Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration
Abstract
1. Introduction to Molecular Signaling and Cell Fates
2. Chronic Inflammation Is Constantly Associated with Neurodegeneration and Cancer
3. Patients Who Survive Cancer Do Not Develop AD
4. WWOX Exhibits Multiple Functional Properties
4.1. WWOX Primary Structure and Binding Partners
4.2. WWOX Is Not a Typical Tumor Suppressor
4.3. pY33-WWOX Maintains Normal Mitochondrial Physiology
4.4. Overexpressed pY33-WWOX Induces Apoptosis Which Is Unfavorable for Neurons But Beneficial for Eliminating Cancer Cells In Vivo
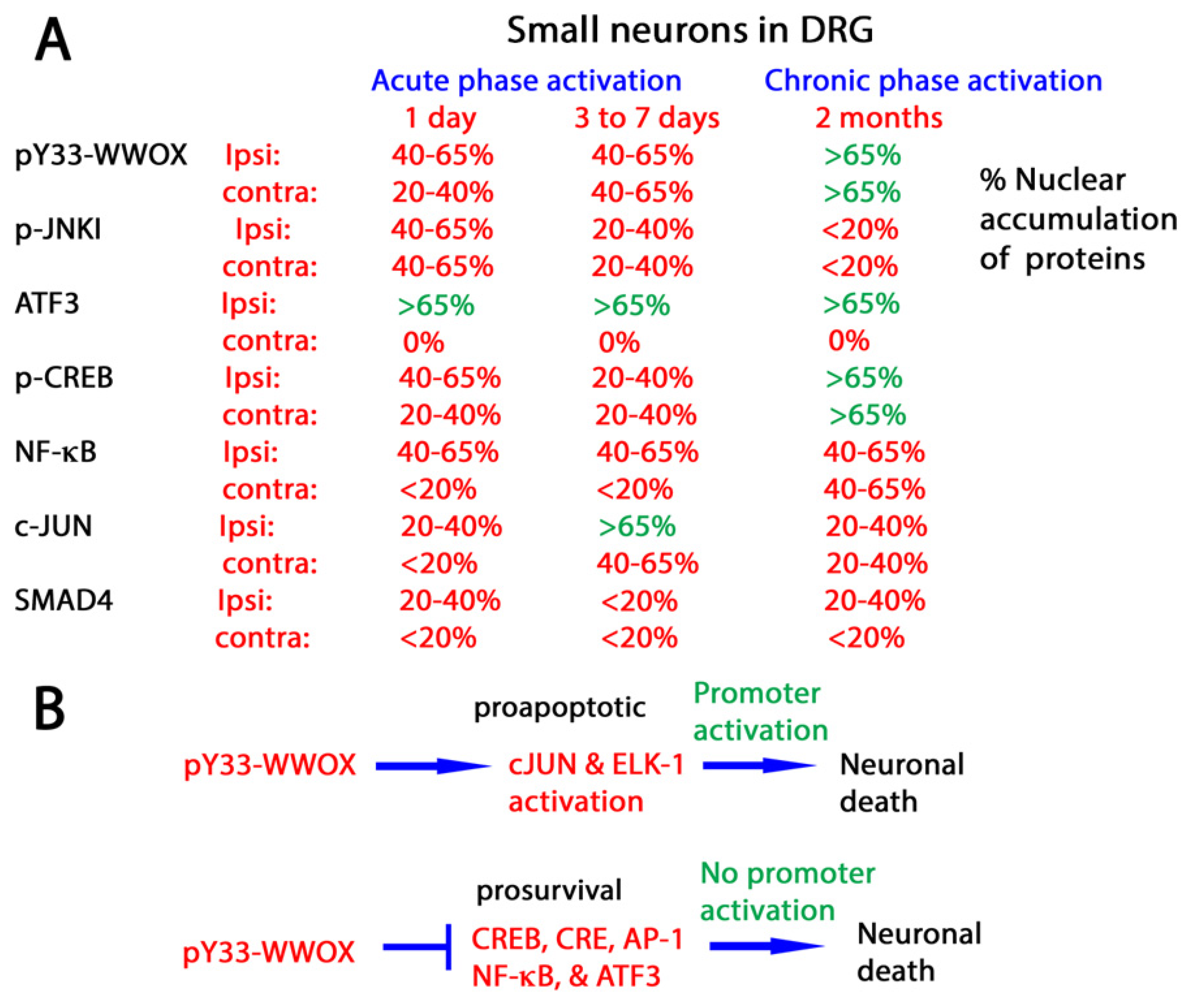
4.5. WWOX Modulates the Activities of Transcription Factors to Determine Neuronal Survival or Death
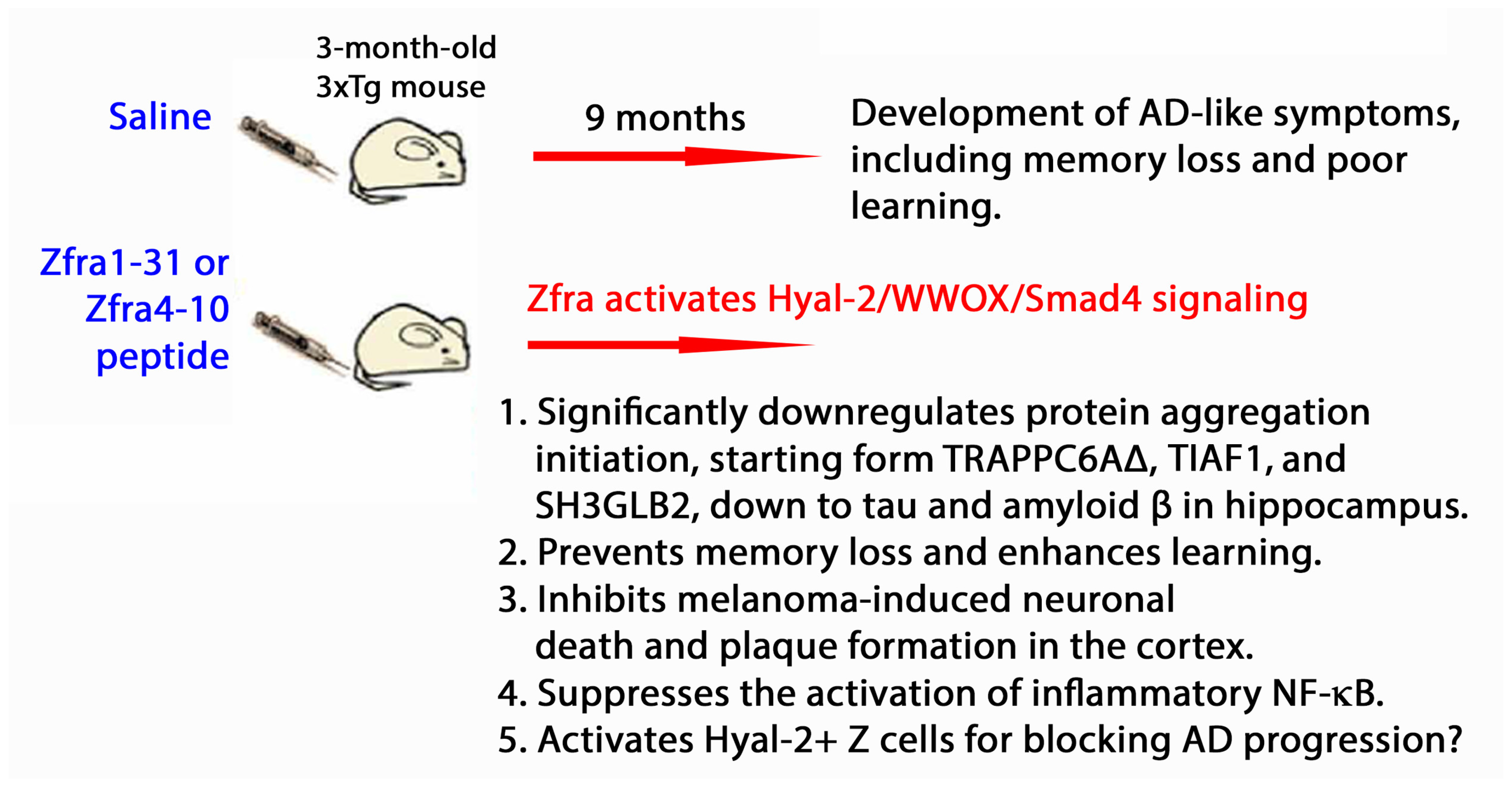
5. Zfra4-10 Activates Hyal-2/WWOX/Smad4 Signaling Pathway for Retardation of Neurodegeneration
