures and risk of childhood acute lymphoblastic leukemia: Findings from the Childhood Leukemia International Consortium. Cancer Causes Control CCC 2015, 26, 1257–1270. [Google Scholar] [CrossRef]
- Ward, M.H.; Colt, J.S.; Deziel, N.C.; Whitehead, T.P.; Reynolds, P.; Gunier, R.B.; Nishioka, M.; Dahl, G.V.; Rappaport, S.M.; Buffler, P.A.; et al. Residential levels of polybrominated diphenyl ethers and risk of childhood acute lymphoblastic leukemia in California. Environ. Health Perspect. 2014, 122, 1110–1116. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Y.; Kamijima, M.; Sakai, K.; Khalequzzaman, M.; Nakajima, T.; Shi, R.; Wang, X.; Chen, D.; Ji, X.; et al. Quantitative assessments of indoor air pollution and the risk of childhood acute leukemia in Shanghai. Environ. Pollut. Barking Essex 2014, 187, 81–89. [Google Scholar] [CrossRef]
- Parodi, S.; Merlo, D.F.; Ranucci, A.; Miligi, L.; Benvenuti, A.; Rondelli, R.; Magnani, C.; Haupt, R.; SETIL Working Group. Risk of neuroblastoma, maternal characteristics and perinatal exposures: The SETIL study. Cancer Epidemiol. 2014, 38, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Glass, D.C.; Greenop, K.R.; Armstrong, B.K.; Kirby, M.; Milne, E.; Fritschi, L. Childhood brain tumours: Associations with parental occupational exposure to solvents. Br. J. Cancer 2014, 111, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Ruckart, P.Z.; Bove, F.J.; Maslia, M. Evaluation of exposure to contaminated drinking water and specific birth defects and childhood cancers at Marine Corps Base Camp Lejeune, North Carolina: A case-control study. Environ. Health Glob. Access Sci. Source 2013, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Gao, Y.; Zhang, Y.; Gao, Y.J.; Zhu, S.; Wang, X.J.; Jin, P.; Tian, Y. Relationship between parental exposure to chemicals and risk of childhood acute leukemia. Chin. J. Ind. Hyg. Occup. Dis. 2013, 31, 413–417. [Google Scholar]
- Brabant, C.; Geerinck, A.; Beaudart, C.; Tirelli, E.; Geuzaine, C.; Bruyère, O. Exposure to magnetic fields and childhood leukemia: A systematic review and meta-analysis of case-control and cohort studies. Rev. Environ. Health 2022, 38, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Amoon, A.T.; Swanson, J.; Magnani, C.; Johansen, C.; Kheifets, L. Pooled analysis of recent studies of magnetic fields and childhood leukemia. Environ. Res. 2022, 204, 111993. [Google Scholar] [CrossRef] [PubMed]
- Seomun, G.; Lee, J.; Park, J. Exposure to extremely low-frequency magnetic fields and childhood cancer: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251628. [Google Scholar] [CrossRef]
- Núñez-Enríquez, J.C.; Correa-Correa, V.; Flores-Lujano, J.; Pérez-Saldivar, M.L.; Jiménez-Hernández, E.; Martín-Trejo, J.A.; Espinoza-Hernández, L.E.; Medina-Sanson, A.; Cárdenas-Cardos, R.; Flores-Villegas, L.V.; et al. Extremely Low-Frequency Magnetic Fields and the Risk of Childhood B-Lineage Acute Lymphoblastic Leukemia in a City With High Incidence of Leukemia and Elevated Exposure to ELF Magnetic Fields. Bioelectromagnetics 2020, 41, 581–597. [Google Scholar] [CrossRef]
- Crespi, C.M.; Swanson, J.; Vergara, X.P.; Kheifets, L. Childhood leukemia risk in the California Power Line Study: Magnetic fields versus distance from power lines. Environ. Res. 2019, 171, 530–535. [Google Scholar] [CrossRef]
- Auger, N.; Bilodeau-Bertrand, M.; Marcoux, S.; Kosatsky, T. Residential exposure to electromagnetic fields during pregnancy and risk of child cancer: A longitudinal cohort study. Environ. Res. 2019, 176, 108524. [Google Scholar] [CrossRef]
- Talibov, M.; Olsson, A.; Bailey, H.; Erdmann, F.; Metayer, C.; Magnani, C.; Petridou, E.; Auvinen, A.; Spector, L.; Clavel, J.; et al. Parental occupational exposure to low-frequency magnetic fields and risk of leukaemia in the offspring: Findings from the Childhood Leukaemia International Consortium [CLIC]. Occup. Environ. Med. 2019, 76, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Amoon, A.T.; Crespi, C.M.; Ahlbom, A.; Bhatnagar, M.; Bray, I.; Bunch, K.J.; Clavel, J.; Feychting, M.; Hémon, D.; Johansen, C.; et al. Proximity to overhead power lines and childhood leukaemia: An international pooled analysis. Br. J. Cancer 2018, 119, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Zhao, C.; Jin, Y.; Lei, Y.; Lu, L.; Chen, G. Association between parental occupational exposure to extremely low frequency magnetic fields and childhood nervous system tumors risk: A meta-analysis. Sci. Total Environ. 2018, 642, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Kheifets, L.; Crespi, C.M.; Hooper, C.; Cockburn, M.; Amoon, A.T.; Vergara, X.P. Residential magnetic fields exposure and childhood leukemia: A population-based case-control study in California. Cancer Causes Control CCC 2017, 28, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Fei, Y.; Wei, X.; Guo, J.; Jiang, X.; Lu, L.; Chen, G. Associations of parental occupational exposure to extremely low-frequency magnetic fields with childhood leukemia risk. Leuk. Lymphoma 2016, 57, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C.M.; Vergara, X.P.; Hooper, C.; Oksuzyan, S.; Wu, S.; Cockburn, M.; Kheifets, L. Childhood leukaemia and distance from power lines in California: A population-based case-control study. Br. J. Cancer 2016, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Bunch, K.J.; Swanson, J.; Vincent, T.J.; Murphy, M.F.G. Epidemiological study of power lines and childhood cancer in the UK: Further analyses. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2016, 36, 437–455. [Google Scholar] [CrossRef]
- Dechent, D.; Driessen, S. Re: Role of Electromagnetic Field Exposure in Childhood Acute Lymphoblastic Leukemia and No Impact of Urinary Alpha- Amylase—A Case Control Study in Tehran, Iran. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 877–878. [Google Scholar] [CrossRef][Green Version]
- Tabrizi, M.M.; Bidgoli, S.A. Increased risk of childhood acute lymphoblastic leukemia [ALL] by prenatal and postnatal exposure to high voltage power lines: A case control study in Isfahan, Iran. Asian Pac. J. Cancer Prev. APJCP 2015, 16, 2347–2350. [Google Scholar] [CrossRef]
- Bunch, K.J.; Swanson, J.; Vincent, T.J.; Murphy, M.F.G. Magnetic fields and childhood cancer: An epidemiological investigation of the effects of high-voltage underground cables. J. Radiol. Prot. Off. J. Soc. Radiol. Prot. 2015, 35, 695–705. [Google Scholar] [CrossRef]
- Pedersen, C.; Johansen, C.; Schüz, J.; Olsen, J.H.; Raaschou-Nielsen, O. Residential exposure to extremely low-frequency magnetic fields and risk of childhood leukaemia, CNS tumour and lymphoma in Denmark. Br. J. Cancer 2015, 113, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Salvan, A.; Ranucci, A.; Lagorio, S.; Magnani, C.; SETIL Research Group. Childhood leukemia and 50 Hz magnetic fields: Findings from the Italian SETIL case-control study. Int. J. Environ. Res. Public Health 2015, 12, 2184–2204. [Google Scholar] [CrossRef] [PubMed]
- Bunch, K.J.; Keegan, T.J.; Swanson, J.; Vincent, T.J.; Murphy, M.F.G. Residential distance at birth from overhead high-voltage powerlines: Childhood cancer risk in Britain 1962–2008. Br. J. Cancer 2014, 110, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, X.; Wang, C.; Yan, K.; Lin, X.; Li, S.; Bao, H.; Liu, X. Magnetic fields exposure and childhood leukemia risk: A meta-analysis based on 11,699 cases and 13,194 controls. Leuk. Res. 2014, 38, 269–274. [Google Scholar] [CrossRef]
- Pedersen, C.; Bräuner, E.V.; Rod, N.H.; Albieri, V.; Andersen, C.E.; Ulbak, K.; Hertel, O.; Johansen, C.; Schüz, J.; Raaschou-Nielsen, O. Distance to high-voltage power lines and risk of childhood leukemia—An analysis of confounding by and interaction with other potential risk factors. PLoS ONE 2014, 9, e107096. [Google Scholar] [CrossRef] [PubMed]
- Sermage-Faure, C.; Demoury, C.; Rudant, J.; Goujon-Bellec, S.; Guyot-Goubin, A.; Deschamps, F.; Hemon, D.; Clavel, J. Childhood leukaemia close to high-voltage power lines—The Geocap study, 2002–2007. Br. J. Cancer 2013, 108, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, L.T.N.; Park, D.; Lee, Y.C. Human Health Impacts of Residential Radon Exposure: Updated Systematic Review and Meta-Analysis of Case-Control Studies. Int. J. Environ. Res. Public Health 2022, 20, 97. [Google Scholar] [CrossRef]
- Moon, J.; Yoo, H. Residential radon exposure and leukemia: A meta-analysis and dose-response meta-analyses for ecological, case-control, and cohort studies. Environ. Res. 2021, 202, 111714. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, L.; Chen, Q.; Wei, J.; Cao, G.; Zhang, J. Domestic radon exposure and risk of childhood leukemia: A meta-analysis. J. BUON Off. J. Balk. Union. Oncol. 2020, 25, 1035–1041. [Google Scholar]
- Nikkilä, A.; Arvela, H.; Mehtonen, J.; Raitanen, J.; Heinäniemi, M.; Lohi, O.; Auvinen, A. Predicting residential radon concentrations in Finland: Model development, validation, and application to childhood leukemia. Scand. J. Work Environ. Health 2020, 46, 278–292. [Google Scholar] [CrossRef]
- Chen, J.; Xie, L. Domestic radon exposure and childhood leukaemia and lymphoma: A population-based study in canada. Radiat. Prot. Dosimetry 2019, 184, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Peckham, E.C.; Scheurer, M.E.; Danysh, H.E.; Lubega, J.; Langlois, P.H.; Lupo, P.J. Residential Radon Exposure and Incidence of Childhood Lymphoma in Texas, 1995–2011. Int. J. Environ. Res. Public Health 2015, 12, 12110–12126. [Google Scholar] [CrossRef] [PubMed]
- Del Risco Kollerud, R.; Blaasaas, K.G.; Claussen, B. Risk of leukaemia or cancer in the central nervous system among children living in an area with high indoor radon concentrations: Results from a cohort study in Norway. Br. J. Cancer 2014, 111, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Hauri, D.; Spycher, B.; Huss, A.; Zimmermann, F.; Grotzer, M.; von der Weid, N.; Weber, D.; Spoerri, A.; Kuehni, C.E.; Röösli, M.; et al. Domestic radon exposure and risk of childhood cancer: A prospective census-based cohort study. Environ. Health Perspect. 2013, 121, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Onyije, F.M.; Olsson, A.; Baaken, D.; Erdmann, F.; Stanulla, M.; Wollschläger, D.; Schüz, J. Environmental Risk Factors for Childhood Acute Lymphoblastic Leukemia: An Umbrella Review. Cancers 2022, 14, 382. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/pr221_E.pdf (accessed on 10 January 2024).
- Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono109-F07.pdf (accessed on 10 January 2024).
- Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100F-24.pdf (accessed on 10 January 2024).
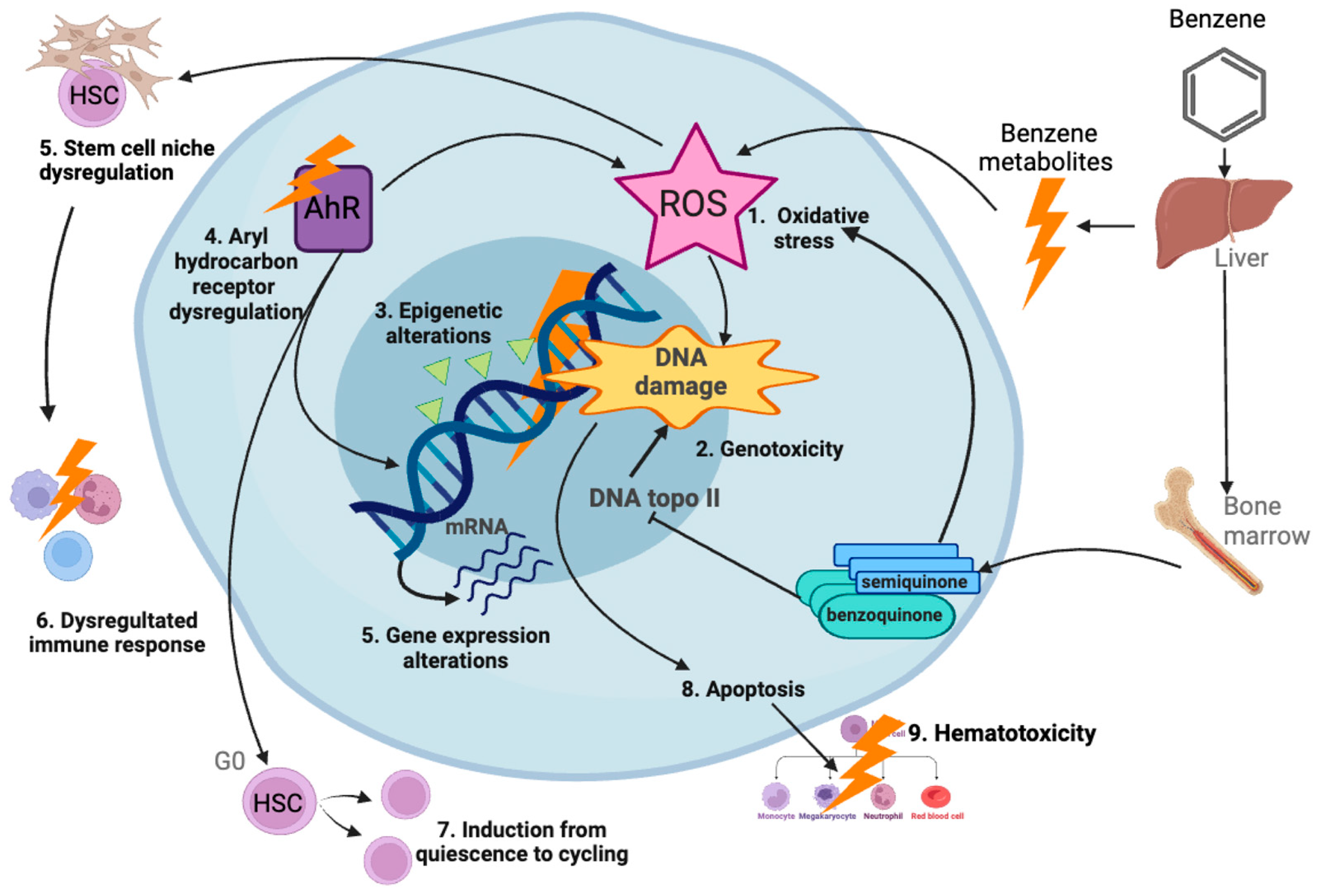
- McHale, C.M.; Zhang, L.; Smith, M.T. Current understanding of the mechanism of benzene-induced leukemia in humans: Implications for risk assessment. Carcinogenesis 2012, 33, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Pech, K.; Pérez-Herrera, N.; Vértiz-Hernández, Á.A.; Lajous, M.; Farías, P. Health Risk Assessment in Children Occupationally and Para-Occupationally Exposed to Benzene Using a Reverse-Translation PBPK Model. Int. J. Environ. Res. Public Health 2023, 20, 2275. [Google Scholar] [CrossRef] [PubMed]
- Gruľová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Gogaľová, Z.; Poráčová, J.; Camele, I.; De Feo, V. Thymol Chemotype Origanum vulgare L. Essential Oil as a Potential Selective Bio-Based Herbicide on Monocot Plant Species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef]
- Ottenbros, I.; Lebret, E.; Huber, C.; Lommen, A.; Antignac, J.P.; Čupr, P.; Šulc, L.; Mikeš, O.; Szigeti, T.; Középesy, S.; et al. Assessment of exposure to pesticide mixtures in five European countries by a harmonized urinary suspect screening approach. Int. J. Hyg. Environ. Health 2023, 248, 114105. [Google Scholar] [CrossRef]
- Available online: https://www.iarc.who.int/wp-content/uploads/2018/07/pr236_E.pdf (accessed on 10 January 2024).
- Navarrete-Meneses, M.d.P.; Pérez-Vera, P. Pyrethroid pesticide exposure and hematological cancer: Epidemiological, biological and molecular evidence. Rev. Environ. Health 2019, 34, 197–210. [Google Scholar] [CrossRef]
- Navarrete-Meneses, M.d.P.; Salas-Labadía, C.; Juárez-Velázquez, M.d.R.; Moreno-Lorenzana, D.; Gómez-Chávez, F.; Olaya-Vargas, A.; Pérez-Vera, P. Exposure to Insecticides Modifies Gene Expression and DNA Methylation in Hematopoietic Tissues In Vitro. Int. J. Mol. Sci. 2023, 24, 6259. [Google Scholar] [CrossRef]
- Nicolella, H.D.; de Assis, S. Epigenetic Inheritance: Intergenerational Effects of Pesticides and Other Endocrine Disruptors on Cancer Development. Int. J. Mol. Sci. 2022, 23, 4671. [Google Scholar] [CrossRef]
- Available online: https://www.who.int/europe/news/item/03-02-2021-world-cancer-day-know-the-facts-tobacco-and-alcohol-both-cause-cancer#:~:text=People%20who%20use%20both%20alcohol,up%20to%2030%20times%20higher (accessed on 10 January 2024).
- Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono100E-6.pdf (accessed on 10 January 2024).
- NCI. Electromagnetic Fields and Cancer. 2022. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/electromagnetic-fields-fact-sheet (accessed on 10 January 2024).
