The Renin–Angiotensin System and Cardiovascular–Kidney–Metabolic Syndrome: Focus on Early-Life Programming
Abstract
1. Introduction
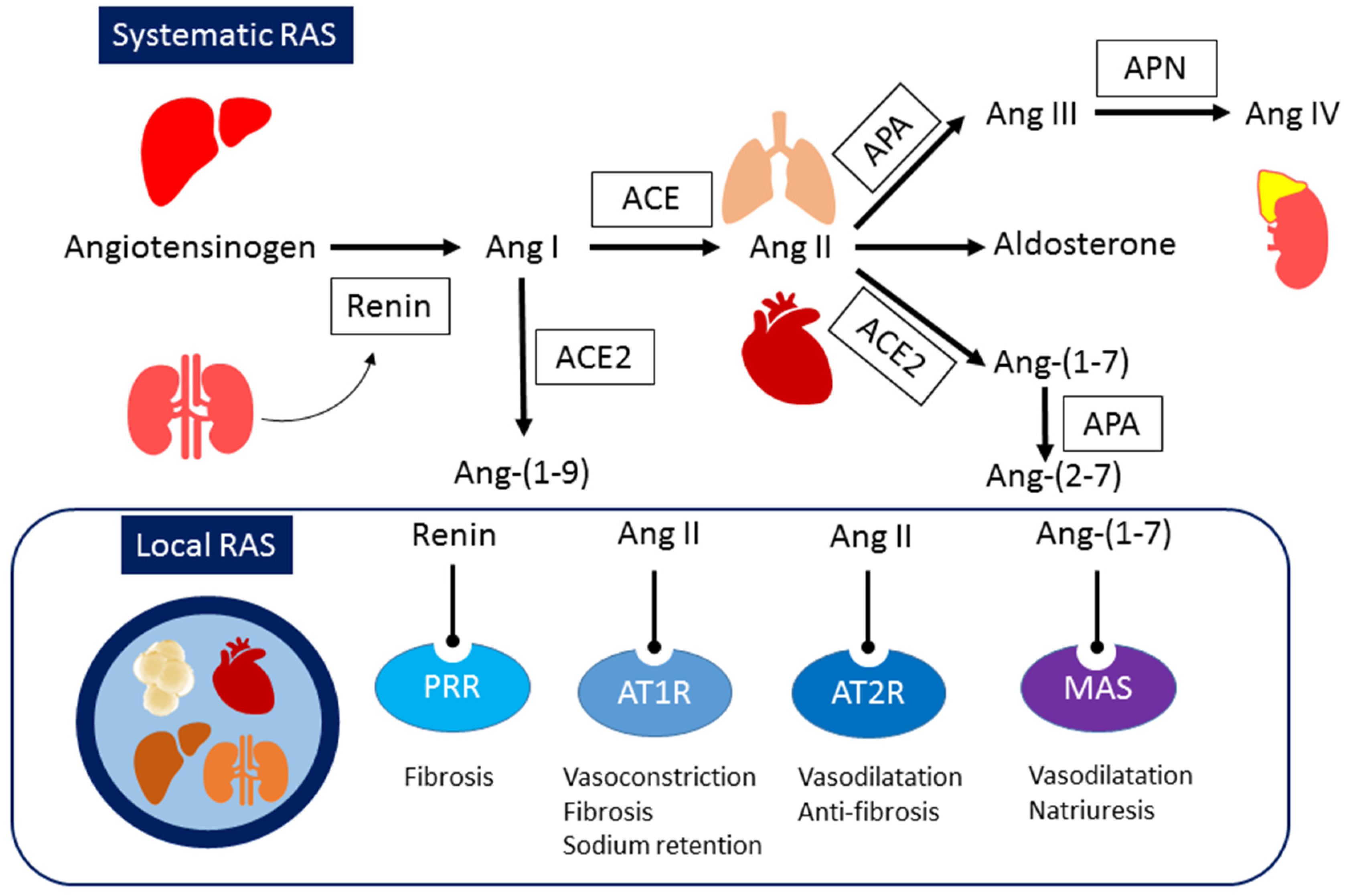
2. Systemic and Local RAS
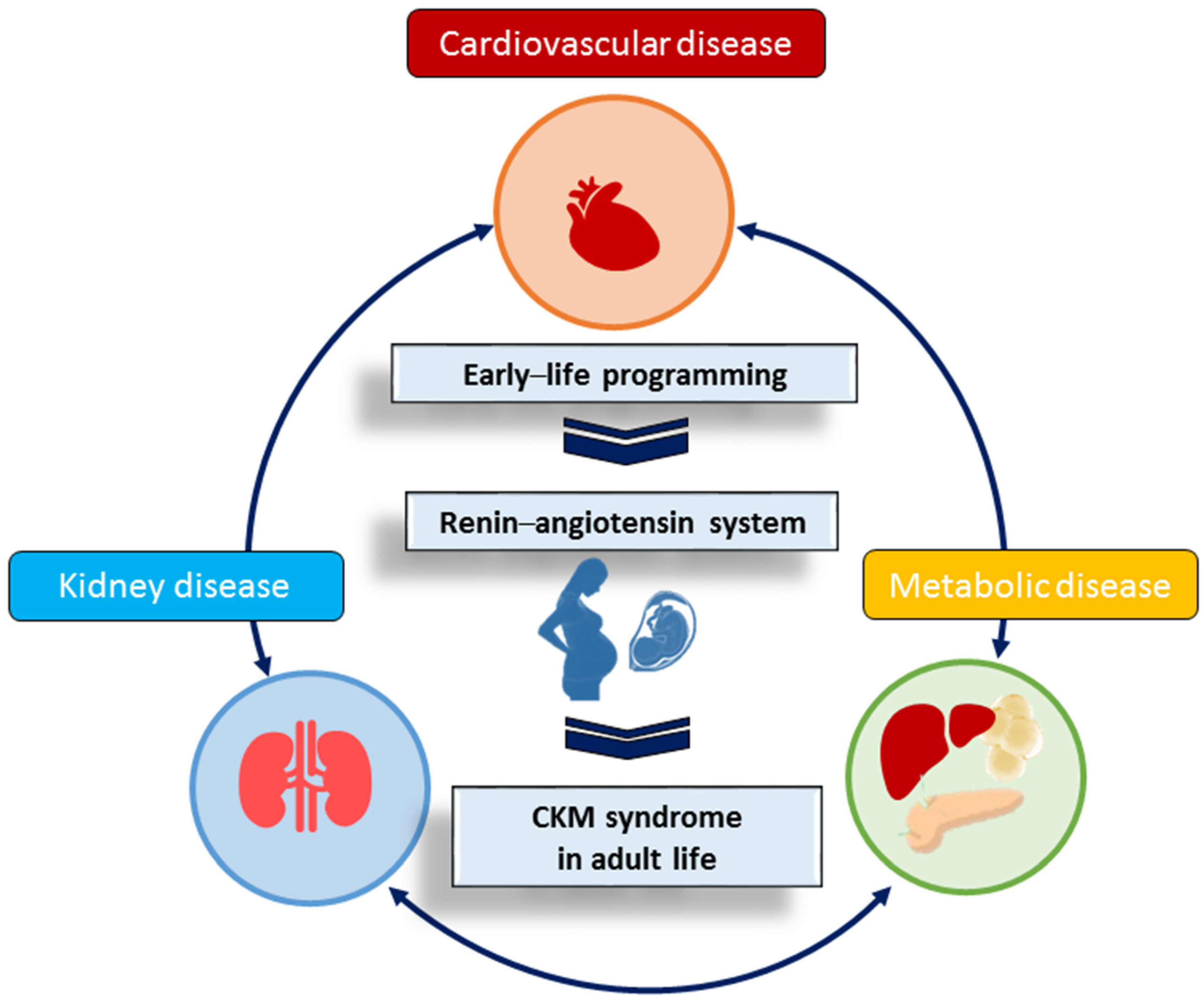
3. CKM Syndrome Is Causal of RAS Perturbation
3.1. Cardiovascular Disease and Hypertension
3.2. Kidney Disease
3.3. Obesity
3.4. Diabetes
3.5. Dyslipidemia and Fatty Liver
4. The RAS in Pregnancy
5. RAS-Related Programming in Animal Models
5.1. Maternal Nutritional Imbalance
5.2. Maternal Illnesses and Conditions
5.3. Drug and Chemical Exposures
6. Targeting the RAS as a Reprogramming Strategy
7. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory From the American Heart Association. Circulation 2023, 148, 1606–1635. [Google Scholar] [CrossRef]
- Jaradat, J.H.; Nashwan, A.J. Cardiovascular-kidney-metabolic syndrome: Understanding the interconnections and the need for holistic intervention. J. Med. Surg. Public Health 2023, 1, 100028. [Google Scholar] [CrossRef]
- Hanson, M.; Gluckman, P. Developmental origins of noncommunicable disease: Population and public health implications. Am. J. Clin. Nutr. 2011, 94, 1754S–1758S. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Fleming, T.P.; Velazquez, M.A.; Eckert, J.J. Embryos, DOHaD and David Barker. J. Dev. Orig. Health Dis. 2015, 6, 377–383. [Google Scholar] [CrossRef]
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- Chevalier, R.L. Evolution, kidney development, and chronic kidney disease. Semin. Cell Dev. Biol. 2019, 91, 119–131. [Google Scholar] [CrossRef]
- Arima, Y.; Fukuoka, H. Developmental origins of health and disease theory in cardiology. J. Cardiol. 2020, 76, 14–17. [Google Scholar] [CrossRef]
- Iturzaeta, A.; Sáenz Tejeira, M.M. Early programming of hypertension. Arch. Argent. Pediatr. 2022, 120, e8–e16. [Google Scholar]
- Saavedra, L.P.J.; Piovan, S.; Moreira, V.M.; Gonçalves, G.D.; Ferreira, A.R.O.; Ribeiro, M.V.G.; Peres, M.N.C.; Almeida, D.L.; Raposo, S.R.; da Silva, M.C.; et al. Epigenetic programming for obesity and noncommunicable disease: From womb to tomb. Rev. Endocr. Metab. Disord. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Tain, Y.L.; Joles, J.A. Reprogramming: A Preventive Strategy in Hypertension Focusing on the Kidney. Int. J. Mol. Sci. 2016, 17, 23. [Google Scholar] [CrossRef]
- Paauw, N.D.; van Rijn, B.B.; Lely, A.T.; Joles, J.A. Pregnancy as a critical window for blood pressure regulation in mother and child: Programming and reprogramming. Acta Physiol. 2017, 219, 241–259. [Google Scholar] [CrossRef]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef]
- Bagby, S.P. Maternal nutrition, low nephron number, and hypertension in later life: Pathways of nutritional programming. J. Nutr. 2007, 137, 1066–1072. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18, 841. [Google Scholar] [CrossRef]
- Goyal, D.; Limesand, S.W.; Goyal, R. Epigenetic responses and the developmental origins of health and disease. J. Endocrinol. 2019, 242, T105–T119. [Google Scholar] [CrossRef]
- Sarkar, A.; Yoo, J.Y.; Valeria Ozorio Dutra, S.; Morgan, K.H.; Groer, M. The Association between Early-Life Gut Microbiota and Long-Term Health and Diseases. J. Clin. Med. 2021, 10, 459. [Google Scholar] [CrossRef]
- Te Riet, L.; van Esch, J.H.; Roks, A.J.; van den Meiracker, A.H.; Danser, A.H. Hypertension: Renin-Angiotensin-aldosterone system alterations. Circ. Res. 2015, 116, 960–975. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-Angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef]
- Hall, J.E.; do Carmo, J.M.; da Silva, A.A.; Wang, Z.; Hall, M.E. Obesity, kidney dysfunction and hypertension: Mechanistic links. Nat. Rev. Nephrol. 2019, 15, 367–385. [Google Scholar] [CrossRef]
- Frigolet, M.E.; Torres, N.; Tovar, A.R. The renin-Angiotensin system in adipose tissue and its metabolic consequences during obesity. J. Nutr. Biochem. 2013, 24, 2003–2015. [Google Scholar] [CrossRef]
- Moreira de Macêdo, S.; Guimarães, T.A.; Feltenberger, J.D.; Sousa Santos, S.H. The role of renin-Angiotensin system modulation on treatment and prevention of liver diseases. Peptides 2014, 62, 189–196. [Google Scholar] [CrossRef]
- Ribeiro-Oliveira, A., Jr.; Nogueira, A.I.; Pereira, R.M.; Boas, W.W.; Dos Santos, R.A.; Simões e Silva, A.C. The renin-angiotensin system and diabetes: An update. Vasc. Health Risk Manag. 2008, 4, 787–803. [Google Scholar]
- Chappell, M.C.; Marshall, A.C.; Alzayadneh, E.M.; Shaltout, H.A.; Diz, D.I. Update on the Angiotensin converting enzyme 2Angiotensin (1-7)-MAS receptor axis: Fetal programing, sex differences, and intracellular pathways. Front. Endocrinol. 2014, 4, 201. [Google Scholar] [CrossRef]
- Cravedi, P.; Ruggenenti, P.; Remuzzi, G. Which antihypertensive drugs are the most nephroprotective and why? Expert Opin. Pharmacother. 2010, 11, 2651–2663. [Google Scholar] [CrossRef]
- Wysocki, J.; Wilsbacher, L.; Batlle, D. Angiotensins and the heart: Is Angiotensin-(1-7) cardioprotective? Hypertension 2015, 66, 260–262. [Google Scholar] [CrossRef]
