Analysis of the Gene Networks and Pathways Correlated with Tissue Differentiation in Prostate Cancer
Abstract
1. Introduction
2. Results
2.1. Demographics and Staging
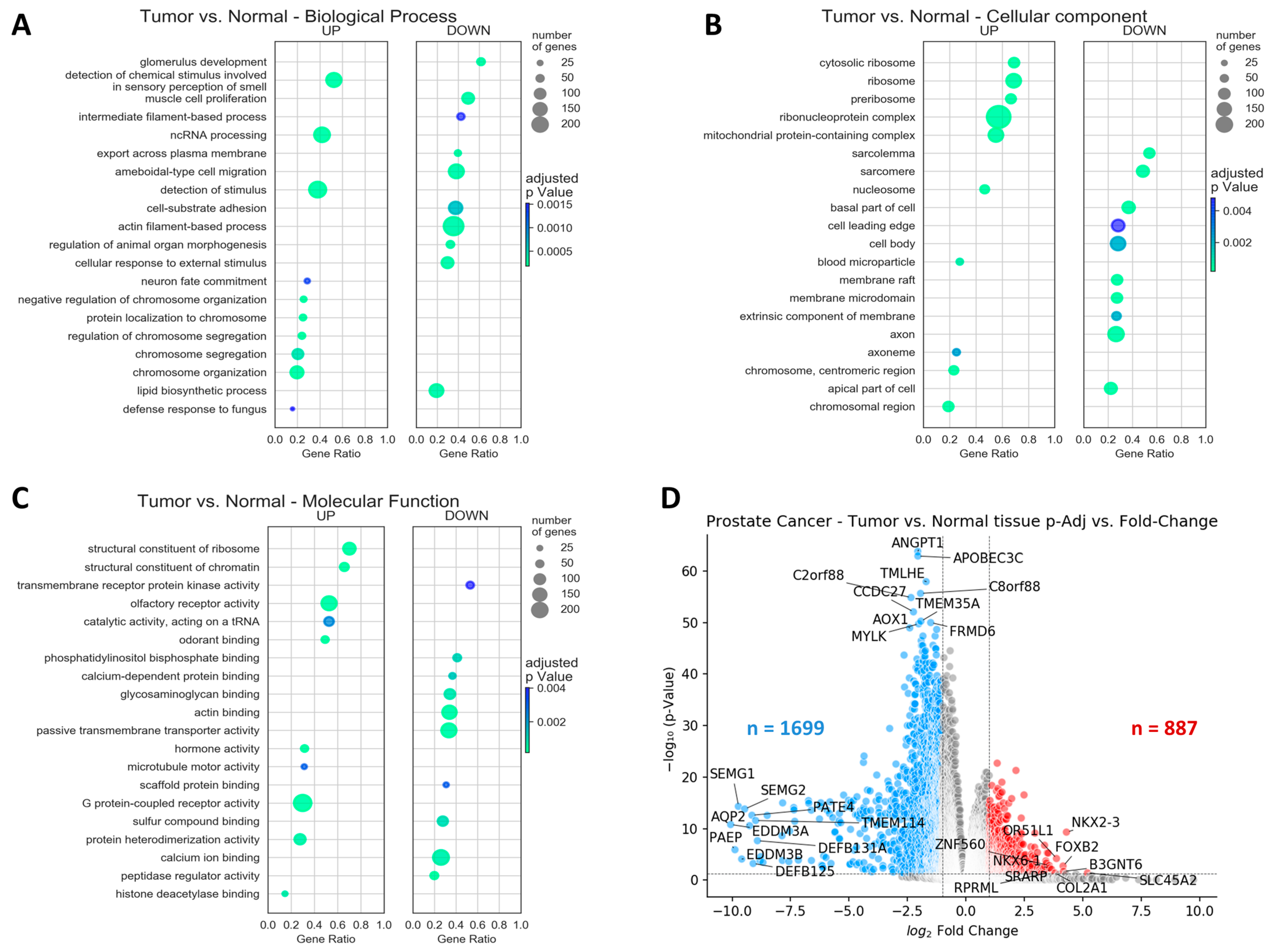
2.2. Lower mRNA Levels Were Observed for a Higher Proportion of Genes in Prostate Tumor Samples Compared to Normal Tissues
2.3. High GS Is Associated with Increased Proliferation and Loss of Cellular Polarization but Also Decreased Vasculature Development
2.4. High GS Is Associated with High Counts of M2 Macrophages
2.5. Protein Networks and Pathways Involved in Gleason Progression
2.6. Selected Gene Signature for Gleason Grade Groups
3. Discussion
4. Materials and Methods
4.1. Data Used
4.2. Differential Expression and Correlations
4.3. Gene Ontology
4.4. Cell Type Enrichment Analysis
4.5. Protein Networks
4.6. Signaling Pathways
4.7. Gene Selection and k-Nearest Neighbors (kNN) Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of Prostate Cancer-Specific Mortality Following Biochemical Recurrence after Radical Prostatectomy. JAMA 2005, 294, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Kreuz, M.; Otto, D.J.; Fuessel, S.; Blumert, C.; Bertram, C.; Bartsch, S.; Loeffler, D.; Puppel, S.H.; Rade, M.; Buschmann, T.; et al. Prostatrend—A Multivariable Prognostic Rna Expression Score for Aggressive Prostate Cancer. Eur. Urol. 2020, 78, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.W.; Bergstralh, E.J.; Blute, M.L.; Slezak, J.M.; Carducci, M.; Han, M.; Epstein, J.I.; Eisenberger, M.A.; Walsh, P.C.; Partin, A.W. Contemporary Identification of Patients at High Risk of Early Prostate Cancer Recurrence after Radical Retropubic Prostatectomy. Urology 2001, 57, 1033–1037. [Google Scholar] [CrossRef] [PubMed]
- Gleason, D.F.; Mellinger, G.T. Prediction of Prognosis for Prostatic Adenocarcinoma by Combined Histological Grading and Clinical Staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Allsbrook, W.C., Jr.; Mangold, K.A.; Johnson, M.H.; Lane, R.B.; Lane, C.G.; Amin, M.B.; Bostwick, D.G.; Humphrey, P.A.; Jones, E.C.; Reuter, V.E.; et al. Interobserver Reproducibility of Gleason Grading of Prostatic Carcinoma: Urologic Pathologists. Hum. Pathol. 2001, 32, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (Isup) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Pierorazio, P.M.; Walsh, P.C.; Partin, A.W.; Epstein, J.I. Prognostic Gleason Grade Grouping: Data Based on the Modified Gleason Scoring System. BJU Int. 2013, 111, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Amin, M.; Boccon-Gibod, L.; Egevad, L.; Humphrey, P.A.; Mikuz, G.; Newling, D.; Nilsson, S.; Sakr, W.; Srigley, J.R.; et al. Prognostic Factors and Reporting of Prostate Carcinoma in Radical Prostatectomy and Pelvic Lymphadenectomy Specimens. Scand. J. Urol. Nephrol. 2005, 39, 34–63. [Google Scholar] [CrossRef]
- Barbieri, C.E.; Baca, S.C.; Lawrence, M.S.; Demichelis, F.; Blattner, M.; Theurillat, J.P.; White, T.A.; Stojanov, P.; Van Allen, E.; Stransky, N.; et al. Exome Sequencing Identifies Recurrent Spop, Foxa1 and Med12 Mutations in Prostate Cancer. Nat. Genet. 2012, 44, 685–689. [Google Scholar] [CrossRef]
