In Vitro Modelling of Osteogenesis Imperfecta with Patient-Derived Induced Mesenchymal Stem Cells
Abstract
:1. Introduction
2. Results
2.1. Morphological Modifications of Induced Pluripotent Stem Cells, Induced Neural Crest Cells, and Induced Mesenchymal Stem Cells
2.2. Characterization and Comparison of Induced Pluripotent Stem Cells to Primary Embryonic Stem Cells
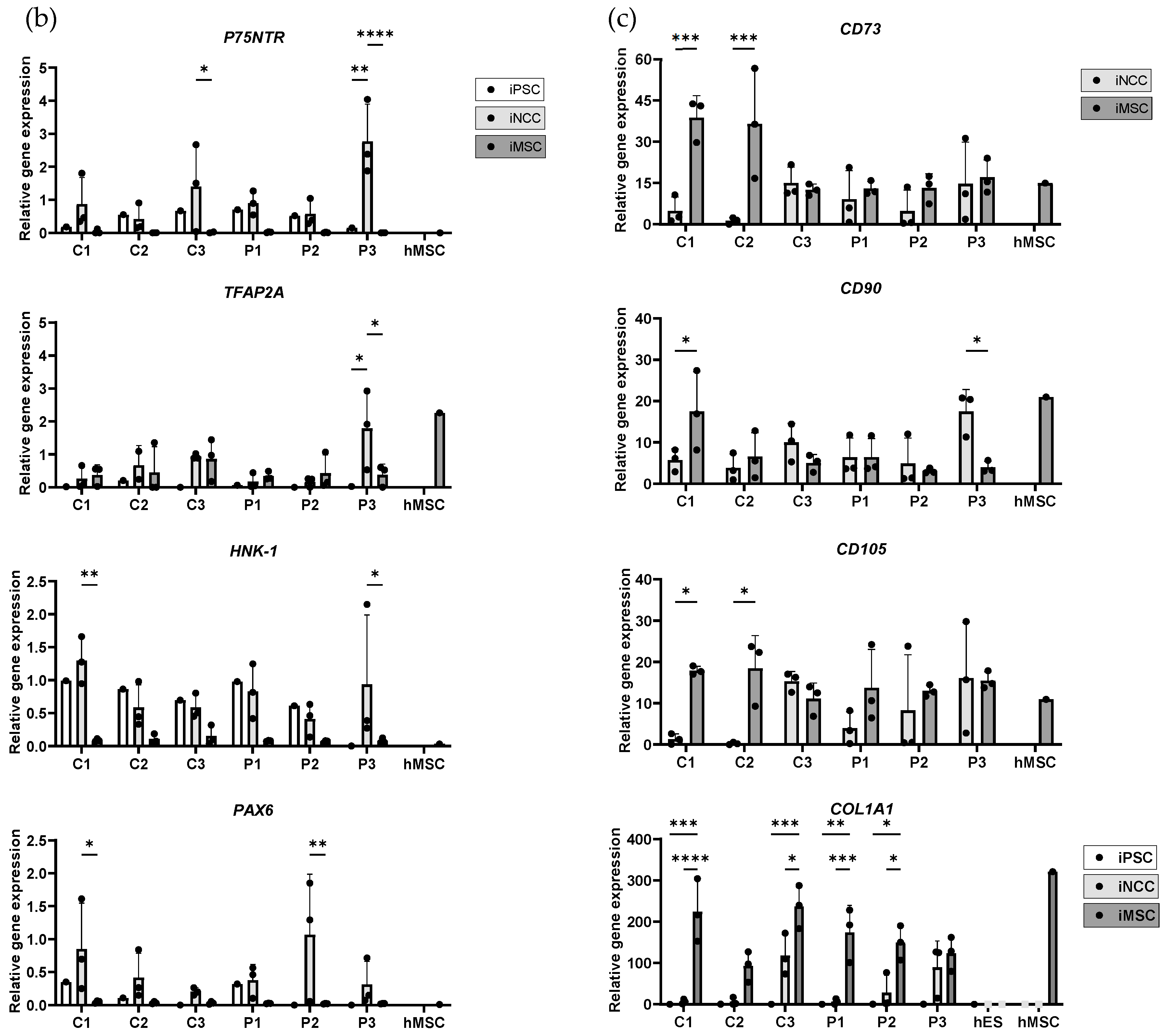
2.3. Characterization of Induced Neural Crest Cells
2.4. Characterization and Comparison of Induced Mesenchymal Stem Cells to Primary Human Mesenchymal Stem Cells and Hematopoietic Stem Cell
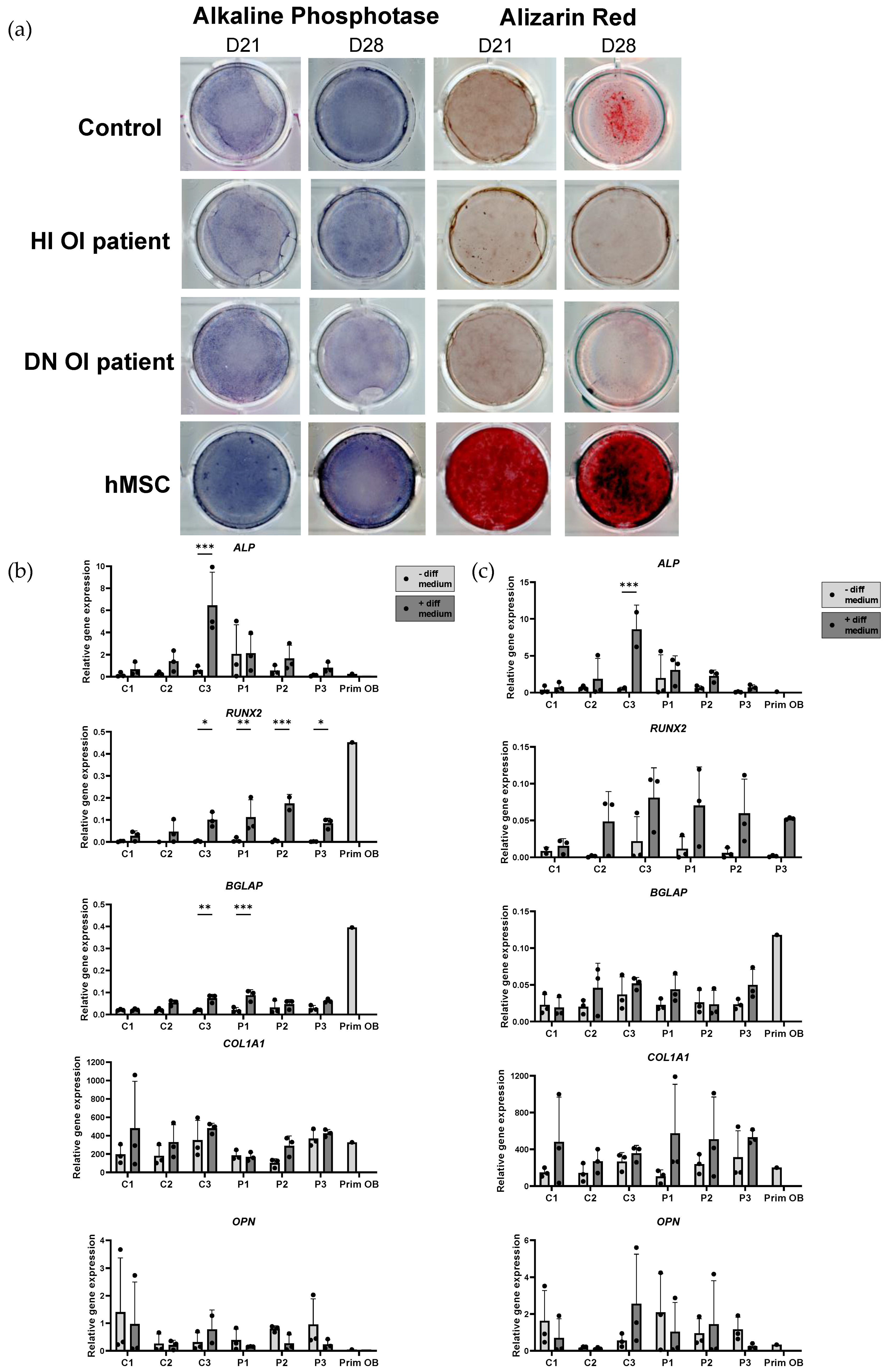
2.5. Osteogenic Induction and Mineralization Assay of OI and Healthy Control Induced Mesenchymal Stem Cells
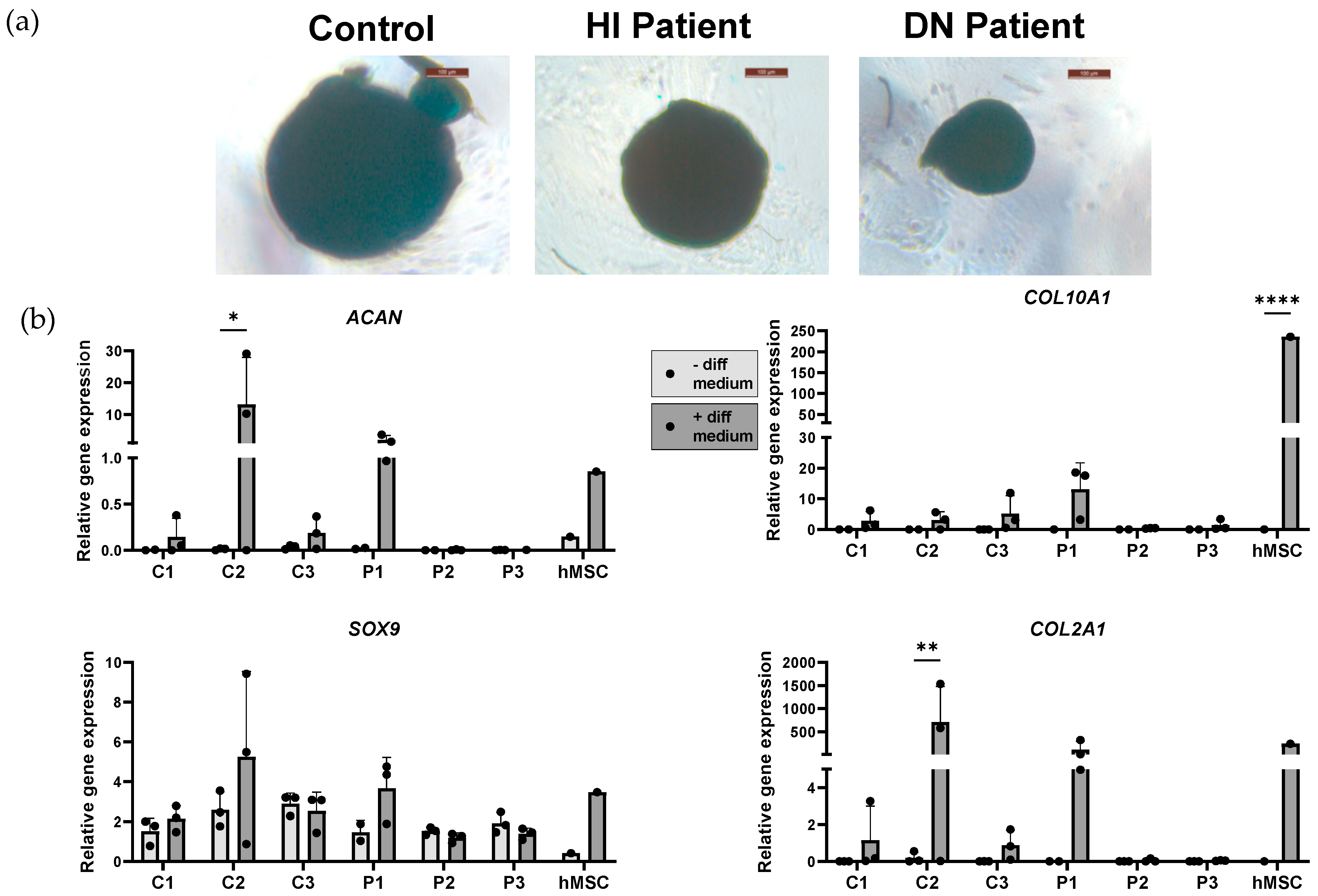
2.6. Adipogenic and Chondrogenic Induction of OI and Healthy Control Induced Mesenchymal Stem Cells
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Induced Pluripotent Stem Cells Induction and Validation
4.3. Induction of Induced Pluripotent Stem Cells into Induced Mesenchymal Stem Cells via Induced Neural Crest Cells’ Stage
4.4. Induction from Induced Mesenchymal Stem Cells to Osteoblast, Adipocyte, and Chondrocyte Cell Stage
4.5. Staining of Osteogenic Differentiation
4.6. Staining of Adipogenic Differentiation
4.7. Staining of Chondrogenic Differentiation
4.8. Gene Expression Analysis
4.9. Flow Cytometry and Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Byers, P.H.; Wallis, G.A.; Willing, M.C. Osteogenesis imperfecta: Translation of mutation to phenotype. J. Med. Genet. 1991, 28, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J. Osteoblast dysfunctions in bone diseases: From cellular and molecular mechanisms to therapeutic strategies. Cell Mol. Life Sci. 2015, 72, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Schipani, E.; Kronenberg, H.M. Adult mesenchymal stem cells. In StemBook; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008. [Google Scholar]
