An Alternative Application of Magnetic-Activated Cell Sorting: CD45 and CD235a Based Purification of Semen and Testicular Tissue Samples
Abstract
1. Introduction
2. Results
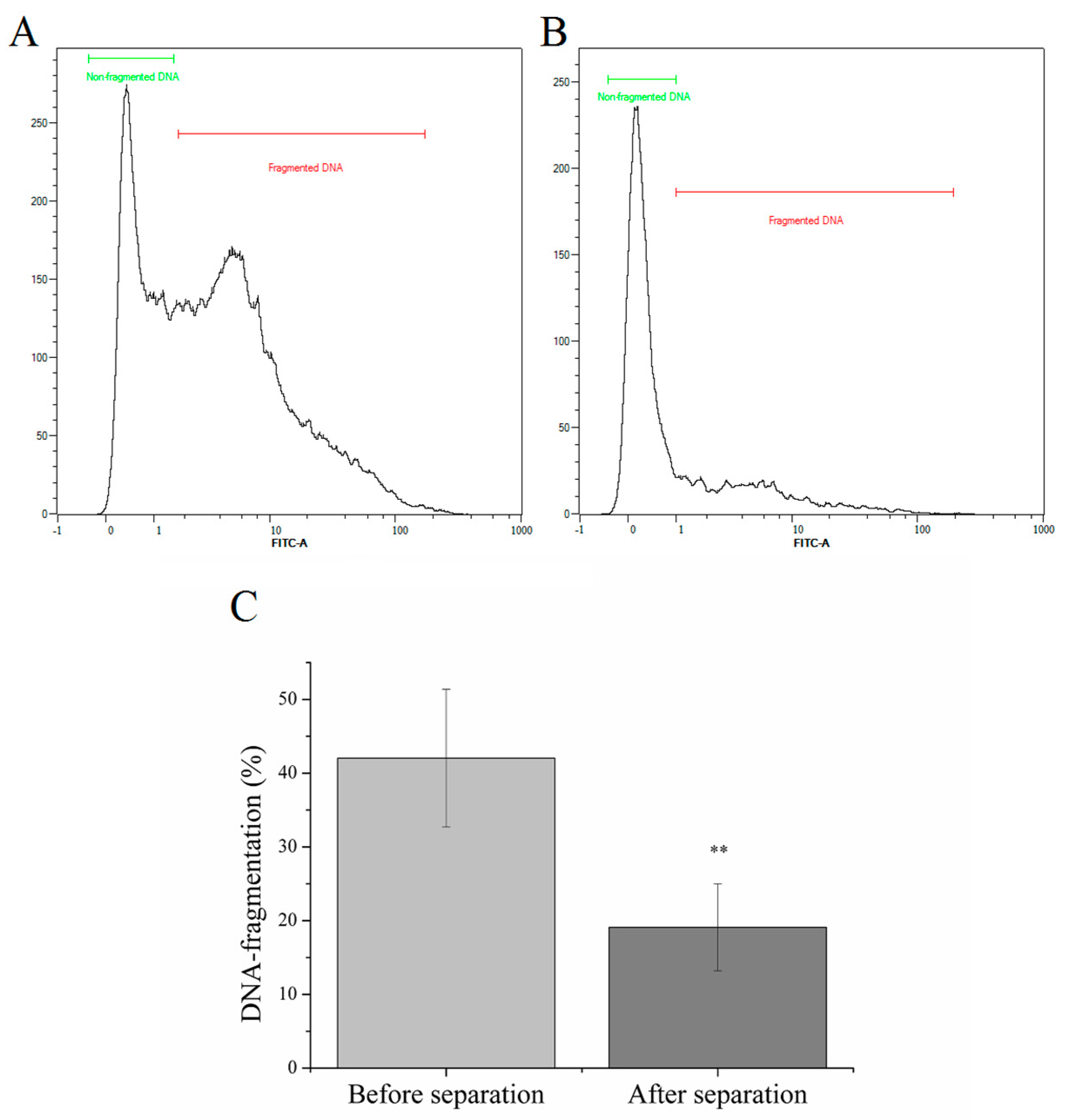
2.1. Magnetic Separation of Sperm Cells with High DNA-fragmentation
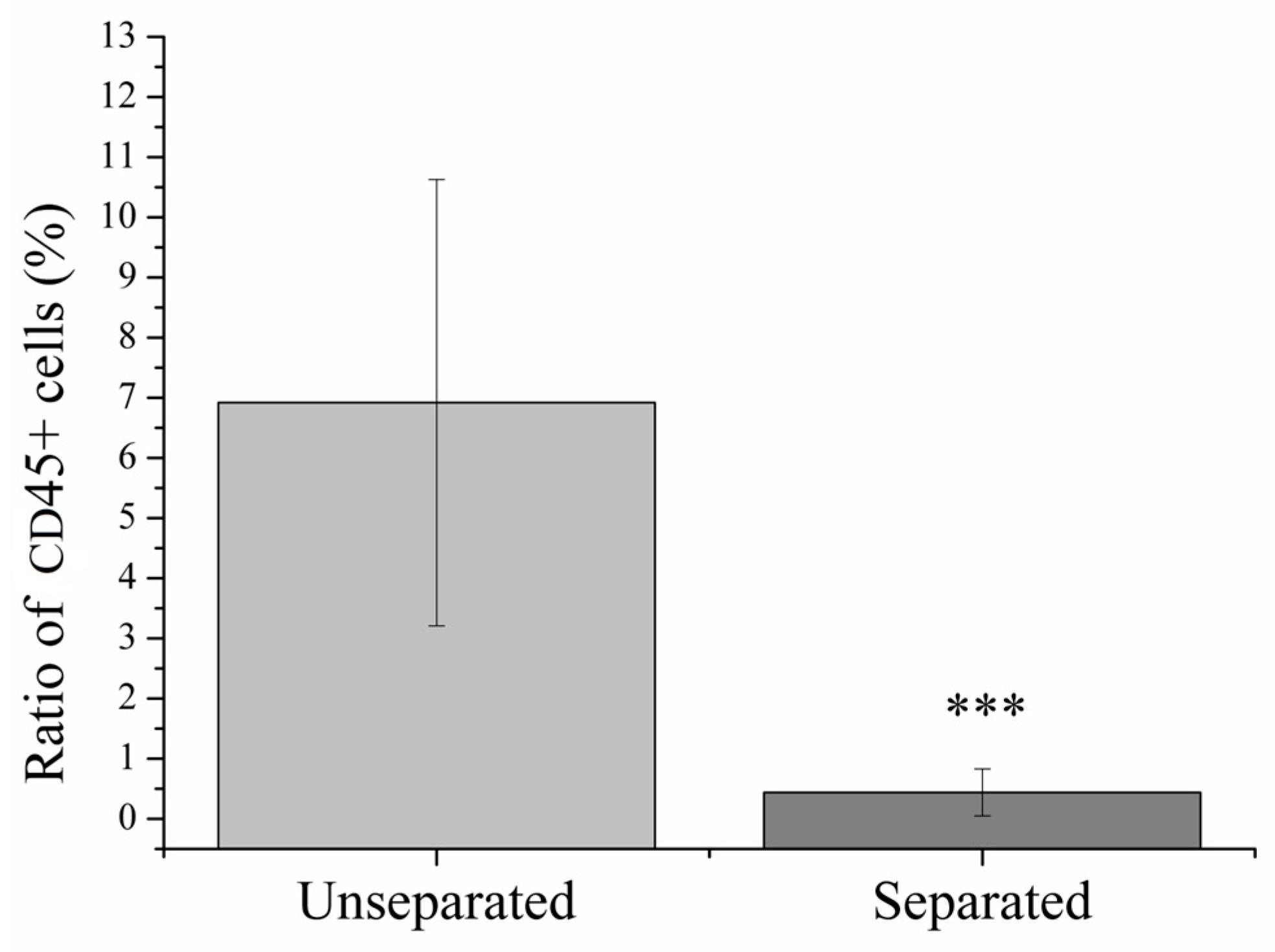
2.2. Magnetic Separation of Leukocytes
2.3. Magnetic Separation of Erythrocytes
3. Discussion
4. Materials and Methods
4.1. Sample Collections
4.2. Magnetic Separation of Apoptotic Sperm Cells for Decreasing DNA-Fragmentation
4.3. Magnetic Separation of Leukocytes
4.4. Magnetic Separation of Erythrocytes
4.5. Chemicals
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cox, C.M.; Thoma, M.E.; Tchangalova, N.; Mburu, G.; Bornstein, M.J.; Johnson, C.L.; Kiarie, J. Infertility Prevalence and the Methods of Estimation from 1990 to 2021: A Systematic Review and Meta-Analysis. Hum. Reprod. Open 2022, 2022, hoac051. [Google Scholar] [CrossRef] [PubMed]
- Kamel, R.M. Assisted Reproductive Technology after the Birth of Louise Brown. J. Reprod. Infertil. 2013, 14, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. 2021 Assisted Reproductive Technology Fertility Clinic and National Summary Report; US Dept of Health and Human Services: Washington, DC, USA, 2023.