- Rodrigues Prestes, T.R.; Rocha, N.P.; Miranda, A.S.; Teixeira, A.L.; Simoes-E-Silva, A.C. The Anti-Inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Curr. Drug Targets 2017, 18, 1301–1313. [Google Scholar] [CrossRef]
- Wu, Z.; Cappiello, M.G.; Scott, B.B.; Bukhtiyarov, Y.; McGeehan, G.M. Purification and characterization of recombinant human renin for X-ray crystallization studies. BMC Biochem. 2008, 9, 19. [Google Scholar] [CrossRef]
- Stanton, A. Potential of renin inhibition in cardiovascular disease. J. Renin-Angiotensin-Aldosterone Syst. 2003, 4, 6–10. [Google Scholar] [CrossRef]
- Brown, M.J. Renin: Friend or foe? Heart 2007, 93, 1026–1033. [Google Scholar] [CrossRef]
- Song, R.; Yosypiv, I.V. (Pro)renin Receptor in Kidney Development and Disease. Int. J. Nephrol. 2011, 2011, 247048. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Z.; Xiao, H.D.; Li, P.; Billet, S.; Lin, C.X.; Fuchs, S.; Bernstein, K.E. Tissue specific expression of Angiotensin converting enzyme: A new way to study an old friend. Int. Immunopharmacol. 2008, 8, 171–176. [Google Scholar] [CrossRef][Green Version]
- Navar, L.G.; Kobori, H.; Prieto, M.C.; Gonzalez-Villalobos, R.A. Intrarenal renin Angiotensin system in hypertension. Hypertension 2011, 57, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Yvan-Charvet, L.; Quignard-Boulangé, A. Role of adipose tissue renin-Angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 2011, 79, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Schwacke, J.H.; Spainhour, J.C.; Ierardi, J.L.; Chaves, J.M.; Arthur, J.M.; Janech, M.G.; Velez, J.C. Network modeling reveals steps in Angiotensin peptide processing. Hypertension 2013, 61, 690–700. [Google Scholar] [CrossRef][Green Version]
- Donoghue, M.; Hsieh, F.; Baronas, E.; Godbout, K.; Gosselin, M.; Stagliano, N.; Donovan, M.; Woolf, B.; Robison, K.; Jeyaseelan, R.; et al. A novel Angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts Angiotensin I to Angiotensin 1–9. Circ. Res. 2000, 87, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.J. Clinical relevance of local Renin Angiotensin systems. Front. Endocrinol. 2014, 5, 113. [Google Scholar] [CrossRef]
- Borghi, F.; Sevá-Pessôa, B.; Grassi-Kassisse, D.M. The adipose tissue and the involvement of the renin-angiotensin-aldosterone system in cardiometabolic syndrome. Cell Tissue Res. 2016, 366, 543–548. [Google Scholar] [CrossRef]
- Bérard, E.; Niel, O.; Rubio, A. Is the renin-Angiotensin system actually hypertensive? Pediatr. Nephrol. 2014, 29, 951–960. [Google Scholar] [CrossRef]
- Esper, R.J.; Nordaby, R.A.; Vilariño, J.O.; Paragano, A.; Cacharrón, J.L.; Machado, R.A. Endothelial dysfunction: A comprehensive appraisal. Cardiovasc. Diabetol. 2006, 5, 4. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Versari, D.; Daghini, E.; Virdis, A.; Ghiadoni, L.; Taddei, S. Endothelium-dependent contractions and endothelial dysfunction in human hypertension. Br. J. Pharmacol. 2009, 157, 527–536. [Google Scholar] [CrossRef]
- Dharmashankar, K.; Widlansky, M.E. Vascular endothelial function and hypertension: Insights and directions. Curr. Hypertens. Rep. 2010, 12, 448–455. [Google Scholar] [CrossRef]
- Hennrikus, M.; Gonzalez, A.A.; Prieto, M.C. The prorenin receptor in the cardiovascular system and beyond. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H139–H145. [Google Scholar] [CrossRef]
- Nguyen, G. Renin, (pro)renin and receptor: An update. Clin. Sci. 2011, 120, 169–178. [Google Scholar] [CrossRef]
- Xiong, J.; Dong, X.; Li, S.; Jiang, F.; Chen, J.; Yu, S.; Dong, B.; Su, Q. Effects of (Pro)renin Receptor on Diabetic Cardiomyopathy Pathological Processes in Rats via the PRR-AMPK-YAP Pathway. Front. Physiol. 2021, 12, 657378. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, K.; Ichihara, A.; Sano, M.; Sun-Wada, G.H.; Wada, Y.; Kurauchi-Mito, A.; Bokuda, K.; Narita, T.; Oshima, Y.; Sakoda, M.; et al. The (pro)renin receptor/ATP6AP2 is essential for vacuolar H+-ATPase assembly in murine cardiomyocytes. Circ. Res. 2010, 107, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, C.; Xiong, J.; Yu, J. Cardiovascular aspects of the (pro)renin receptor: Function and significance. FASEB J. 2022, 36, e22237. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J. Angiotensin II-dependent superoxide: Effects on hypertension and vascular dysfunction. Hypertension 2008, 52, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-Angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Gubler, M.C.; Antignac, C. Renin-Angiotensin system in kidney development: Renal tubular dysgenesis. Kidney Int. 2010, 77, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Yosypiv, I.V. Renin-Angiotensin system in ureteric bud branching morphogenesis: Insights into the mechanisms. Pediatr. Nephrol. 2011, 26, 1499–1512. [Google Scholar] [CrossRef] [PubMed]
