Detection of Sensitization Profiles with Cellular In Vitro Tests in Wheat Allergy Dependent on Augmentation Factors (WALDA)
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Total and Specific IgE
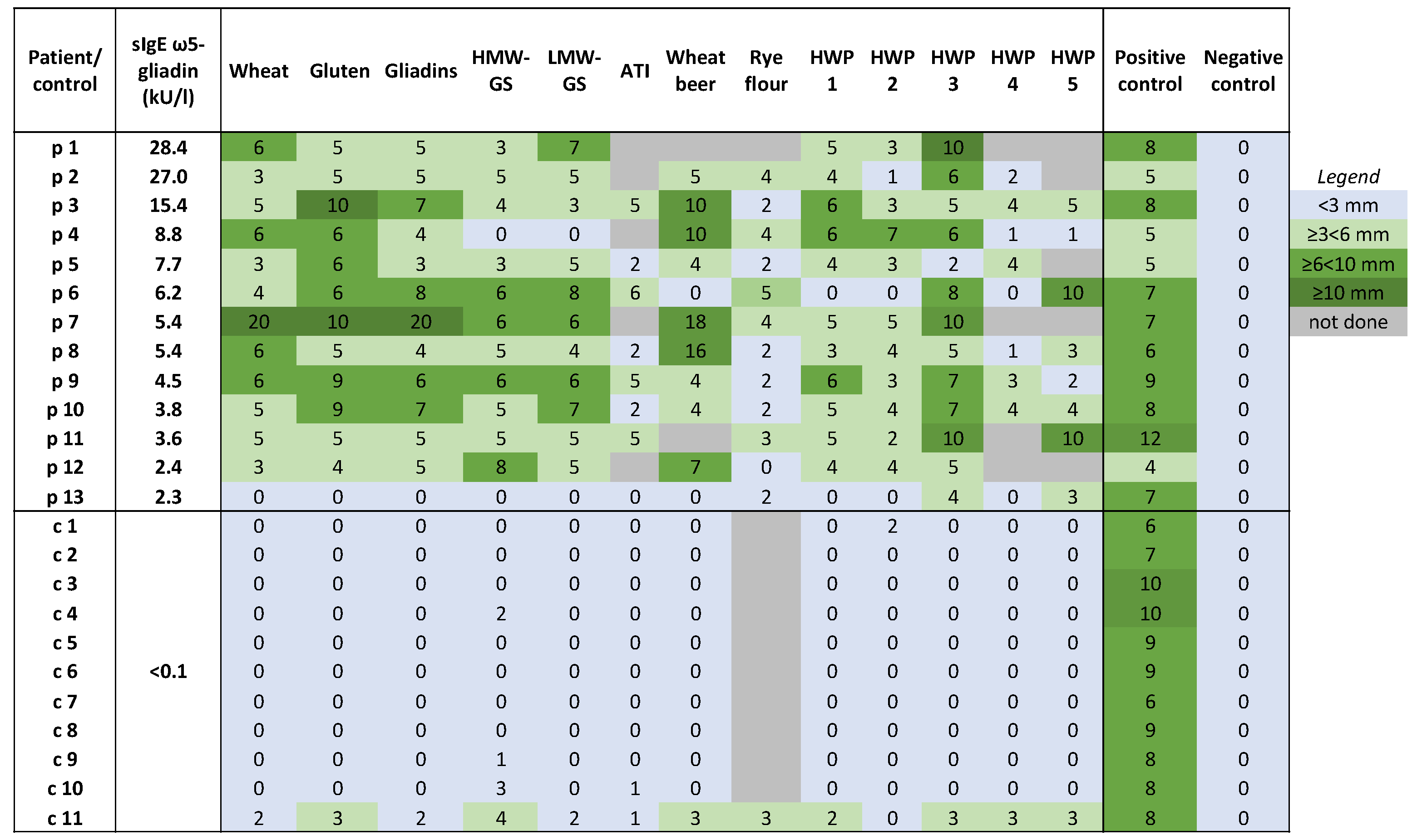
2.3. Skin Prick Tests (SPTs)
2.4. Basophil Activation Tests (BATs)
2.5. Active Basophil Histamine-Release Assay (aBHRA)
2.6. Passive Basophil Histamine-Release Assay (pBHRA)
2.7. Correlations between Applied Tests and Clinical Characteristics
2.8. Responses to Different Test Substance Concentrations in the Cellular In Vitro Tests
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Clinical History, IgE Diagnostics, Skin Prick Tests, and Oral Challenge Tests
4.3. Allergen Test Solutions for In Vitro Tests
4.4. Basophil Activation Tests (BATs)
4.5. Active Basophil Histamine-Release Assay (aBHRA)
4.6. Passive Basophil Histamine-Release Assay (pBHRA)
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faihs, V.; Kugler, C.; Schmalhofer, V.; Scherf, K.A.; Lexhaller, B.; Mortz, C.G.; Bindslev-Jensen, C.; Biedermann, T.; Brockow, K. Wheat-dependent exercise-induced anaphylaxis: Subtypes, diagnosis, and management. J. Dtsch. Dermatol. Ges. 2023, 21, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Faihs, V.; Kugler, C.; Mortz, C.G.; Biedermann, T. Correct nomenclature of wheat allergy dependent on augmentation factors. J. Allergy Clin. Immunol. Pract. 2023, 11, 3562–3563. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Dolle-Bierke, S.; Renaudin, J.M.; Rueff, F.; Hofmeier, K.S.; Treudler, R.; Pfohler, C.; Hawranek, T.; Poziomkowska-Gesicka, I.; Jappe, U.; et al. Wheat Anaphylaxis in Adults Differs from Reactions to Other Types of Food. J. Allergy Clin. Immunol. Pract. 2021, 9, 2844–2852.e5. [Google Scholar] [CrossRef] [PubMed]
- Le, T.A.; Al Kindi, M.; Tan, J.A.; Smith, A.; Heddle, R.J.; Kette, F.E.; Hissaria, P.; Smith, W.B. The clinical spectrum of omega-5-gliadin allergy. Intern. Med. J. 2016, 46, 710–716. [Google Scholar] [CrossRef]
- Dolle-Bierke, S.; Hofer, V.; Francuzik, W.; Naher, A.F.; Bilo, M.B.; Cichocka-Jarosz, E.; Lopes de Oliveira, L.C.; Fernandez-Rivas, M.; Garcia, B.E.; Hartmann, K.; et al. Food-Induced Anaphylaxis: Data from the European Anaphylaxis Registry. J. Allergy Clin. Immunol. Pract. 2023, 11, 2069–2079.e7. [Google Scholar] [CrossRef]
- Du, Z.; Gao, X.; Li, J.; Li, L.; Liu, J.; Yin, J. Clinical features and outcomes of patients with wheat-dependent exercise-induced anaphylaxis: A retrospective study. Allergy Asthma Clin. Immunol. 2022, 18, 61. [Google Scholar] [CrossRef]
- Brockow, K.; Kneissl, D.; Valentini, L.; Zelger, O.; Grosber, M.; Kugler, C.; Werich, M.; Darsow, U.; Matsuo, H.; Morita, E.; et al. Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 2015, 135, 977–984.e4. [Google Scholar] [CrossRef]