- Schüz, J.; Erdmann, F. Environmental Exposure and Risk of Childhood Leukemia: An Overview. Arch. Med. Res. 2016, 47, 607–614. [Google Scholar] [CrossRef]
- Onyije, F.M.; Olsson, A.; Bouaoun, L.; Schüz, J. Synthesized evidence for childhood acute lymphoblastic leukemia. Front. Pediatr. 2023, 11, 1209330. [Google Scholar] [CrossRef]





| Searching Commands | Number of Papers Retrieved |
|---|---|
| “pollution” “childhood” “cancer” | 323 |
| “children” “cancer” “pollution” | 1788 |
| “children” “cancer” “pesticides” | 376 |
| “children” “cancer” “magnetic” “fields” | 172 |
| “children” “cancer” “benzene” | 98 |
| “smoke” “children” “cancer” | 2121 |
| “childhood” “cancer” “water” “pollution” | 17 |
| “childhood” “cancer” “air” “pollution” | 187 |
| “childhood” “cancer” “pollution” | 324 |
| “childhood” “cancer” “pollutants” | 766 |
| Reference | Pollutant | Cancer | Population | Results OR 95% CI | Type of Study |
|---|---|---|---|---|---|
| Zhong, C., 2023 [12] | Air pollution and artificial light at night (ALAN) | ALL | California | ALAN 1.15, 1.01–1.32; PM2.5 1.24, 0.98–1.56 | Large population-based case–control: 2782 ALL cases and 139,100 controls |
| Malavolti, M., 2023 [13] | Residential proximity to petrol stations | Leukemia | Italy | RR was 2.2, 0.5–9.4 for children living <50 m from the nearest petrol station. Associations were stronger for the ALL subtype RR 2.9, 0.6–13.4. | Population-based case–control: 182 cases and 726 controls |
| Kreis, C., 2022 [14] | Traffic-related air pollution | ALL | Switzerland | HR NO2 and ALL 1.00, 0.88–1.13; AML 1.31, 1.00–1.71; benzene and ALL 1.03, 0.86–1.23; AML 1.29, 0.86–1.95 | Cohort study: 2960 cases |
| Lee, 2022 [15] | Particulate matter | Childhood cancer | Korea | HR PM2.5 3.02, 1.63-5.59 | Retrospective cohort: 1725 patients |
| Mazzei, 2022 [16] | Residential proximity to petrol stations and benzene | Childhood cancer | Switzerland | All cancers 1.29, 0.84–1.98; Leukemia 1.08, 0.46–2.51; CNS 1.30 [0.51–3.35] | Case–control: 6129 cases |
| Asenjo, S., 2022 [17] | Cadmium and lead topsoil levels | Leukemia | Spain | A unit increase on the topsoil level for cadmium and leukemia 1.11, 1.00–1.24; for lead and leukemia 1.10, 0.99–1.21 | Case–control: 2897 cases |
| Onyije, 2021 [18] | Residential proximity to petroleum facilities | Leukemia | France | Petroleum and childhood leukemia, summary effect size [ES] 1.90, 1.34–2.70 | Systematic review and meta-analysis |
| Ribeiro, A., 2021 [19] | Residential traffic exposure | Lympho hematopoietic | Sao Paulo | Lymphoid leukemia and traffic density, IRR 1.21, 1.06–1.39 and 1.3, 1.13–1.68 for NO2 for the lower SES group. In the higher group, 1.06, 1.00–1.14 traffic density and 1.37, 1.16–1.62 for NO2 | Retrospective population-based registry |
| Lavigne, E., 2020 [20] | Ultrafine particle concentrations [UFP] | Childhood cancer | Toronto | First trimester exposure to UFPs per 10,000/cm3 increases HR 1.13, 1.03–1.22 cancer. | Population-based cohort: 1066 cases |
| Hvidtfeldt, 2020 [21] | Residential exposure to PM2.5 components | Non-Hodgkin’s lymphoma | Denmark | PM2.5, 2.05, 1.10–3.83 and for BC, 1.22, 1.02–1.46, SIA 1.73, 0.68–4.41, and NHL | Case–control: 170 cases |
| Volk, J., 2019 [22] | Parental occupational exposure to diesel engine exhaust | Leukemia and CNS | Denmark | Maternal exposure to diesel engine and CNS cancer 1.31, 0.99–1.74, and astrocytoma 1.49, 1.04–2.14 | Register-based nested case–control: 1673 cases |
| Peckham-Gregory, 2019 [23] | Maternal residential proximity to major roadways | Leukemia | Texas | No association mothers who lived ≤500 m to a major roadway and ALL No association mothers who lived in areas characterized by high roadway density and ALL or AML | Case–control study: 2030 cases |
| García-Pérez, 2019 [24] | Vicinity of pollution sources | Childhood cancer | Spain | Proximity [≤2 km] to specific industrial areas and leukemias 1.31, 1.04–1.65, and 1.28, 1.00–1.53 for urban areas, and neuroblastoma 2.12, 1.18–3.83 for both industrial and urban areas, and renal 2.02, 1.16–3.52 for industrial areas | Population-based case–control |
| Gong, 2019 [25] | Traffic-related air pollution | Leukemia | Traffic density 1.01, 0.98–1.04, high traffic density 1.04, 0.91–1.17, moderate exposure to NO2 1.02, 0.93–1.10, and benzene 1.04, 0.71–1.37, with risk of leukemia | Meta-analysis | |
| Filippini, 2019 [26] | Outdoor air pollution | Leukemia | Traffic density RR 1.09, 1.00–1.20; Benzene 1.27, 1.03–1.56; NO2 1.04, 0.90–1.19; PM2:5 1.05 0.94–1.16; PM10 1.20, 0.70–2.04; 1,3—Butadiene 1.45, 1.08–1.95 with leukemia | Systematic review and dose–response meta-analysis | |
| Seifi, M., 2019 [27] | Exposure to ambient air pollution | Childhood cancer | Tehran, Iran | Cancer and PM10 1.008, 1.001–1.015; NO2 1.05, 0.98–1.01 | Case–control: 161 cases |
| Hall, C., 2019 [28] | Prenatal exposure to air toxics | Malignant germ cell tumors | California | Prenatal exposure to 1,3-butadiene during the second trimester and GCT, 1.51, 0.01–2.26 and meta/para-xylene 1.56, 1.10–2.21]. Elevated ORs for yolk sac tumors. | Case–control: 243 cases |
| Iavarone, 2018 [29] | Living in industrially contaminated sites | Childhood cancer | Italy | Contaminated areas, especially of industrial origin and CNS cancer in the age-group <1 year SIR 3.2, 1.4–6.3; soft tissue sarcoma in the age-group 0-14 years SIR 1.6, 1.1–2.3; AML in the age group 0-14 years SIR 1.7, 1.1–2.4; | Cohort: 1050 cases |
| Kirkeleit, 2018 [30] | Exposure to gasoline exhaust | Leukemia | Norway | Gasoline or exhaust and increased risk of leukemia HR 2.59, 1.03–6.48 and ALL HR 2.71, 0.97–7.58 | Prospective population-based cohort study |
| Kumar, S., 2018 [31] | Maternal residential proximity to major roads | Childhood cancer | Texas | Embryonal tumor in children born to mothers living within 500 m of a major roadway 1.24, 1.00–1.54. For unilateral retinoblastoma 2.57, 1.28–5.15, for every kilometer closer the mother lived to the nearest major roadway. | Case–control |
| Ortega-García, 2017 [32] | Proximity to air-polluting industries | Childhood cancer | Spain | SNST was around energy-generating chemical industries, another of NHL was around residue-valorization plants, and, finally, one cluster of HL was around building materials | Retrospective cohort |
| Janitz, A.E., 2017 [33] | Benzene | Leukemia | Oklahoma | Cases born from 2005 to 2010 had a three-fold increased unadjusted odds of elevated exposure to benzene and leukemia compared to controls born in this same time 3.53, 1.35–9.27. | Case–control |
| Ramis, R., 2017 [34] | Exposure to industrial/urban environment | CNS | Spain | Children living in industrial and urban areas and CNS tumors. Urban areas 0.90, 0.65–1.24, and industrial areas 0.96, 0.81–1.77 | Case–control: 714 cases |
| Lavigne, E., 2017 [35] | Maternal exposure to ambient air pollution | Childhood cancer | Ontario | PM2.5, over the entire pregnancy, and during the first trimester and astrocytoma HR per 3.9 μg/m3 1.01–1.88, and HR per 4.0 μg/m3 = 1.40, 1.05–1.86, respectively. First trimester NO2 and ALL HR 1.20, 1.02-1.41 per IQR [13.3 ppb]. | Population-based: 2044 cases |
| Spycher, D., 2017 [36] | Parental occupational exposure to benzene | Childhood cancer | Switzerland | Maternal exposure to benzene and leukemia HR 1.73, 1.12–2.67, and ALL 1.88, 1.16–3.04. | Case–control: 1520 cases |
| García-Pérez, 2017 [37] | Residential proximity to industrial and urban areas | Bone tumors | Spain | Close to industrial facilities as a whole 2.33, 1.17–4.63, at 3 km. Surface treatment of metals 2.50, 1.13–5.56, at 2 km; production and processing of metals 3.30, 1.41–7.77, at 2.5 km; urban waste–water treatment plants 4.41, 1.62–11.98, at 2 km; hazardous waste 4.63, 1.37–15.61, at 2 km; disposal or recycling of animal waste 4.73, 1.40–15.97, at 2 km; cement and lime 3.89, 1.19–12.77, at 2.5km; and combustion installations 3.85, 1.39–10.66, at 3 km, and bone tumors | Case–control: 114 cases |
| Janitz, E., 2017 [38] | Maternal and paternal occupational exposures | Hepatoblastoma | USA | Both paternal and maternal exposure to paints and hepatoblastoma: paternal 1.71, 1.04–2.81; maternal 3.29, 0.32–33.78]. | Case–control: 383 cases |
| Janitz, A., 2016 [39] | Traffic-related air pollution | Leukemia | Oklahoma | NO2 [11.19–19.89 ppb] and AML with a positive [4th quartile] 5.25, 1.09–25.26 | Case–control: 307 cases |
| Danysh, E., 2016 [40] | Maternal residential proximity to major roadways | CNS | Texas | Mothers living ≤500 m from a major roadway were 31%, 1.0–1.8 more likely to have offspring with any CNS tumor and 3.1-times 0.9–10.4 more likely to have offspring with an ependymoma compared to mothers living >500 m from the nearest major roadway. Comparing mothers living in areas with low roadway density vs. areas with high roadway density were 51%, 1.1–2.1 more likely to have offspring with any CNS tumor and 4.2-times 1.2–14.9 more likely to have offspring with an ependymoma. | Case–control: 315 cases |
| Von Ehrenstein, O., 2016 [41] | In utero and early-life exposure to ambient air toxics | Brain tumors | California | Prenatal exposure to acetaldehyde and primitive neuroectodermal tumors 2.30, 1.44–3.67, 1,3-butadiene 2.23, 1.28–3.88, and exposure during the first year of life to ortho-dichlorobenzene 3.27, 1.17–9.14, 1,3-butadiene 3.15, 1.57–6.32. Medulloblastoma was associated with prenatal exposure to polycyclic aromatic hydrocarbons 1.44, 1.15–1.80. Exposures to lead and some PAHs during the first year of life and astrocytoma 1.40, 0.97–2.03. | Population-based case–control: 183 cases |
