Treatment with Rasburicase in Hospitalized Patients with Cardiorenal Syndrome: Old Treatment, New Scenario
Abstract
:1. Introduction
2. Results
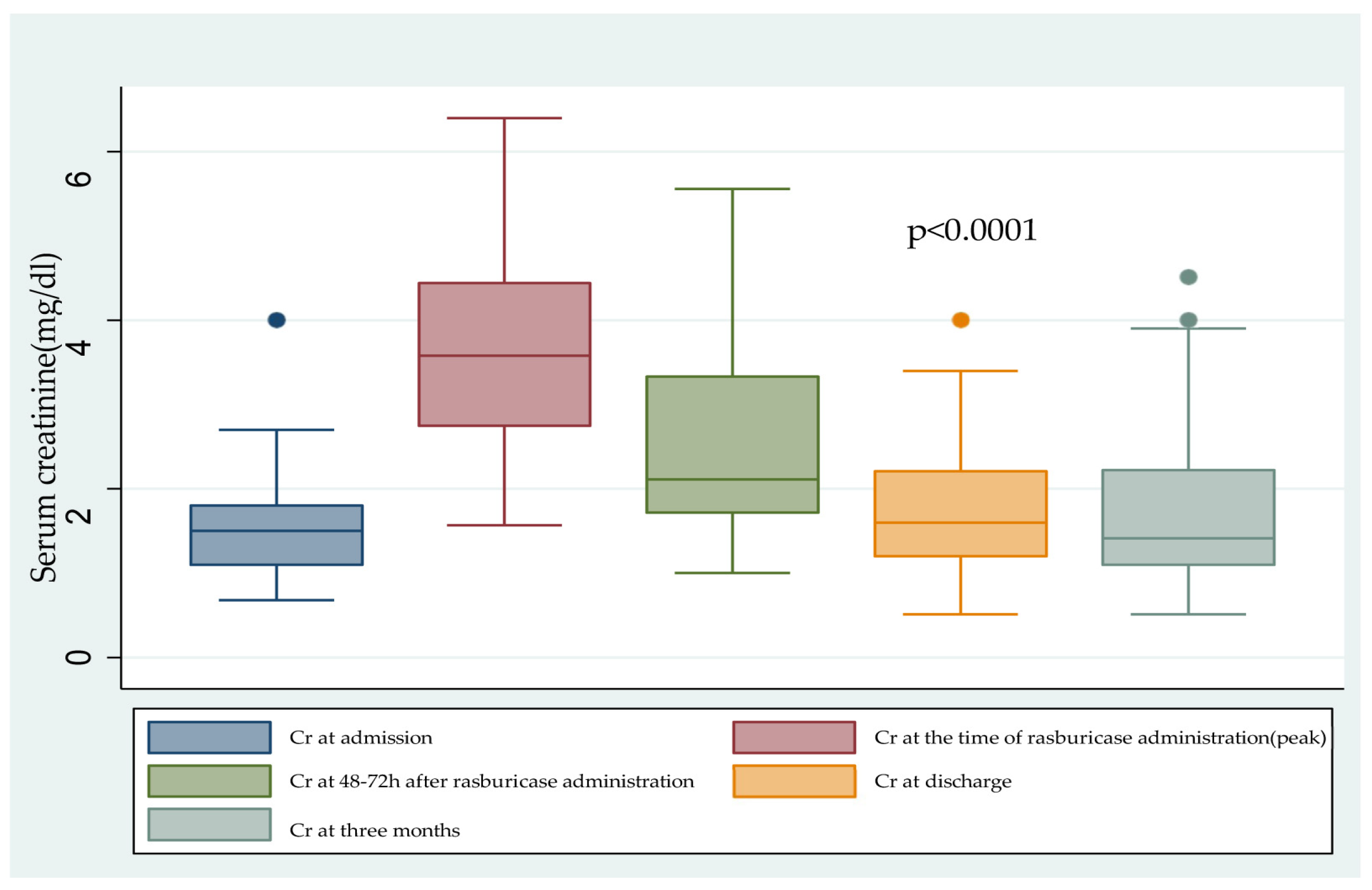
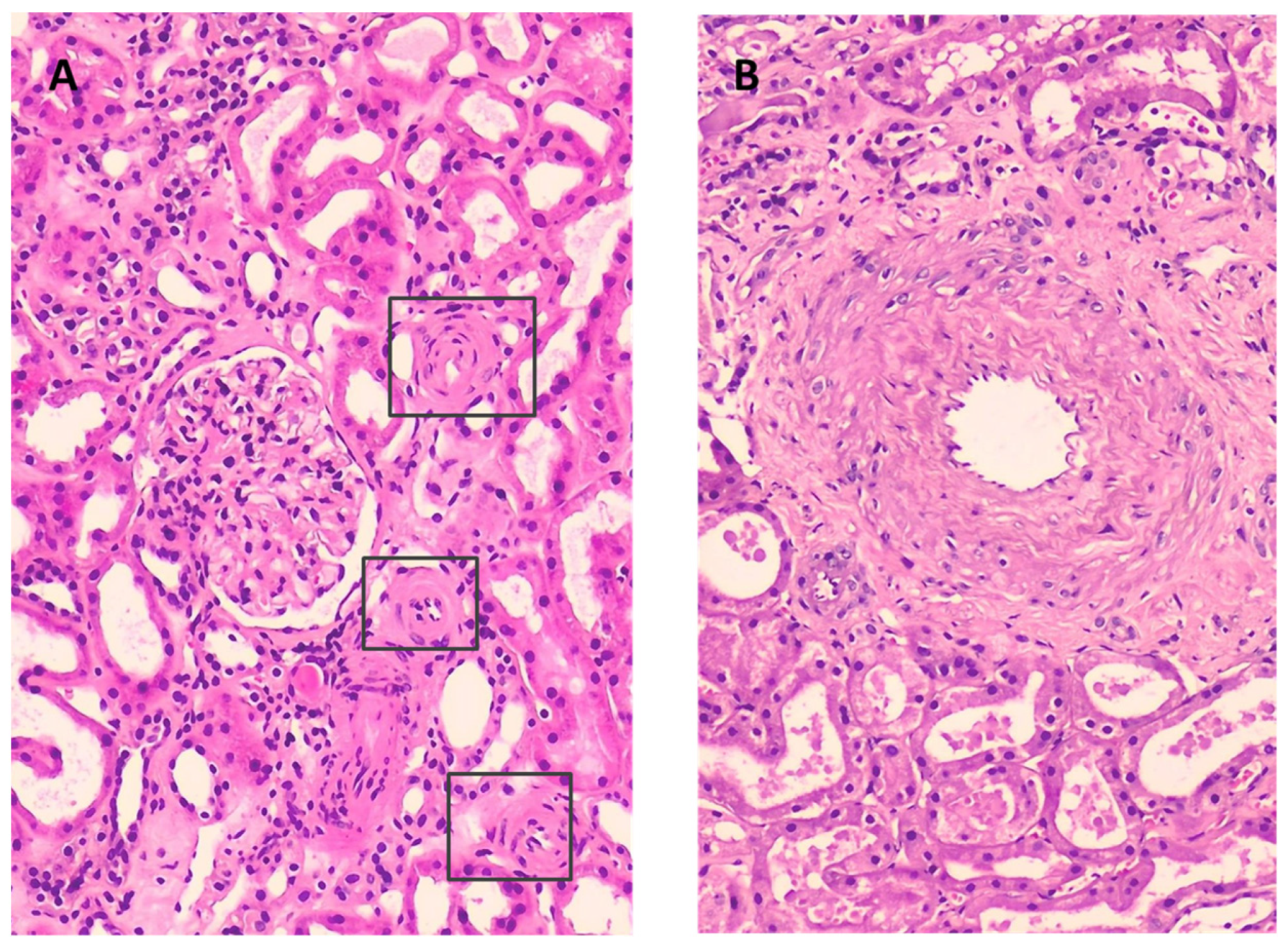
2.1. Renal Fuction
2.2. Cardiac Function
2.3. Inflammation and Mortality
3. Discussion
4. Materials and Methods
4.1. Study Design
Single-Centre, Observational, Retrospective Study
- History of allergy to rasburicase or any of its components;
- Confirmed or suspected glucose-6-phosphate dehydrogenase deficiency or other metabolic disorders causing haemolytic anaemia.
