Changes in the Phenotype of Intramural Inhibitory Neurons of the Porcine Descending Colon Resulting from Glyphosate Administration
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a century herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Dill, G.M. Glyphosate-resistant crops: History, status and future. Pest Manag. Sci. 2005, 61, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Krüger, M.; Schledorn, P.; Schrödl, W.; Hoppe, H.W.; Lutz, W.; Shehata, A.A. Detection of glyphosate residues in animals and humans. J. Environ. Anal. Toxicol. 2014, 4, 2. [Google Scholar]
- Manservisi, F.; Lesseur, C.; Panzacchi, S.; Mandrioli, D.; Falcioni, L.; Bua, L.; Manservigi, M.; Spinaci, M.; Galeati, G.; Mantovani, A.; et al. The Ramazzini institute 13-week pilot study glyphosate-based herbicides administered at human-equivalent dose to Sprague Dawley rats: Effects on development and endocrine system. Environ. Health 2019, 18, 15. [Google Scholar] [CrossRef]
- Cattani, D.; de Liz Oliveira Cavalli, V.L.; Heinz-Rieg, C.L.; Domingues, J.T.; Dal-Cim, T.; Tasca, C.I.; Silva, F.R.M.; Zamoner, A. Mechanisms underlying the neurotoxicity induced by glyphosate-based herbicide in immature rat hippocampus: Involvement of glutamate excitotoxicity. Toxicology 2014, 320, 34–45. [Google Scholar] [CrossRef]
- Panza, S.B.; Vargas, R.; Balbo, S.L.; Bonfleur, M.L.; Granzotto, D.C.T.; Sant’Ana, D.M.G.; Nogueira-Melo, G.A. Perinatal exposure to low doses of glyphosate-based herbicide combined with a high-fat diet in adulthood causes changes in the jejunums of mice. Life Sci. 2021, 275, 119350. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef]
- Brewster, D.W.; Warren, J.; Hopkins, W.E. Metabolism of glyphosate in Sprague-Dawley rats: Tissue distribution, identification, and quantitation of glyphosate-derived materials following a single oral dose. Fundam. Appl. Toxicol. 1991, 17, 43–51. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Clerc, N.; Furness, J.B. Intrinsic primary afferent neurones of the digestive tract. Neurogastroenterol. Motil. 2004, 16, 24–27. [Google Scholar] [CrossRef]
- Nezami, B.G.; Srinivasan, S. Enteric Nervous System in the Small Intestine: Pathophysiology and Clinical Implications. Curr. Gastroenterol. Rep. 2010, 12, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The organisation of the autonomic nervous system: Peripheral connections. Auton. Neurosci. 2006, 130, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, V.P. Vasoactive intestinal polypeptide (VIP) as a cholinergic co-transmitter: Some recent results. Cell Biol. Int. Rep. 1989, 13, 1039–1051. [Google Scholar] [CrossRef]