- Baca, S.C.; Prandi, D.; Lawrence, M.S.; Mosquera, J.M.; Romanel, A.; Drier, Y.; Park, K.; Kitabayashi, N.; MacDonald, T.Y.; Ghandi, M.; et al. Punctuated Evolution of Prostate Cancer Genomes. Cell 2013, 153, 666–677. [Google Scholar] [CrossRef]
- Cooper, C.S.; Eeles, R.; Wedge, D.C.; Van Loo, P.; Gundem, G.; Alexandrov, L.B.; Kremeyer, B.; Butler, A.; Lynch, A.G.; Camacho, N.; et al. Analysis of the Genetic Phylogeny of Multifocal Prostate Cancer Identifies Multiple Independent Clonal Expansions in Neoplastic and Morphologically Normal Prostate Tissue. Nat. Genet. 2015, 47, 367–372. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M.; The Cancer Genome Atlas Research Network. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A Pathology Atlas of the Human Cancer Transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Bjorling, E.; Agaton, C.; Szigyarto, C.A.; Amini, B.; Andersen, E.; Andersson, A.C.; Angelidou, P.; Asplund, A.; Asplund, C.; et al. A Human Protein Atlas for Normal and Cancer Tissues Based on Antibody Proteomics. Mol. Cell Proteom. 2005, 4, 1920–1932. [Google Scholar] [CrossRef]
- Thurtle, D.; Rossi, S.H.; Berry, B.; Pharoah, P.; Gnanapragasam, V.J. Models Predicting Survival to Guide Treatment Decision-Making in Newly Diagnosed Primary Non-Metastatic Prostate Cancer: A Systematic Review. BMJ Open 2019, 9, e029149. [Google Scholar] [CrossRef]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate Cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate Cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Yimamu, Y.; Yang, X.; Chen, J.; Luo, C.; Xiao, W.; Guan, H.; Wang, D. The Development of a Gleason Score-Related Gene Signature for Predicting the Prognosis of Prostate Cancer. J. Clin. Med. 2022, 11, 7164. [Google Scholar] [CrossRef]
- Mohammad, T.; Singh, P.; Jairajpuri, D.S.; Al-Keridis, L.A.; Alshammari, N.; Adnan, M.; Dohare, R.; Hassan, M.I. Differential Gene Expression and Weighted Correlation Network Dynamics in High-Throughput Datasets of Prostate Cancer. Front. Oncol. 2022, 12, 881246. [Google Scholar] [CrossRef]
- Meng, J.; Guan, Y.; Wang, B.; Chen, L.; Chen, J.; Zhang, M.; Liang, C. Risk Subtyping and Prognostic Assessment of Prostate Cancer Based on Consensus Genes. Commun. Biol. 2022, 5, 233. [Google Scholar] [CrossRef]
- Niu, W.; Zhang, T.; Ma, L. Correlation Analysis between Immune-Related Genes and Cell Infiltration Revealed Prostate Cancer Immunotherapy Biomarkers Linked to T Cells Gamma Delta. Sci. Rep. 2023, 13, 2459. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Lin, X.; Kapoor, A.; Li, T.; Major, P.; Tang, D. Effective Prediction of Prostate Cancer Recurrence through the Iqgap1 Network. Cancers 2021, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Blume-Jensen, P.; Berman, D.M.; Rimm, D.L.; Shipitsin, M.; Putzi, M.; Nifong, T.P.; Small, C.; Choudhury, S.; Capela, T.; Coupal, L.; et al. Development and Clinical Validation of an in Situ Biopsy-Based Multimarker Assay for Risk Stratification in Prostate Cancer. Clin. Cancer Res. 2015, 21, 2591–2600. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, D.; Goddard, A.D.; Natraj, N.; Cherbavaz, D.B.; Clark-Langone, K.M.; Snable, J.; Watson, D.; Falzarano, S.M.; Magi-Galluzzi, C.; Klein, E.A.; et al. Analytical Validation of the Oncotype Dx Prostate Cancer Assay—A Clinical Rt-Pcr Assay Optimized for Prostate Needle Biopsies. BMC Genom. 2013, 14, 690. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Swanson, G.P.; Fisher, G.; Brothman, A.R.; Berney, D.M.; Reid, J.E.; Mesher, D.; Speights, V.O.; Stankiewicz, E.; Foster, C.S.; et al. Prognostic Value of an Rna Expression Signature Derived from Cell Cycle Proliferation Genes in Patients with Prostate Cancer: A Retrospective Study. Lancet Oncol. 2011, 12, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Karnes, R.J.; Bergstralh, E.J.; Davicioni, E.; Ghadessi, M.; Buerki, C.; Mitra, A.P.; Crisan, A.; Erho, N.; Vergara, I.A.; Lam, L.L.; et al. Validation of a Genomic Classifier That Predicts Metastasis Following Radical Prostatectomy in an at Risk Patient Population. J. Urol. 2013, 190, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Ozer, B.; Sezerman, U. Analysis of the Interplay between Methylation and Expression Reveals Its Potential Role in Cancer Aetiology. Funct. Integr. Genom. 2017, 17, 53–68. [Google Scholar] [CrossRef]
- Cao, S.; Wang, J.R.; Ji, S.; Yang, P.; Dai, Y.; Guo, S.; Montierth, M.D.; Shen, J.P.; Zhao, X.; Chen, J.; et al. Estimation of Tumor Cell Total Mrna Expression in 15 Cancer Types Predicts Disease Progression. Nat. Biotechnol. 2022, 40, 1624–1633. [Google Scholar] [CrossRef]
- Soerohardjo, I.; Widodo, I.; Heriyanto, D.S.; Zulfiqqar, A.; Anwar, S.L. Down-Regulation of Rb1 and Tp53 as Potential Predicting Biomarkers for Castration-Resistant Prostate Cancer (Crpc): Indonesian Retrospective Cohort Study. Ann. Med. Surg. 2020, 60, 549–554. [Google Scholar] [CrossRef]
- Nyquist, M.D.; Corella, A.; Coleman, I.; De Sarkar, N.; Kaipainen, A.; Ha, G.; Gulati, R.; Ang, L.; Chatterjee, P.; Lucas, J.; et al. Combined Tp53 and Rb1 Loss Promotes Prostate Cancer Resistance to a Spectrum of Therapeutics and Confers Vulnerability to Replication Stress. Cell Rep. 2020, 31, 107669. [Google Scholar] [CrossRef]
- Jhun, M.A.; Geybels, M.S.; Wright, J.L.; Kolb, S.; April, C.; Bibikova, M.; Ostrander, E.A.; Fan, J.B.; Feng, Z.; Stanford, J.L. Gene Expression Signature of Gleason Score Is Associated with Prostate Cancer Outcomes in a Radical Prostatectomy Cohort. Oncotarget 2017, 8, 43035–43047. [Google Scholar] [CrossRef]
- Kukurba, K.R.; Montgomery, S.B. Rna Sequencing and Analysis. Cold Spring Harb. Protoc. 2015, 2015, 951–969. [Google Scholar] [CrossRef]