| Symanski, E., 2016 [42] | Air toxics | ALL | Texas | Polycyclic organic matter and leukemia, 1.11, 0.94–1.32, 1.17, 0.98-1.39 for benzene, and 1.29, 1.08–1.52 for 1,3-butadiene. | Population-based case–control |
| Magnani, C., 2016 [43] | Road traffic pollution | Leukemia | Italy | Closeness of the house to traffic lights and to the passage of trucks [Road traffic pollution] and ANLL 6.35, 2.59–15.6. | Nationwide case–control: 648 cases |
| García-Pérez, 2016 [44] | Residential proximity to environmental pollution | Renal tumors | Spain | Children living near [≤2.5 km] industrial installations and renal tumors 1.97, 1.13–3.42. | Case–control: 213 cases |
| García-Pérez, 2016 [45] | Residential proximity to environmental pollution | Rare tumors | Spain | Retinoblastoma and proximity to industries involved in glass and mineral fibers 2.49, 1.01–6.12 at 3 km, and organic chemical industries 2.54, 1.10–5.90 at 2 km. | Population-based case–control: 557 cases |
| García-Pérez, 2016 [46] | Residential proximity to industrial and urban sites | Neuroblastoma | Spain | Neuroblastoma and intersection between industrial and urban areas: 2.52, 1.20–5.30 for industrial distance of 1 km, and 1.99, 1.17–3.37 for industrial distance of 2 km. | Population-based case–control: 398 cases |
| Carlos-Walace, 2016 [47] | Parental, in utero and early-life exposure to benzene | Leukemia | Occupational and household product exposure and RR leukemia was 1.96, 1.53, 2.52. RR was higher for AML 2.34, 1.72–3.18 than for ALL 1.57; 1.21–2.05. Traffic density or traffic–related air pollution RR 1.48, 1.10–1.99; it was higher for AML 2.07, 1.34–3.20 than for ALL 1.49, 1.07–2.08 | Meta-analysis | |
| Filippini, T., 2015 [48] | Outdoor air pollution | Leukemia | NO2 and benzene 1.21, 0.97–1.52 and 1.64, 0.91–2.95 respectively. Stratifying by leukemia type, upon NO2 were 1.21, 1.04–1.41 for ALL, and 1.06, 0.51–2.21 for AML; upon benzene were 1.09, 0.67–1.77 for ALL, and 2.28, 1.09–4.75 for AML. | Meta-analysis | |
| Spycher, B., 2015 [49] | Residential exposure to highways | Childhood cancer | Switzerland | Comparing children living <100 m from a highway with unexposed children [≥500 m] was 1.43, 0.79–2.61. Associations were similar for ALL RR 1.64, 1.10–2.43, and stronger for leukemia in children aged <5 years RR 1.92, 1.22–3.04. | Cohort |
| Malagoli, C., 2015 [50] | Living in urban areas | Leukemia | Italy | Benzene and PM10 and leukemia, associated with residence in a highly urbanized area and residential area 1.4, 0.8–2.4 and 1.3, 0.8–2.2, respectively. | Case–control: 111 cases |
| Heck, J., 2015 [51] | Exposure to toxics in perinatal period | Retinoblastoma | California | Pregnancy exposure to benzene and retinoblastoma 1.67, 1.06–2.64. Pregnancy exposure to chloroform 1.35, 1.07–1.70, chromium 1.29, 1.04–1.60, para–dichlorobenzene 1.24, 1.04–1.49, nickel 1.4, 1.08–2.01, and, in the first year of life, acetaldehyde 1.62, 1.06–2.48 and retinoblastoma | Case–control: 103 cases |
| Houot, J., 2015 [52] | Residential proximity to heavy-traffic roads | Leukemia | France | A 300 m increase in major road length within 150 m of the geocoded address and AML 1.2, 1.0–1.4. | Case–control: 2760 cases |
| Greenop, K., 2015 [53] | Vehicle refuelling, use of domestic wood heaters | Brain tumors | Australia | Paternal refueling ≥ 4 times/month and risk of CBT 1.59, 1.11–2.29. Wood heaters before 1.51, 1.05–2.15 and after 1.44, 1.03–2.01 the child’s birth and risk of CBT. | Case–control: 306 cases |
| García-Pérez, 2015 [54] | Residential proximity to industrial and urban sites | Leukemia | Spain | Leukemia associated with living near [≤2.5 km] industries 1.31, 1.03–1.67—particularly glass and mineral fibers 2.42, 1.49–3.92, surface treatment using organic solvents 1.87, 1.24–2.83, galvanization 1.86, 1.07–3.21, production and processing of metals 1.69, 1.22–2.34, and surface treatment of metals 1.62, 1.22–2.15. | Population-based case–control: 638 cases |
| Zhou, 2014 [55] | Maternal benzene exposure during pregnancy | ALL | Solvent 1.25, 1.09–1.45; paint 1.23, 1.02–1.47; petroleum exposure 1.42, 1.10–1.84, and maternal smoking during pregnancy 0.99, 0.93–1.06 associated with ALL | Meta-analysis | |
| Shrestha, A., 2014 [56] | Prenatal exposure to air toxics | Wilms tumor | California | Prenatally exposed to formaldehyde, polycyclic aromatic hydrocarbons, perchloroethylene, or acetaldehyde in the third trimester and Wilms’ tumor per interquartile increase in concentration 1.28, 1.12–1.45; 1.10, 0.99–1.22; 1.09, 1.00–1.18; 1.25, 1.07–1.45, respectively. | Case–control: 337 cases |
| Boothe, V., 2014 [57] | Residential traffic exposure | Leukemia | Residential traffic exposure and leukemia [postnatal exposure window] 1.53, 1.12–2.10 | Systematic review and meta-analysis | |
| Heck, J., 2014 [58] | Exposure to ambient toxics during pregnancy and early childhood | Leukemia | California | Polycyclic aromatic hydrocarbons [third trimester exposure] and ALL 1.16, 1.04–1.29; arsenic 1.33, 1.02–1.73; benzene 1.50, 1.08–2.09; AML associated to chloroform [third trimester exposure] and 1.30, 1.00–1.69; benzene 1.30, 1.00–1.69 | Case–control: 69 cases |
| Badaloni, C., 2013 [59] | Air pollution | Leukemia | Italy | All ORs, independent of the method of assessment and the exposure windows, were close to the null value. | Nationwide case–control: 620 cases |
| Heck, J., 2013 [60] | Traffic-related air pollution | Childhood cancer | California | Traffic–related pollution during the first trimester and ALL 0.05, 1.01–1.10; germ cell tumors 1.1, 1.04–1.29, particularly teratomas 1.26, 1.12–1.41; and retinoblastoma 1.11, 1.01–1.21, particularly bilateral retinoblastoma 1.16, 1.02–1.33 | Case–control: 3590 cases |
| Ghosh, J., 2013 [61] | Prenatal exposure to traffic-related air pollution | Childhood cancer | California | ALL and higher air pollution exposures by 9% per 25 ppb increase in nitric oxide 1.09, 1.01–1.18. For every 25 ppb increase in third-trimester pollutant concentrations, increase risk of bilateral retinoblastoma 15%, 1.01–1.31, and 13%, 0.99–1.29 for nitric oxide and nitrogen dioxide, respectively. | Case–control: 4015 cases |
| Peters, S., 2013 [62] | Occupational exposure to engine exhausts | Brain tumors | Australia | Maternal exposure to diesel exhaust and brain tumors any time before the child's birth 2.03, 1.09–3.81 and paternal exposure around the time of the child’s conception 1.62, 1.12–2.34. | Population-based case–control: 306 cases |
| Heck, E., 2013 [63] | Ambient air toxics exposure in pregnancy | Neuroblastoma | California | Maternal exposure to carbon tetrachloride and neuroblastoma 2.65, 1.07–6.53, and polycyclic aromatic hydrocarbons [indeno[1,2,3-cd], pyrene and dibenz[a,h]anthracene] 1.39, 1.05–1.84. Hexavalent chromium and neuroblastoma 1.32, 1.00–1.74, at 5 km. | Case–control: 75 cases |
| Reference | Pollutant | Cancer | Population | Results OR 95% CI | Type of Study |
|---|---|---|---|---|---|
| Ward, 2023 [64] | Pesticides [glyphosate] | ALL | California | No association of occupational pesticide exposure, and ALL | Case–control: 181 cases and 225 controls |
| Rafeeinia, 2023 [65] | Pesticides [organochlorines] | ALL | Iran | 76.2% of CDKN2B promoters, and 85.1% of MGMT promoters were hypermethylated in children with ALL | Case–control: 72 cases and 141 controls |
| Rossides, M., 2022 [66] | Maternal and paternal exposure to pesticides | Childhood cancer | Sweden | Aromatic HSC and N-HL 1.64, 1.05–2.58; aliphatic/alicyclic HCS and GCT 1:52, 0.89–2.59; gasoline/diesel EEF and astrocytoma 1:40, 1.04–1.88, ML 1:53, 0.84–2.81, HL 1:60, 0.85–3.02, EEF and HL 1:21, 1.01–1.44; STS 1:22, 1.00–1.48 | Large population-based case–control: 17,313 cancer cases |
| Thompson, S., 2022 [67] | Prenatal exposure to pesticides | Retinoblastoma | California | EEF and HL 1:21, 1.01–1.44; STS 1:22, 1.00–1.48 | Case–control study: 335 cases and 123,166 controls |
| Khan, 2022 [68] | Prenatal exposure to pesticides | Neuroblastoma | Prenatal pesticide exposure and neuroblastoma 1.6, 1.1–2.3, while after birth, 1.0, 0.8–1.3. | Systematic review and meta-analysis | |
| El-Helaly, 2022 [69] | Pesticides, radiation, hazardous chemicals, and smoking | Bone cancer | Egypt | Nitrose compounds among children, paternal smoking, and consanguinity are predictors of bone cancer. | Retrospective case–control study: 51 cases and 67 controls |
| Khan, 2022 [70] | Pesticides | Wilms tumor | Parental pesticide exposure and Wilms’ tumor. A strong association between organophosphate herbicides/insecticides and pediatric cancer was found. | Meta-analysis and systematic review | |
| Bamouni, 2022 [71] | Residential proximity to croplands at birth | Leukemia | France | No association between croplands density and acute leukemia. | Case–control: 8747 cases and 19,809,700 controls |
| Onyije, 2022 [72] | Parental exposure to pesticides and other agents | Leukemia | Europe [UK, France, Spain, Lithuania, Norway, and Greece] | High paternal occupational exposure to crystalline silica and ALL 2.20, 1.60–3.01, and for AML 2.03, 1.04–3.97. For ALL, high paternal occupational exposure to chromium 1.23, 0.77–1.96, and diesel engine exhaust 1.21, 0.82–1.77. | Case–control study: 3362 cases and 6268 controls |