- Sundler, F.; Ekblad, E.; Grunditz, T.; Håkanson, R.; Uddman, R. Vasoactive intestinal peptide in the peripheral nervous system. Ann. N. Y. Acad. Sci. 1988, 527, 143–167. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Całka, J. Changes in pituitary adenylate cyclase-activating Peptide 27-like immunoreactive nervous structures in the porcine descending colon during selected pathological processes. J. Mol. Neurosci. 2012, 48, 777–787. [Google Scholar] [CrossRef]
- Schleiffer, R.; Raul, F. Nitric oxide and the digestive system in mammals and nonmammalian vertebrates. Comp. Biochem. Physiol. 1997, 118A, 965–974. [Google Scholar] [CrossRef]
- Lang, R.; Gundlach, A.L.; Kofler, B. The galanin peptide family: Receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol. Ther. 2007, 115, 177–207. [Google Scholar] [CrossRef]
- Tang, Q.; Tang, J.; Ren, X.; Li, C. Glyphosate exposure induces inflammatory responses in the small intestine and alters gut microbial composition in rats. Environ. Pollut. 2020, 261, 114129. [Google Scholar] [CrossRef]
- Smith, A.C.; Swindle, M.M. Preparation of swine for the laboratory. ILAR J. 2006, 47, 358–363. [Google Scholar] [CrossRef]
- Swindle, M.M.; Smith, A.C. Comparative anatomy and physiology of the pig. Scand. J. Lab. Anim. Sci. 1998, 25, 11–21. [Google Scholar]
- Makowska, K.; Fagundes, K.R.C.; Gonkowski, S. Influence of bisphenol A and its analog bisphenol S on cocaine- and amphetamine-regulated transcript peptide-positive enteric neurons in the mouse gastrointestinal tract. Front. Mol. Neurosci. 2023, 16, 1234841. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Obremski, K.; Calka, J. The Influence of Low Doses of Zearalenone on Distribution of Selected Active Substances in Nerve Fibers within the Circular Muscle Layer of Porcine Ileum. J. Mol. Neurosci. 2015, 56, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Czajkowska, M.; Całka, J. Neurochemistry of Enteric Neurons Following Prolonged Indomethacin Administration in the Porcine Duodenum. Front. Pharmacol. 2020, 11, 564457. [Google Scholar] [CrossRef] [PubMed]