- Gabler, A.M.; Gebhard, J.; Eberlein, B.; Biedermann, T.; Scherf, K.A.; Brockow, K. The basophil activation test differentiates between patients with wheat-dependent exercise-induced anaphylaxis and control subjects using gluten and isolated gluten protein types. Clin. Transl. Allergy 2021, 11, e12050. [Google Scholar] [CrossRef]
- Altenbach, S.B.; Tanaka, C.K.; Pineau, F.; Lupi, R.; Drouet, M.; Beaudouin, E.; Morisset, M.; Denery-Papini, S. Assessment of the Allergenic Potential of Transgenic Wheat (Triticum aestivum) with Reduced Levels of omega5-Gliadins, the Major Sensitizing Allergen in Wheat-Dependent Exercise-Induced Anaphylaxis. J. Agric. Food Chem. 2015, 63, 9323–9332. [Google Scholar] [CrossRef]
- Morita, E.; Matsuo, H.; Kohno, K.; Yokooji, T.; Yano, H.; Endo, T. A Narrative Mini Review on Current Status of Hypoallergenic Wheat Development for IgE-Mediated Wheat Allergy, Wheat-Dependent Exercise-Induced Anaphylaxis. Foods 2023, 12, 954. [Google Scholar] [CrossRef]
- Gabler, A.M.; Gebhard, J.; Norwig, M.C.; Eberlein, B.; Biedermann, T.; Brockow, K.; Scherf, K.A. Basophil Activation to Gluten and Non-Gluten Proteins in Wheat-Dependent Exercise-Induced Anaphylaxis. Front. Allergy 2022, 3, 822554. [Google Scholar] [CrossRef]
- Wang, J.; Golden, D.B.K.; Khan, D.A. The Value of Current Laboratory Tests in Diagnosing Food, Venom, and Drug Allergies. J. Allergy Clin. Immunol. Pract. 2023, 11, 2973–2981. [Google Scholar] [CrossRef]
- Aoki, Y.; Yagami, A.; Sakai, T.; Ohno, S.; Sato, N.; Nakamura, M.; Futamura, K.; Suzuki, K.; Horiguchi, T.; Nakata, S.; et al. Alpha/Beta Gliadin MM1 Is a Novel Antigen for Wheat-Dependent Exercise-Induced Anaphylaxis. Int. Arch. Allergy Immunol. 2023, 184, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Alpan, O.; Hoffmann, H.J. Basophil activation test: Mechanisms and considerations for use in clinical trials and clinical practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.F.; Juel-Berg, N.; Hansen, K.S.; Mills, E.N.C.; van Ree, R.; Poulsen, L.K.; Jensen, B.M. A comparative study on basophil activation test, histamine release assay, and passive sensitization histamine release assay in the diagnosis of peanut allergy. Allergy 2018, 73, 137–144. [Google Scholar] [CrossRef]
- Lehto, M.; Palosuo, K.; Varjonen, E.; Majuri, M.L.; Andersson, U.; Reunala, T.; Alenius, H. Humoral and cellular responses to gliadin in wheat-dependent, exercise-induced anaphylaxis. Clin. Exp. Allergy 2003, 33, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Aihara, M.; Miyazawa, H.; Osuna, H.; Tsubaki, K.; Ikebe, T.; Aihara, Y.; Ikezawa, Z. Food-dependent exercise-induced anaphylaxis: Influence of concurrent aspirin administration on skin testing and provocation. Br. J. Dermatol. 2002, 146, 466–472. [Google Scholar] [CrossRef]