- Schreuder, M.F.; Bueters, R.R.; Huigen, M.C.; Russel, F.G.; Masereeuw, R.; van den Heuvel, L.P. Effect of drugs on renal development. Clin. J. Am. Soc. Nephrol. 2011, 6, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Okubo, S.; Niimura, F.; Matsusaka, T.; Fogo, A.; Hogan, B.L.; Ichikawa, I. Angiotensinogen gene null-mutant mice lack homeostatic regulation of glomerular filtration and tubular reabsorption. Kidney Int. 1998, 53, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Matsusaka, T.; Chen, X.; Okubo, S.; Niimura, F.; Nishimura, H.; Fogo, A.; Utsunomiya, H.; Inagami, T.; Ichikawa, I. Murine double nullizygotes of the Angiotensin type 1A and 1B receptor genes duplicate severe abnormal phenotypes of Angiotensinogen nullizygotes. J. Clin. Investig. 1998, 101, 755–760. [Google Scholar] [CrossRef]
- Woods, L.L.; Rasch, R. Perinatal ANG II programs adult blood pressure, glomerular number and renal function in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1998, 275, R1593–R1599. [Google Scholar] [CrossRef]
- Lai, K.N.; Leung, J.C.; Lai, K.B.; To, W.Y.; Yeung, V.T.; Lai, F.M. Gene expression of the renin-Angiotensin system in human kidney. J. Hypertens. 1998, 16, 91–102. [Google Scholar] [CrossRef]
- Konoshita, T.; Wakahara, S.; Mizuno, S.; Motomura, M.; Aoyama, C.; Makino, Y.; Kawai, Y.; Kato, N.; Koni, I.; Miyamori, I.; et al. Tissue gene expression of renin-angiotensin system in human type 2 diabetic nephropathy. Diabetes Care 2006, 29, 848–852. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.K.; Leehey, D.J. A novel mechanism for Angiotensin II formation in streptozotocin-diabetic rat glomeruli. Am. J. Physiol. Renal Physiol. 2005, 288, F1183–F1190. [Google Scholar] [CrossRef]
- Sasser, J.M.; Moningka, N.C.; Tsarova, T.; Baylis, C. Nebivolol does not protect against 5/6 ablation/infarction induced chronic kidney disease in rats -comparison with Angiotensin II receptor blockade. Life Sci. 2012, 91, 54–63. [Google Scholar] [CrossRef][Green Version]
- Tain, Y.L.; Yang, H.W.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Anti-Hypertensive Property of an NO Nanoparticle in an Adenine-Induced Chronic Kidney Disease Young Rat Model. Antioxidants 2023, 12, 513. [Google Scholar] [CrossRef] [PubMed]
- Mulrow, P.J. The intrarenal renin-Angiotensin system. Curr. Opin. Nephrol. Hypertens. 1993, 2, 41–44. [Google Scholar] [CrossRef] [PubMed]
- AlQudah, M.; Hale, T.M.; Czubryt, M.P. Targeting the renin-Angiotensin-aldosterone system in fibrosis. Matrix Biol. 2020, 91–92, 92–108. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Lamas, S.; Ortiz, A. Antifibrotic Agents for the Management of CKD: A Review. Am. J. Kidney Dis. 2022, 80, 251–263. [Google Scholar] [CrossRef]
- Kalupahana, N.S.; Moustaid-Moussa, N. The adipose tissue renin-Angiotensin system and metabolic disorders: A review of molecular mechanisms. Crit. Rev. Biochem. Mol. Biol. 2012, 47, 379–390. [Google Scholar] [CrossRef]
- Rüster, C.; Wolf, G. The role of the renin-Angiotensin-aldosterone system in obesity-related renal diseases. Semin. Nephrol. 2013, 33, 44–53. [Google Scholar] [CrossRef]
- Lelis, D.F.; Freitas, D.F.; Machado, A.S.; Crespo, T.S.; Santos, S.H.S. Angiotensin-(1-7), Adipokines and Inflammation. Metabolism 2019, 95, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Rein, J.; Bader, M. Renin-Angiotensin System in Diabetes. Protein Pept. Lett. 2017, 24, 833–840. [Google Scholar] [CrossRef]
- Tikellis, C.; Cooper, M.E.; Thomas, M.C. Role of the renin-Angiotensin system in the endocrine pancreas: Implications for the development of diabetes. Int. J. Biochem. Cell Biol. 2006, 38, 737–751. [Google Scholar] [CrossRef]
- Kamper, M.; Tsimpoukidi, O.; Chatzigeorgiou, A.; Lymberi, M.; Kamper, E.F. The antioxidant effect of Angiotensin II receptor blocker, losartan, in streptozotocin-induced diabetic rats. Transl. Res. 2010, 156, 26–36. [Google Scholar] [CrossRef]
- Yuan, L.; Li, X.; Xu, G.L.; Qi, C.J. Effects of renin-Angiotensin system blockade on islet function in diabetic rats. J. Endocrinol. Investig. 2010, 33, 13–19. [Google Scholar] [CrossRef]
- Shao, C.; Yu, L.; Gao, L. Activation of Angiotensin type 2 receptors partially ameliorates streptozotocin-induced diabetes in male rats by islet protection. Endocrinology 2014, 155, 793–804. [Google Scholar] [CrossRef]
- Putnam, K.; Shoemaker, R.; Yiannikouris, F.; Cassis, L.A. The renin-Angiotensin system: A target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1219–H1230. [Google Scholar] [CrossRef]
- Golovchenko, I.; Goalstone, M.L.; Watson, P.; Brownlee, M.; Draznin, B. Hyperinsulinemia enhances transcriptional activity of nuclear factor-kappaB induced by Angiotensin II, hyperglycemia, and advanced glycosylation end products in vascular smooth muscle cells. Circ. Res. 2000, 87, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Jung, O.; Strehlow, K.; Zolk, O.; Linz, W.; Scholkens, B.A.; Bohm, M. Hypercholesterolemia is associated with enhanced Angiotensin AT1-receptor expression. Am. J. Physiol. Heart Circ. Physiol. 1997, 272, H2701–H2707. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Wu, P.S.; Lin, H.C. Pathogenesis and treatment of non-alcoholic steatohepatitis and its fibrosis. Clin. Mol. Hepatol. 2023, 29, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Borém, L.M.A.; Neto, J.F.R.; Brandi, I.V.; Lelis, D.F.; Santos, S.H.S. The role of the Angiotensin II type I receptor blocker telmisartan in the treatment of non-alcoholic fatty liver disease: A brief review. Hypertens. Res. 2018, 41, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.R.V.; Costa, L.B.; Ferreira, G.C.; Ferreira, A.M.; Reis, F.M.; Simões, E.; Silva, A.C. Renin-Angiotensin system in normal pregnancy and in preeclampsia: A comprehensive review. Pregnancy Hypertens. 2022, 28, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Yart, L.; Roset Bahmanyar, E.; Cohen, M.; Martinez de Tejada, B. Role of the uteroplacental renin-Angiotensin system in placental development and function, and its implication in the preeclampsia pathogenesis. Biomedicines 2021, 9, 1332. [Google Scholar] [CrossRef] [PubMed]