- Bulc, M.; Palus, K.; Dąbrowski, M.; Całka, J. Hyperglycaemia-induced downregulation in expression of nNOS intramural neurons of the small intestine in the pig. Int. J. Mol. Sci. 2019, 20, 1681. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Burliński, P.; Skobowiat, C.; Majewski, M.; Całka, J. Inflammation- and axotomy-induced changes in galanin-like immunoreactive (GAL-LI) nerve structures in the porcine descending colon. Acta Vet. Hung. 2010, 58, 91–103. [Google Scholar] [CrossRef]
- Palus, K.; Makowska, K.; Całka, J. Acrylamide-induced alterations in the cocaine-and amphetamine-regulated peptide transcript (CART)-like immunoreactivity within the enteric nervous system of the porcine small intestines. Ann. Anat. 2018, 219, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Reding, M.A. Evaluation of the Impact of Glyphosate Residues in Food on Human Health. Monsanto Brussels. Available online: https://ec.europa.eu/environment/archives/ppps/pdf/ma_reding_annex4.pdf (accessed on 17 May 2018).
- Horvath, G.; Opper, B.; Reglodi, D. The Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) is Protective in Inflammation and Oxidative Stress-Induced Damage in the Kidney. Int. J. Mol. Sci. 2019, 20, 4944. [Google Scholar] [CrossRef]
- Arciszewski, M.B.; Sand, E.; Ekblad, E. Vasoactive intestinal peptide rescues cultured rat myenteric neurons from lipopolysaccharide induced cell death. Regul. Pept. 2008, 146, 218–223. [Google Scholar] [CrossRef]
- Greenwood-Van Meerveld, B.; Johnson, A.C.; Grundy, D. Gastrointestinal Physiology and Function. Handb. Exp. Pharmacol. 2017, 239, 1–16. [Google Scholar]
- Sandgren, K.; Lin, Z.; Fex Svenningsen, A.; Ekblad, E. Vasoactive intestinal peptide and nitric oxide promote survival of adult rat myenteric neurons in culture. J. Neurosci. Res. 2003, 72, 595–602. [Google Scholar] [CrossRef]
- Jansen, M.I.; Broome, S.T.; Castorina, A. Targeting the neurological comorbidities of multiple sclerosis: The beneficial effects of VIP and PACAP neuropeptides. J. Integr. Neurosci. 2022, 21, 33. [Google Scholar] [CrossRef]
- Vaudry, D.; Pamantung, T.F.; Basille, M.; Rousselle, C.; Fournier, A.; Vaudry, H.; Beauvillain, J.C.; Gonzalez, B.J. PACAP protects cerebellar granule neurons against oxidative stress-induced apoptosis. Eur. J. Neurosci. 2002, 15, 1451–1460. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J.; Zielonka, Ł.; Nowicki, M. Diabetes affects the pituitary adenylate cyclase-activating polypeptide (PACAP)-like immunoreactive enteric neurons in the porcine digestive tract. Int. J. Mol. Sci. 2021, 22, 5727. [Google Scholar] [CrossRef]
- Shah, V.; Lyford, G.; Gores, G.; Farrugia, G. Nitric oxide in gastrointestinal health and disease. Gastroenterology 2004, 126, 903–913. [Google Scholar] [CrossRef]