5.1. Zfra Induces Activation of Spleen Hyal-2+ CD3- CD19- Z Cell
5.2. Zfra Restores Memory Loss in Triple Transgenic Mice for AD
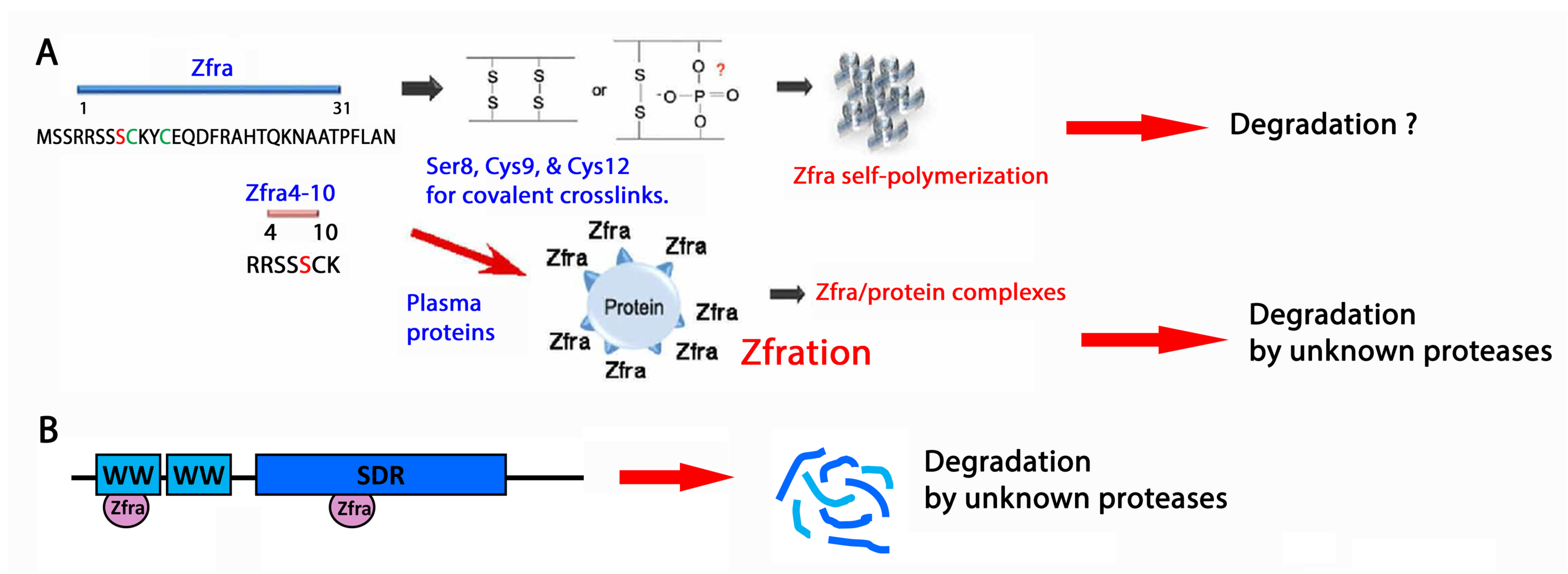
5.3. Zfra Physically Interacts with Endogenous Proteins for Degradation
5.4. Zfra4-10 Covalently Interacts with WWOX7-21
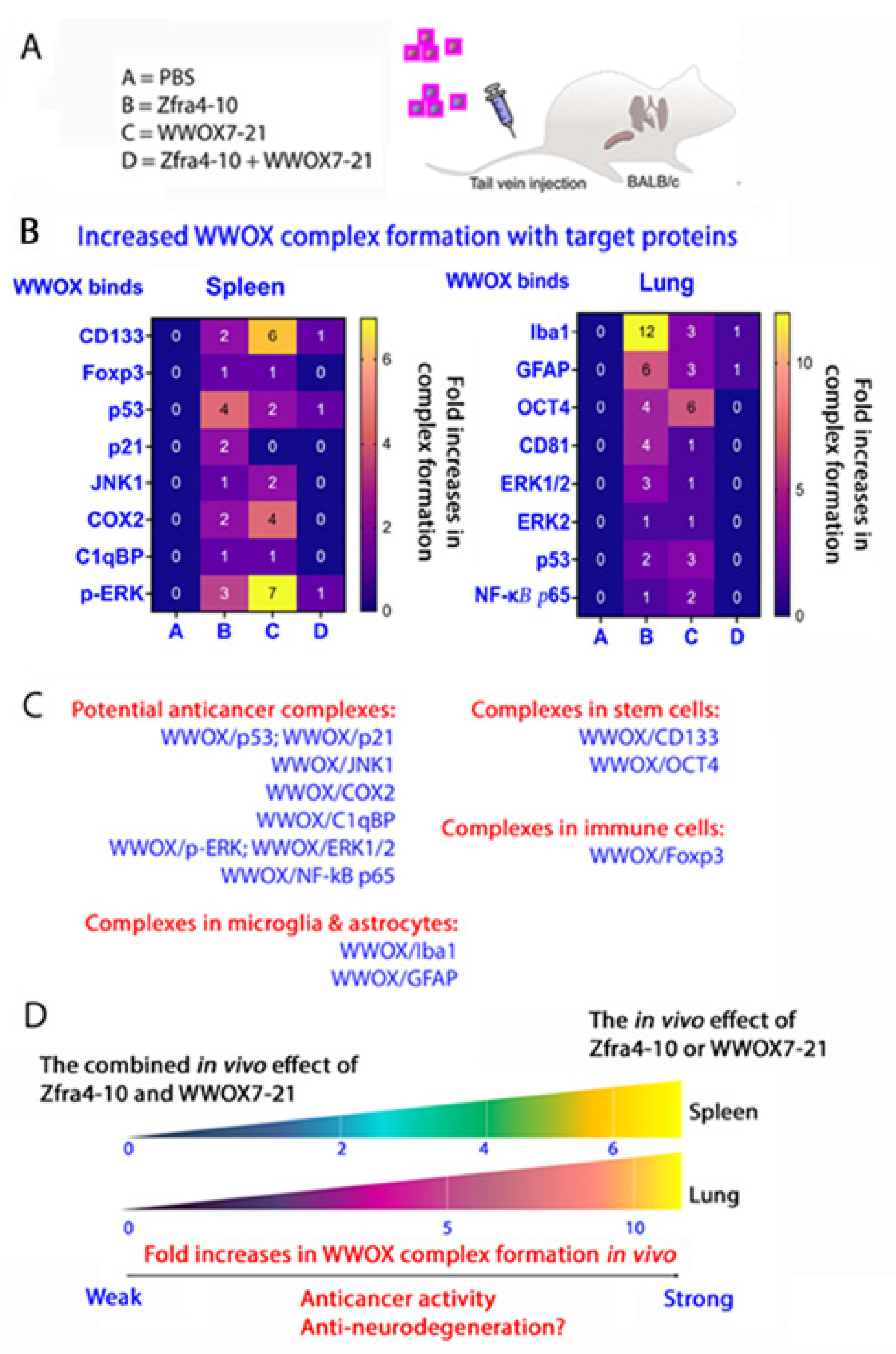
5.5. Zfra4-10 and WWOX7-21 Strengthen the Binding of Endogenous WWOX with Target Proteins in Stem Cells, Microglia Cells, Astrocytes, and Treg Cells and in Exosomes In Vivo
5.6. Inhibition of S14 Phosphorylation in WWOX by Zfra4-10 or WWOX7-21 Leads to Reduced Neurodegeneration
6. Dramatic Upregulation of pY33- and pY287-WWOX in the Brain Cortex of Heterozygous Wwox Mice
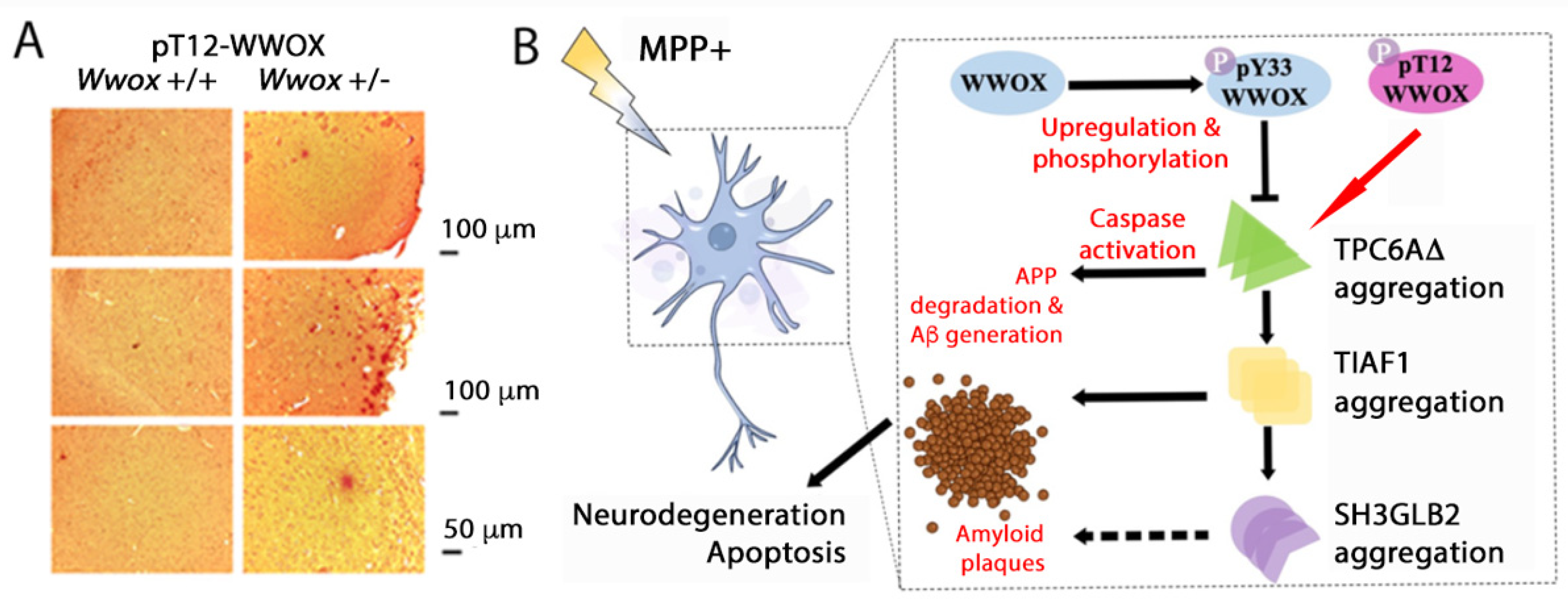
6.1. pT12-WWOX Is an Aggregated form in the AD Brain Lesions
6.2. pT12- and pS14-WWOX in Controlling Protein Aggregation?
7. WWOX in Embryonic Development and AD Progression
8. WWOX Antagonizes p53 for Inducing Neurodegeneration In Vivo
9. Membrane Epitopes WWOX7-21 and WWOX286-299 and Functional Implications
9.1. WWOX7-21 Epitope Confers Cancer Suppression and Probably Blocks AD Progression
9.2. WWOX7-21 and WWOX286-299, along with Membrane Type II TGFβ Receptor (TβRII), Control Cell Migration and Cell-Cell Recognition
9.3. WWOX in Cortical Neuron Migration and Loss of WWOX Causes Neuronal Heterotopia
10. Z Cells Have Memory Function in Eliminating Cancer Cells
10.1. Z Cell Activation by Zfra4-10, WWOX7-21, and Sonicated Hyaluronan HAson8 to Kill Cancer Cells and Retard AD Progression
10.2. Zfra4-10 Activates Z Cells in Immune Deficient NOD-SCID Mice
10.3. Zfra1-31 for Treating Hyperglycemia/Diabetes-Associated Neurodegeneration
11. Summary and Concluding Remarks
11.1. pT12-WWOX as an Initiator of Protein Aggregation Cascade?