- Winters, B.R.; Walsh, T.J. The Epidemiology of Male Infertility. Urol. Clin. N. Am. 2014, 41, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S.; Pacey, A.A. Sperm Transport in the Female Reproductive Tract. Hum. Reprod. Update 2006, 12, 23–37. [Google Scholar] [CrossRef]
- Sánchez-Calabuig, M.J.; López-Cardona, A.P.; Fernández-González, R.; Ramos-Ibeas, P.; Fonseca Balvís, N.; Laguna-Barraza, R.; Pericuesta, E.; Gutiérrez-Adán, A.; Bermejo-Álvarez, P. Potential Health Risks Associated to ICSI: Insights from Animal Models and Strategies for a Safe Procedure. Front. Public Health 2014, 2, 241. [Google Scholar] [CrossRef]
- Pinto, S.; Carrageta, D.F.; Alves, M.G.; Rocha, A.; Agarwal, A.; Barros, A.; Oliveira, P.F. Sperm Selection Strategies and Their Impact on Assisted Reproductive Technology Outcomes. Andrologia 2021, 53, e13725. [Google Scholar] [CrossRef]
- McDowell, S.; Kroon, B.; Ford, E.; Hook, Y.; Glujovsky, D.; Yazdani, A. Advanced Sperm Selection Techniques for Assisted Reproduction. Cochrane Database Syst. Rev. 2014, CD010461. [Google Scholar] [CrossRef]
- Oseguera-López, I.; Ruiz-Díaz, S.; Ramos-Ibeas, P.; Pérez-Cerezales, S. Novel Techniques of Sperm Selection for Improving IVF and ICSI Outcomes. Front. Cell Dev. Biol. 2019, 7, 298. [Google Scholar] [CrossRef]
- Pesce, M.; De Felici, M. Purification of Mouse Primordial Germ Cells by MiniMACS Magnetic Separation System. Dev. Biol. 1995, 170, 722–725. [Google Scholar] [CrossRef]
- Govers, C.; Berrevoets, C.; Treffers-Westerlaken, E.; Broertjes, M.; Debets, R. Magnetic-Activated Cell Sorting of TCR-Engineered T Cells, Using TCD34 as a Gene Marker, but Not Peptide-MHC Multimers, Results in Significant Numbers of Functional CD4+ and CD8+ T Cells. Hum. Gene Ther. Methods 2012, 23, 213–224. [Google Scholar] [CrossRef]
- Ravelo, K.M.; Andersen, N.D.; Monje, P. V Magnetic-Activated Cell Sorting for the Fast and Efficient Separation of Human and Rodent Schwann Cells from Mixed Cell Populations. Methods Mol. Biol. 2018, 1739, 87–109. [Google Scholar] [CrossRef]
- Weil, M.-T.; Schulz-Ëberlin, G.; Mukherjee, C.; Kuo-Elsner, W.P.; Schäfer, I.; Müller, C.; Simons, M. Isolation and Culture of Oligodendrocytes. Methods Mol. Biol. 2019, 1936, 79–95. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, S.; Chen, M.; Tian, D.-S.; Qin, C. Isolation of Mouse Primary Microglia by Magnetic-Activated Cell Sorting in Animal Models of Demyelination. J. Vis. Exp. 2022, 182, e63511. [Google Scholar] [CrossRef]
- Frauchiger, D.A.; Tekari, A.; May, R.D.; Džafo, E.; Chan, S.C.W.; Stoyanov, J.; Bertolo, A.; Zhang, X.; Guerrero, J.; Sakai, D.; et al. Fluorescence-Activated Cell Sorting Is More Potent to Fish Intervertebral Disk Progenitor Cells Than Magnetic and Beads-Based Methods. Tissue Eng. Part C. Methods 2019, 25, 571–580. [Google Scholar] [CrossRef]