- Ross-Adams, H.; Lamb, A.D.; Dunning, M.J.; Halim, S.; Lindberg, J.; Massie, C.M.; Egevad, L.A.; Russell, R.; Ramos-Montoya, A.; Vowler, S.L.; et al. Integration of Copy Number and Transcriptomics Provides Risk Stratification in Prostate Cancer: A Discovery and Validation Cohort Study. EBioMedicine 2015, 2, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Ashkani, J.; Naidoo, K.J. Glycosyltransferase Gene Expression Profiles Classify Cancer Types and Propose Prognostic Subtypes. Sci. Rep. 2016, 6, 26451. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.; McDowell, C.; Yang, Q.; He, Y.; Zhao, Y.; Hauck, J.S.; Zhou, Y.; Zhang, H.; Armstrong, A.J.; George, D.J.; et al. Rewiring of the N-Glycome with Prostate Cancer Progression and Therapy Resistance. NPJ Precis. Oncol. 2023, 7, 22. [Google Scholar] [CrossRef]
- Shand, R.L.; Gelmann, E.P. Molecular Biology of Prostate-Cancer Pathogenesis. Curr. Opin. Urol. 2006, 16, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Malkowicz, S.B. The Role of Diethylstilbestrol in the Treatment of Prostate Cancer. Urology 2001, 58, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.C.; Redman, B.G.; Flaherty, L.E.; Li, L.; Strawderman, M.; Pienta, K.J. A Phase Ii Trial of Oral Diethylstilbesterol as a Second-Line Hormonal Agent in Advanced Prostate Cancer. Urology 1998, 52, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Nelles, J.L.; Hu, W.Y.; Prins, G.S. Estrogen Action and Prostate Cancer. Expert. Rev. Endocrinol. Metab. 2011, 6, 437–451. [Google Scholar] [CrossRef]
- Han, X.; Liehr, J.G.; Bosland, M.C. Induction of a DNA Adduct Detectable by 32p-Postlabeling in the Dorsolateral Prostate of Nbl/Cr Rats Treated with Estradiol-17 Beta and Testosterone. Carcinogenesis 1995, 16, 951–954. [Google Scholar] [CrossRef]
- Bonkhoff, H. Estrogen Receptor Signaling in Prostate Cancer: Implications for Carcinogenesis and Tumor Progression. Prostate 2018, 78, 2–10. [Google Scholar] [CrossRef]
- Carruba, G. Estrogen and Prostate Cancer: An Eclipsed Truth in an Androgen-Dominated Scenario. J. Cell Biochem. 2007, 102, 899–911. [Google Scholar] [CrossRef]
- Dey, P.; Barros, R.P.; Warner, M.; Strom, A.; Gustafsson, J.A. Insight into the Mechanisms of Action of Estrogen Receptor Beta in the Breast, Prostate, Colon, and Cns. J. Mol. Endocrinol. 2013, 51, T61–T74. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Gerhardt, H.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis. Cell 2011, 146, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Crawford, Y.; Ferrara, N. Vegf Inhibition: Insights from Preclinical and Clinical Studies. Cell Tissue Res. 2009, 335, 261–269. [Google Scholar] [CrossRef]
- Ebos, J.M.; Kerbel, R.S. Antiangiogenic Therapy: Impact on Invasion, Disease Progression, and Metastasis. Nat. Rev. Clin. Oncol. 2011, 8, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, C.; Goswami, S.; Basu, S.; Chakroborty, D. Angiogenesis Inhibition in Prostate Cancer: An Update. Cancers 2020, 12, 2382. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, C.; Muthana, M.; Coffelt, S.B.; Lewis, C.E. The Role of Myeloid Cells in the Promotion of Tumour Angiogenesis. Nat. Rev. Cancer 2008, 8, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Poh, A.R.; Ernst, M. Targeting Macrophages in Cancer: From Bench to Bedside. Front. Oncol. 2018, 8, 49. [Google Scholar] [CrossRef]