11.2. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Navas, L.E.; Carnero, A. NAD(+) metabolism, stemness, the immune response, and cancer. Signal Transduct. Target. Ther. 2021, 6, 2. [Google Scholar] [CrossRef]
- Alberti, S.; Dormann, D. Liquid-Liquid Phase Separation in Disease. Annu. Rev. Genet. 2019, 53, 171–194. [Google Scholar] [CrossRef]
- Taniue, K.; Akimitsu, N. Aberrant phase separation and cancer. FEBS J. 2022, 289, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.C.C.; Rebouças, C.; Oliveira, L.F.; Cardoso, F.D.S.; Nascimento, T.S.; Oliveira, A.V.; Lima, M.P.P.; de Andrade, G.M.; de Castro Brito, G.A.; de Barros Viana, G.S. The role of gut-brain axis in a rotenone-induced rat model of Parkinson’s disease. Neurobiol. Aging 2023, 132, 185–197. [Google Scholar] [CrossRef]
- Sun, N.; Victor, M.B.; Park, Y.P.; Xiong, X.; Scannail, A.N.; Leary, N.; Prosper, S.; Viswanathan, S.; Luna, X.; Boix, C.A.; et al. Human microglial state dynamics in Alzheimer’s disease progression. Cell 2023, 186, 4386–4403. [Google Scholar] [CrossRef]
- Shi, X.; Li, L.; Liu, Z.; Wang, F.; Huang, H. Exploring the mechanism of metformin action in Alzheimer’s disease and type 2 diabetes based on network pharmacology, molecular docking, and molecular dynamic simulation. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231187493. [Google Scholar] [CrossRef]
- Gulen, M.F.; Samson, N.; Keller, A.; Schwabenland, M.; Liu, C.; Glück, S.; Thacker, V.V.; Favre, L.; Mangeat, B.; Kroese, L.J.; et al. cGAS-STING drives ageing-related inflammation and neurodegeneration. Nature 2023, 620, 374–380. [Google Scholar] [CrossRef]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef] [PubMed]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.F.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H.; Nabavi, S.M. Molecular targets of curcumin for cancer therapy: An updated review. Tumour Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Chen, T.H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.F. Pharmacological properties and underlying mechanisms of curcumin and prospects in medicinal potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef] [PubMed]
- Juric, M.; Rawat, V.; Amaradhi, R.; Zielonka, J.; Ganesh, T. Novel NADPH Oxidase-2 Inhibitors as Potential Anti-Inflammatory and Neuroprotective Agents. Antioxidants 2023, 12, 1660. [Google Scholar] [CrossRef] [PubMed]
- Shamsnia, H.; Samanian, A.; Sabbagh Kashani, A.; Khayatan, D.; Momtaz, S.; Johnston, T.P.; Majeed, M.; Jamialahmadi, T.; Abdolghaffari, A.H.; Sahebkar, A. An Overview of the Pharmacological Properties of Calebin-A. Curr. Med. Chem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Mueller, A.L.; Brockmueller, A.; Kunnumakkara, A.B.; Shakibaei, M. Calebin A, a Compound of Turmeric, Down-Regulates Inflammation in Tenocytes by NF-κB/Scleraxis Signaling. Int. J. Mol. Sci. 2022, 23, 1695. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, D.S. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: A drug discovery effort against Alzheimer’s disease. J. Nat. Prod. 2002, 65, 1227–1231. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, J.Y. Total synthesis of Calebin-A, preparation of its analogues, and their neuronal cell protectivity against beta-amyloid insult. Bioorg. Med. Chem. Lett. 2001, 11, 2541–2543. [Google Scholar] [CrossRef]
- Lanni, C.; Masi, M.; Racchi, M.; Govoni, S. Cancer and Alzheimer’s disease inverse relationship: An age-associated diverging derailment of shared pathways. Mol. Psychiatry 2021, 26, 280–295. [Google Scholar] [CrossRef]
- Zabłocka, A.; Kazana, W.; Sochocka, M.; Stańczykiewicz, B.; Janusz, M.; Leszek, J.; Orzechowska, B. Inverse Correlation between Alzheimer’s Disease and Cancer: Short Overview. Mol. Neurobiol. 2021, 58, 6335–6349. [Google Scholar] [CrossRef]
- Driver, J.A. Inverse association between cancer and neurodegenerative disease: Review of the epidemiologic and biological evidence. Biogerontology 2014, 15, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Chang, N.S. WWOX dysfunction induces sequential aggregation of TRAPPC6AΔ, TIAF1, tau and amyloid β, and causes apoptosis. Cell Death. Discov. 2015, 1, 15003. [Google Scholar] [CrossRef]
- Sze, C.I.; Su, M.; Pugazhenthi, S.; Jambal, P.; Hsu, L.J.; Heath, J.; Schultz, L.; Chang, N.S. Down-regulation of WW domain-containing oxidoreductase induces Tau phosphorylation in vitro. A potential role in Alzheimer’s disease. J. Biol. Chem. 2004, 279, 30498–30506. [Google Scholar] [CrossRef] [PubMed]
- Pfoertner, S.; Jeron, A.; Probst-Kepper, M.; Guzman, C.A.; Hansen, W.; Westendorf, A.M.; Toepfer, T.; Schrader, A.J.; Franzke, A.; Buer, J.; et al. Signatures of human regulatory T cells: An encounter with old friends and new players. Genome Biol. 2006, 7, R54. [Google Scholar] [CrossRef]
- Chang, J.Y.; Chiang, M.F.; Lin, S.R.; Lee, M.H.; He, H.; Chou, P.Y.; Chen, S.J.; Chen, Y.A.; Yang, L.Y.; Lai, F.J.; et al. TIAF1 self-aggregation in peritumor capsule formation, spontaneous activation of SMAD-responsive promoter in p53-deficient environment, and cell death. Cell Death. Dis. 2012, 3, e302. [Google Scholar] [CrossRef]
- Lee, M.H.; Lin, S.R.; Chang, J.Y.; Schultz, L.; Heath, J.; Hsu, L.J.; Kuo, Y.M.; Hong, Q.; Chiang, M.F.; Gong, C.X.; et al. TGF-β induces TIAF1 self-aggregation via type II receptor-independent signaling that leads to generation of amyloid β plaques in Alzheimer’s disease. Cell Death. Dis. 2010, 1, e110. [Google Scholar] [CrossRef]