- Zhao, C.; Ikeya, M. Generation and Applications of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 9601623. [Google Scholar] [CrossRef] [PubMed]
- Eto, S.; Goto, M.; Soga, M.; Kaneko, Y.; Uehara, Y.; Mizuta, H.; Era, T. Mesenchymal stem cells derived from human iPS cells via mesoderm and neuroepithelium have different features and therapeutic potentials. PLoS ONE 2018, 13, e0200790. [Google Scholar]
- Wagner, W.; Bork, S.; Horn, P.; Krunic, D.; Walenda, T.; Diehlmann, A.; Benes, V.; Blake, J.; Huber, F.X.; Eckstein, V.; et al. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS ONE 2009, 4, e5846. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Wang, Y.M.; Zhang, H.; Li, J.; Wang, W.; Wei, Y.J.; Hu, S.S. Aging adversely impacts biological properties of human bone marrow-derived mesenchymal stem cells: Implications for tissue engineering heart valve construction. Artif. Organs 2010, 34, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Claeys, L.; Bravenboer, N.; Eekhoff, E.M.W.; Micha, D. Human Fibroblasts as a Model for the Study of Bone Disorders. Front. Endocrinol. 2020, 11, 494761. [Google Scholar] [CrossRef] [PubMed]
- Wruck, W.; Graffmann, N.; Spitzhorn, L.-S.; Adjaye, J. Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Acquire Rejuvenation and Reduced Heterogeneity. Front. Cell Dev. Biol. 2021, 9, 717772. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, M.; Liao, J.; Chang, C.; Liu, Y.; Padhiar, A.A.; Zhou, Y.; Zhou, G. Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Hold Lower Heterogeneity and Great Promise in Biological Research and Clinical Applications. Front. Cell Dev. Biol. 2021, 9, 716907. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.K.; Kim, H.; Kim, T.M. Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 3119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.D.; Bao, X.; Kaneda, G.; Avalos, P.; Behrens, P.; Salehi, K.; Da, X.; Chen, A.; Castaneda, C.; Nakielski, P.; et al. iPSC-neural crest derived cells embedded in 3D printable bio-ink promote cranial bone defect repair. Sci. Rep. 2022, 12, 18701. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.; Kim, K.W.; Kim, B.M.; Shin, Y.J.; Luo, K.; Quan, Y.; Cui, S.; Ko, E.J.; Chung, B.H.; Yang, C.W. Alleviation of renal ischemia/reperfusion injury by exosomes from induced pluripotent stem cell-derived mesenchymal stem cells. Korean J. Intern. Med. 2022, 37, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Hynes, K.; Menicanin, D.; Mrozik, K.; Gronthos, S.; Bartold, P.M. Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cells Dev. 2014, 23, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Loh, J.-K.; Wang, M.-L.; Cheong, S.-K.; Tsai, F.-T.; Huang, S.-H.; Wu, J.-R.; Yang, Y.-P.; Chiou, S.-H.; Ong, A.H.-K. The study of cancer cell in stromal environment through induced pluripotent stem cell–derived mesenchymal stem cells. J. Chin. Med. Assoc. 2022, 85, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Sheyn, D.; Ben-David, S.; Shapiro, G.; De Mel, S.; Bez, M.; Ornelas, L.; Sahabian, A.; Sareen, D.; Da, X.; Pelled, G.; et al. Human Induced Pluripotent Stem Cells Differentiate Into Functional Mesenchymal Stem Cells and Repair Bone Defects. Stem Cells Transl. Med. 2016, 5, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Era, T.; Nakao, K.; Kondo, S.; Kasuga, M.; Smith, A.G.; Nishikawa, S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell 2007, 129, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.; Chambers, S.M.; Tomishima, M.J.; Studer, L. Derivation of neural crest cells from human pluripotent stem cells. Nat. Protoc. 2010, 5, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Shakhova, O.; Sommer, L. Neural crest-derived stem cells. In StemBook; Harvard Stem Cell Institute: Cambridge, MA, USA, 2008. [Google Scholar]
- Mayor, R.; Theveneau, E. The neural crest. Development 2013, 140, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Menendez, L.; Yatskievych, T.A.; Antin, P.B.; Dalton, S. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc. Natl. Acad. Sci. USA 2011, 108, 19240. [Google Scholar] [CrossRef] [PubMed]