- Han, C.; Deng, Y.; Xu, W.; Liu, Z.; Wang, T.; Wang, S.; Liu, J.; Liu, X. The Roles of Tumor-Associated Macrophages in Prostate Cancer. J. Oncol. 2022, 2022, 8580043. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef]
- Martori, C.; Sanchez-Moral, L.; Paul, T.; Pardo, J.C.; Font, A.; Ruiz de Porras, V.; Sarrias, M.R. Macrophages as a Therapeutic Target in Metastatic Prostate Cancer: A Way to Overcome Immunotherapy Resistance? Cancers 2022, 14, 440. [Google Scholar] [CrossRef] [PubMed]
- Yu, H. Cdc20: A Wd40 Activator for a Cell Cycle Degradation Machine. Mol. Cell 2007, 27, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Sun, Y.; Chen, J.; Li, H.; Yao, K.; Liu, Y.; Liu, Q.; Lu, J. The Oncogenic Role of Apc/C Activator Protein Cdc20 by an Integrated Pan-Cancer Analysis in Human Tumors. Front. Oncol. 2021, 11, 721797. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Mao, Y.; Lu, L.; Hu, C.; Wang, D.; Si-Tu, J.; Lu, M.; Peng, S.; Qiu, J.; Gao, X. Silencing of Cdc20 Suppresses Metastatic Castration-Resistant Prostate Cancer Growth and Enhances Chemosensitivity to Docetaxel. Int. J. Oncol. 2016, 49, 1679–1685. [Google Scholar] [CrossRef]
- Wu, F.; Wang, M.; Zhong, T.; Xiao, C.; Chen, X.; Huang, Y.; Wu, M.; Yu, J.; Chen, D. Inhibition of Cdc20 Potentiates Anti-Tumor Immunity through Facilitating Gsdme-Mediated Pyroptosis in Prostate Cancer. Exp. Hematol. Oncol. 2023, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W.; Schmidt, M.; Gekeler, V.; Denkert, C.; Stephan, C.; Jung, K.; Loening, S.; Dietel, M.; Kristiansen, G. Polo-Like Kinase 1 Is Overexpressed in Prostate Cancer and Linked to Higher Tumor Grades. Prostate 2004, 60, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Rokhlin, O.W.; Taghiyev, A.F.; Bayer, K.U.; Bumcrot, D.; Koteliansk, V.E.; Glover, R.A.; Cohen, M.B. Calcium/Calmodulin-Dependent Kinase Ii Plays an Important Role in Prostate Cancer Cell Survival. Cancer Biol. Ther. 2007, 6, 732–742. [Google Scholar] [CrossRef]
- Fleischmann, A.; Rocha, C.; Schobinger, S.; Seiler, R.; Wiese, B.; Thalmann, G.N. Androgen Receptors Are Differentially Expressed in Gleason Patterns of Prostate Cancer and Down-Regulated in Matched Lymph Node Metastases. Prostate 2011, 71, 453–460. [Google Scholar] [CrossRef]
- Mamaeva, O.A.; Kim, J.; Feng, G.; McDonald, J.M. Calcium/Calmodulin-Dependent Kinase Ii Regulates Notch-1 Signaling in Prostate Cancer Cells. J. Cell Biochem. 2009, 106, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Mognol, G.P.; Carneiro, F.R.; Robbs, B.K.; Faget, D.V.; Viola, J.P. Cell Cycle and Apoptosis Regulation by Nfat Transcription Factors: New Roles for an Old Player. Cell Death Dis. 2016, 7, e2199. [Google Scholar] [CrossRef] [PubMed]