- Advani, D.; Gupta, R.; Tripathi, R.; Sharma, S.; Ambasta, R.K.; Kumar, P. Protective role of anticancer drugs in neurodegenerative disorders: A drug repurposing approach. Neurochem. Int. 2020, 140, 104841. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, A.K.; Laflin, K.J.; Daniel, R.L.; Liao, Q.; Hawkins, K.A.; Aldaz, C.M. WWOX, a novel WW domain-containing protein mapping to human chromosome 16q23.3-24.1, a region frequently affected in breast cancer. Cancer Res. 2000, 60, 2140–2145. [Google Scholar]
- Ried, K.; Finnis, M.; Hobson, L.; Mangelsdorf, M.; Dayan, S.; Nancarrow, J.K.; Woollatt, E.; Kremmidiotis, G.; Gardner, A.; Venter, D.; et al. Common chromosomal fragile site FRA16D sequence: Identification of the FOR gene spanning FRA16D and homozygous deletions and translocation breakpoints in cancer cells. Hum. Mol. Genet. 2000, 9, 1651–1663. [Google Scholar] [CrossRef]
- Chang, N.S.; Pratt, N.; Heath, J.; Schultz, L.; Sleve, D.; Carey, G.B.; Zevotek, N. Hyaluronidase induction of a WW domain-containing oxidoreductase that enhances tumor necrosis factor cytotoxicity. J. Biol. Chem. 2001, 276, 3361–3370. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Pan, W.; Yu, Y.; Huang, W.; Gao, J.; Zhang, Y.; Zhang, S. WWOX promotes apoptosis and inhibits autophagy in paclitaxel-treated ovarian carcinoma cells. Mol. Med. Rep. 2021, 23, 115. [Google Scholar] [CrossRef]
- Taouis, K.; Driouch, K.; Lidereau, R.; Lallemand, F. Molecular Functions of WWOX Potentially Involved in Cancer Development. Cells 2021, 10, 1051. [Google Scholar] [CrossRef]
- Iliopoulos, D.; Guler, G.; Han, S.Y.; Druck, T.; Ottey, M.; McCorkell, K.A.; Huebner, K. Roles of FHIT and WWOX fragile genes in cancer. Cancer Lett. 2006, 232, 27–36. [Google Scholar] [CrossRef]
- Smith, D.I.; McAvoy, S.; Zhu, Y.; Perez, D.S. Large common fragile site genes and cancer. Semin. Cancer Biol. 2007, 17, 31–41. [Google Scholar] [CrossRef]
- Chang, N.S.; Hsu, L.J.; Lin, Y.S.; Lai, F.J.; Sheu, H.M. WW domain-containing oxidoreductase: A candidate tumor suppressor. Trends Mol. Med. 2007, 13, 12–22. [Google Scholar] [CrossRef]
- Gardenswartz, A.; Aqeilan, R.I. WW domain-containing oxidoreductase’s role in myriad cancers: Clinical significance and future implications. Exp. Biol. Med. 2014, 239, 253–263. [Google Scholar] [CrossRef]
- Aldaz, C.M.; Hussain, T. WWOX Loss of Function in Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 8922. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Joy-Dodson, E.; Schueler-Furman, O.; Aqeilan, R.I. Pleiotropic Functions of Tumor Suppressor WWOX in Normal and Cancer Cells. J. Biol. Chem. 2015, 290, 30728–30735. [Google Scholar] [CrossRef] [PubMed]
- Bonin, F.; Taouis, K.; Azorin, P.; Petitalot, A.; Tariq, Z.; Nola, S.; Bouteille, N.; Tury, S.; Vacher, S.; Bièche, I.; et al. VOPP1 promotes breast tumorigenesis by interacting with the tumor suppressor WWOX. BMC Biol. 2018, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Gharghabi, M.; Reczek, C.R.; Plow, R.; Yungvirt, C.; Aldaz, C.M.; Huebner, K. Wwox Binding to the Murine Brca1-BRCT Domain Regulates Timing of Brip1 and CtIP Phospho-Protein Interactions with This Domain at DNA Double-Strand Breaks, and Repair Pathway Choice. Int. J. Mol. Sci. 2022, 23, 3729. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Sun, L.; Mu, Z.; Huang, Y.; Fu, C.; Hu, B. Ectopic WWOX Expression Inhibits Growth of 5637 Bladder Cancer Cell In Vitro and In Vivo. Cell Biochem. Biophys. 2015, 73, 417–425. [Google Scholar] [CrossRef]
- Liu, C.W.; Chen, P.H.; Yu, T.J.; Lin, K.J.; Chang, L.C. WWOX Modulates ROS-Dependent Senescence in Bladder Cancer. Molecules 2022, 27, 7388. [Google Scholar] [CrossRef]
- Cheng, H.C.; Huang, P.H.; Lai, F.J.; Jan, M.S.; Chen, Y.L.; Chen, S.Y.; Chen, W.L.; Hsu, C.K.; Huang, W.; Hsu, L.J. Loss of fragile WWOX gene leads to senescence escape and genome instability. Cell. Mol. Life Sci. 2023, 80, 338. [Google Scholar] [CrossRef]
- Gao, K.; Yin, J.; Dong, J. Deregulated WWOX is involved in a negative feedback loop with microRNA-214-3p in osteosarcoma. Int. J. Mol. Med. 2016, 38, 1850–1856. [Google Scholar] [CrossRef]
- Saigo, C.; Kito, Y.; Takeuchi, T. Cancerous Protein Network That Inhibits the Tumor Suppressor Function of WW Domain-Containing Oxidoreductase (WWOX) by Aberrantly Expressed Molecules. Front. Oncol. 2018, 8, 350. [Google Scholar] [CrossRef]
- Shaukat, Q.; Hertecant, J.; El-Hattab, A.W.; Ali, B.R.; Suleiman, J. West syndrome, developmental and epileptic encephalopathy, and severe CNS disorder associated with WWOX mutations. Epileptic Disord. 2018, 20, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Ho, P.C.; Lee, I.T.; Chen, Y.A.; Chu, C.H.; Teng, C.C.; Wu, S.N.; Sze, C.I.; Chiang, M.F.; Chang, N.S. WWOX Phosphorylation, Signaling, and Role in Neurodegeneration. Front. Neurosci. 2018, 12, 563. [Google Scholar] [CrossRef]
- Chang, N.S.; Doherty, J.; Ensign, A. JNK1 physically interacts with WW domain-containing oxidoreductase (WOX1) and inhibits WOX1-mediated apoptosis. J. Biol. Chem. 2003, 278, 9195–9202. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.S.; Doherty, J.; Ensign, A.; Schultz, L.; Hsu, L.J.; Hong, Q. WOX1 is essential for tumor necrosis factor-, UV light-, staurosporine-, and p53-mediated cell death, and its tyrosine 33-phosphorylated form binds and stabilizes serine 46-phosphorylated p53. J. Biol. Chem. 2005, 280, 43100–43108. [Google Scholar] [CrossRef] [PubMed]