| Feulefack, 2021 [73] | Prenatal exposure to pesticides | Brain cancer | Pesticides prenatal exposure and brain tumors 1.32; 1.17–1.49. After birth exposure 1.22, 1.03–1.45, and residential exposure to pesticides 1.31, 1.11–1.54. Parental occupational exposure and CBT 1.17, 0.99–1.38. | Meta-analysis | |
| Nguyen, 2021 [74] | Proximity of residence at birth to outdoor plant nurseries | Leukemia | USA | For birth residences less than 75 m from plant nurseries and leukemia 2.40, 0.99–5.82; stronger for AML 3.09, 1.14–8.34. | Case–control: 5788 cases and 5788 controls |
| Madrigal, 2021 [75] | Pesticides | ALL | USA | No association of permethrin, chlorpyrifos, diazinon, and carbaryl with ALL. | 252 cases and 306 controls |
| Coste, A., 2020 [76] | Parental occupational exposure to pesticides | Childhood cancer | Switzerland | No association with maternal or paternal exposure to pesticides and any cancer subtype. | 1891 cases of cancer |
| Patel, 2020 [77] | Parental occupational exposures to pesticides, animals, and organic dust | Leukemia and CNS | Australia, Denmark, Israel, Norway, and UK | Paternal exposures to pesticides and animals and risk for AML herbicides HR = 3.22, 0.97–10.68; insecticides 2.86, 0.99–8.23; animals 3.89, 1.18–12.90. Paternal exposure to organic dust and AML 2.38, 1.12–5.07. | 329,658 participants from birth cohorts in five countries with ALL (129), AML (31), and CNS tumors (158) |
| Park, 2020 [78] | Pesticide exposure in pregnancy | Leukemia | USA | Exposure to any carcinogenic pesticide and risks for ALL 2.83, 1.67–4.82, diuron 2.38, 1.57–3.60, phosmet 2.10, 1.46–3.02, kresoxim-methyl 1.77, 1.14–2.75, and propanil 2.58, 1.44–4.63. Elevated risks for the group of 2,6-dinitroanilines 2.50, 1.56–3.99, anilides 2.16, 1.38–3.36, and ureas 2.18, 1.42–3.34. | Case–control: 162 cases and 9805 controls |
| Mavoungou, 2020 [79] | Pesticide exposure during pregnancy | Lymphoma | France | Insecticides association with Burkitt lymphoma and mixed cellularity classical HL 1.3, 1.0–1.7. | Case–control: 328 cases with Hodgkin’s lymphoma, 305 with non-Hodgkin’s lymphoma, and 2415 controls |
| Rios, 2020 [80] | Pesticides and other agents | Wilms tumor | France | Maternal use of pesticide during pregnancy associated with the risk of Wilms tumor 1.6, 1.1–2.3. Association was stronger when they were used more often than once a month 1.9, 1.2–3.0. | Case–control: 117 cases and 1100 controls |
| Coste, 2020 [81] | Agricultural crop density | Leukemia | France | Viticulture density and the incidence of ALL, SIRR = 1.03, 1.00–1.06. | Registry of cases: 11,487 cases |
| Patel, 2020 [82] | Residential proximity to crops and animals during pregnancy | Leukemia and CNS | Denmark | Mothers with increasing crop area near their home and leukemia highest tertile > 24 ha HR: 2.0, 1.02–3.8; after adjustment for animals [within 1000 m] HR: 2.6, 1.02–6.8. We also observed increased risk for grass/clover highest tertile > 1.1 ha HR: 3.1, 1.2–7.7, peas > 0 HR: 2.4, 1.02–5.4, and maize > 0 HR: 2.8, 1.1–6.9 in animal-adjusted models. | Case–control |
| Van Maele-Fabry, 2019 [83] | Domestic pesticide exposure | Leukemia | Association between residential pesticide exposure and leukemia SOR: 1.57, 1.27–1.95. | Meta-analysis | |
| Bunch, 2019 [84] | Paternal occupational exposure to pesticides and other agents | Lymphoma | Great Britain | Paternal exposure to ceramics and/or glass and lymphoma risk both before and after adjustment for social class 2.33, 1.19–4.59 and 2.45, 1.22–4.95, respectively; specifically for both HL and NHL 4.00, 1.08–22.09 and 2.80, 1.01–7.77, respectively. BL risk and paternal exposure to lead 2.67, 1.24–5.74. | Case–control |
| Georgakis, 2019 [85] | Instrument-assisted delivery and other agents including pesticides | Brain tumors | Greece | Instrument-assisted delivery and risk of brain tumors. Maternal alcohol consumption during pregnancy 2.35, 1.45–3.81, and history of living in a farm 4.98, 2.40–10.32 and brain tumors. | Case–control |
| Hyland, 2018 [86] | Maternal residential pesticide uses before and after chld´s birth | ALL | Costa Rica | Maternal insecticide uses inside the home in the year before pregnancy, during pregnancy, and while breastfeeding associated with ALL among boys 1.63, 1.05–2.53, 1.75, 1.13–2.73, and 1.75, 1.12–2.73, respectively. | Case–control |
| Ferri, 2018 [87] | Parental occupations, pesticide use, environmental factors, and genetic polymorphism [CYP2D6*4] | Leukemia | Italy | Prenatal maternal use of insecticides/rodenticides 1.87, 1.04–3.33, with subjects living <100 m from pesticide-treated fields 3.21, 1.37–7.53, and with a paternal occupation as traffic warden/policeman 4.02, 1.63–9.87, and increased risk for leukemia. Associations were found with genetic polymorphism of CYP2D6*4 for homozygous alleles [mutant type/mutant type 6.39, 1.17–34.66]. | Case–control |
| Vidart d’Egurbide Bagazgoïtia, 2018 [88] | Maternal residential use of pesticides | Brain tumors | France | Brain tumors with the maternal home use of pesticides during pregnancy 1.4, 1.2–1.8, more specifically, with insecticide 1.4, 1.2–1.8. | Case–control |
| Boffetta, 2018 [89] | Permethirn | Childhood cancer | An increased risk of multiple myeloma was found among AHS members with the highest tertile of estimated permethrin exposure 5.01, 2.41–10.42. | Systematic review | |
| Van Maele-Fabry, 2017 [90] | Residential, household, and domestic exposure to pesticides | Brain tumors | Associations were observed with CBT after combining all studies with exposure to non-agricultural pesticides 1.26, 1.13–1.40. | Meta-analysis | |
| Gunier, 2017 [91] | Parental occupational pesticide exposure from the year before pregnancy to the child's third year of life | ALL | USA | Increased risk of ALL for paternal occupational exposure to any pesticides 1.7, 1.2, 2.5, diagnosed before five years of age 2.3, 1.3, 4.1. | Case–control |
| Erjaee, 2017 [92] | Pesticides and other agents related to cancer | Childhood cancer | Iran | Association between the patients’ allergy [mostly food allergy] and obtaining a malignancy 2.09, 1.04–4.22. Parental smoking and risk of malignancy 1.56, 0.73–3.36. Living near high-voltage electricity lines 2.12, 1.17–3.84: Lymphoma [11.8%] osteosarcoma [9.8%], neuroblastoma [6.7%], and ALL [6.4%]. Contact with domestic animals [mostly hens, roosters, and sheep] 2.24, 1.43–3.50: adrenocortical tumors [75%], hepatoblastoma [50%], brain tumors [37%], rhabdomyosarcoma [28.6%], and ALL [28%]. Exposure to chemical pesticides and fertilizers 2.27, 1.26–4.08: adrenocortical tumors [50%], hepatoblastoma [40%], brain tumor [22%], and Ewing’s sarcoma [20%]. | Case–control |
| Rios, 2017 [93] | Maternal use of household pesiticides during pregnancy | Neuroblastoma in mother’s offspring | France | Maternal use of any type of pesticide during pregnancy was associated with neuroblastoma 1.5, 1.2–1.9, most commonly insecticides 1.4, 1.1–1.9 or with other pesticides 2.0, 1.1–3.4. | Case–control study |
| Omidakhsh, 2017 [94] | Parental pesticide exposure | Retinoblastoma | USA | Unilateral retinoblastoma associated with parental insecticide use 2.8, 1.1–6.7; professional lawn or landscape services 2.8, 1.0–8.2. | Case–control |
| Febvey, 2016 [95] | Parental pesticide exposure | CNS | France, Germany, and the UK | Around conception, paternal occupational pesticide exposure and CNS tumors 0.71, 0.53 to 0.95. | Case–control |
| Gómez-Barroso, 2016 [96] | Pesticides | Childhood cancer | Spain | Living in the proximity of cultivated land could be associated with many types of cancer. | Population-based case–control |
| Malagoli, 2016 [97] | Passive exposure to agricultural pesticides | Leukemia | Italy | Residing close to arable crops and leukemia 2.04, 0.50–8.35, and such excess risk was further enhanced among children aged <5 years. | Case–control |
| Chen, 2016 [98] | Pyrethoid metabolites | Brain tumors | China | trans-DCCA, 3-PBA, and total metabolites associated with risk of CBT 2.58, 1.38–4.80; 3-PBA 3.26, 1.73–6.14; total metabolites 3.60, 1.87–6.93. Exposure to both mosquitocide and cockroach killer was related to the increased risk of CBT [mosquitocide, 1.68, 1.06–2.67; cockroach killer 1.83, 1.13–2.95, respectively]. | Case–control |
| Chen, 2015 [99] | Residential childhood pesticide exposures and childhood cancers | [Leukemia and lymphoma] | Exposure to indoor residential insecticides was associated with risk of leukemia 1.47, 1.26–1.72, and lymphomas 1.43, 1.15–1.78. Leukemia was associated with herbicide exposure 1.26, 1.10–1.44. | Meta-analysis | |
| Maryam, 2015 [100] | Pesticides | Leukemia | Iran | Increased risk for farmers 14.7, 5.6–38.4 and for fathers of the pediatric cases 5.4, 3.0–9.9. | Case–control |
| Zhang, 2015 [101] | Household exposure to pesticides | Leukemia | China | The household use of mosquito repellent and AL 1.9, 1.2–3.1. | Case–control: 248 cases and 111 controls |
| Chen, 2015 [102] | Pesticides and other agents related to cancer | Leukemia | China | Chemical exposure during childhood 4.76, 1.34–16.89, maternal exposure to chemicals 4.51, 1.65–12.33, household insecticides use during 0–3 years of child 2.90, 1.31–6.39, and renovating after their children's birth 3.12, 1.26–7.74 were associated with an increased risk of AL | Case–control: 66 cases |