4.2. Data Collection
- The following variables were collected from the hospital records and the electronic prescription software:
- Demographics (age, sex, and weight);
- Underlying diseases: arterial hypertension (HTA), diabetes mellitus (DM), HF, rhabdomyolisis, kidney or heart transplant;
- Treatment: iodinated contrasts and/or vancomycin; anticalcineurin agents described as nephrotoxic drugs; use of inotropic drugs (dobutamine or levosimendan), vasopressors (noradrenaline, adrenaline or vasopressin) and diuretics (loop diuretics, thiazides, carbonic anhydrase inhibitors and aldosterone antagonists); and chronic treatment with xanthine oxidase inhibitors (XOis). Need for controlled ventilatory support (CVS) and/or ventricular assist devices (VADs) during hospital admission was also compiled;
- Renal function: serum creatinine and estimated glomerular filtration rate (eGFR) were calculated by the CKD-EPI equation at baseline, at 48–72 h after rasburicase administration, at hospital discharge and at least three months after hospital discharge. The need for RRT and its duration were recorded, as well as whether there was recovery of the diuretic rhythm, defined as a doubling of the diuretic rhythm with respect to baseline, or >100 cc/h;
- Cardiac function at the time of rasburicase: lactic acid, LVEF (preserved > 40%, reduced < 40%) and tricuspid annular systolic displacement (TAPSE) by echocardiography. Intravascular congestion was assessed by the presence or absence of >50% collapse of the inferior vena cava by ultrasound measurement and Nt-ProBNP levels at the time of rasburicase administration and at discharge;
- Inflammation and mortality: CRP levels were measured at the time of HU and maximum creatinine and at discharge. Intrahospital mortality rate and the causes of death were also assessed;
- Rasburicase adverse events (AEs), including allergy, headache, gastrointestinal symptoms, fever, skin rash, haemolysis, haemolytic anaemia, metahaemoglobinaemia and seizures.
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ronco, C.; McCullough, P.; Anker, S.D.; Anand, I.; Aspromonte, N.; Bagshaw, S.M.; Bellomo, R.; Berl, T.; Bobek, I.; Cruz, D.N.; et al. Cardio-renal syndromes: Report from the consensus conference of the Acute Dialysis Quality Initiative. Eur. Heart J. 2009, 31, 703–711. [Google Scholar] [CrossRef]
- Junho, C.V.C.; Trentin-Sonoda, M.; Panico, K.; dos Santos, R.S.N.; Abrahão, M.V.; Vernier, I.C.S.; Fürstenau, C.R.; Carneiro-Ramos, M.S. Cardiorenal syndrome: Long road between kidney and heart. Heart Fail. Rev. 2022, 27, 2137–2153. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, Y.; Yamamoto, T.; Tsutsumi, Z.; Takahashi, S.; Hada, T. Effects of angiotensin II infusion on renal ex-cretion of purine bases and oxypurinol. Metabolism 2002, 51, 893–895. [Google Scholar] [CrossRef]
- Kanbay, M.; Solak, Y.; Afsar, B.; Nistor, I.; Aslan, G.; Çağlayan, O.H.; Aykanat, A.; Donciu, M.D.; Lanaspa, M.A.; Ejaz, A.A.; et al. Serum Uric Acid and Risk for Acute Kidney Injury Following Contrast. Angiology 2017, 68, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, A.A.; Dass, B.; Lingegowda, V.; Shimada, M.; Beaver, T.M.; Ejaz, N.I.; Abouhamze, A.S.; Johnson, R.J. Effect of uric acid lowering therapy on the prevention of acute kidney injury in cardiovascular surgery. Int. Urol. Nephrol. 2012, 45, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Akcay, A.; Huddam, B.; Usluogullari, C.; Arat, Z.; Ozdemir, F.; Haberal, M. Influence of Cyclosporine and Tacrolimus on Serum Uric Acid Levels in Stable Kidney Transplant Recipients. Transplant. Proc. 2005, 37, 3119–3120. [Google Scholar] [CrossRef] [PubMed]
- Sancho Bueso, T.; Bernardino de la Serna, I.; Garcia Puig, J. A patient with hiperuricemia. Med. Integral 2000, 35, 110–111. [Google Scholar]
- Skoczyńska, M.; Chowaniec, M.; Szymczak, A.; Langner-Hetmańczuk, A.; Maciążek-Chyra, B.; Wiland, P. Patho-physiology of hyperuricemia and its clinical significance—A narrative review. Reumatologia 2020, 58, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Hahn, K.; Kanbay, M.; Lanaspa, M.A.; Johnson, R.J.; Ejaz, A.A. Serum uric acid and acute kidney injury: A mini review. J. Adv. Res. 2017, 8, 529–536. [Google Scholar] [CrossRef]