- Li, M.Y.; Lai, F.J.; Hsu, L.J.; Lo, C.P.; Cheng, C.L.; Lin, S.R.; Lee, M.H.; Chang, J.Y.; Subhan, D.; Tsai, M.S.; et al. Dramatic co-activation of WWOX/WOX1 with CREB and NF-kappaB in delayed loss of small dorsal root ganglion neurons upon sciatic nerve transection in rats. PLoS ONE 2009, 4, e7820. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Chuang, J.I.; Cheng, C.L.; Hsu, L.J.; Chang, N.S. Light-induced retinal damage involves tyrosine 33 phosphorylation, mitochondrial and nuclear translocation of WW domain-containing oxidoreductase in vivo. Neuroscience 2005, 130, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, M.; Salah, Z.; Herbel, C.; Hofmann, T.G.; Aqeilan, R.I. WWOX, the common fragile site FRA16D gene product, regulates ATM activation and the DNA damage response. Proc. Natl. Acad. Sci. USA 2014, 111, E4716–E4725. [Google Scholar] [CrossRef] [PubMed]
- Abu-Odeh, M.; Hereema, N.A.; Aqeilan, R.I. WWOX modulates the ATR-mediated DNA damage checkpoint response. Oncotarget 2016, 7, 4344–4355. [Google Scholar] [CrossRef][Green Version]
- Chen, S.J.; Lin, P.W.; Lin, H.P.; Huang, S.S.; Lai, F.J.; Sheu, H.M.; Hsu, L.J.; Chang, N.S. UV irradiation/cold shock-mediated apoptosis is switched to bubbling cell death at low temperatures. Oncotarget 2015, 6, 8007–8018. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.S. Bubbling cell death: A hot air balloon released from the nucleus in the cold. Exp. Biol. Med. 2016, 241, 1306–1315. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.J.; Hong, Q.; Chen, S.T.; Kuo, H.L.; Schultz, L.; Heath, J.; Lin, S.R.; Lee, M.H.; Li, D.Z.; Li, Z.L.; et al. Hyaluronan activates Hyal-2/WWOX/Smad4 signaling and causes bubbling cell death when the signaling complex is overexpressed. Oncotarget 2017, 8, 19137–19155. [Google Scholar] [CrossRef]
- Chen, Y.A.; Sie, Y.D.; Liu, T.Y.; Kuo, H.L.; Chou, P.Y.; Chen, Y.J.; Lee, K.T.; Chen, P.J.; Chen, S.T.; Chang, N.S. Normal cells repel WWOX-negative or -dysfunctional cancer cells via WWOX cell surface epitope 286–299. Commun. Biol. 2021, 4, 753. [Google Scholar] [CrossRef]
- Chang, N.S.; Doherty, J.; Ensign, A.; Lewis, J.; Heath, J.; Schultz, L.; Chen, S.T.; Oppermann, U. Molecular mechanisms underlying WOX1 activation during apoptotic and stress responses. Biochem. Pharmacol. 2003, 66, 1347–1354. [Google Scholar] [CrossRef]
- Chang, N.S.; Schultz, L.; Hsu, L.J.; Lewis, J.; Su, M.; Sze, C.I. 17beta-Estradiol upregulates and activates WOX1/WWOXv1 and WOX2/WWOXv2 in vitro: Potential role in cancerous progression of breast and prostate to a premetastatic state in vivo. Oncogene 2005, 24, 714–723. [Google Scholar] [CrossRef]
- Lin, H.P.; Chang, J.Y.; Lin, S.R.; Lee, M.H.; Huang, S.S.; Hsu, L.J.; Chang, N.S. Identification of an In Vivo MEK/WOX1 Complex as a Master Switch for Apoptosis in T Cell Leukemia. Genes Cancer 2011, 2, 550–562. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, S.S.; Su, W.P.; Lin, H.P.; Kuo, H.L.; Wei, H.L.; Chang, N.S. Role of WW Domain-containing Oxidoreductase WWOX in Driving T Cell Acute Lymphoblastic Leukemia Maturation. J. Biol. Chem. 2016, 291, 17319–17331. [Google Scholar] [CrossRef]
- Gaudio, E.; Palamarchuk, A.; Palumbo, T.; Trapasso, F.; Pekarsky, Y.; Croce, C.M.; Aqeilan, R.I. Physical association with WWOX suppresses c-Jun transcriptional activity. Cancer Res. 2006, 66, 11585–11589. [Google Scholar] [CrossRef]
- Aderca, I.; Moser, C.D.; Veerasamy, M.; Bani-Hani, A.H.; Bonilla-Guerrero, R.; Ahmed, K.; Shire, A.; Cazanave, S.C.; Montoya, D.P.; Mettler, T.A.; et al. The JNK inhibitor SP600129 enhances apoptosis of HCC cells induced by the tumor suppressor WWOX. J. Hepatol. 2008, 49, 373–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, S.J.; Buchthal, B.; Lau, D.; Hayer, S.; Dick, O.; Schwaninger, M.; Veltkamp, R.; Zou, M.; Weiss, U.; Bading, H. A signaling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage. J. Neurosci. 2011, 31, 4978–4990. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, Q.; Li, F.; Pang, R.; Chen, C.; Li, P.; Wang, X.; Xuan, W.; Yu, W. The multifaceted roles of activating transcription factor 3 (ATF3) in inflammatory responses—Potential target to regulate neuroinflammation in acute brain injury. J. Cereb. Blood Flow Metab. 2023, 43, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Piao, M.; Liu, N.; Gu, W.; Feng, C. Sevoflurane Exposure Induces Neuronal Cell Ferroptosis Initiated by Increase of Intracellular Hydrogen Peroxide in the Developing Brain via ER Stress ATF3 Activation. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Hashiuchi, E.; Watanabe, H.; Kimura, K.; Oshima, Y.; Tsuchiya, K.; Murai, S.; Takahashi, C.; Matsumoto, M.; Kitajima, S.; et al. The transcription factor ATF3 switches cell death from apoptosis to necroptosis in hepatic steatosis in male mice. Nat. Commun. 2023, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Wang, W.J.; Chang, J.Y.; Ho, P.C.; Liu, T.Y.; Wen, K.Y.; Kuo, H.L.; Chen, Y.J.; Huang, S.S.; Subhan, D.; et al. Therapeutic Zfra4-10 or WWOX7-21 Peptide Induces Complex Formation of WWOX with Selective Protein Targets in Organs that Leads to Cancer Suppression and Spleen Cytotoxic Memory Z Cell Activation In Vivo. Cancers 2020, 12, 2189. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.J.; Schultz, L.; Hong, Q.; Van Moer, K.; Heath, J.; Li, M.Y.; Lai, F.J.; Lin, S.R.; Lee, M.H.; Lo, C.P.; et al. Transforming growth factor beta1 signaling via interaction with cell surface Hyal-2 and recruitment of WWOX/WOX1. J. Biol. Chem. 2009, 284, 16049–16059. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Su, W.P.; Wang, W.J.; Lin, S.R.; Lu, C.Y.; Chen, Y.A.; Chang, J.Y.; Huang, S.S.; Chou, P.Y.; Ye, S.R.; et al. Zfra activates memory Hyal-2+ CD3- CD19- spleen cells to block cancer growth, stemness, and metastasis in vivo. Oncotarget 2015, 6, 3737–3751. [Google Scholar] [CrossRef]
- Lee, M.H.; Shih, Y.H.; Lin, S.R.; Chang, J.Y.; Lin, Y.H.; Sze, C.I.; Kuo, Y.M.; Chang, N.S. Zfra restores memory deficits in Alzheimer’s disease triple-transgenic mice by blocking aggregation of TRAPPC6AΔ, SH3GLB2, tau, and amyloid β, and inflammatory NF-κB activation. Alzheimers. Dement. 2017, 3, 189–204. [Google Scholar] [CrossRef]