- Tamanna, S.; Lumbers, E.R.; Morosin, S.K.; Delforce, S.J.; Pringle, K.G. ACE2: A key modulator of the renin-angiotensin system and pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R833–R843. [Google Scholar] [CrossRef]
- Morosin, S.K.; Lochrin, A.J.; Delforce, S.J.; Lumbers, E.R.; Pringle, K.G. The (pro)renin receptor ((P)RR) and soluble (pro)renin receptor (s(P)RR) in pregnancy. Placenta 2021, 116, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Grigore, D.; Ojeda, N.B.; Robertson, E.B.; Dawson, A.S.; Huffman, C.A.; Bourassa, E.A.; Speth, R.C.; Brosnihan, K.B.; Alexander, B.T. Placental insufficiency results in temporal alterations in the renin Angiotensin system in male hypertensive growth restricted offspring. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R804–R811. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Bertagnolli, M. Preterm Birth and Renin-Angiotensin-Aldosterone System: Evidences of Activation and Impact on Chronic Cardiovascular Disease Risks. Protein Pept. Lett. 2017, 24, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Li, J.; Wang, Z.N.; Reichetzeder, C.; Xu, H.; Gong, J.; Chen, G.J.; Pfab, T.; Xiao, X.M.; Hocher, B. Renin Angiotensin aldosterone system and glycemia in pregnancy. Clin. Lab. 2012, 58, 527–533. [Google Scholar] [PubMed]
- Świątkowska-Stodulska, R.; Kmieć, P.; Stefańska, K.; Sworczakm, K. Renin-Angiotensin-Aldosterone System in the Pathogenesis of Pregnancy-Induced Hypertension. Exp. Clin. Endocrinol. Diabetes 2018, 126, 362–366. [Google Scholar] [CrossRef]
- Merrill, D.; Karoly, M.; Chen, K.; Ferrario, C.; Brosnihan, K.B. Angiotensin-(1-7) in normal and preeclamptic pregnancy. Endocrine 2002, 18, 239–245. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lu, Y.P.; Li, J.; Liu, Z.W.; Chen, W.J.; Liang, X.J. Fetal and maternal Angiotensin (1-7) are associated with preterm birth. J. Hypertens. 2014, 32, 1833–1841. [Google Scholar] [CrossRef]
- Nogueira, A.I.; Souza Santos, R.A.; Simões e Silva, A.C.; Cabral, A.C.V.; Vieira, R.L.P.; Drumond, T.C. The pregnancy-induced increase of plasma Angiotensin-(1-7) is blunted in gestational diabetes. Regul. Pept. 2007, 141, 55–60. [Google Scholar] [CrossRef]
- Alexander, B.T.; South, A.M.; August, P.; Bertagnolli, M.; Ferranti, E.P.; Grobe, J.L.; Jones, E.J.; Loria, A.S.; Safdar, B.; Sequeira-Lopez, M.L.S.; et al. Appraising the Preclinical Evidence of the Role of the Renin-Angiotensin-Aldosterone System in Antenatal Programming of Maternal and Offspring Cardiovascular Health Across the Life Course: Moving the Field Forward: A Scientific Statement From the American Heart Association. Hypertension 2023, 80, e75–e89. [Google Scholar]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal L-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Franco Mdo, C.; Ponzio, B.F.; Gomes, G.N.; Gil, F.Z.; Tostes, R.; Carvalho, M.H.; Fortes, Z.B. Micronutrient prenatal supplementation prevents the development of hypertension and vascular endothelial damage induced by intrauterine malnutrition. Life Sci. 2009, 85, 327–333. [Google Scholar] [CrossRef]
- Holemans, K.; Verhaeghe, J.; Dequeker, J.; Van Assche, F.A. Insulin sensitivity in adult female rats subjected to malnutrition during the perinatal period. J. Soc. Gynecol. Investig. 1996, 3, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, S.E.; Smith, G.D.; Tikerpae, J.; Hales, C.N. Altered regulation of hepatic glucose output in the male offspring of protein-malnourished rat dams. Am. J. Physiol. 1996, 270, E559–E564. [Google Scholar] [CrossRef] [PubMed]
- Sahajpal, V.; Ashton, N. Increased glomerular Angiotensin II binding in rats exposed to a maternal low protein diet in utero. J. Physiol. 2005, 563, 193–201. [Google Scholar] [CrossRef]
- Cambonie, G.; Comte, B.; Yzydorczyk, C.; Ntimbane, T.; Germain, N.; Lê, N.L.; Pladys, P.; Gauthier, C.; Lahaie, I.; Abran, D.; et al. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1236–R1245. [Google Scholar] [CrossRef] [PubMed]
- de Bem, G.F.; da Costa, C.A.; de Oliveira, P.R.; Cordeiro, V.S.; Santos, I.B.; de Carvalho, L.C.; Souza, M.A.; Ognibene, D.T.; Daleprane, J.B.; Sousa, P.J.; et al. Protective effect of Euterpe oleracea Mart (açaí) extract on programmed changes in the adult rat offspring caused by maternal protein restriction during pregnancy. J. Pharm. Pharmacol. 2014, 66, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Ching, R.H.; Yeung, L.O.; Tse, I.M.; Sit, W.H.; Li, E.T. Supplementation of bitter melon to rats fed a high-fructose diet during gestation and lactation ameliorates fructose-induced dyslipidemia and hepatic oxidative stress in male offspring. J. Nutr. 2011, 141, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.F.; Dickerson, J.; Kechichian, T.B.; Yin, H.; Gamble, P.; Salazar, A.; Patrikeev, I.; Motamedi, M.; Saade, G.R.; Costantine, M.M. High-fructose diet in pregnancy leads to fetal programming of hypertension, insulin resistance, and obesity in adult offspring. Am. J. Obstet. Gynecol. 2016, 215, 378.e1–378.e6. [Google Scholar] [CrossRef]