- Yoshimatsu, M.; Ohnishi, H.; Zhao, C.; Hayashi, Y.; Kuwata, F.; Kaba, S.; Okuyama, H.; Kawai, Y.; Hiwatashi, N.; Kishimoto, Y.; et al. In vivo regeneration of rat laryngeal cartilage with mesenchymal stem cells derived from human induced pluripotent stem cells via neural crest cells. Stem Cell Res. 2021, 52, 102233. [Google Scholar] [CrossRef]
- Chambers, S.M.; Fasano, C.A.; Papapetrou, E.P.; Tomishima, M.; Sadelain, M.; Studer, L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009, 27, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Madhu, V.; Dighe, A.S.; Cui, Q.; Deal, D.N. Dual Inhibition of Activin/Nodal/TGF-β and BMP Signaling Pathways by SB431542 and Dorsomorphin Induces Neuronal Differentiation of Human Adipose Derived Stem Cells. Stem Cells Int. 2016, 2016, 1035374. [Google Scholar] [CrossRef] [PubMed]
- Horbelt, D.; Boergermann, J.H.; Chaikuad, A.; Alfano, I.; Williams, E.; Lukonin, I.; Timmel, T.; Bullock, A.N.; Knaus, P. Small Molecules Dorsomorphin and LDN-193189 Inhibit Myostatin/GDF8 Signaling and Promote Functional Myoblast Differentiation*. J. Biol. Chem. 2015, 290, 3390–3404. [Google Scholar] [CrossRef] [PubMed]
- Chijimatsu, R.; Ikeya, M.; Yasui, Y.; Ikeda, Y.; Ebina, K.; Moriguchi, Y.; Shimomura, K.; Hart, D.A.; Hideki, Y.; Norimasa, N. Characterization of Mesenchymal Stem Cell-Like Cells Derived From Human iPSCs via Neural Crest Development and Their Application for Osteochondral Repair. Stem Cells Int. 2017, 2017, 1960965. [Google Scholar] [CrossRef] [PubMed]
- Betters, E.; Liu, Y.; Kjaeldgaard, A.; Sundström, E.; García-Castro, M.I. Analysis of early human neural crest development. Dev. Biol. 2010, 344, 578–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.A.; Cheung, M. Neural crest stem cells and their potential therapeutic applications. Dev. Biol. 2016, 419, 199–216. [Google Scholar] [CrossRef] [PubMed]
- Curchoe, C.L.; Maurer, J.; McKeown, S.J.; Cattarossi, G.; Cimadamore, F.; Nilbratt, M.; Snyder, E.Y.; Bronner-Fraser, M.; Terskikh, A.V. Early acquisition of neural crest competence during hESCs neuralization. PLoS ONE 2010, 5, e13890. [Google Scholar] [CrossRef]