- Yokooji, T.; Kurihara, S.; Murakami, T.; Chinuki, Y.; Takahashi, H.; Morita, E.; Harada, S.; Ishii, K.; Hiragun, M.; Hide, M.; et al. Characterization of causative allergens for wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat proteins in facial soap. Allergol. Int. 2013, 62, 435–445. [Google Scholar] [CrossRef]
- Hiragun, M.; Ishii, K.; Hiragun, T.; Shindo, H.; Mihara, S.; Matsuo, H.; Hide, M. The sensitivity and clinical course of patients with wheat-dependent exercise-induced anaphylaxis sensitized to hydrolyzed wheat protein in facial soap—Secondary publication. Allergol. Int. 2013, 62, 351–358. [Google Scholar] [CrossRef]
- Chinuki, Y.; Kaneko, S.; Dekio, I.; Takahashi, H.; Tokuda, R.; Nagao, M.; Fujisawa, T.; Morita, E. CD203c expression-based basophil activation test for diagnosis of wheat-dependent exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 2012, 129, 1404–1406. [Google Scholar] [CrossRef]
- Hofmann, S.C.; Fischer, J.; Eriksson, C.; Gref, O.B.; Biedermann, T.; Jakob, T. IgE detection to alpha/beta/gamma-gliadin and its clinical relevance in wheat-dependent exercise-induced anaphylaxis. Allergy 2012, 67, 1457–1460. [Google Scholar] [CrossRef]
- Bouchez-Mahiout, I.; Snegaroff, J.; Tylichova, M.; Pecquet, C.; Branlard, G.; Lauriere, M. Low molecular weight glutenins in wheat-dependant, exercise-induced anaphylaxis: Allergenicity and antigenic relationships with omega 5-gliadins. Int. Arch. Allergy Immunol. 2010, 153, 35–45. [Google Scholar] [CrossRef]
- Stoffersen, P.; Skov, P.S.; Poulsen, L.K.; Jensen, B.M. The Allergen-Specific IgE Concentration Is Important for Optimal Histamine Release From Passively Sensitized Basophils. Front. Allergy 2022, 3, 875119. [Google Scholar] [CrossRef]
- Morita, E.; Chinuki, Y.; Takahashi, H. Recent advances of in vitro tests for the diagnosis of food-dependent exercise-induced anaphylaxis. J. Dermatol. Sci. 2013, 71, 155–159. [Google Scholar] [CrossRef]
- Takahashi, H.; Matsuo, H.; Chinuki, Y.; Kohno, K.; Tanaka, A.; Maruyama, N.; Morita, E. Recombinant high molecular weight-glutenin subunit-specific IgE detection is useful in identifying wheat-dependent exercise-induced anaphylaxis complementary to recombinant omega-5 gliadin-specific IgE test. Clin. Exp. Allergy 2012, 42, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Mamone, G.; Cutignano, A.; Fontana, A.; Zurlo, L.; Addeo, F.; Ferranti, P. Proteomics, peptidomics, and immunogenic potential of wheat beer (Weissbier). J. Agric. Food Chem. 2015, 63, 3579–3586. [Google Scholar] [CrossRef] [PubMed]