- Hsu, C.N.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y.; Tain, Y.L. Aliskiren Administration during Early Postnatal Life Sex-Specifically Alleviates Hypertension Programmed by Maternal High Fructose Consumption. Front. Physiol. 2016, 7, 299. [Google Scholar] [CrossRef]
- Seong, H.Y.; Cho, H.M.; Kim, M.; Kim, I. Maternal High-Fructose Intake Induces Multigenerational Activation of the Renin-Angiotensin-Aldosterone System. Hypertension 2019, 74, 518–525. [Google Scholar] [CrossRef]
- Wu, K.L.; Wu, C.W.; Tain, Y.L.; Chao, Y.M.; Hung, C.Y.; Tsai, P.C.; Wang, W.S.; Shih, C.D. Effects of high fructose intake on the development of hypertension in the spontaneously hypertensive rats: The role of AT1R/gp91PHOX signaling in the rostral ventrolateral medulla. J. Nutr. Biochem. 2017, 41, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Hou, C.Y.; Chan, J.Y.H.; Lee, C.T.; Tain, Y.L. Hypertension Programmed by Perinatal High-Fat Diet: Effect of Maternal Gut Microbiota-Targeted Therapy. Nutrients 2019, 11, 2908. [Google Scholar] [CrossRef]
- Tain, Y.L.; Lin, Y.J.; Sheen, J.M.; Lin, I.C.; Yu, H.R.; Huang, L.T.; Hsu, C.N. Resveratrol prevents the combined maternal plus postweaning high-fat-diets-induced hypertension in male offspring. J. Nutr. Biochem. 2017, 48, 120–127. [Google Scholar] [CrossRef]
- Tsai, T.A.; Tsai, C.K.; Huang, L.T.; Sheen, J.M.; Tiao, M.M.; Tain, Y.L.; Chen, C.C.; Lin, I.C.; Lai, Y.J.; Tsai, C.C.; et al. Maternal Resveratrol Treatment Re-Programs and Maternal High-Fat Diet-Induced Retroperitoneal Adiposity in Male Offspring. Int. J. Environ. Res. Public Health 2020, 17, 2780. [Google Scholar] [CrossRef]
- Sheen, J.M.; Yu, H.R.; Tain, Y.L.; Tsai, W.L.; Tiao, M.M.; Lin, I.C.; Tsai, C.C.; Lin, Y.J.; Huang, L.T. Combined maternal and postnatal high-fat diet leads to metabolic syndrome and is effectively reversed by resveratrol: A multiple-organ study. Sci. Rep. 2018, 8, 5607. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chenier, I.; Tran, S.; Scotcher, M.; Chang, S.Y.; Zhang, S.L. Maternal diabetes programs hypertension and kidney injury in offspring. Pediatr. Nephrol. 2010, 25, 1319–1329. [Google Scholar] [CrossRef]
- Wichi, R.B.; Souza, S.B.; Casarini, D.E.; Morris, M.; Barreto-Chaves, M.L.; Irigoyen, M.C. Increased blood pressure in the offspring of diabetic mothers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1129–R1133. [Google Scholar] [CrossRef]
- Thaeomor, A.; Teangphuck, P.; Chaisakul, J.; Seanthaweesuk, S.; Somparn, N.; Roysommuti, S. Perinatal Taurine Supplementation Prevents Metabolic and Cardiovascular Effects of Maternal Diabetes in Adult Rat Offspring. Adv. Exp. Med. Biol. 2017, 975, 295–305. [Google Scholar]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Tain, Y.L. Dietary Supplementation with Cysteine during Pregnancy Rescues Maternal Chronic Kidney Disease-Induced Hypertension in Male Rat Offspring: The Impact of Hydrogen Sulfide and Microbiota-Derived Tryptophan Metabolites. Antioxidants 2022, 11, 483. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Protective Role of Taurine on Rat Offspring Hypertension in the Setting of Maternal Chronic Kidney Disease. Antioxidants 2023, 12, 2059. [Google Scholar] [CrossRef]
- Wlodek, M.E.; Mibus, A.; Tan, A.; Siebel, A.L.; Owens, J.A.; Moritz, K.M. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J. Am. Soc. Nephrol. 2007, 18, 1688–1696. [Google Scholar] [CrossRef]
- Wlodek, M.E.; Westcott, K.; Siebel, A.L.; Owens, J.A.; Moritz, K.M. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 2008, 74, 187–195. [Google Scholar] [CrossRef]
- Nüsken, K.D.; Dötsch, J.; Rauh, M.; Rascher, W.; Schneider, H. Uteroplacental insufficiency after bilateral uterine artery ligation in the rat: Impact on postnatal glucose and lipid metabolism and evidence for metabolic programming of the offspring by sham operation. Endocrinology 2008, 149, 1056–1063. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, X.; Xue, Q.; Zhang, L. Antenatal hypoxia induces programming of reduced arterial blood pressure response in female rat offspring: Role of ovarian function. PLoS ONE 2014, 9, e98743. [Google Scholar] [CrossRef]
- Vargas, V.E.; Gurung, S.; Grant, B.; Hyatt, K.; Singleton, K.; Myers, S.M.; Saunders, D.; Njoku, C.; Towner, R.; Myers, D.A. Gestational hypoxia disrupts the neonatal leptin surge and programs hyperphagia and obesity in male offspring in the Sprague-Dawley rat. PLoS ONE 2017, 12, e0185272. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Chen, C.C.; Sheen, J.M.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Melatonin attenuates prenatal dexamethasoneinduced blood pressure increase in a rat model. J. Am. Soc. Hypertens. 2014, 8, 216–226. [Google Scholar] [CrossRef]