- Tsujisaka, Y.; Hatani, T.; Okubo, C.; Ito, R.; Kimura, A.; Narita, M.; Chonabayashi, K.; Funakoshi, S.; Lucena-Cacace, A.; Toyoda, T.; et al. Purification of Human IPSC-Derived Cells at Large Scale Using MicroRNA Switch and Magnetic-Activated Cell Sorting. Stem Cell Rep. 2022, 17, 1772–1785. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, D.G.; Yang, L.; Qin, S.; Li, K.; Fitch, A.; Huang, L.; McVerry, B.J.; Hand, T.W.; Methé, B.A.; Morris, A. Magnetic-Activated Cell Sorting Identifies a Unique Lung Microbiome Community. Microbiome 2023, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, H.; Alipour, M.; Valcheva, R.; Bording-Jorgensen, M.; Jovel, J.; Zaidi, D.; Shah, P.; Lou, Y.; Ebeling, C.; Mason, A.L.; et al. Host Immunoglobulin G Selectively Identifies Pathobionts in Pediatric Inflammatory Bowel Diseases. Microbiome 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Shamloo, A.; Yazdani, A.; Saghafifar, F. Investigation of a Two-Step Device Implementing Magnetophoresis and Dielectrophoresis for Separation of Circulating Tumor Cells from Blood Cells. Eng. Life Sci. 2020, 20, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, S.; Paasch, U.; Glander, H.J. Enrichment of Non-Apoptotic Human Spermatozoa after Cryopreservation by Immunomagnetic Cell Sorting. Cell Tissue Bank. 2001, 2, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of Phosphatidylserine on the Surface of Apoptotic Lymphocytes Triggers Specific Recognition and Removal by Macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Garrido, N.; Gil Juliá, M. The Use of Non-Apoptotic Sperm Selected by Magnetic Activated Cell Sorting (MACS) to Enhance Reproductive Outcomes: What the Evidence Says. Biology 2024, 13, 30. [Google Scholar] [CrossRef]
- Hichri, R.; Amor, H.; Khammari, M.; Harzallah, M.; El Fekih, S.; Saad, A.; Ajina, M.; Ben Ali, H. Apoptotic Sperm Biomarkers and the Correlation between Conventional Sperm Parameters and Clinical Characteristics. Andrologia 2018, 50, e12813. [Google Scholar] [CrossRef]
- Said, T.M.; Agarwal, A.; Grunewald, S.; Rasch, M.; Glander, H.-J.; Paasch, U. Evaluation of Sperm Recovery Following Annexin V Magnetic-Activated Cell Sorting Separation. Reprod. Biomed. Online 2006, 13, 336–339. [Google Scholar] [CrossRef]
- Said, T.; Agarwal, A.; Grunewald, S.; Rasch, M.; Baumann, T.; Kriegel, C.; Li, L.; Glander, H.-J.; Thomas, A.J.J.; Paasch, U. Selection of Nonapoptotic Spermatozoa as a New Tool for Enhancing Assisted Reproduction Outcomes: An in Vitro Model. Biol. Reprod. 2006, 74, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-H.; Liu, C.-H.; Shih, Y.-T.; Tsao, H.-M.; Huang, C.-C.; Chen, H.-H.; Lee, M.-S. Magnetic-Activated Cell Sorting for Sperm Preparation Reduces Spermatozoa with Apoptotic Markers and Improves the Acrosome Reaction in Couples with Unexplained Infertility. Hum. Reprod. 2010, 25, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Tavalaee, M.; Deemeh, M.R.; Azadi, L.; Fazilati, M.; Nasr-Esfahani, M.H. Zeta Potential vs Apoptotic Marker: Which Is More Suitable for ICSI Sperm Selection? J. Assist. Reprod. Genet. 2013, 30, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Lepine, S.; McDowell, S.; Searle, L.M.; Kroon, B.; Glujovsky, D.; Yazdani, A. Advanced Sperm Selection Techniques for Assisted Reproduction. Cochrane database Syst. Rev. 2019, 7, CD010461. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Chen, L.-J.; Zhu, X.-X.; Yu, W.; Gao, Q.-Q.; Sun, H.-X.; Ding, L.-J.; Wang, J.-X. Magnetic-Activated Cell Sorting of Nonapoptotic Spermatozoa with a High DNA Fragmentation Index Improves the Live Birth Rate and Decreases Transfer Cycles of IVF/ICSI. Asian J. Androl. 2022, 24, 367–372. [Google Scholar] [CrossRef] [PubMed]