- Spada, V.; Di Stasio, L.; Picascia, S.; Messina, B.; Gianfrani, C.; Mamone, G.; Picariello, G. Immunogenic Potential of Beer Types Brewed with Hordeum and Triticum spp. Malt Disclosed by Proteomics. Front. Nutr. 2020, 7, 98. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Baumert, J.L.; Downs, M.L. Compositional and immunogenic evaluation of fractionated wheat beers using mass spectrometry. Food Chem. 2020, 333, 127379. [Google Scholar] [CrossRef] [PubMed]
- Herzinger, T.; Kick, G.; Ludolph-Hauser, D.; Przybilla, B. Anaphylaxis to wheat beer. Ann. Allergy Asthma Immunol. 2004, 92, 673–675. [Google Scholar] [CrossRef] [PubMed]
- Terao, H.; Kishikawa, R.; Kato, M.; Noda, K.; Iwanaga, T.; Shouji, S.; Nishima, S. [A case of food-dependent exercise-induced anaphylaxis caused by various foods which contained flour for thirty years]. Arerugi 2004, 53, 1119–1122. [Google Scholar] [PubMed]
- Pita, J.S.; Sousa, N.; Bartolome, B.; Loureiro, C.; Bom, A.T. Beer: An uncommon cause of anaphylaxis. BMJ Case Rep. 2019, 12, e227723. [Google Scholar] [CrossRef]
- Geisslitz, S.; Weegels, P.; Shewry, P.; Zevallos, V.; Masci, S.; Sorrells, M.; Gregorini, A.; Colomba, M.; Jonkers, D.; Huang, X.; et al. Wheat amylase/trypsin inhibitors (ATIs): Occurrence, function and health aspects. Eur. J. Nutr. 2022, 61, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Sander, I.; Rihs, H.P.; Doekes, G.; Quirce, S.; Krop, E.; Rozynek, P.; van Kampen, V.; Merget, R.; Meurer, U.; Bruning, T.; et al. Component-resolved diagnosis of baker’s allergy based on specific IgE to recombinant wheat flour proteins. J. Allergy Clin. Immunol. 2015, 135, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Pastorello, E.A.; Farioli, L.; Conti, A.; Pravettoni, V.; Bonomi, S.; Iametti, S.; Fortunato, D.; Scibilia, J.; Bindslev-Jensen, C.; Ballmer-Weber, B.; et al. Wheat IgE-mediated food allergy in European patients: Alpha-amylase inhibitors, lipid transfer proteins and low-molecular-weight glutenins. Allergenic molecules recognized by double-blind, placebo-controlled food challenge. Int. Arch. Allergy Immunol. 2007, 144, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Gabler, A.M.; Scherf, K.A. Comparative Characterization of Gluten and Hydrolyzed Wheat Proteins. Biomolecules 2020, 10, 1227. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, A.R.; Castan, L.; Larsen, J.M.; Piras, C.; Villemin, C.; Andersen, D.; Madsen, C.B.; Roncada, P.; Brix, S.; Denery-Papini, S.; et al. Acid Hydrolysis of Gluten Enhances the Skin Sensitizing Potential and Drives Diversification of IgE Reactivity to Unmodified Gluten Proteins. Mol. Nutr. Food Res. 2021, 65, e2100416. [Google Scholar] [CrossRef] [PubMed]