- Nottoli, T.; Hagopian-Donaldson, S.; Zhang, J.; Perkins, A.; Williams, T. AP-2-null cells disrupt morphogenesis of the eye, face, and limbs in chimeric mice. Proc. Natl. Acad. Sci. USA 1998, 95, 13714–13719. [Google Scholar] [CrossRef]
- Fan, Z.; Yamaza, T.; Lee, J.S.; Yu, J.; Wang, S.; Fan, G.; Shi, S.; Wang, C.Y. BCOR regulates mesenchymal stem cell function by epigenetic mechanisms. Nat. Cell Biol. 2009, 11, 1002–1009. [Google Scholar] [CrossRef]
- Lin, X.; Yang, H.; Wang, L.; Li, W.; Diao, S.; Du, J.; Wang, S.; Dong, R.; Li, J.; Fan, Z. AP2a enhanced the osteogenic differentiation of mesenchymal stem cells by inhibiting the formation of YAP/RUNX2 complex and BARX1 transcription. Cell Prolif. 2019, 52, e12522. [Google Scholar] [CrossRef]
- Nishino, K.; Arai, Y.; Takasawa, K.; Toyoda, M.; Yamazaki-Inoue, M.; Sugawara, T.; Akutsu, H.; Nishimura, K.; Ohtaka, M.; Nakanishi, M.; et al. Epigenetic-scale comparison of human iPSCs generated by retrovirus, Sendai virus or episomal vectors. Regen. Ther. 2018, 9, 71–78. [Google Scholar] [CrossRef]
- Fekete, N.; Gadelorge, M.; Fürst, D.; Maurer, C.; Dausend, J.; Fleury-Cappellesso, S.; Mailänder, V.; Lotfi, R.; Ignatius, A.; Sensebé, L.; et al. Platelet lysate from whole blood-derived pooled platelet concentrates and apheresis-derived platelet concentrates for the isolation and expansion of human bone marrow mesenchymal stromal cells: Production process, content and identification of active components. Cytotherapy 2012, 14, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Jonsdottir-Buch, S.M.; Lieder, R.; Sigurjonsson, O.E. Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells. PLoS ONE 2013, 8, e68984. [Google Scholar] [CrossRef]
- Xu, M.; Shaw, G.; Murphy, M.; Barry, F. Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells Are Functionally and Genetically Different From Bone Marrow-Derived Mesenchymal Stromal Cells. Stem Cells 2019, 37, 754–765. [Google Scholar] [CrossRef] [PubMed]