- Lin, Y.H.; Shih, Y.H.; Yap, Y.V.; Chen, Y.W.; Kuo, H.L.; Liu, T.Y.; Hsu, L.J.; Kuo, Y.M.; Chang, N.S. Zfra inhibits the TRAPPC6AΔ-initiated pathway of neurodegeneration. Int. J. Mol. Sci. 2022, 23, 14510. [Google Scholar] [CrossRef]
- Hong, Q.; Hsu, L.J.; Schultz, L.; Pratt, N.; Mattison, J.; Chang, N.S. Zfra affects TNF-mediated cell death by interacting with death domain protein TRADD and negatively regulates the activation of NF-kappaB, JNK1, p53 and WOX1 during stress response. BMC Mol. Biol. 2007, 8, 50. [Google Scholar] [CrossRef]
- Wang, W.J.; Ho, P.C.; Nagarajan, G.; Chen, Y.A.; Kuo, H.L.; Subhan, D.; Su, W.P.; Chang, J.Y.; Lu, C.Y.; Chang, K.T.; et al. WWOX possesses N-terminal cell surface-exposed epitopes WWOX(7-21) and WWOX(7-11) for signaling cancer growth suppression and prevention in vivo. Cancers 2019, 11, 1818. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, Z.; Cui, Y. Unconventional T cells in brain homeostasis, injury and neurodegeneration. Front. Immunol. 2023, 14, 1273459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, X.; Li, H.; Wang, B.; Chen, P.; Meng, J. The roles of macrophage migration inhibitory factor in retinal diseases. Neural Regen. Res. 2024, 19, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.M.; Taher, N.A.; Doherty, D.G.; Molloy, E.J. The role of lymphocytes in neonatal encephalopathy. Brain Behav. Immun. Health 2021, 18, 100380. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.J.; Rinaldi, S.; Costigan, M.; Oh, S.B. Cytotoxic Immunity in Peripheral Nerve Injury and Pain. Front. Neurosci. 2020, 14, 142. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, N.P.; Whang, Y.E.; Mohler, J.L.; Earp, H.S. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: Role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer Res. 2005, 65, 10514–10523. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Park, J.H.; Park, J.H.; Lee, K.B.; Oh, S.M. Pc2-mediated SUMOylation of WWOX is essential for its suppression of DU145 prostate tumorigenesis. FEBS Lett. 2015, 589, 3977–3988. [Google Scholar] [CrossRef]
- Sze, C.I.; Kuo, Y.M.; Hsu, L.J.; Fu, T.F.; Chiang, M.F.; Chang, J.Y.; Chang, N.S. A cascade of protein aggregation bombards mitochondria for neurodegeneration and apoptosis under WWOX deficiency. Cell Death Dis. 2015, 6, e1881. [Google Scholar] [CrossRef]
- Chang, J.Y.; Lee, M.H.; Lin, S.R.; Yang, L.Y.; Sun, H.S.; Sze, C.I.; Hong, Q.; Lin, Y.S.; Chou, Y.T.; Hsu, L.J.; et al. Trafficking protein particle complex 6A delta (TRAPPC6AΔ) is an extracellular plaque-forming protein in the brain. Oncotarget 2015, 6, 3578–3589. [Google Scholar] [CrossRef]
- Piard, J.; Hawkes, L.; Milh, M.; Villard, L.; Borgatti, R.; Romaniello, R.; Fradin, M.; Capri, Y.; Héron, D.; Nougues, M.C.; et al. The phenotypic spectrum of WWOX-related disorders: 20 additional cases of WOREE syndrome and review of the literature. Genet. Med. 2019, 21, 1308–1318. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Chou, Y.T.; Lai, F.J.; Jan, M.S.; Chang, T.H.; Jou, I.M.; Chen, P.S.; Lo, J.Y.; Huang, S.S.; Chang, N.S.; et al. Wwox deficiency leads to neurodevelopmental and degenerative neuropathies and glycogen synthase kinase 3β-mediated epileptic seizure activity in mice. Acta Neuropathol. Commun. 2020, 8, 6. [Google Scholar] [CrossRef]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef]
- Liu, G.; Peng, J.; Liao, Z.; Locascio, J.J.; Corvol, J.C.; Zhu, F.; Dong, X.; Maple-Grødem, J.; Campbell, M.C.; Elbaz, A.; et al. Genome-wide survival study identifies a novel synaptic locus and polygenic score for cognitive progression in Parkinson’s disease. Nat. Genet. 2021, 53, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Juo, L.I.; Lin, Y.T.; Hsiao, M.; Lin, J.T.; Tsai, C.H.; Tzeng, Y.H.; Chuang, Y.C.; Chang, N.S.; Yang, C.N.; et al. WW domain-containing oxidoreductase promotes neuronal differentiation via negative regulation of glycogen synthase kinase 3β. Cell Death Differ. 2012, 19, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Lin, S.R.; Lee, M.H.; Schultz, L.; Sze, C.I.; Chang, N.S. A p53/TIAF1/WWOX triad exerts cancer suppression but may cause brain protein aggregation due to p53/WWOX functional antagonism. Cell Commun. Signal. 2019, 17, 76. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Ge, L.; Ding, X.; Chen, Y.; Zhu, H.; Ward, T.; Wu, F.; Cao, X.; Wang, Q.; Yao, X. PKA-mediated protein phosphorylation regulates ezrin-WWOX interaction. Biochem. Biophys. Res. Commun. 2006, 341, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.Y.; Lai, F.J.; Chen, Y.A.; Sie, Y.D.; Kuo, H.L.; Su, W.P.; Wu, C.Y.; Liu, T.Y.; Wen, K.Y.; Hsu, L.J.; et al. Strategies by which WWOX-deficient metastatic cancer cells utilize to survive via dodging, compromising, and causing damage to WWOX-positive normal microenvironment. Cell Death Discov. 2019, 5, 97. [Google Scholar] [CrossRef] [PubMed]
- Oliver, K.L.; Trivisano, M.; Mandelstam, S.A.; De Dominicis, A.; Francis, D.I.; Green, T.E.; Muir, A.M.; Chowdhary, A.; Hertzberg, C.; Goldhahn, K.; et al. WWOX developmental and epileptic encephalopathy: Understanding the epileptology and the mortality risk. Epilepsia 2023, 64, 1351–1367. [Google Scholar] [CrossRef]
- Al Baradie, R.; Mir, A.; Alsaif, A.; Ali, M.; Al Ghamdi, F.; Bashir, S.; Howsawi, Y. Epilepsy in patients with WWOX-related epileptic encephalopathy (WOREE) syndrome. Epileptic Disord. 2022, 24, 697–712. [Google Scholar] [CrossRef] [PubMed]