- Dai, Y.; Kou, H.; Gui, S.; Guo, X.; Liu, H.; Gong, Z.; Sun, X.; Wang, H.; Guo, Y. Prenatal dexamethasone exposure induced pancreatic β-cell dysfunction and glucose intolerance of male offspring rats: Role of the epigenetic repression of ACE2. Sci. Total Environ. 2022, 826, 154095. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Tiao, M.M.; Sheen, J.M.; Huang, L.T.; Tain, Y.L.; Lin, I.C.; Lin, Y.J.; Lai, Y.J.; Chen, C.C.; Chang, K.A.; et al. Obesity programmed by prenatal dexamethasone and postnatal high-fat diet leads to distinct alterations in nutrition sensory signals and circadian-clock genes in visceral adipose tissue. Lipids Health Dis. 2019, 18, 19. [Google Scholar] [CrossRef]
- O’Regan, D.; Kenyon, C.J.; Seckl, J.R.; Holmes, M.C. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E863–E870. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Rodriguez, M.; Loyse, N.; Bourdon, C.; Arab, S.; Pausova, Z. Effect of prenatal exposure to nicotine on kidney glomerular mass and AT1R expression in genetically diverse strains of rats. Toxicol. Lett. 2012, 213, 228–234. [Google Scholar] [CrossRef]
- Xiao, D.; Huang, X.; Li, Y.; Dasgupta, C.; Wang, L.; Zhang, L. Antenatal Antioxidant Prevents Nicotine-Mediated Hypertensive Response in Rat Adult Offspring. Biol. Reprod. 2015, 93, 66. [Google Scholar] [CrossRef] [PubMed]
- Conceição, E.P.; Peixoto-Silva, N.; Pinheiro, C.R.; Oliveira, E.; Moura, E.G.; Lisboa, P.C. Maternal nicotine exposure leads to higher liver oxidative stress and steatosis in adult rat offspring. Food Chem. Toxicol. 2015, 78, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.M.; Chou, H.C.; Huang, L.T. Maternal nicotine exposure during gestation and lactation induces kidney injury and fibrosis in rat offspring. Pediatr. Res. 2015, 77, 56–63. [Google Scholar] [CrossRef][Green Version]
- Gray, S.P.; Denton, K.M.; Cullen-McEwen, L.; Bertram, J.F.; Moritz, K.M. Prenatal exposure to alcohol reduces nephron number and raises blood pressure in progeny. J. Am. Soc. Nephrol. 2010, 21, 1891–1902. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.T.; Steane, S.E.; Moritz, K.M.; Akison, L.K. Prenatal alcohol exposure programmes offspring disease: Insulin resistance in adult males in a rat model of acute exposure. J. Physiol. 2019, 597, 5619–5637. [Google Scholar] [CrossRef]
- Aragon, A.C.; Kopf, P.G.; Campen, M.J.; Huwe, J.K.; Walker, M.K. In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: Effects on fetal and adult cardiac gene expression and adult cardiac and renal morphology. Toxicol. Sci. 2008, 101, 321–330. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hung, C.H.; Hou, C.Y.; Chang, C.I.; Tain, Y.L. Perinatal Resveratrol Therapy to Dioxin-Exposed Dams Prevents the Programming of Hypertension in Adult Rat Offspring. Antioxidants 2021, 10, 1393. [Google Scholar] [CrossRef]
- Wei, Z.; Song, L.; Wei, J.; Chen, T.; Chen, J.; Lin, Y.; Xia, W.; Xu, B.; Li, X.; Chen, X.; et al. Maternal exposure to di-(2-ethylhexyl) phthalate alters kidney development through the renin-Angiotensin system in offspring. Toxicol. Lett. 2012, 212, 212–221. [Google Scholar] [CrossRef]
- Rajagopal, G.; Bhaskaran, R.S.; Karundevi, B. Maternal di-(2-ethylhexyl) phthalate exposure alters hepatic insulin signal transduction and glucoregulatory events in rat F1 male offspring. J. Appl. Toxicol. 2019, 39, 751–763. [Google Scholar] [CrossRef]
- Zhu, Y.P.; Chen, L.; Wang, X.J.; Jiang, Q.H.; Bei, X.Y.; Sun, W.L.; Xia, S.J.; Jiang, J.T. Maternal exposure to di-n-butyl phthalate (DBP) induces renal fibrosis in adult rat offspring. Oncotarget 2017, 8, 31101–31111. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Hsu, C.N. Resveratrol Butyrate Ester Supplementation Blunts the Development of Offspring Hypertension in a Maternal Di-2-ethylhexyl Phthalate Exposure Rat Model. Nutrients 2023, 15, 697. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.N.; Tain, Y.L. Animal Models for DOHaD Research: Focus on Hypertension of Developmental Origins. Biomedicines 2021, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- McMillen, I.C.; Robinson, J.S. Developmental origins of the metabolic syndrome: Prediction, plasticity, and programming. Physiol. Rev. 2005, 85, 571–633. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Parhofer, K.G.; Woenckhaus, M.; Wrede, C.E.; Kunz-Schughart, L.A.; Schölmerich, J.; Bollheimer, L.C. Defining high-fat-diet rat models: Metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 2006, 36, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Ganu, R.S.; Harris, R.A.; Collins, K.; Aagaard, K.M. Early origins of adult disease: Approaches for investigating the programmable epigenome in humans, nonhuman primates, and rodents. ILAR J. 2012, 53, 306–321. [Google Scholar] [CrossRef]
- Cheng, Z.; Zheng, L.; Almeida, F.A. Epigenetic reprogramming in metabolic disorders: Nutritional factors and beyond. J. Nutr. Biochem. 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Padmanabhan, V.; Cardoso, R.C.; Puttabyatappa, M. Developmental Programming, a Pathway to Disease. Endocrinology 2016, 157, 1328–1340. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Lin, C.Y.; Huang, L.T.; Lau, Y.T. Aliskiren prevents hypertension and reduces asymmetric dimethylarginine in young spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 670, 561–565. [Google Scholar] [CrossRef]