- Diederichs, S.; Tuan, R.S. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev. 2014, 23, 1594–1610. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.R.; Kim, S.; Shin, S.; Jeong, S.Y.; Lee, D.W.; Lim, S.U.; Kang, J.Y.; Son, M.Y.; Lee, C.; Yu, K.R.; et al. iPSC-Derived MSCs Are a Distinct Entity of MSCs with Higher Therapeutic Potential than Their Donor-Matched Parental MSCs. Int. J. Mol. Sci. 2023, 24, 881. [Google Scholar] [CrossRef] [PubMed]
- Glaeser, J.D.; Behrens, P.; Stefanovic, T.; Salehi, K.; Papalamprou, A.; Tawackoli, W.; Metzger, M.F.; Eberlein, S.; Nelson, T.; Arabi, Y.; et al. Neural crest-derived mesenchymal progenitor cells enhance cranial allograft integration. Stem Cells Transl. Med. 2021, 10, 797–809. [Google Scholar] [CrossRef]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef]
- Gioia, R.; Panaroni, C.; Besio, R.; Palladini, G.; Merlini, G.; Giansanti, V.; Scovassi, I.A.; Villani, S.; Villa, I.; Villa, A.; et al. Impaired osteoblastogenesis in a murine model of dominant osteogenesis imperfecta: A new target for osteogenesis imperfecta pharmacological therapy. Stem Cells 2012, 30, 1465–1476. [Google Scholar] [CrossRef]
- Kaneto, C.M.; Pereira Lima, P.S.; Prata, K.L.; Dos Santos, J.L.; de Pina Neto, J.M.; Panepucci, R.A.; Noushmehr, H.; Covas, D.T.; de Paula, F.J.A.; Silva, W.A., Jr. Gene expression profiling of bone marrow mesenchymal stem cells from Osteogenesis Imperfecta patients during osteoblast differentiation. Eur. J. Med. Genet. 2017, 60, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Liu, Y.; Jing, Y.; Zhao, Y.; Shao, C.; Fu, T.; Wang, Z.; Li, G. Impaired proliferation of growth plate chondrocytes in a model of osteogenesis imperfecta. Biochem. Biophys. Res. Commun. 2022, 613, 146–152. [Google Scholar] [CrossRef]
- Claeys, L.; Zhytnik, L.; Wisse, L.E.; van Essen, H.W.; Eekhoff, E.M.W.; Pals, G.; Bravenboer, N.; Micha, D. Exploration of the skeletal phenotype of the Col1a1 (+/Mov13) mouse model for haploinsufficient osteogenesis imperfecta type 1. Front. Endocrinol. 2023, 14, 1145125. [Google Scholar] [CrossRef]