- Chinuki, Y.; Morita, E. Wheat-dependent exercise-induced anaphylaxis sensitized with hydrolyzed wheat protein in soap. Allergol. Int. 2012, 61, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Tranquet, O.; Gaudin, J.C.; Patil, S.; Steinbrecher, J.; Matsunaga, K.; Teshima, R.; Sakai, S.; Larre, C.; Denery-Papini, S. A chimeric IgE that mimics IgE from patients allergic to acid-hydrolyzed wheat proteins is a novel tool for in vitro allergenicity assessment of functionalized glutens. PLoS ONE 2017, 12, e0187415. [Google Scholar] [CrossRef]
- Yagami, A.; Aihara, M.; Ikezawa, Z.; Hide, M.; Kishikawa, R.; Morita, E.; Chinuki, Y.; Fukutomi, Y.; Urisu, A.; Fukushima, A.; et al. Outbreak of immediate-type hydrolyzed wheat protein allergy due to a facial soap in Japan. J. Allergy Clin. Immunol. 2017, 140, 879–881.e7. [Google Scholar] [CrossRef]
- Kennard, L.; Thomas, I.; Rutkowski, K.; Azzu, V.; Yong, P.F.K.; Kasternow, B.; Hunter, H.; Cabdi, N.M.O.; Nakonechna, A.; Wagner, A. A Multicenter Evaluation of Diagnosis and Management of Omega-5 Gliadin Allergy (Also Known as Wheat-Dependent Exercise-Induced Anaphylaxis) in 132 Adults. J. Allergy Clin. Immunol. Pract. 2018, 6, 1892–1897. [Google Scholar] [CrossRef]
- Palosuo, K.; Alenius, H.; Varjonen, E.; Kalkkinen, N.; Reunala, T. Rye g-70 and g-35 secalins and barley g-3 hordein cross-react with v-5 gliadin, a major allergen in wheat-dependent, exercise-induced anaphylaxis. Clin. Exp. Allergy 2001, 3, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Snegaroff, J.; Bouchez-Mahiout, I.; Pecquet, C.; Branlard, G.; Lauriere, M. Study of IgE antigenic relationships in hypersensitivity to hydrolyzed wheat proteins and wheat-dependent exercise-induced anaphylaxis. Int. Arch. Allergy Immunol. 2006, 139, 201–208. [Google Scholar] [CrossRef]
- Faihs, V.; Kugler, C.; Bent, R.; Biedermann, T.; Brockow, K. Omega-5-gliadin-specific-IgE-positive, but wheat-specific-IgE-negative wheat allergy dependent on augmentation factors (WALDA)—A frequent presentation. Explor. Asthma Allergy 2023, 1, 230–238. [Google Scholar] [CrossRef]
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K.A. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS ONE 2017, 12, e0172819. [Google Scholar] [CrossRef] [PubMed]
- Baumann, K.; Skov, P.S.; Eller, E.; Kristensen, B.; Bindslev-Jensen, C. Detection of Circulating Peanut Components in Serum after Ingestion. Int. Arch. Allergy Immunol. 2022, 183, 706–713. [Google Scholar] [CrossRef] [PubMed]




| Allergen Test Solution | Concentrations in BATs | Concentrations in aBHRA | Concentrations in pBHRA |
|---|---|---|---|
| Gluten | 4000/2000/800 µg/mL | 4000/1142.9/326.5/93.3/26.5/7.6/2.6/0.6/0.16/0.04/0.01/0.004 µg/mL | 3600/1028/293/84/24/6.9/0.2/0.6/0.2/0.05/0.01/0.004 µg/mL |