- Erho, N.; Crisan, A.; Vergara, I.A.; Mitra, A.P.; Ghadessi, M.; Buerki, C.; Bergstralh, E.J.; Kollmeyer, T.; Fink, S.; Haddad, Z.; et al. Discovery and Validation of a Prostate Cancer Genomic Classifier That Predicts Early Metastasis Following Radical Prostatectomy. PLoS ONE 2013, 8, e66855. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, A.; Lewinger, J.P.; Ren, J.; April, C.; Sherrod, A.E.; Hacia, J.G.; Daneshmand, S.; Gill, I.; Pinski, J.K.; Fan, J.B.; et al. Novel Gene Expression Signature Predictive of Clinical Recurrence after Radical Prostatectomy in Early Stage Prostate Cancer Patients. Prostate 2016, 76, 1239–1256. [Google Scholar] [CrossRef] [PubMed]
- Rubicz, R.; Zhao, S.; Wright, J.L.; Coleman, I.; Grasso, C.; Geybels, M.S.; Leonardson, A.; Kolb, S.; April, C.; Bibikova, M.; et al. Gene Expression Panel Predicts Metastatic-Lethal Prostate Cancer Outcomes in Men Diagnosed with Clinically Localized Prostate Cancer. Mol. Oncol. 2017, 11, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Cooperberg, M.R.; Simko, J.P.; Cowan, J.E.; Reid, J.E.; Djalilvand, A.; Bhatnagar, S.; Gutin, A.; Lanchbury, J.S.; Swanson, G.P.; Stone, S.; et al. Validation of a Cell-Cycle Progression Gene Panel to Improve Risk Stratification in a Contemporary Prostatectomy Cohort. J. Clin. Oncol. 2013, 31, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Cuzick, J.; Berney, D.M.; Fisher, G.; Mesher, D.; Moller, H.; Reid, J.E.; Perry, M.; Park, J.; Younus, A.; Gutin, A.; et al. Prognostic Value of a Cell Cycle Progression Signature for Prostate Cancer Death in a Conservatively Managed Needle Biopsy Cohort. Br. J. Cancer 2012, 106, 1095–1099. [Google Scholar] [CrossRef]
- Cuzick, J.; Stone, S.; Fisher, G.; Yang, Z.H.; North, B.V.; Berney, D.M.; Beltran, L.; Greenberg, D.; Moller, H.; Reid, J.E.; et al. Validation of an Rna Cell Cycle Progression Score for Predicting Death from Prostate Cancer in a Conservatively Managed Needle Biopsy Cohort. Br. J. Cancer 2015, 113, 382–389. [Google Scholar] [CrossRef]
- Bishoff, J.T.; Freedland, S.J.; Gerber, L.; Tennstedt, P.; Reid, J.; Welbourn, W.; Graefen, M.; Sangale, Z.; Tikishvili, E.; Park, J.; et al. Prognostic Utility of the Cell Cycle Progression Score Generated from Biopsy in Men Treated with Prostatectomy. J. Urol. 2014, 192, 409–414. [Google Scholar] [CrossRef]
- Bibikova, M.; Chudin, E.; Arsanjani, A.; Zhou, L.; Garcia, E.W.; Modder, J.; Kostelec, M.; Barker, D.; Downs, T.; Fan, J.B.; et al. Expression Signatures That Correlated with Gleason Score and Relapse in Prostate Cancer. Genomics 2007, 89, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, W. Foxs1 Promotes Prostate Cancer Progression through the Hedgehog/Gli1 Pathway. Biochem. Pharmacol. 2023, 218, 115893. [Google Scholar] [CrossRef]