- Deyle, D.R.; Khan, I.F.; Ren, G.; Wang, P.R.; Kho, J.; Schwarze, U.; Russell, D.W. Normal collagen and bone production by gene-targeted human osteogenesis imperfecta iPSCs. Mol. Ther. 2012, 20, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Duangchan, T.; Tawonsawatruk, T.; Angsanuntsukh, C.; Trachoo, O.; Hongeng, S.; Kitiyanant, N.; Supokawej, A. Amelioration of osteogenesis in iPSC-derived mesenchymal stem cells from osteogenesis imperfecta patients by endoplasmic reticulum stress inhibitor. Life Sci. 2021, 278, 119628. [Google Scholar] [CrossRef] [PubMed]
- Fus-Kujawa, A.; Mendrek, B.; Bajdak-Rusinek, K.; Diak, N.; Strzelec, K.; Gutmajster, E.; Janelt, K.; Kowalczuk, A.; Trybus, A.; Rozwadowska, P.; et al. Gene-repaired iPS cells as novel approach for patient with osteogenesis imperfecta. Front. Bioeng. Biotechnol. 2023, 11, 1205122. [Google Scholar] [CrossRef]
- Takeyari, S.; Kubota, T.; Ohata, Y.; Fujiwara, M.; Kitaoka, T.; Taga, Y.; Mizuno, K.; Ozono, K. 4-Phenylbutyric acid enhances the mineralization of osteogenesis imperfecta iPSC-derived osteoblasts. J. Biol. Chem. 2021, 296, 100027. [Google Scholar] [CrossRef]
- Mabotuwana, N.S.; Rech, L.; Lim, J.; Hardy, S.A.; Murtha, L.A.; Rainer, P.P.; Boyle, A.J. Paracrine Factors Released by Stem Cells of Mesenchymal Origin and their Effects in Cardiovascular Disease: A Systematic Review of Pre-clinical Studies. Stem Cell Rev. Rep. 2022, 18, 2606–2628. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Yan, J.; Yao, Z.; Zhang, C.; Li, X.; Mao, H.Q. Effects of Mesenchymal Stem Cell-Derived Paracrine Signals and Their Delivery Strategies. Adv. Healthc. Mater. 2021, 10, e2001689. [Google Scholar] [CrossRef] [PubMed]
- Nadadhur, A.G.; Alsaqati, M.; Gasparotto, L.; Cornelissen-Steijger, P.; van Hugte, E.; Dooves, S.; Harwood, A.J.; Heine, V.M. Neuron-Glia Interactions Increase Neuronal Phenotypes in Tuberous Sclerosis Complex Patient iPSC-Derived Models. Stem Cell Rep. 2019, 12, 42–56. [Google Scholar] [CrossRef]