- Macdonald, G. Harrison’s Internal Medicine, 17th edition.—By A. S. Fauci, D.L. Kasper, D.L. Longo, E. Braunwald, S.L. Hauser, J.L. Jameson and J. Loscalzo. Intern. Med. J. 2008, 38, 932. [Google Scholar] [CrossRef]
- Ejaz, A.A.; Mohandas, R.; Beaver, T.M.; Johnson, R.J. A Crystal-Independent Role for Uric Acid in AKI Associated with Tumor Lysis Syndrome. J. Am. Soc. Nephrol. 2023, 34, 175. [Google Scholar] [CrossRef]
- Wang, K.; Liao, Q.; Chen, X. Research progress on the mechanism of renal interstitial fibrosis in obstructive nephropathy. Heliyon 2023, 9, e18723. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, K.; Jiang, P.; Chang, C.; Xu, L.; Xu, L.; Shi, Y.; Guo, S.; Xue, Y.; He, D. Inflammatory Response to Regulated Cell Death in Gout and Its Functional Implications. Front. Immunol. 2022, 13, 888306. [Google Scholar] [CrossRef]
- Mulay, S.R.; Evan, A.; Anders, H.J. Molecular mechanisms of crystal-related kidney inflammation and injury. Implications for cholesterol embolism, crystalline nephropathies and kidney stone disease. Nephrol. Dial. Transplant. 2014, 29, 507–514. [Google Scholar] [CrossRef]
- Li, D.; Yuan, S.; Deng, Y.; Wang, X.; Wu, S.; Chen, X.; Li, Y.; Ouyang, J.; Lin, D.; Quan, H.; et al. The dysregulation of immune cells induced by uric acid: Mechanisms of inflammation associated with hyperuricemia and its complications. Front. Immunol. 2023, 14, 1282890. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lozada, L.G.; Tapia, E.; Santamaría, J.; Avila-Casado, C.; Soto, V.; Nepomuceno, T.; Rodríguez-Iturbe, B.; Johnson, R.J.; Herrera-Acosta, J. Mild hyperu-ricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005, 67, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Messerli, F.H.; Frohlich, E.D.; Dreslinski, G.R.; Suarez, D.H.; Aristimuno, G.G. Serum Uric Acid in Essential Hypertension: An Indicator of Renal Vascular Involvement. Ann. Intern. Med. 1980, 93, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Mazzali, M.; Hughes, J.; Kim, Y.-G.; Jefferson, J.A.; Kang, D.-H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated Uric Acid Increases Blood Pressure in the Rat by a Novel Crystal-Independent Mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Huang, X.R.; Suga, S.; Mazzali, M.; Tang, D.; Metz, C.; Bucala, R.; Kivlighn, S.; Johnson, R.J.; Lan, H.Y. Involvement of macrophage migration inhib-itory factor (MIF) in experimental uric acid nephropathy. Mol. Med. 2000, 6, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.Y.; Lan, R.Y.; Zeng, J.; Bai, X.; Wang, J.T.; Yin, X.L.; Qu, R.J.; Qu, M.H.; Jiang, H.; Li, W.L.; et al. Effect of High-Concentration Uric Acid on Nitric Ox-ide. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2023, 45, 666–671. [Google Scholar] [PubMed]
- Johnson, R.J.; Segal, M.S.; Srinivas, T.; Ejaz, A.; Mu, W.; Roncal, C.; Sánchez-Lozada, L.G.; Gersch, M.; Rodriguez-Iturbe, B.; Kang, D.H.; et al. Essential hypertension, progressive renal disease, and uric acid: A pathogenetic link? J. Am. Soc. Nephrol. 2005, 16, 1909–1919. [Google Scholar] [CrossRef]
- He, C.; Lin, P.; Liu, W.; Fang, K. Prognostic value of hyperuricemia in patients with acute coronary syndrome: A meta-analysis. Eur. J. Clin. Investig. 2019, 49, e13074. [Google Scholar] [CrossRef]
- Raos, D.; Prkačin, I.; Delalić, Đ.; Bulum, T.; Lovrić Benčić, M.; Jug, J. Postoperative Hyperuricemia—A Risk Factor in Elective Cardiosurgical Patients. Metabolites 2023, 13, 590. [Google Scholar] [CrossRef]
- Huang, G.; Qin, J.; Deng, X.; Luo, G.; Yu, D.; Zhang, M.; Zhou, S.; Wang, L. Prognostic value of serum uric acid in patients with acute heart failure: A meta-analysis. Medicine 2019, 98, e14525. [Google Scholar] [CrossRef] [PubMed]
- Cairo, M.S.; Bishop, M. Tumour lysis syndrome: New therapeutic strategies and classification. Br. J. Haematol. 2004, 127, 3–11. [Google Scholar] [CrossRef]