| Bailey, 2015 [103] | Pesticides | Leukemia | Childhood Leukemia International Consortium | ALL associated with any pesticide exposure shortly before conception, during pregnancy and after birth were 1.39, 1.25–1.55, 1.43, 1.32–1.54, and 1.36, 1.23–1.51, respectively. Risk of AML were 1.49, 1.02–2.16, 1.55, 1.21–1.99, and 1.08, 0.76–1.53, respectively. | Case–control |
| Zheng, R., 2015 [104] | Occupational exposure to pentachlorophenol | Lymphoma Leukemia | Lymphoma and workers' occupational exposing to PCP 2.57, 1.52–4.35. | Systematic review | |
| Kunkle, 2014 [105] | Farm-related pesticide exposures | Brain tumors. | CBT and farm-related exposures during pregnancy RR 1.48, 1.18–1.84. CBT and maternal exposure to non-agricultural pesticides [e.g., home extermination, pest strips] during pregnancy RR 1.36, 1.10–1.68, and risk of CBT and agricultural activities RR 1.32, 1.04–1.67. Paternal exposure to pesticides during preconception 2.29, 1.39–3.78. | Meta-analysis | |
| Kumar, 2014 [106] | Exposure to pesticides and other agents related to cancer especially during pregnancy | Leukemia | India | Leukemia with mother's education [p = 0.001], occupation [p = 0.0005], and pesticides exposure [p = 0.005] during pregnancy were found. However, there were no significant links with maternal age [p = 0.090], history of fetal loss [0.85], history of radiography during pregnancy [p = 0.400], history of drug intake [p = 0.689], and infection [p = 0.696] during pregnancy. | Case–control: 132 cases |
| Bailey, 2014 [107] | Parental occupational pesticide exposure | Leukemia | Maternal pesticide exposure during pregnancy and the risk of ALL was 1.01, 0.78–1.30, and for paternal exposure around conception 1.20, 1.06–1.38. For AML, for maternal exposure during pregnancy was 1.94, 1.19–3.18, and for paternal exposure around conception 0.91, 0.66–1.24. | 13 case–control studies | |
| Van Maele-Fabry, G., 2013 [108] | Parental occupational exposure to pesticides [mostly farm/agricultural jobs] | Brain tumors | Statistically significant association between parental occupational exposure to pesticides and brain tumors. | Meta-analysis | |
| Ferreira, 2013 [109] | Pesticide exposure during pregnancy | Leukemia | Brazil | Pesticides during pregnancy and ALL 2.10, 1.14–3.86, and AML 5.01, 1.97–12.7 in children 0–11 months of age, and with ALL 1.88, 1.05–5.23 at 12–23 months of age. Maternal exposure to permethrin, for children 0–11 months of age 2.47, 1.17–5.25 for ALL; and 7.28; 2.60–20.38 for AML. Maternal pesticide exposure related to agricultural activities 5.25, 1.83–15.08 for ALL, and 7.56, 1.83–31.23 for AML. | Case–control: mothers of 252 cases |
| Greenop, 2013 [110] | Exposure to pesticides before pregnancy, during pregnancy, and during childhood | Brain tumors | Australia | Professional pest control treatments in the home in the year before the index pregnancy, during the pregnancy, and after the child’s birth were 1.54, 1.07–2.22, 1.52, 0.99–2.34, and 1.04, 0.75–1.43, respectively. Treatments exclusively before pregnancy and during pregnancy were 1.90, 1.08–3.36, and 1.02, 0.35–3.00, respectively. Paternal occupational exposure in the year before the child’s conception was 1.36, 0.66–2.80. | Case–control: 374 cases |
| Metayer, 2013 [111] | Herbicides | ALL | USA | ALL and dust levels of chlorthal; for the first, second, and third tertiles were 1.49, 0.82–2.72, 1.49, 0.83–2.67, and 1.57, 0.90–2.73, respectively. | Case–control: 269 cases |
| Abdolah, 2013 [112] | Paternal occupational exposure to pesticides and other agents | Retinoblastoma | USA | Paternal pesticide exposure in the 10 years prior to conception 1.64, 1.08–2.50, as well as in the year before conception 2.12, 1.25–3.61. An increased risk was also observed for non-welding metal exposure during the 10 years prior to conception in the full 1.35, 0.86–2.12. | Case–control: 198 cases |
| Reference | Pollutant | Cancer | Population | Results OR 95% CI | Type of Study |
|---|---|---|---|---|---|
| Wimberly, 2023 [113] | Prenatal exposure to alcohol, tobacco, and illicit drugs | Childhood cancer | USA | Prenatal illicit drug associated intracranial embryonal tumors PR 1.94, 1.05–3.58, including medulloblastoma PR 1.82, and supratentorial primitive neuroectodermal tumors PR 2.66, and retinoblastoma PR 3.11, 1.20–8.08. Moderate to heavy alcohol consumption and non-Hodgkin’s lymphoma PR 5.94, 1.84–19.21. | Case-only: 3145 families |
| Xu, K., 2021 [114] | Prenatal tobacco smoke exposure | ALL | California | Association between DNA methylation at AHRR CpG cg05575921 and B-ALL [284 cases] with a ratio of means [RM] 1.31, 1.02–1.69. Polyepigenetic smoking score was positively associated with B-ALL [482 cases], RM 1.31, 1.09–1.57 | Case-only: 482 B ALL |
| Alyahya, M., 2020 [115] | Parental smoking behavior | Childhood cancer | Jordan | Women who had past exposure to smoke were more likely to have a child with cancer, 2.9 | Case–control: 200 cases |
| Frederiksen, 2020 [116] | Tobacco smoking | Leukemia | Costa Rica | Paternal smoking before conception, during pregnancy, and after birth was associated with an increased risk of AML 2.51, 1.21–5.17; 3.21, 1.56–6.60; and 2.83, 1.36–5.90, respectively. | Population-based case–control: 252 ALL and 40 AML |
| Rios, 2020 [80] | Parental habits in the perinatal period | Wilms tumor | France | Maternal use of any type of pesticide during pregnancy was associated with the risk of Wilms tumor 1.6, 1.1–2.3. Insecticides 1.7, 1.1–2.6. The association was stronger when they were used more often than once a month 1.9, 1.2–3.0. | Case–control: 117 cases |
| Doganis, D., 2020 [117] | Maternal lifestyle characteristics | Wilms tumor | Greece | Positive association 5.31, 2.00–14.10 was found for mothers who consumed alcohol only before pregnancy and Wilms tumor. | Systematic review and meta-analysis |
| Medina-Sanson, 2020 [118] | Tobacco smoking and pesticides | ALL | Mexico | Gene-environment interaction analysis showed that NAT2 rs1799929 TT genotype confers high risk to ALL under exposure to fertilizers, insecticides, hydrocarbon derivatives, and parental tobacco smoking. | Case–control: 478 cases |
| Cao, Y., 2020 [119] | Paternal smoking before conception and during pregnancy | ALL | RR for smoking before conception [RR = 1.15, 95% confidence interval: 1.04–1.27] and during pregnancy [RR = 1.20, 95% confidence interval: 1.12–1.28] | Systematic review and meta-analysis | |
| Chunxia, D., 2019 [120] | Tobacco smoke exposure | ALL, AML | Paternal smoking with ALL 1.15, 1.038–1.275. Paternal daily smoking and AML 1.242, 1.031–1.496. | Meta-analysis | |
| Rios, P., 2019 [121] | Parental smoking, and maternal alcohol consumption during pregnancy | Neuroblastoma | French | Both parents reported having smoked during pregnancy 1.5, 1.1–2.1. | Case–control: 357 cases |
| Kessous, R., 2019 [122] | Smoking during pregnancy | Childhood cancer | Israel | Maternal smoking during pregnancy and increased risk for benign tumors HR 2.5, 1.57–3.83. | Population-based cohort study |
| Milne, E., 2018 [123] | Maternal consumption of coffee and tea during pregnancy | ALL | Childhood Leukemia International Consortium | No association was seen with ‘any’ maternal coffee consumption during pregnancy and ALL | Case–control: 2553 cases for maternal coffee and 2982 cases for tea intake |
| J de Smith, A., 2017 [124] | Prenatal and early-life tobacco smoke | Leukemia | California | Deletions per case was positively associated with tobacco smoke exposure, in particular, for maternal ever-smoking RM 1.31, 1.08–1.59, maternal smoking during pregnancy 1.48, 1.12–1.94, and during breastfeeding RM 2.11, 1.48–3.02. The total number of deletions was also associated with DNA methylation at the AHRR epigenetic biomarker RM 1.32, 1.02–1.69. | Case-only: 559 cases |
| Tettamanti, G., 2016 [125] | Maternal smoking during pregnancy | Brain tumor | Sweden | Positive associations were found among 5–9-year-old children. In this age interval, maternal smoking during pregnancy and increased risk of brain tumors RR 1.50, 0.96–2.34, astrocytoma in male RR 2.00, 1.02–3.91, and female children RR 1.80, 0.85–3.82. | Population-based cohort: 1039 cases |
| Vienneau, D., 2016 [126] | Prenatal exposures | Brain tumors | Denmark, Sweden, Norway, and Switzerland | Little evidence of prenatal exposures and brain tumor. | Multinational case–control |
| Azary, S., 2016 [127] | Parental smoking and alcohol consumption | Retinoblastoma | USA | Maternal smoking before and during pregnancy contributed to unilateral retinoblastoma: year before pregnancy 8.9; 1.5–51; month before or during pregnancy 3.3, 0.5–20.8. | Case–control: 488 cases |
| Metayer, C., 2016 [128] | Parental tobacco smoking | AML | Smoking during pregnancy and ALL in Hispanics 2.08, 1.20–3.61. Paternal lifetime smoking 1.34, 1.11–1.62. | Meta-analysis | |
| Chu, 2016 [129] | Maternal smoking during pregnancy | Neuroblastoma | Possible association between maternal smoking during pregnancy and risk of neuroblastoma 1.28, 1.01–1.62. | Meta-analysis | |
| Orsi, L., 2015 [130] | Parental smoking, maternal alcohol, coffee, and tea consumption during pregnancy | Leukemia | France | Pre-conception paternal smoking and ALL 1.2, 1.1–1.5, and AML 1.5, 1.0–2.3. High consumption of coffee [>2 cups/day] was significantly associated with ALL 1.3, 1.0–1.8. | Case–control: 747 cases |