- Holmes, D.B.; Heine, V.M. Simplified 3D protocol capable of generating early cortical neuroepithelium. Biol. Open 2017, 6, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Cayami, F.K.; Claeys, L.; de Ruiter, R.; Smilde, B.J.; Wisse, L.; Bogunovic, N.; Riesebos, E.; Eken, L.; Kooi, I.; Sistermans, E.A.; et al. Osteogenic transdifferentiation of primary human fibroblasts to osteoblast-like cells with human platelet lysate. Sci. Rep. 2022, 12, 14686. [Google Scholar] [CrossRef]


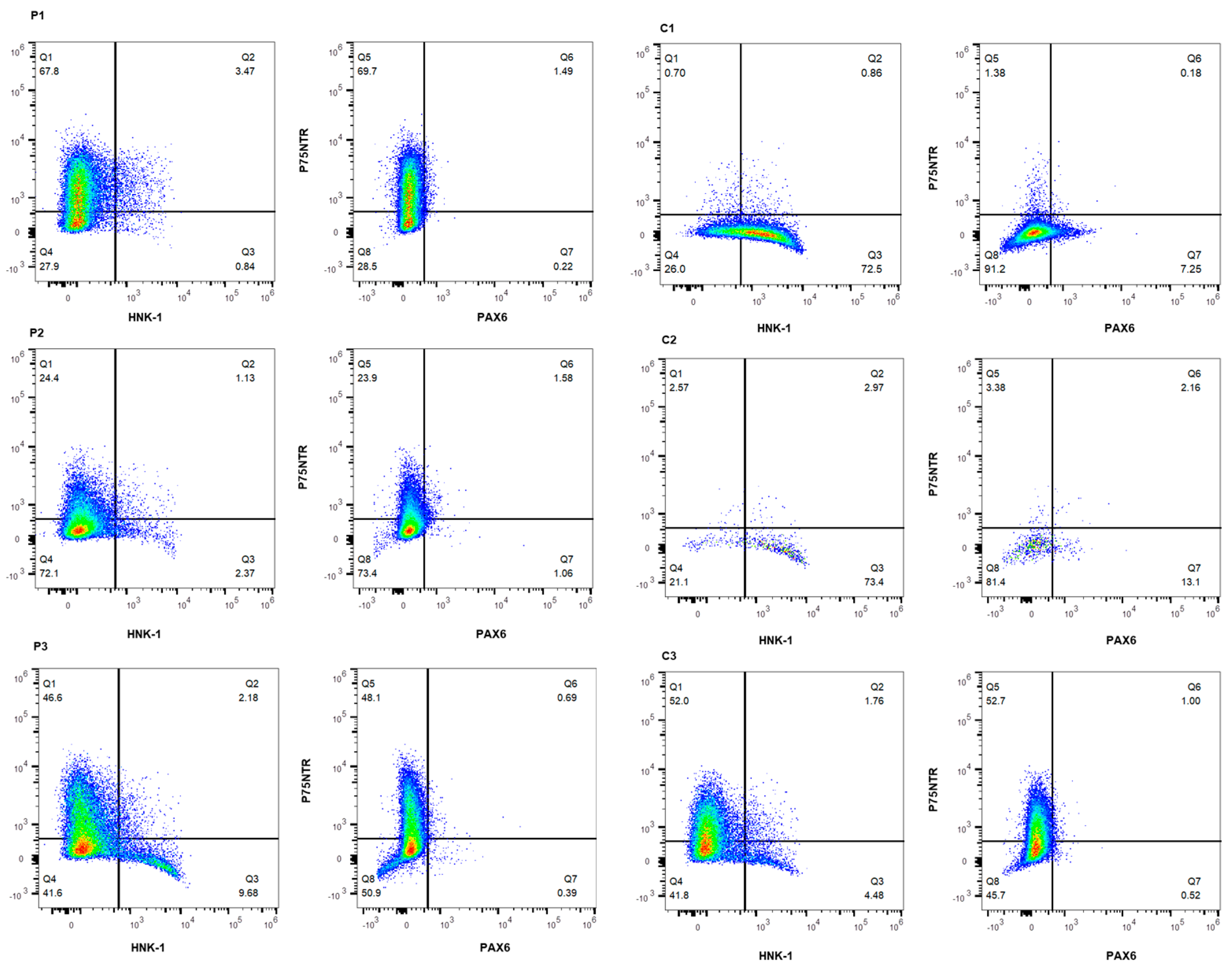

| Cell Line | P75NTR+ | P75NTR+/HNK-1+ | HNK-1+ | P75NTR-/HNK-1− |
| C1 | 0.70% | 0.86% | 72.5% | 26.0% |
| C2 | 2.57% | 2.97% | 73.4% | 21.1% |
| C3 | 52.0% | 1.76% | 4.48% | 41.8% |
| P1 | 67.8% | 3.47% | 0.84% | 27.9% |
| P2 | 24.4% | 1.13% | 2.37% | 72.1% |
| P3 | 46.6% | 2.18% | 9.68% | 41.6% |
| Cell Line | P75NTR+ | P75NTR+/PAX6+ | PAX6+ | P75NTR-/PAX6− |
| C1 | 1.38% | 0.18% | 7.25% | 91.2% |
| C2 | 3.38% | 2.16% | 13.1% | 81.4% |
| C3 | 52.7% | 1.00% | 0.52% | 45.7% |
| P1 | 69.7% | 1.49% | 0.22% | 28.5% |
| P2 | 23.9% | 1.58% | 1.06% | 73.4% |
| P3 | 48.1% | 0.69% | 0.39% | 50.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claeys, L.; Zhytnik, L.; Ventura, L.; Wisse, L.E.; Eekhoff, E.M.W.; Pals, G.; Bravenboer, N.; Heine, V.M.; Micha, D. In Vitro Modelling of Osteogenesis Imperfecta with Patient-Derived Induced Mesenchymal Stem Cells. Int. J. Mol. Sci. 2024, 25, 3417. https://doi.org/10.3390/ijms25063417
Claeys L, Zhytnik L, Ventura L, Wisse LE, Eekhoff EMW, Pals G, Bravenboer N, Heine VM, Micha D. In Vitro Modelling of Osteogenesis Imperfecta with Patient-Derived Induced Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2024; 25(6):3417. https://doi.org/10.3390/ijms25063417
Chicago/Turabian StyleClaeys, Lauria, Lidiia Zhytnik, Laura Ventura, Lisanne E. Wisse, Elisabeth M. W. Eekhoff, Gerard Pals, Nathalie Bravenboer, Vivi M. Heine, and Dimitra Micha. 2024. "In Vitro Modelling of Osteogenesis Imperfecta with Patient-Derived Induced Mesenchymal Stem Cells" International Journal of Molecular Sciences 25, no. 6: 3417. https://doi.org/10.3390/ijms25063417