- Adeyinka, A.; Bashir, K. Tumor Lysis Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Shimada, M.; Johnson, R.J.; May, W.S.; Lingegowda, V.; Sood, P.; Nakagawa, T.; Van, Q.C.; Dass, B.; Ejaz, A.A. A novel role for uric acid in acute kidney injury associated with tumour lysis syndrome. Nephrol. Dial. Transplant. 2009, 24, 2960–2964. [Google Scholar] [CrossRef]
- Mullens, W.; Damman, K.; Harjola, V.P.; Mebazaa, A.; Brunner-La Rocca, H.P.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuret-ics in heart failure with congestion—A position statement from the Heart Failure Association of the Euro-pean Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 137–155. [Google Scholar] [CrossRef] [PubMed]
- A McDonagh, T.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. Corrigendum to: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 4901. [Google Scholar] [CrossRef]
- Ronco, C.; Inguaggiato, P.; Bordoni, V.; De Cal, M.; Bonello, M.; Andrikos, E.; Assuman, Y.; Rattanarat, R.; Bellomo, R. Rasburicase therapy in acute hyperuricemia and renal dysfunction. Contrib. Nephrol. 2005, 147, 115–123. [Google Scholar]
- Patte, C.; Sakiroglu, C.; Ansoborlo, S.; Baruchel, A.; Plouvier, E.; Pacquement, H.; Babin-Boilletot, A.; Société Française d’Oncologie Pédiatrique. Urate-oxidase in the preven-tion and treatment of metabolic complications in patients with B-cell lymphoma and leukemia, treated in the Société Française d’Oncologie Pédiatrique LMB89 protocol. Ann. Oncol. 2002, 13, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.; Gugger, M.; Frey, F.J. Rasburicase (Fasturtec). Ther. Umsch. 2004, 61, 579–582. [Google Scholar] [CrossRef]
- Brogard, J.M.; Coumaros, D.; Franckhauser, J.; Stahl, A.; Stahl, J. Enzymatic uricolysis: A study of the effect of a fungal urate-oxydase. Rev. Eur. Etud. Clin. Biol. 1972, 17, 890–895. [Google Scholar] [PubMed]
- Russo, E.; Viazzi, F.; Pontremoli, R.; Barbagallo, C.M.; Bombelli, M.; Casiglia, E.; Cicero, A.F.G.; Cirillo, M.; Cirillo, P.; Desideri, G.; et al. Serum Uric Acid and Kidney Disease Measures Independently Predict Cardiovascular and Total Mortality: The Uric Acid Right for Heart Health (URRAH) Project. Front. Cardiovasc. Med. 2021, 8, 713652. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Fonarow, G.C. Quality of care and outcomes in acute decompensated heart failure: The ADHERE registry. Curr. Heart Fail. Rep. 2004, 1, 121–128. [Google Scholar] [CrossRef]
- Gallo, G.; Lanza, O.; Savoia, C. New Insight in Cardiorenal Syndrome: From Biomarkers to Therapy. Int. J. Mol. Sci. 2023, 24, 5089. [Google Scholar] [CrossRef]
- Husain-Syed, F.; Gröne, H.; Assmus, B.; Bauer, P.; Gall, H.; Seeger, W.; Ghofrani, A.; Ronco, C.; Birk, H. Congestive nephropathy: A neglected entity? Proposal for diagnostic criteria and future perspectives. ESC Heart Fail. 2020, 8, 183–203. [Google Scholar] [CrossRef] [PubMed]
- Nørregaard, R.; Mutsaers, H.A.M.; Frøkiær, J.; Kwon, T.H. Obstructive nephropathy and molecular pathophysi-ology of renal interstitial fibrosis. Physiol. Rev. 2023, 103, 2827–2872. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S. Obstructive nephropathy. Intern. Med. 2000, 39, 355–361. [Google Scholar] [CrossRef]
- Sato, M.; Muragaki, Y.; Saika, S.; Roberts, A.B.; Ooshima, A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J. Clin. Investig. 2003, 112, 1486–1494. [Google Scholar] [CrossRef]
- Lytvyn, Y.M.; Yang, G.K.; Yip, P.M.; Perkins, B.A.; Cherney, D.Z. Glucosuria-mediated urinary uric acid excretion in patients with uncomplicated type 1 diabetes mellitus. Am. J. Physiol. Renal Physiol. 2015, 308, F77–F83. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Segura Torres, P.; Borrego Utiel, F.J.; Pérez Del Barrio, P.; Gil Cunquero, J.M.; Pérez Bañasco, V. Efficacy of rasbu-ricase therapy in obstructive renal failure secondary to urolithiasis: A novel therapeutic option. Nefrologia 2008, 28, 102–105. [Google Scholar]
- Kim, G.-H.; Jun, J.-B. Altered Serum Uric Acid Levels in Kidney Disorders. Life 2022, 12, 1891. [Google Scholar] [CrossRef]
- Shabaka Fernández, A.; Cases Corona, C.; Fernández Juarez, G. Diuréticos. Lorenzo, V., López Gómez, J.M., Eds.; Available online: https://www.nefrologiaaldia.org/217 (accessed on 18 February 2024).