- Aytes, A.; Giacobbe, A.; Mitrofanova, A.; Ruggero, K.; Cyrta, J.; Arriaga, J.; Palomero, L.; Farran-Matas, S.; Rubin, M.A.; Shen, M.M.; et al. Nsd2 Is a Conserved Driver of Metastatic Prostate Cancer Progression. Nat. Commun. 2018, 9, 5201. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Vasciaveo, A.; Karagiannis, D.; Sun, Z.; Chen, X.; Socciarelli, F.; Frankenstein, Z.; Zou, M.; Pannellini, T.; Chen, Y.; et al. Nsd2 Maintains Lineage Plasticity and Castration-Resistance in Neuroendocrine Prostate Cancer. bioRxiv 2023. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, T.; Gao, G.; Xu, J.; Lin, R.; Pan, Z.; Liu, J.; Feng, W. Cell Division Cycle 42 Effector Protein 4 Inhibits Prostate Cancer Progression by Suppressing Erk Signaling Pathway. Biomol. Biomed. 2023. [CrossRef] [PubMed]
- Richmond, C.S.; Oldenburg, D.; Dancik, G.; Meier, D.R.; Weinhaus, B.; Theodorescu, D.; Guin, S. Glycogen Debranching Enzyme (Agl) Is a Novel Regulator of Non-Small Cell Lung Cancer Growth. Oncotarget 2018, 9, 16718–16730. [Google Scholar] [CrossRef][Green Version]
- Worst, T.S.; Meyer, Y.; Gottschalt, M.; Weis, C.A.; von Hardenberg, J.; Frank, C.; Steidler, A.; Michel, M.S.; Erben, P. Rab27a, Rab27b and Vps36 Are Downregulated in Advanced Prostate Cancer and Show Functional Relevance in Prostate Cancer Cells. Int. J. Oncol. 2017, 50, 920–932. [Google Scholar] [CrossRef]
- Xu, N.; Wu, Y.P.; Ke, Z.B.; Liang, Y.C.; Cai, H.; Su, W.T.; Tao, X.; Chen, S.H.; Zheng, Q.S.; Wei, Y.; et al. Identification of Key DNA Methylation-Driven Genes in Prostate Adenocarcinoma: An Integrative Analysis of Tcga Methylation Data. J. Transl. Med. 2019, 17, 311. [Google Scholar] [CrossRef]
- Qi, C.; Li, B.; Yang, Y.; Yang, Y.; Li, J.; Zhou, Q.; Wen, Y.; Zeng, C.; Zheng, L.; Zhang, Q.; et al. Glipizide Suppresses Prostate Cancer Progression in the Tramp Model by Inhibiting Angiogenesis. Sci. Rep. 2016, 6, 27819. [Google Scholar] [CrossRef]
- McKinney, W. Data Structures for Statistical Computing in Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; van der Walt, S., Millman, J., Eds.; pp. 56–61. [Google Scholar]
- Hunter, J.D. Matplotlib: A 2d Graphics Environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Waskom, M.L. Seaborn: Statistical Data Visualization. J. Open Source Softw. 2021, 6, 3021. [Google Scholar] [CrossRef]
- Seabold, S.; Perktold, J. Statsmodels: Econometric and Statistical Modeling with Python. In Proceedings of the 9th Python in Science Conference, Austin, TX, USA, 28 June–3 July 2010; pp. 57–61. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucl. Acids Res. 2019, 47, D607–D613. [Google Scholar] [PubMed]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor Package for Pathway-Based Data Integration and Visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed]








| Normal Tissues | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |
|---|---|---|---|---|---|---|
| Number | 52 | 45 | 146 | 101 | 64 | 141 |
| Age (mean + stdev) | 60.3 ± 7.3 | 58.2 ± 7.6 | 59.8 ± 6.8 | 61.4 ± 6.5 | 61.7 ± 7 | 62.7 ± 6.3 |
| Ethnicity | ||||||
| Asian | 0 (0%) | 0 (0%) | 3 (2.1%) | 3 (3%) | 1 (1.6%) | 5 (3.5%) |
| African American | 7 (13.5%) | 11 (24.4%) | 26 (17.8%) | 12 (11.9%) | 2 (3.1%) | 6 (4.3%) |