- Repudi, S.; Steinberg, D.J.; Elazar, N.; Breton, V.L.; Aquilino, M.S.; Saleem, A.; Abu-Swai, S.; Vainshtein, A.; Eshed-Eisenbach, Y.; Vijayaragavan, B.; et al. Neuronal deletion of Wwox, associated with WOREE syndrome, causes epilepsy and myelin defects. Brain 2021, 144, 3061–3077. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B. Neuronal migration disorders. Radiol. Technol. 2018, 89, 279–295. [Google Scholar]
- Francis, F.; Cappello, S. Neuronal migration and disorders—An update. Curr. Opin. Neurobiol. 2021, 66, 57–68. [Google Scholar] [CrossRef]
- Hussain, T.; Kil, H.; Hattiangady, B.; Lee, J.; Kodali, M.; Shuai, B.; Attaluri, S.; Takata, Y.; Shen, J.; Abba, M.C.; et al. Wwox deletion leads to reduced GABA-ergic inhibitory interneuron numbers and activation of microglia and astrocytes in mouse hippocampus. Neurobiol. Dis. 2019, 121, 163–176. [Google Scholar] [CrossRef]
- McAvoy, S.; Zhu, Y.; Perez, D.S.; James, C.D.; Smith, D.I. Disabled-1 is a large common fragile site gene, inactivated in multiple cancers. Genes Chromosomes Cancer 2008, 47, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Hergenreder, T.; Ye, B. Analysis of mouse brain sections by live-cell time-lapse confocal microscopy. Bio-Protocol 2023, 13, e4648. [Google Scholar] [CrossRef]
- Honda, T.; Hirota, Y.; Nakajima, K. Heterozygous Dab1 null mutation disrupts neocortical and hippocampal development. eNeuro 2023, 10, ENEURO.0433-22.2023. [Google Scholar] [CrossRef]
- Kounoupa, Z.; Tivodar, S.; Theodorakis, K.; Kyriakis, D.; Denaxa, M.; Karagogeos, D. Rac1 and Rac3 GTPases and TPC2 are required for axonal outgrowth and migration of cortical interneurons. J. Cell Sci. 2023, 136, jcs260373. [Google Scholar] [CrossRef]
- Carvalho, C.; Correia, S.C.; Seiça, R.; Moreira, P.I. WWOX inhibition by Zfra1-31 restores mitochondrial homeostasis and viability of neuronal cells exposed to high glucose. Cell Mol Life Sci. 2022, 79, 487. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Aqeilan, R.I. Tumor suppressor WWOX regulates glucose metabolism via HIF1alpha modulation. Cell Death Differ. 2014, 21, 1805–1814. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Aqeilan, R.I. The tumor suppressor WW domain-containing oxidoreductase modulates cell metabolism. Exp. Biol. Med. 2015, 240, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Seewaldt, V.L.; Aqeilan, R.I. WWOX loss activates aerobic glycolysis. Mol. Cell Oncol. 2014, 2, e965640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janczar, S.; Nautiyal, J.; Xiao, Y.; Curry, E.; Sun, M.; Zanini, E.; Paige, A.J.; Gabra, H. WWOX sensitises ovarian cancer cells to paclitaxel via modulation of the ER stress response. Cell Death Dis. 2017, 8, e2955. [Google Scholar] [CrossRef] [PubMed]
- Abu-Remaileh, M.; Abu-Remaileh, M.; Akkawi, R.; Knani, I.; Udi, S.; Pacold, M.E.; Tam, J.; Aqeilan, R.I. WWOX somatic ablation in skeletal muscles alters glucose metabolism. Mol. Metab. 2019, 22, 132–140. [Google Scholar] [CrossRef]
- Baryła, I.; Styczeń-Binkowska, E.; Płuciennik, E.; Kośla, K.; Bednarek, A.K. The WWOX/HIF1A Axis Downregulation Alters Glucose Metabolism and Predispose to Metabolic Disorders. Int. J. Mol. Sci. 2022, 23, 3326. [Google Scholar] [CrossRef] [PubMed]
- Wittekind, D.A.; Scholz, M.; Kratzsch, J.; Löffler, M.; Horn, K.; Kirsten, H.; Witte, V.; Villringer, A.; Kluge, M. Genome-wide association and transcriptome analysis suggests total serum ghrelin to be linked with GFRAL. Eur. J. Endocrinol. 2021, 184, 847–856. [Google Scholar] [CrossRef]
- Baryla, I.; Pluciennik, E.; Kośla, K.; Wojcik, M.; Zieleniak, A.; Zurawska-Klis, M.; Cypryk, K.; Wozniak, L.A.; Bednarek, A.K. Identification of a novel association for the WWOX/HIF1A axis with gestational diabetes mellitus (GDM). PeerJ. 2021, 9, e10604. [Google Scholar] [CrossRef]
- Permana, S.; Lukman, H.; Norahmawati, E.; Eka Puspita, O.; Faisal Moh Al Zein, D.; Kawamoto, Y.; Tri Endharti, A. East Asian Genome-wide association study derived loci in relation to type 2 diabetes in the Han Chinese population. Acta Biochim. Pol. 2019, 66, 679–686. [Google Scholar]
- Chang, Y.C.; Chiu, Y.F.; Liu, P.H.; Shih, K.C.; Lin, M.W.; Sheu, W.H.; Quertermous, T.; Curb, J.D.; Hsiung, C.A.; Lee, W.J.; et al. Replication of genome-wide association signals of type 2 diabetes in Han Chinese in a prospective cohort. Clin. Endocrinol. 2012, 76, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Saadane, A.; Du, Y.; Thoreson, W.B.; Miyagi, M.; Lessieur, E.M.; Kiser, J.; Wen, X.; Berkowitz, B.A.; Kern, T.S. Photoreceptor Cell Calcium Dysregulation and Calpain Activation Promote Pathogenic Photoreceptor Oxidative Stress and Inflammation in Prodromal Diabetic Retinopathy. Am. J. Pathol. 2021, 191, 1805–1821. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-A.; Liu, T.-Y.; Wen, K.-Y.; Hsu, C.-Y.; Sze, C.-I.; Chang, N.-S. Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration. Int. J. Mol. Sci. 2024, 25, 3507. https://doi.org/10.3390/ijms25063507
Chen Y-A, Liu T-Y, Wen K-Y, Hsu C-Y, Sze C-I, Chang N-S. Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration. International Journal of Molecular Sciences. 2024; 25(6):3507. https://doi.org/10.3390/ijms25063507
Chicago/Turabian StyleChen, Yu-An, Tsung-Yun Liu, Kuan-Yu Wen, Che-Yu Hsu, Chun-I Sze, and Nan-Shan Chang. 2024. "Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration" International Journal of Molecular Sciences 25, no. 6: 3507. https://doi.org/10.3390/ijms25063507
APA StyleChen, Y.-A., Liu, T.-Y., Wen, K.-Y., Hsu, C.-Y., Sze, C.-I., & Chang, N.-S. (2024). Zfra Overrides WWOX in Suppressing the Progression of Neurodegeneration. International Journal of Molecular Sciences, 25(6), 3507. https://doi.org/10.3390/ijms25063507