- Kjellstrand, C.M.; Campbell, D.C.; von Hartitzsch, B.; Buselmeier, T.J. Hyperuricemic Acute Renal Failure. Arch. Intern. Med. 1974, 133, 349–359. [Google Scholar] [CrossRef]
- Roncal, C.A.; Mu, W.; Croker, B.; Reungjui, S.; Ouyang, X.; Tabah-Fisch, I.; Johnson, R.J.; Ejaz, A.A. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am. J. Physiol. Physiol. 2007, 292, F116–F122. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, L.; Zhang, M.; Zhou, C.; Lin, N. Uric acid enhances PKC-dependent eNOS phosphorylation and mediates cellular ER stress: A mechanism for uric acid-induced endothelial dysfunction. Int. J. Mol. Med. 2016, 37, 989–997. [Google Scholar] [CrossRef]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Park, S.K.; Lee, I.K.; Johnson, R.J. Uric acid-induced C-reactive protein expression: Implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 2005, 16, 3553–3562. [Google Scholar] [CrossRef]
- McDonnell, A.M.; Lenz, K.L.; Frei-Lahr, D.A.; Hayslip, J.; Hall, P.D. Single-dose rasburicase 6 mg in the manage-ment of tumor lysis syndrome in adults. Pharmacotherapy 2006, 26, 806–812. [Google Scholar] [CrossRef] [PubMed]
| Demographics | |
| Age (years), mean (SD) | 67.5 (15.4) |
| Sex, male, n (%) | 23 (65.7) |
| Underlying diseases | |
| Arterial hypertension, n (%) | 30 (85.7) |
| Diabetes mellitus, n (%) | 13 (37.1) |
| Heart failure, n (%) | 34 (97.1) |
| Rhabdomyolysis, n (%) | 4 (11.4) |
| Kidney or heart transplant, n (%) | 8 (22.8) |
| Cr at admission (mg/dL), mean (SD) | 1.58 (0.68) |
| eGFR CKD-EPI at admission (mL/min/1.73 m2), mean (SD) | 43.41 (19) |
| Treatment | |
| Contrast, n (%) | 11 (31.4) |
| Nephrotoxic drugs, n (%) | 21 (60) |
| Inotropic drugs, n (%) | 15 (42.8) |
| Vasopressor drugs, n (%) | 16 (45.7) |
| Loop diuretics, n (%) | 35 (100) |
| Two or more diuretics, n (%) | 16 (45.7) |
| XOis, n (%) | 13 (37.14) |
| CVS, n (%) | 6 (16.7) |
| VAD, n (%) | 6 (16.7) |
| Renal function | |
| AKI II, n (%) | 18 (51.4) |
| AKI III, n (%) | 17 (48.6) |
| RRT, n (%) | 5 (14.2) |
| Cardiac function | |
| Lactic acid (mg/dL), mean (SD) | 1.55 (0.4) |
| TAPSE (cm) (SD) | 17.3 (5.1) |
| LVEF < 40%, n (%) | 15 (42.8) |
| Absence of >50% collapse of the inferior vena cava, n (%) | 27 (77.1) |
| Value at the Time of Rasburicase Administration (Peak) | Value at Discharge | p | |
|---|---|---|---|
| Cr (mg/dL), mean (SD) | 3.65 (1.27) | 1.8 (0.8) | <0.0001 |
| eFGR CKD-EPI (mL/min/1.73 m2), mean (SD) | 17 (8) | 41 (20) | <0.0001 |
| Nt-proBNP (pg/mL), median (IQR) | 24,298 (7122–35,000) | 6034 (1973–11,191) | <0.0001 |
| CRP (mg/L), median (IQR) | 10 (3.4–39.27) | 4.3 (1.0–10.2) | <0.0001 |
| RRT (n = 5) | No RRT (n = 30) | p | |
|---|---|---|---|
| Age (years), mean (SD) | 56 (16) | 70 (14) | 0.047 |