- Rivera, L.R.; Poole, D.P.; Thacker, M.; Furness, J.B. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol. Motil. 2011, 23, 980–988. [Google Scholar] [CrossRef]
- Bulc, M.; Palus, K.; Zielonka, Ł.; Gajecka, M.; Całka, J. Changes in expression of inhibitory substances in the intramural neurons of the stomach following streptozotocin- induced diabetes in the pig. World J. Gastroenterol. 2017, 23, 6088–6099. [Google Scholar] [CrossRef]
- Palus, K.; Makowska, K.; Całka, J. Alterations in galanin-like immunoreactivity in the enteric nervous system of the porcine stomach following acrylamide supplementation. Int. J. Mol. Sci. 2019, 20, 3345. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Glyphosate affects the neurochemical phenotype of the intramural neurons in the duodenum in the pig. Neurogastroenterol. Motil. 2023, 35, e14507. [Google Scholar] [CrossRef]
- Vasiluk, L.; Pinto, L.J.; Moore, M.M. Oral bioavailability of glyphosate: Studies using two intestinal cell lines. Environ. Toxicol. Chem. 2005, 24, 153–160. [Google Scholar] [CrossRef]
- Ding, W.; Shangguan, Y.; Zhu, Y. Negative impacts of microcystin- LR and glyphosate on zebrafish intestine: Linked with gut microbiota and microRNAs? Environ. Pollut. 2021, 286, 117685. [Google Scholar] [CrossRef]
- Qiu, S.; Fu, H.; Zhou, R.; Yang, Z.; Bai, G.; Shi, B. Toxic effects of glyphosate on intestinal morphology, antioxidant capacity and barrier function in weaned piglets. Ecotoxicol. Environ. Saf. 2020, 187, 109846. [Google Scholar] [CrossRef]
- Martínez, M.A.; Ares, I.; Rodríguez, J.L.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A. Neurotransmitter changes in rat brain regions following glyphosate exposure. Environ. Res. 2018, 161, 212–219. [Google Scholar] [CrossRef]
- Larsen, K.; Najle, R.; Lifschitz, A.; Virkel, G. Effects of sub-lethal exposure of rats to the herbicide glyphosate in drinking water: Glutathione transferase enzyme activities, levels of reduced glutathione and lipid peroxidation in liver, kidneys and small intestine. Environ. Toxicol. Pharmacol. 2012, 34, 811–818. [Google Scholar] [CrossRef]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases III: Manganese, neurological diseases, and associated pathologies. Surg. Neurol. Int. 2015, 6, 45. [Google Scholar]


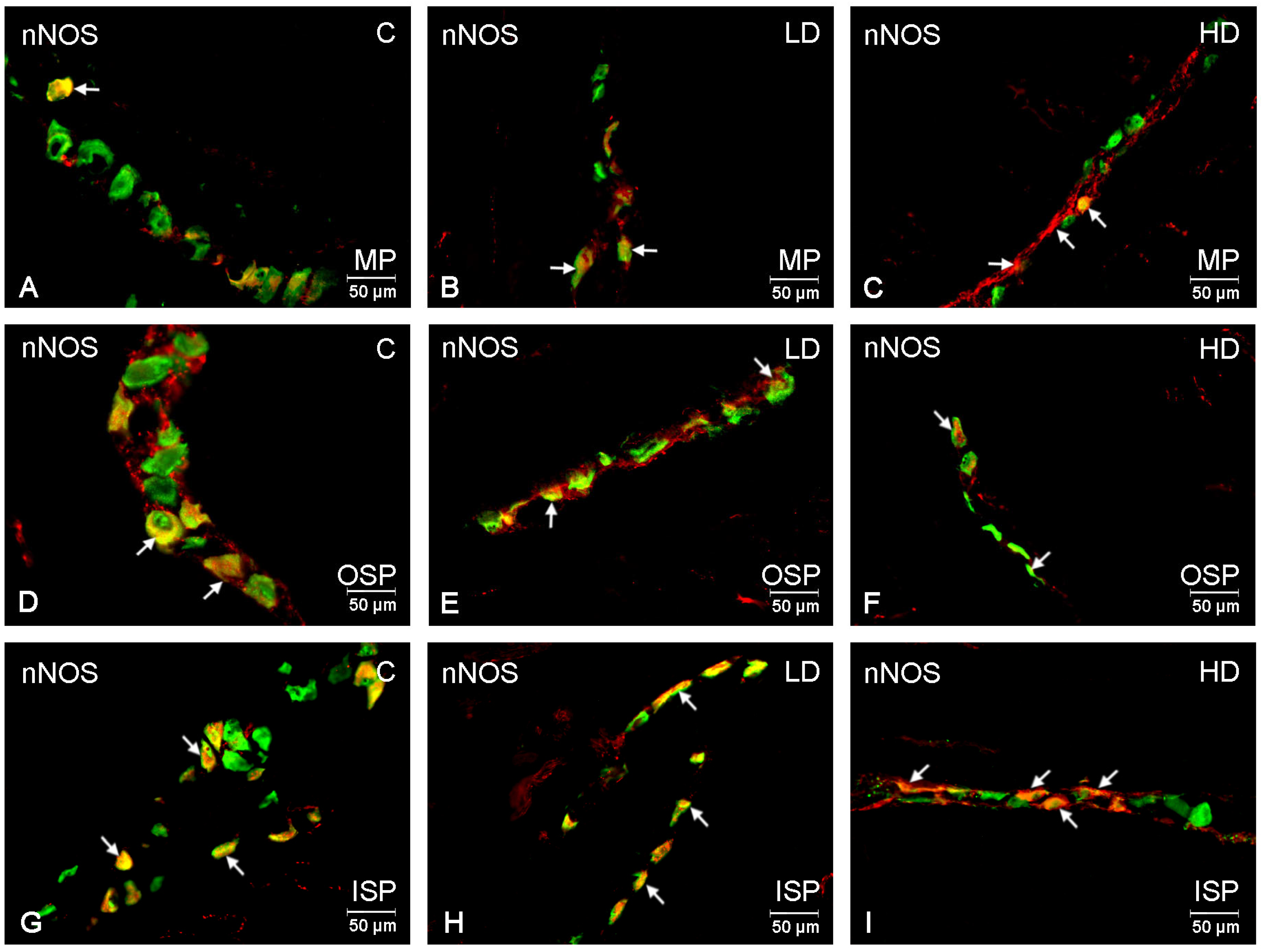
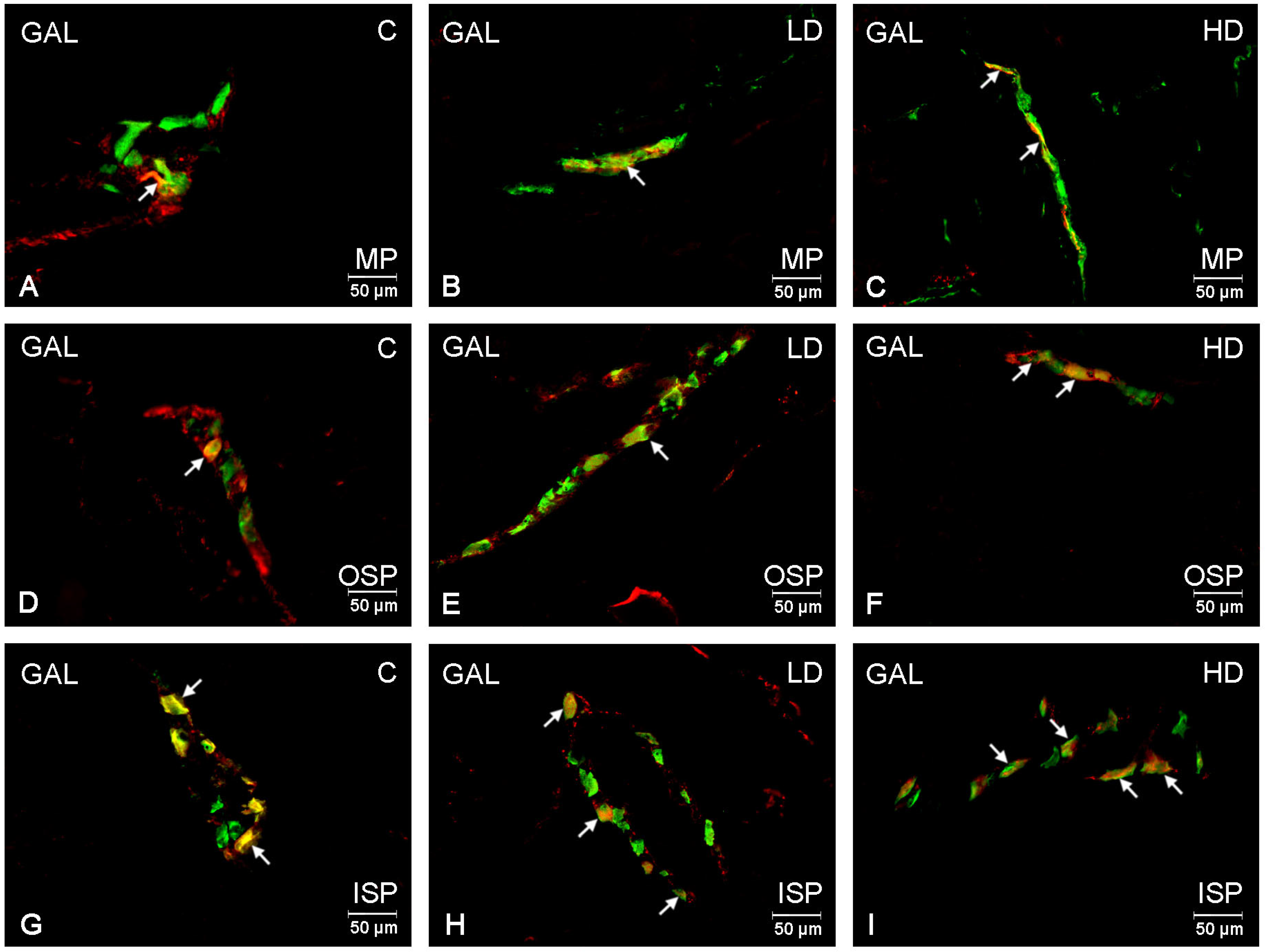