| Momen, 2015 [131] | Exposure to maternal smoking during pregnancy | Childhood cancer | Danish | Increased risk of cancer among children whose mothers reported smoking cessation in pregnancy 1.46, 1.01–2.10. | Prospective, national-registry-based |
| Mattioli, 2014 [132] | Tobacco smoke | Leukemia | Italy | Paternal smoke in the conception period was associated with ANLL for ≥11 cigarettes/day = 1.79, 1.01–3.15. | Case–control: 82 cases |
| Grufferman, S., 2014 [133] | Parental military service, Agent Orange exposure | Rhabdomyosarcoma | USA | Paternal exposure to AO and RMS 1.72, 0.55–5.41 | Case–control: 319 cases |
| Farioli, A., 2014 [134] | Tobacco smoke exposure | ALL | Italy | No association of parental active smoking and ALL | Large population-based case–control: 602 |
| Greenop, 2014 [135] | Maternal consumption of coffee and tea during pregnancy | Brain tumors | Australia | Among children aged under 5 years, any coffee consumption during pregnancy was 1.7, 1.09–2.84, and, for ≥2 cups per day during pregnancy, was 2.52, 1.26–5.04, and brain tumors. | Case–control: 293 cases |
| Huang Yi, 2014 [136] | Parental smoking | Brain tumors | Maternal smoking during pregnancy, paternal smoking during pregnancy, maternal smoking before pregnancy, and paternal smoking before pregnancy and brain tumors 0.96, 0.86–1.07, 1.09, 0.97–1.22, 0.93, 0.85–1.00, and 1.09, 1.00–1.20, respectively. | Systematic review and meta-analysis | |
| Barrington-Trimis, 2013 [137] | Parental smoking and functional polymorphisms in polycyclic aromatic hydrocarbon metabolism genes | Brain tumor | USA | A dose–response pattern for paternal smoking was observed among children with the EPHX1 H139R high-risk genotype 1.0; OR [≤3 h/day] = 1.32, 0.52–3.34; OR [>3 h/day] = 3.18, 0.92–11.0 and brain tumors. | Population-based case–control: 202 cases |
| Metayer, C., 2013 [138] | Tobacco smoke exposure | ALL AML | California | Paternal prenatal smoking combined with postnatal passive smoking and risk of ALL 1.01–2.23. This joint effect was seen for B-cell precursor ALL with t[12;21] 2.08. Similarly, child’s passive smoking was associated with an elevated risk of AML with chromosome structural changes 2.76, 1.01–7.58. | Case–control: 767 cases |
| Milne, E., 2013 [139] | Parental smoking | Brain tumors | Australia | No association of parental smoking before or during pregnancy with CBT risk. | Case–control: 302 cases |
| Reference | Pollutant | Cancer | Population | Results OR 95% CI | Type of Study |
|---|---|---|---|---|---|
| Rossides, M., 2022 [140] | Parents occupationally exposed to hydrocarbon solvents and engine exhaust fumes | Childhood cancer | Sweden | Maternal exposure was associated with non-Hodgkin’s lymphoma, germ cell tumors, astrocytoma, myeloid leukemia, lymphomas, and epithelial tumors. Paternal exposure associated with Hodgkin’s lymphoma. | Case–control: 9653 cases of maternal and 12,521 cases of paternal exposure |
| Zhang, 2021 [141] | Indoor volatile organic compounds exposures | Leukemia | Shanghai | Styrene 2.33, 1.07–5.07, and butyl alcohol 2.51, 1.19–5.28 with increased risk of AL | Case–control: 97 cases |
| Stayner, 2021 [142] | Exposure to nitrate from drinking water | Childhood cancer | Denmark | CNC and the highest category of nitrate exposure [>25 mg/L nitrate] was observed for preconception 1.82, 1.09–3.04, prenatal 1.65, 0.97–2.81, and postnatal exposure 1.48, 0.82–2.68. | Case–control: 596 cases |
| Hvidtfeldt, 2020 [143] | Air pollution exposure at the residence | Childhood cancer | Denmark | All cancers combined 0·97, 0·94–1·01 per 10 µg/m3 NO2, 0·89, 0·82–0·98 per 5 µg/m3 PM2.5, and 0·94, 0·88–1·01 per 1 µg/m3 BC. AP exposure and NHL 1·21, 0·94–1·55 per 10 µg/m3 NO2, 2·11, 1·10–4·01 per 5 µg/m3 PM2.5, and 1·68, 1·06–2·66 per 1 µg/m3 BC | Case–control: 5045 cases |
| Wang, 2019 [144] | Maternal prenatal exposure to pesticides and several environmental factors | ALL | China | Maternal prenatal exposure to interior housing renovation 2.98, 1.51–5.86 or pesticides 1.48, 1.67–2.28 and increased the risk of ALL. | Case–control: 345 cases |
| Raaschou-Nielsen, 2018 [145] | Ambient benzene at the residence | Leukemia, lymphoma, and CNS tumor | Denmark | Benzene and ALL, and AML 1.0, 0.6–1.7 and 1.9, 0.3–11.1 | Case–control: 1989 cases |
| Ghosh, S., 2018 [146] | Polychlorinated biphenyls | Childhood cancer | Slovakia | PCB exposures, even at the early age of these children, may have lifelong consequences for the future development of chronic diseases. | Cohort: 175 children |
| Eerjaee, 2017 [92] | Diverse environmental factors | Childhood cancers | Iran | Maternal oral contraceptive pill uses during pregnancy 1.85, 0.67–5.15; radiation during pregnancy 2.15, 0.74–4.61; parental smoking 1.56, 0.73–3.36; residence near high-voltage electricity lines 2.12, 1.17–3.84; pesticides and fertilizers 2.27, 1.26–4.08; patient allergy 2.09, 1.04–4.22; contact with domestic animals 2.24, 1.43–3.50 associated with cancer | Case–control: 300 cases |
| Park, S., 2017 [147] | Exposure to ambient dichloromethane in pregnancy | Childhood cancer | California | Dichloromethane in pregnancy and germ cell tumors 1.52, 1.11–2.08, particularly teratomas 2.08, 1.38–3.13, and possible increased risk for AML 1.64, 1.15–2.32 | Case–control: 13,636 cases |
| Whitehead, P., 2017 [148] | Home remodeling | Leukemia | Construction in the home between birth and diagnosis associated with ALL 1.52, 1.14–2.02 | Case- control. 609 ALL and 89 AML | |
| Metayer, C., 2016 [149] | Paretnal occupational exposure to organic solvents | Leukemia | California | Exposure to any organic solvents in Latino fathers and ALL 1.48, 1.01–2.16. In multivariable analyses, for chlorinated hydrocarbons was 2.28, 0.97–5.37. Combustion exhaust/polycyclic aromatic hydrocarbons [PAHs] and ALL 1.70, 1.16–2.57, and 1.46, 0.94–2.26 with and without adjustment for chlorinated hydrocarbons, respectively. | Case–control: 774 cases |
| Jiang, W.C., 2016 [150] | Paint, maternal chemical exposure during pregnancy, paternal diesel or gasoline exposure, paternal dye exposure, trash burning near the child's residence, benzene, and formaldehyde | ALL | China | Benzene exposure 1.09, 1.00–1.19, home painting in the past 10 years 3.56, 1.20–10.53, and paternal diesel or gasoline exposure 3.75, 1.06–13.22 were associated with increased risk of cALL. | Case–control: 71 cases |
| Bailey, D., 2015 [151] | Home paint exposures | Leukemia | France | Home paint exposure in the 1–3 months before conception and risk of ALL was 1.54, 1.28–1.85; for exposure in the year before conception, it was 1.00, 0.86–1.17. For exposure during pregnancy, was 1.14, 1.04–1.25, and for exposure after birth, was 1.22, 1.07–1.39. | Case–control: 3002 cases |
| Ward, H., 2014 [152] | Residential levels of polybrominated diphenyl ethers [PBDEs] | ALL | California | Homes in the highest PBDES concentration [nanograms per gram] and ALL risk. For PBDE-196 2.1, 1.1–3.8, PBDE-203 2.0, 1.1–3.6; PBDE-206 2.1, 1.1–3.9, and PBDE-207 2.0, 1.03–3.8 | Case–control: 167 cases |
| Gao Yu, 2014 [153] | Indoor air pollution | Leukemia | Shanghai | Higher concentrations of NO2 5.87, 2.25–15.30, benzene 2.56, 1.04–6.28, toluene 2.46, 1.02–5.93, styrene 4.39, 1.90–10.17, chloroform 30.00, 4.09–219.99, butyl alcohol 2.19, 1.03–4.66, methyl ethyl ketone 3.89, 1.55–9.78, and methyl isobutyl ketone 5.32, 1.61–17.58 were related to ALL | Case–control: 105 cases |
| Parodi, S., 2014 [154] | Perinatal exposures | Neuroblastoma | Italy | Exposure in pregnancy to chemical products for domestic work and to hair dye; the latter was higher among 0–17-month-old children 5.5, 1.0–29.3 and neuroblastoma. Mothers with exposure in the preconception period to solvents 2.0, 1.0–4.1, particularly to aromatic hydrocarbons 9.2, 2.4–34.3. A higher risk was found among children with congenital malformations 4.9, 1.8–13.6. | Case–control: 153 cases |
| Peters, S., 2014 [155] | Benzene, other aromatics, aliphatics, and chlorinated solvents in key time periods relative to the birth of their child | Brain tumors | Australia | Increased risk of CBT with maternal occupational exposures to chlorinated solvents 8.59, 0.94–78.9. Paternal exposure to solvents in the year before conception and CBT risk 1.55, 0.99–2.43. This increased risk appeared to be mainly attributable to exposure to aromatic solvents 2.72, 0.94–7.86, for benzene and 1.76, 1.10–2.82 for other aromatics compounds. | Case–control: 306 cases |
| Ruckart, P.Z., 2013 [156] | Water contaminated with trichloroethylene, tetrachloroethylene, benzene, vinyl chloride, and trans-1,2-dichloroethylene | Hematopoietic cancers | North Carolina, USA | Tetrachloroethylene exposure and any vinyl chloride exposure and hematopoietic cancers 1.69, 0.5–4.8, and 1.6, 0.5–4.7, respectively. | Case–control: 106 cases |
| Shi, 2013 [157] | Parental exposure to chemicals | Leukemia | China | Maternal exposure to total chemicals [diesel oil, gasoline, paints, insecticides, pesticides, herbicides, and chemical fertilizers] from 3 months before pregnancy to the end of pregnancy 2.9, 1.1–7.8, paternal exposure to insecticides 10.1, 1.2–82.9, and chemical fertilizers 9.5, 1.1–79.6 and leukemia | Case–control: 201 cases |
| Reference | Pollutant | Cancer | Population | Results OR 95% CI | Type of Study |
|---|---|---|---|---|---|