| CRP (mg/L), median (IQR) | 80.5 (1.6–186) | 10.9 (3.5–29.4) | ns |
| eGFR CKD-EPI (mL/min/1.73 m2), mean (SD) | 49.6 (14) | 43 (20) | ns |
| Arterial hypertension, n (%) | 4 (80) | 26 (86.6) | ns |
| Diabetes mellitus, n (%) | 2 (40) | 11 (36) | ns |
| LVEF (%), mean (SD) | 42 (13) | 39 (14) | ns |
| TAPSE (cm), mean (SD) | 16.2 (4.34) | 17.5 (5.4) | ns |
| Absence of >50% collapse of the inferior vena cava, n (%) | 5 (18.5) | 22 (81.5) | ns |
| Lactic acid (mg/dL), mean (SD) | 1.7 (0.5) | 1.5 (0.4) | ns |
| Nt-proBNP (pg/mL), median (IQR) | 24,429 (9201–35,000) | 24,158 (6806–35,000) | ns |
| VAD, n (%) | 2 (40) | 4 (13.3) | ns |
| CVS, n (%) | 2 (40) | 4 (13.3) | ns |
| Inotropic drugs, n (%) | 5 (100) | 10 (33.3) | 0.009 |
| Conditions That Favour Severe HU in CRS |
|---|
| Hypoxemia |
| ATP degradation |
| Decrease in glomerular filtration rate |
| Activation of the renin–angiotensin–aldosterone system |
| Decrease in effective circulating volume |
| Diuretics |
| Acetylsalicylic acid |
| Cyclosporine and tacrolimus |
| Lactic acid production and ketoacidosis |
| Surgery with general anaesthesia |
| Post-surgery rewarming |
| Ischaemia–reperfusion |
| Haemolysis (VAD, BiAo, and Impella) |
| Rhabdomyolysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melero, R.; Torroba-Sanz, B.; Goicoechea, M.; Sousa-Casasnovas, I.; Barrio, J.M.; García-Prieto, A.M.; Rodriguez-Benitez, P.; García-González, X.; Sanjurjo-Sáez, M. Treatment with Rasburicase in Hospitalized Patients with Cardiorenal Syndrome: Old Treatment, New Scenario. Int. J. Mol. Sci. 2024, 25, 3329. https://doi.org/10.3390/ijms25063329
Melero R, Torroba-Sanz B, Goicoechea M, Sousa-Casasnovas I, Barrio JM, García-Prieto AM, Rodriguez-Benitez P, García-González X, Sanjurjo-Sáez M. Treatment with Rasburicase in Hospitalized Patients with Cardiorenal Syndrome: Old Treatment, New Scenario. International Journal of Molecular Sciences. 2024; 25(6):3329. https://doi.org/10.3390/ijms25063329
Chicago/Turabian StyleMelero, Rosa, Beatriz Torroba-Sanz, Marian Goicoechea, Iago Sousa-Casasnovas, Jose María Barrio, Ana María García-Prieto, Patrocinio Rodriguez-Benitez, Xandra García-González, and María Sanjurjo-Sáez. 2024. "Treatment with Rasburicase in Hospitalized Patients with Cardiorenal Syndrome: Old Treatment, New Scenario" International Journal of Molecular Sciences 25, no. 6: 3329. https://doi.org/10.3390/ijms25063329
APA StyleMelero, R., Torroba-Sanz, B., Goicoechea, M., Sousa-Casasnovas, I., Barrio, J. M., García-Prieto, A. M., Rodriguez-Benitez, P., García-González, X., & Sanjurjo-Sáez, M. (2024). Treatment with Rasburicase in Hospitalized Patients with Cardiorenal Syndrome: Old Treatment, New Scenario. International Journal of Molecular Sciences, 25(6), 3329. https://doi.org/10.3390/ijms25063329