| Caucasian | 44 (84.6%) | 30 (66.7%) | 116 (79.5%) | 82 (81.2%) | 59 (92.2%) | 126 (89.4%) |
| Other or not reported | 1 (1.9%) | 4 (8.9%) | 1 (0.7%) | 4 (4%) | 2 (3.1%) | 4 (2.8%) |
| Pathologic T | ||||||
| T2a | - | 5 (11.4%) | 7 (4.9%) | 1 (1%) | 0 (0%) | 0 (0%) |
| T2b | - | 2 (4.5%) | 4 (2.8%) | 0 (0%) | 3 (4.8%) | 1 (0.7%) |
| T2c | - | 25 (56.8%) | 84 (58.3%) | 31 (31%) | 16 (25.8%) | 8 (5.7%) |
| T3a | - | 11 (25%) | 40 (27.8%) | 45 (45%) | 23 (37.1%) | 39 (27.9%) |
| T3b | - | 1 (2.3%) | 8 (5.6%) | 21 (21%) | 19 (30.6%) | 86 (61.4%) |
| T4 | - | 0 (0%) | 1 (0.7%) | 2 (2%) | 1 (1.6%) | 6 (4.3%) |
| Pathologic N | ||||||
| N0 | - | 23 (100%) | 112 (95.7%) | 83 (90.2%) | 47 (77%) | 80 (61.1%) |
| N1 | - | 0 (0%) | 5 (4.3%) | 9 (9.8%) | 14 (23%) | 51 (38.9%) |
| Clinical M | ||||||
| M0 | - | 38 (100%) | 136 (100%) | 93 (100%) | 58 (98.3%) | 130 (98.5%) |
| M1a | - | 0 (0%) | 0 (0%) | 0 (0%) | 1 (1.7%) | 0 (0%) |
| M1b | - | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.8%) |
| M1c | - | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0.8%) |
| Gene Symbol | Gene Name | Function in PCa/Cancer | References |
|---|---|---|---|
| FOXS1 | Forkhead Box Protein S1 | Mutated in PCa; required for cell growth and survival | [72] |
| NSD2 | Nuclear Receptor Binding SET Domain Protein 2 | Drives metastatic progression, maintains neuroendocrine phenotype of PCa | [73,74] |
| CDC42 EP4 | Cell Division Cycle 42 Effector Protein 4 | Inhibits proliferation and invasion | [75] |
| AGL | Amylo-Alpha-1, 6-Glucosidase, 4-Alpha Glucanotransferase/glycogen debranching enzyme | Involved in glycogen degradation * | [76] |
| VPS36 | Vacuolar Protein Sorting 36 Homolog | Predictive for reduced BCR-free survival; associated with distant metastasis | [77] |
| TMLHE | Trimethyllysine Hydroxylase, Epsilon | Methylated in PCa | [78] |
| ANGPT1 | Angiopoietin 1 | Involved in angiogenesis * | [79] |
| C22orf23 | Chromosome 22 Open Reading Frame 23 | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippi, A.; Aurelian, J.; Mocanu, M.-M. Analysis of the Gene Networks and Pathways Correlated with Tissue Differentiation in Prostate Cancer. Int. J. Mol. Sci. 2024, 25, 3626. https://doi.org/10.3390/ijms25073626
Filippi A, Aurelian J, Mocanu M-M. Analysis of the Gene Networks and Pathways Correlated with Tissue Differentiation in Prostate Cancer. International Journal of Molecular Sciences. 2024; 25(7):3626. https://doi.org/10.3390/ijms25073626
Chicago/Turabian StyleFilippi, Alexandru, Justin Aurelian, and Maria-Magdalena Mocanu. 2024. "Analysis of the Gene Networks and Pathways Correlated with Tissue Differentiation in Prostate Cancer" International Journal of Molecular Sciences 25, no. 7: 3626. https://doi.org/10.3390/ijms25073626
APA StyleFilippi, A., Aurelian, J., & Mocanu, M.-M. (2024). Analysis of the Gene Networks and Pathways Correlated with Tissue Differentiation in Prostate Cancer. International Journal of Molecular Sciences, 25(7), 3626. https://doi.org/10.3390/ijms25073626