| HMW-GS | 4000/2000/800 µg/mL | 4000/1142.9/326.5/93.3/26.5/7.6/2.6/0.6/0.16/0.04/0.01/0.004 µg/mL | 3600/1028/293/84/24/6.9/0.2/0.6/0.2/0.05/0.01/0.004 µg/mL |
| ATIs | 400/200/80/40 µg/mL | 326.5/93.3/26.5/7.6/2.6/0.6/0.16/0.04/0.01/0.004 µg/mL | 293/84/24/6.9/0.2/0.6/0.2/0.05/0.01/0.004 µg/mL |
| Alcohol-free wheat beer | 1:10/1:100 | 1:12.2/1:42.9/1:150.1/1:525.2/1:1838.8/1:6430.7/1:22,517.4/1:78,710.9/1:275,487.5/1:964,706.8 | 1:12.2/1:42.9/1:150.1/1:525.2/1:1838.8/1:6430.7/1:22,517.4/1:78,710.9/1:275,487.5/1:964,706.8 |
| eHWP | 1:5/1:10/1:50 | 1:12.2/1:42.9/1:150.1/1:525.2/1:1838.8/1:6430.7/1:22,517.4/1:78,710.9/1:275,487.5/1:964,706.8 | 1:12.2/1:42.9/1:150.1/1:525.2/1:1838.8/1:6430.7/1:22,517.4/1:78,710.9/1:275,487.5/1:964,706.8 |
| sHWP | 1:5/1:10/1:50 | 1:12.2/1:42.9/1:150.1/1:525.2/1:1838.8/1:6430.7/1:22,517.4/1:78,710.9/1:275,487.5/1:964,706.8 | 1:12.2/1:42.9/1:150.1/1:525.2/1:1838.8/1:6430.7/1:22,517.4/1:78,710.9/1:275,487.5/1:964,706.8 |
| Rye gluten | 4000/2000/800 µg/mL | 4000/1142.9/326.5/93.3/26.5/7.6/2.6/0.6/0.16/0.04/0.01/0.004 µg/mL | not tested |
| Rye secalins | 800 µg/mL | 26.5/7.6/2.6/0.6/0.16/0.04/0.01/0.004 µg/mL | 24/6.9/0.2/0.6/0.2/0.05/0.01/0.004 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faihs, V.; Schmalhofer, V.; Kugler, C.; Bent, R.K.; Scherf, K.A.; Lexhaller, B.; Mortz, C.G.; Bindslev-Jensen, C.; Biedermann, T.; Skov, P.S.; et al. Detection of Sensitization Profiles with Cellular In Vitro Tests in Wheat Allergy Dependent on Augmentation Factors (WALDA). Int. J. Mol. Sci. 2024, 25, 3574. https://doi.org/10.3390/ijms25073574
Faihs V, Schmalhofer V, Kugler C, Bent RK, Scherf KA, Lexhaller B, Mortz CG, Bindslev-Jensen C, Biedermann T, Skov PS, et al. Detection of Sensitization Profiles with Cellular In Vitro Tests in Wheat Allergy Dependent on Augmentation Factors (WALDA). International Journal of Molecular Sciences. 2024; 25(7):3574. https://doi.org/10.3390/ijms25073574
Chicago/Turabian StyleFaihs, Valentina, Viktoria Schmalhofer, Claudia Kugler, Rebekka K. Bent, Katharina A. Scherf, Barbara Lexhaller, Charlotte G. Mortz, Carsten Bindslev-Jensen, Tilo Biedermann, Per S. Skov, and et al. 2024. "Detection of Sensitization Profiles with Cellular In Vitro Tests in Wheat Allergy Dependent on Augmentation Factors (WALDA)" International Journal of Molecular Sciences 25, no. 7: 3574. https://doi.org/10.3390/ijms25073574
APA StyleFaihs, V., Schmalhofer, V., Kugler, C., Bent, R. K., Scherf, K. A., Lexhaller, B., Mortz, C. G., Bindslev-Jensen, C., Biedermann, T., Skov, P. S., Eberlein, B., & Brockow, K. (2024). Detection of Sensitization Profiles with Cellular In Vitro Tests in Wheat Allergy Dependent on Augmentation Factors (WALDA). International Journal of Molecular Sciences, 25(7), 3574. https://doi.org/10.3390/ijms25073574