- Hsu, C.N.; Lee, C.T.; Huang, L.T.; Tain, Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin Aldosterone Syst. 2015, 16, 506–513. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Tan, G.; Binger, K.J.; Sutton, L.; McMaster, K.; Deliyanti, D.; Perera, G.; Campbell, D.J.; Miller, A.G. Aliskiren reduces vascular pathology in diabetic retinopathy and oxygen-induced retinopathy in the transgenic (mRen-2)27 rat. Diabetologia 2011, 54, 2724–2735. [Google Scholar] [CrossRef]
- Manning, J.; Vehaskari, V.M. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R80–R84. [Google Scholar] [CrossRef] [PubMed]
- Zicha, J.; Dobesová, Z.; Kunes, J. Late blood pressure reduction in SHR subjected to transient captopril treatment in youth: Possible mechanisms. Physiol. Res. 2008, 57, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.C.; Langley-Evans, S.C. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin. Sci. 2000, 98, 269–275. [Google Scholar] [CrossRef]
- Mansuri, A.; Elmaghrabi, A.; Legan, S.K.; Gattineni, J.; Baum, M. Transient Exposure of Enalapril Normalizes Prenatal Programming of Hypertension and Urinary Angiotensinogen Excretion. PLoS ONE 2015, 10, e0146183. [Google Scholar] [CrossRef] [PubMed]
- Harrap, S.B.; Nicolaci, J.A.; Doyle, A.E. Persistent effects on blood pressure and renal haemodynamics following chronic Angiotensin converting enzyme inhibition with perindopril. Clin. Exp. Pharmacol. Physiol. 1986, 13, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.C.; Langley-Evans, S.C. Early administration of Angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin. Sci. 1998, 94, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Klimas, J.; Olvedy, M.; Ochodnicka-Mackovicova, K.; Kruzliak, P.; Cacanyiova, S.; Kristek, F.; Krenek, P.; Ochodnicky, P. Perinatally administered losartan augments renal ACE2 expression but not cardiac or renal Mas receptor in spontaneously hypertensive rats. J. Cell Mol. Med. 2015, 19, 1965–1974. [Google Scholar] [CrossRef]
- Walton, S.L.; Mazzuca, M.Q.; Tare, M.; Parkington, H.C.; Wlodek, M.E.; Moritz, K.M.; Gallo, L.A. Angiotensin receptor blockade in juvenile male rat offspring: Implications for long-term cardio-renal health. Pharmacol. Res. 2018, 134, 320–331. [Google Scholar] [CrossRef]
- Iyer, S.N.; Lu, D.; Katovich, M.J.; Raizada, M.K. Chronic control of high blood pressure in the spontaneously hypertensive rat by delivery of Angiotensin type 1 receptor antisense. Proc. Natl. Acad. Sci. USA 1996, 93, 9960–9965. [Google Scholar] [CrossRef] [PubMed]
- Bessa, A.S.M.; Jesus, É.F.; Nunes, A.D.C.; Pontes, C.N.R.; Lacerda, I.S.; Costa, J.M.; Souza, E.J.; Lino-Júnior, R.S.; Biancardi, M.F.; Dos Santos, F.C.A.; et al. Stimulation of the ACE2/Ang-(1-7)/Mas axis in hypertensive pregnant rats attenuates cardiovascular dysfunction in adult male offspring. Hypertens. Res. 2019, 42, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age with Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Ichihara, A.; Hayashi, M.; Kaneshiro, Y.; Suzuki, F.; Nakagawa, T.; Tada, Y.; Koura, Y.; Nishiyama, A.; Okada, H.; Uddin, M.N.; et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J. Clin. Investig. 2004, 114, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sullivan, M.N.; Zhang, S.; Worker, C.J.; Xiong, Z.; Speth, R.C.; Feng, Y. Intracerebroventricular infusion of the (Pro)renin receptor antagonist PRO20 attenuates deoxycorticosterone acetate-salt-induced hypertension. Hypertension 2015, 65, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Krop, M.; Lu, X.; Danser, A.H.; Meima, M.E. The (pro)renin receptor. A decade of research: What have we learned? Pflugers Arch. 2013, 465, 87–97. [Google Scholar] [CrossRef]
- Pravenec, M.; Kurtz, T.W. Recent advances in genetics of the spontaneously hypertensive rat. Curr. Hypertens. Rep. 2010, 12, 5–9. [Google Scholar] [CrossRef]
- South, A.M.; Shaltout, H.A.; Washburn, L.K.; Hendricks, A.S.; Diz, D.I.; Chappell, M.C. Fetal programming and the Angiotensin-(1-7) axis: A review of the experimental and clinical data. Clin. Sci. 2019, 133, 55–74. [Google Scholar] [CrossRef]
| Experimental Model | Early-Life Exposure | CKM Phenotype | References |
|---|---|---|---|
| Maternal nutritional imbalance | Caloric restriction | Hypertension, insulin resistance, and kidney disease | [92,93,94] |
| Protein restriction | Hypertension, insulin resistance, and kidney disease | [95,96,97,98] | |
| High-fructose diet | Hypertension, insulin resistance, obesity, and dyslipidemia | [99,100,101,102,103] | |
| High-fat diet | Hypertension, insulin resistance, obesity, dyslipidemia, and kidney disease | [104,105,106,107] | |
| Maternal illnesses and conditions | Maternal diabetes | Hypertension, insulin resistance, obesity, dyslipidemia, and kidney disease | [108,109,110] |
| Maternal chronic kidney disease | Hypertension and kidney disease | [111,112] | |