| Investigate Substance | Control Group | Low-Dose Group | High-Dose Group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MP | OSP | ISP | MP | OSP | ISP | MP | OSP | ISP | |
| VIP | 8.56 | 10.34 | 12.77 | 8.68 | 16.07 * | 17.83 * | 19.43 *** | 21.78 *** | 23.14 *** |
| ±SEM | 0.30 | 0.47 | 0.89 | 0.44 | 0.72 | 1.05 | 1.88 | 1.33 | 0.89 |
| PACAP | 9.83 | 7.54 | 6.56 | 9.50 | 12.34 ** | 6.49 | 15.88 ** | 22.98 *** | 13.60 ** |
| ±SEM | 0.23 | 0.56 | 0.84 | 0.88 | 1.56 | 0.93 | 1.57 | 2.04 | 0.99 |
| nNOS | 31.98 | 33.15 | 38.29 | 36.65 * | 31.98 | 39.01 | 44.78 ** | 40.14 * | 41.58 * |
| ±SEM | 1.54 | 1.96 | 1.80 | 2.06 | 1.74 | 2.55 | 2.12 | 1.95 | 2.77 |
| GAL | 7.12 | 5.87 | 9.74 | 6.60 | 5.56 | 13.99 * | 14.90 ** | 11.98 *** | 19.09 *** |
| ±SEM | 0.80 | 1.08 | 0.62 | 0.45 | 0.96 | 0.71 | 1.06 | 0.84 | 1.56 |
| Primary Antibodies | ||||
|---|---|---|---|---|
| Antigen | Host Species | Catalogue Number | Final Dilution | Supplier |
| Hu C/D | mouse | A-21271 | 1:1000 | Thermo Fisher Scientific. Waltham, MA, USA |
| VIP | rabbit | VA1285 | 1:6000 | Biomol, Hamburg, Germany |
| PACAP | guinea pig | T-5039 | 1:3000 | Peninsula, San Carlos, CA, USA |
| nNOS | rabbit | AB5380 | 1:2000 | Sigma-Aldrich, Saint Louis, MO, USA |
| GAL | rabbit | AB 2233 | 1:1000 | Millipore, Billerica, MA, USA |
| Secondary Antibodies | ||||
| Alexa Fluor 488 anti-mouse | donkey | A21202 | 1:1000 | Thermo Fisher Scientific. Waltham, MA, USA |
| Alexa Fluor 488 anti-guinea pig | donkey | A11074 | 1:1000 | Thermo Fisher Scientific. Waltham, MA, USA |
| Alexa Fluor 488 anti-rabbit | goat | A11010 | 1:1000 | Thermo Fisher Scientific. Waltham, MA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulc, M.; Całka, J.; Palus, K. Changes in the Phenotype of Intramural Inhibitory Neurons of the Porcine Descending Colon Resulting from Glyphosate Administration. Int. J. Mol. Sci. 2023, 24, 16998. https://doi.org/10.3390/ijms242316998
Bulc M, Całka J, Palus K. Changes in the Phenotype of Intramural Inhibitory Neurons of the Porcine Descending Colon Resulting from Glyphosate Administration. International Journal of Molecular Sciences. 2023; 24(23):16998. https://doi.org/10.3390/ijms242316998
Chicago/Turabian StyleBulc, Michał, Jarosław Całka, and Katarzyna Palus. 2023. "Changes in the Phenotype of Intramural Inhibitory Neurons of the Porcine Descending Colon Resulting from Glyphosate Administration" International Journal of Molecular Sciences 24, no. 23: 16998. https://doi.org/10.3390/ijms242316998