| Brabant, C., 2022 [158] | Magnetic fields | Leukemia | Leukemia and ELF-MF 1.26, 1.06–1.49. Magnetic flux density threshold associated with leukemia. Living within 50 m and 200 m of power lines were 1.11, 0.81–1.52 and 0.98, 0.85–1.12, respectively. Living within 50 m of power lines and ALL analyzed separately was 1.44, 0.72–2.88. Finally, the risk of leukemia was increased after exposure to electric blankets 2.75, 1.71–4.42 and, to a lesser extent, electric clocks 1.27, 1.01–1.60. | Systematic review meta-analysis | |
| Amoon, A., 2022 [159] | Magnetic fields | Leukemia | No increased risk of leukemia among children exposed to greater MF | Pooled analysis | |
| Seoumun, G., 2021 [160] | Extremely low-frequency magnetic fields | Childhood cancer | 0.2, 0.3, and 0.4 μT ELF-MFs had a 1.26, 1.06–1.49, 1.22, 0.93–1.61, and 1.72, 1.25–2.35-times higher odds of leukemia. In brain tumors, children exposed to 0.2 μT had a 0.95, 0.59–1.56-times higher odds, and those exposed to 0.4 μT ELF-MFs had a 1.25, 0.93–1.61. Children exposed to 0.2 and 0.4 μT ELF-MFs had a 1.10, 0.70–1.75 and 2.01, 0.89–4.52-times higher odds of any cancer. | Systematic review meta-analysis | |
| Núñez-Enríquez, 2020 [161] | Extremely low-frequency magnetic fields | ALL | Mexico | ELF-MF exposure as a continuous variable [per 0.2 μT intervals] was associated with B-ALL risk 1.06, 1.01–1.12. | Case–control: 290 cases |
| Crespi, C., 2019 [162] | Magnetic fields from power lines | Leukemia | California | No close proximity to high-voltage lines alone nor exposure to high calculated fields alone were associated with leukemia. Group that was both very close to high-voltage lines [<50 m] and had high calculated fields [≥0.4 μT] 4.06, 1.16–14.3. | Case–control |
| Auger, N., 2019 [163] | Residential exposure to electromagnetic fields | Childhood cancer | Canada | Residential proximity to transformer stations associated with cancer. Compared with 200 m, a distance of 80 m from a transformer station was associated with a hazard ratio of 1.08, 0.98–1.20 for any cancer, 1.04, 0.88–1.23 for hematopoietic cancer, and 1.11, 0.99–1.25 for solid tumors. | Cohort: 1114 cases |
| Talibov, M., 2019 [164] | Parental occupaitonal exposure to low-frequency magnetic fields | Leukemia | Did not find any associations between parental occupational ELF-MF exposure and leukemia. | Meta-analysis | |
| Amoon, A., 2018 [165] | Proximity to overhead power lines | Leukemia | No association between leukemia and distance to nearest overhead power line of any voltage. Among children living < 50 m from 200+ kV power lines, for leukemia observed 1.33, 0.92–1.93. | Pooled analysis | |
| Su, L., 2018 [166] | Parental occupational exposure to extremely low-frequency magnetic fields | CNS | Parental occupational ELF-MF exposure and increased risk of CNS tumors 1.11, 1.02–1.21. Increased risk of CNS tumors was associated with maternal 1.16, 1.06–1.26 but not paternal 1.15, 0.98–1.34 occupational ELF-MF exposure. | Meta-analysis | |
| Kheifets, L., 2017 [167] | Residential magnetic fields | Leukemia | California | Slight risk deficit in two intermediate exposure groups of RMF and a small excess risk in the highest exposure group 1.50, 0.70–3.23, associated with leukemia | Population-based case–control: 5788 cases |
| Su, L., 2016 [168] | Parental occupational exposure to extremely low-frequency magnetic fields | Leukemia | No maternal or paternal occupational exposure was associated with leukemia. | Meta-analysis | |
| Crespi, C., 2016 [169] | Distance from power lines | Leukemia | California | For leukemia, there was a slight excess of cases within 50 m of a transmission line over 200 kV, 1.4, 0.7–2.7. | Population-based case–control: 5788 cases |
| Bunch, K.J., 2016 [170] | High-voltage power lines, electromagnetic fields | Leukemia, CNS | UK | Elevated risks for childhood leukemia and overhead power lines may be higher for older age at diagnosis. | |
| Dechent, D., 2016 [171] | Electromagnetic fields | Leukemia | Iran | Exposure to high-voltage power lines 3.651, 1.692–7.878 and leukemia. | Case–control |
| Tabrizi, 2015 [172] | Electromagnetic field exposure | ALL | Tehran, Iran | Exposure to high-voltage power lines 3.651, 1.692–7.878 and ALL. | Case–control: 22 cases |
| Bunch, J., 2015 [173] | High-voltage underground cables | Childhood cancer | England | No indications of an association of risk with distance or of trend in risk with increasing magnetic field for leukemia, and no convincing pattern of risks for any other cancer. | Case–control: 52,525 cases |
| Pedersen, C., 2015 [174] | Extremely low-frequency magnetic fields | Childhood leukemia, CNS tumor, and lymphoma | Denmark | RR was 0.88, 0.32–2.42, and, for the total period [1968–2003], it was 1.63, 0.77–3.46 for leukemia, CNS tumour, and malignant lymphoma combined for exposures ≥0.4 μT compared with <0.1 μT. | Case–control: 3277 cases |
| Salvan, A., 2015 [175] | 50 Hz magnetic fields | Leukemia | Italy | Our results may be affected by several sources of bias, and they are noninformative at exposure levels >0.3 μT. | Case–control: 412 cases |
| Bunch, K.J., 2014 [176] | Distance at birth from overhead high-voltage powerlines | Childhood cancer | Britain | RR for leukemia, 0–199 m compared with >1000 m, all voltages: 1960s 4.50, 0.97–20.83, 2000s 0.71, 0.49–1.03. | Case–control: 53,515 children |
| Zhao, L., 2013 [177] | Magnetic fields | Leukemia | Positive association between magnetic field intensity ≥0.2 μT and leukemia 1.31, 1.06–1.61. For total leukemia: 1.57, 1.03–2.40; for ALL 2.43, 1.30–4.55. | 11,699 cases | |
| Pedersen, C., 2014 [178] | Distance from residence to power line | Leukemia | Denmark | Children living 0–199 m of a power line and being exposed to domestic radon >42 Bq/m3 and leukemia RR 2.88, 1.01–8.27. Children living 200–599 m from a power line and being exposed to domestic radon <42 Bq/m3 had a lower risk, 0.24, 0.07–0.83 | Case–control: 1698 cases |
| Sermage-Faure, C., 2013 [179] | High-voltage power lines | Leukemia | France | Living within 50 m of a VHV-HVOL and AL 1.7, 0.9–3.6 | Case–control: 2779 cases |
| Reference | Pollutant | Cancer | Population | Results OR 95% CI | Type of Study |
|---|---|---|---|---|---|
| Ngoc, 2022 [180] | Radon | Leukemia | Europe | Pooled OR 1.43, 1.19–1.72 | Systematic review and meta-analysis |
| Moon, J., 2021 [181] | Residential radon exposure | Leukemia | For case–control studies, pooled OR 1.0308, 1.0050–1.0573 increase for each 100 Bq/m3 of radon dose. The pooled OR 1.0361, 1.0014–1.0720 increase for each 100 Bq/m3 of radon dose for lymphoid leukemia subgroup. | Meta-analysis | |
| Lu, Y., 2020 [182] | Domestic radon exposure | Leukemia | Radon exposure and leukemia 1.22, 1.01–1.42. Cohort studies HR 0.97, 0.81–1.1. | Meta-analysis | |
| Nikkilä, A., 2019 [183] | Residential radon concentration | Leukemia | Finland | Radon and leukemia, second quartile 1.08, 0.77–1.50, third quartile 1.10, 0.79–1.53, and 1.29, 0.93–1.77 for the highest quartile. | Case–control |
| Chen, J., 2019 [184] | Domestic radon exposure | Leukemia and lymphoma | Canada | AML incidence in group of 5–9 years male with increased radon exposure R2 = 0.53, p = 0.026. For females, NHL incidence rates and average radon concentrations occurred in the 5–9 years age group R2 = 0.387, p = 0.041. | Population-based registry |
| Peckham, E., 2015 [185] | Residential radon exposure | Lymphoma | Texas | No evidence that residential radon exposure was positively associated with lymphoma overall, HL, or BL. Areas with radon concentrations >75th percentile had a marginal increase in DLBCL incidence [aIRR = 1.73, 95% CI: 1.03–2.91]. | Population-based: 2147 cases |
| Kollerud, R., 2014 [186] | High indoor radon concentrations | Leukemia and CNS tumors | Norway | Small increased risk of both leukemia and CNS among children under 1 year of age in the highest radon exposure group 1.26, 1.05–1.52, and similar for CNS alone 1.34, 1.04–1.73 | Cohort: 864 cases |
| Hauri, D., 2013 [187] | Domestic radon exposure | Childhood cancer | Switzerland | They did not find evidence that domestic radon exposure is associated with childhood cancer | Prospective census-based cohort: 997 cases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete-Meneses, M.d.P.; Salas-Labadía, C.; Gómez-Chávez, F.; Pérez-Vera, P. Environmental Pollution and Risk of Childhood Cancer: A Scoping Review of Evidence from the Last Decade. Int. J. Mol. Sci. 2024, 25, 3284. https://doi.org/10.3390/ijms25063284
Navarrete-Meneses MdP, Salas-Labadía C, Gómez-Chávez F, Pérez-Vera P. Environmental Pollution and Risk of Childhood Cancer: A Scoping Review of Evidence from the Last Decade. International Journal of Molecular Sciences. 2024; 25(6):3284. https://doi.org/10.3390/ijms25063284
Chicago/Turabian StyleNavarrete-Meneses, María del Pilar, Consuelo Salas-Labadía, Fernando Gómez-Chávez, and Patricia Pérez-Vera. 2024. "Environmental Pollution and Risk of Childhood Cancer: A Scoping Review of Evidence from the Last Decade" International Journal of Molecular Sciences 25, no. 6: 3284. https://doi.org/10.3390/ijms25063284
APA StyleNavarrete-Meneses, M. d. P., Salas-Labadía, C., Gómez-Chávez, F., & Pérez-Vera, P. (2024). Environmental Pollution and Risk of Childhood Cancer: A Scoping Review of Evidence from the Last Decade. International Journal of Molecular Sciences, 25(6), 3284. https://doi.org/10.3390/ijms25063284