| Uteroplacental insufficiency | Hypertension, dyslipidemia, insulin resistance, and kidney disease | [83,113,114,115] | |
| Maternal hypoxia | Obesity and hypertension | [116,117] | |
| Drug and chemical exposures | Prenatal glucocorticoid exposure | Hypertension, obesity, insulin resistance, and kidney disease | [92,118,119,120,121] |
| Prenatal nicotine exposure | Hypertension, hyperlipidemia, steatosis, and kidney disease | [122,123,124,125] | |
| Prenatal ethanol exposure | Hypertension, insulin resistance, and kidney disease | [126,127] | |
| Maternal TCDD exposure | Hypertension, cardiac hypertrophy, and kidney disease | [128,129] | |
| Maternal DEHP exposure | Hypertension, insulin resistance, and kidney disease | [130,131,132,133] |
| Intervention | Experimental Model | Species | Age at Evaluation (Weeks) | Protective Effects | Ref. |
|---|---|---|---|---|---|
| Renin inhibitor | |||||
| Administration of aliskiren at doses of 10 or 30 mg/kg/day between the ages of 4 and 10 weeks | Genetic hypertension model | SHR/M | 10 | Hypertension was prevented | [141] |
| Administration of aliskiren at a dosage of 10 mg/kg/day between the ages of 2 and 4 weeks | Maternal caloric restriction | SD rat/M | 12 | Hypertension was prevented | [142] |
| Administration of aliskiren at a dosage of 10 mg/kg/day between the ages of 2 and 4 weeks | Maternal high-fructose diet | SD rat/M & F | 12 | Hypertension was prevented | [101] |
| Aliskiren was administered at a dosage of 10 mg/kg/day using a pump from postnatal days 12 to 18 | STZ-induced diabetes | TGR (mREN)27 rat/M | 16 | Diabetic retinopathy was prevented and hypertension was attenuated | [143] |
| ACEI | |||||
| Administration of captopril at a dosage of 100 mg/kg/day between the ages of 2 and 4 weeks | Maternal protein restriction | Wistar rat/M | 12 | Hypertension was prevented | [144] |
| Administration of captopril at a dosage of 100 mg/kg/day between the ages of 4 and 10 weeks | Genetic hypertension model | SHR/M | 30 | Hypertension was attenuated | [145] |
| Enalapril was administered at a concentration of 100 mg/L in the drinking water between the ages of 3 and 6 weeks | Maternal protein restriction | SD rat/M | 16 | Hypertension was prevented | [146] |
| Enalapril was administered at a concentration of 100 mg/L in the drinking water between the ages of 3 and 6 weeks | Maternal protein restriction | SD rat/M | 24 | Hypertension and albuminuria were prevented | [147] |
| Lisinopril was administered at a dosage of 10 mg/kg/day through the drinking water from postnatal days 12 to 18 | STZ-induced diabetes | TGR (mREN)27 rat/M | 16 | Hypertension was prevented and diabetic retinopathy was attenuated | [143] |
| Perindopril was administered at a dosage of 3 mg/kg/day between the ages of 4 and 16 weeks | Genetic hypertension model | SHR/M | 28 | Hypertension and renal dysfunction were attenuated | [148] |
| ARB | |||||
| Losartan was administered at a concentration of 100 mg/L in the drinking water between the ages of 2 and 4 weeks | Maternal protein restriction | Wistar rat/M | 12 | Hypertension was prevented | [149] |
| Losartan was administered at a dosage of 20 mg/kg/day between the ages of 2 and 4 weeks | Maternal caloric restriction | SD rat/M | 12 | Hypertension was prevented | [150] |
| Losartan was administered at a dosage of 20 mg/kg/day between the ages of 4 and 9 weeks | Genetic hypertension model | SHR/M | 10 | Hypertension was prevented | [141] |
| Losartan was administered at a concentration of 30 mg/L in the drinking water between the ages of 5 and 8 weeks | Uteroplacental insufficiency | WKY rat/M | 26 | Hypertension, vascular dysfunction, and kidney disease were prevented | [151] |
| AT1R antisense | |||||
| AT1R antisense was delivered at 5 days of age | Genetic hypertension model | SHR/M | 12 | Hypertension was prevented | [152] |
| ACE2 activator | |||||
| Diminazene aceturate was administered in pregnancy | Maternal hypertension | SHR/M | 16 | Hypertension and kidney fibrosis were attenuated | [153] |
| ANG-(1-7) was administered in pregnancy | Maternal hypertension | SHR/M | 16 | Hypertension and kidney fibrosis were attenuated | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Hsu, C.-N. The Renin–Angiotensin System and Cardiovascular–Kidney–Metabolic Syndrome: Focus on Early-Life Programming. Int. J. Mol. Sci. 2024, 25, 3298. https://doi.org/10.3390/ijms25063298
Tain Y-L, Hsu C-N. The Renin–Angiotensin System and Cardiovascular–Kidney–Metabolic Syndrome: Focus on Early-Life Programming. International Journal of Molecular Sciences. 2024; 25(6):3298. https://doi.org/10.3390/ijms25063298
Chicago/Turabian StyleTain, You-Lin, and Chien-Ning Hsu. 2024. "The Renin–Angiotensin System and Cardiovascular–Kidney–Metabolic Syndrome: Focus on Early-Life Programming" International Journal of Molecular Sciences 25, no. 6: 3298. https://doi.org/10.3390/ijms25063298
APA StyleTain, Y.-L., & Hsu, C.-N. (2024). The Renin–Angiotensin System and Cardiovascular–Kidney–Metabolic Syndrome: Focus on Early-Life Programming. International Journal of Molecular Sciences, 25(6), 3298. https://doi.org/10.3390/ijms25063298