- El Fekih, S.; Tous, C.; Gueganic, N.; Brugnon, F.; Ben Ali, H.; Bujan, L.; Moinard, N.; Caire-Tetauru, E.; Ajina, M.; Douet-Guilbert, N.; et al. Decrease of Spermatozoa with an Unbalanced Chromosome Content after Cell Sorting in Men Carrying a Structural Chromosomal Abnormality. Andrology 2020, 8, 181–190. [Google Scholar] [CrossRef]
- Aziz, N.; Said, T.; Paasch, U.; Agarwal, A. The Relationship between Human Sperm Apoptosis, Morphology and the Sperm Deformity Index. Hum. Reprod. 2007, 22, 1413–1419. [Google Scholar] [CrossRef]
- de Vantéry Arrighi, C.; Lucas, H.; Chardonnens, D.; de Agostini, A. Removal of Spermatozoa with Externalized Phosphatidylserine from Sperm Preparation in Human Assisted Medical Procreation: Effects on Viability, Motility and Mitochondrial Membrane Potential. Reprod. Biol. Endocrinol. 2009, 7, 1. [Google Scholar] [CrossRef]
- Esbert, M.; Godo, A.; Soares, S.R.; Florensa, M.; Amorós, D.; Ballesteros, A.; Vidal, F. Spermatozoa with Numerical Chromosomal Abnormalities Are More Prone to Be Retained by Annexin V-MACS Columns. Andrology 2017, 5, 807–813. [Google Scholar] [CrossRef]
- Romany, L.; Garrido, N.; Motato, Y.; Aparicio, B.; Remohí, J.; Meseguer, M. Removal of Annexin V-Positive Sperm Cells for Intracytoplasmic Sperm Injection in Ovum Donation Cycles Does Not Improve Reproductive Outcome: A Controlled and Randomized Trial in Unselected Males. Fertil. Steril. 2014, 102, 1567–1575.e1. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Kuchakulla, M.; Narasimman, M.; Khosravizadeh, Z.; Ali, A.; Brackett, N.; Ibrahim, E.; Ramasamy, R. Laboratory and Clinical Management of Leukocytospermia and Hematospermia: A Review. Ther. Adv. Reprod. Health 2020, 14, 2633494120922511. [Google Scholar] [CrossRef]
- Wolff, H. The Biologic Significance of White Blood Cells in Semen. Fertil. Steril. 1995, 63, 1143–1157. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Mongioì, L.M.; Alamo, A.; Calogero, A.E.; Compagnone, M.; Giacone, F.; Cannarella, R.; La Vignera, S.; Condorelli, R.A. Evaluation of Seminal Fluid Leukocyte Subpopulations in Patients with Varicocele. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420925719. [Google Scholar] [CrossRef] [PubMed]
- Close, C.E.; Roberts, P.L.; Berger, R.E. Cigarettes, Alcohol and Marijuana Are Related to Pyospermia in Infertile Men. J. Urol. 1990, 144, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Agarwal, A.; Kandirali, E.; Sharma, R.K.; Thomas, A.J.; Nada, E.A.; Evenson, D.P.; Alvarez, J.G. Leukocytospermia Is Associated with Increased Reactive Oxygen Species Production by Human Spermatozoa. Fertil. Steril. 2002, 78, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Derbel, R.; Sellami, H.; Sakka, R.; Ben Slima, A.; Mkaddem, I.; Gdoura, R.; Mcelreavey, E.; Ammar-Keskes, L. Relationship between Nuclear DNA Fragmentation, Mitochondrial DNA Damage and Standard Sperm Parameters in Spermatozoa of Infertile Patients with Leukocytospermia. J. Gynecol. Obstet. Hum. Reprod. 2021, 50, 102101. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Zhao, Y.; Zhang, Z.; Wang, H.; Hou, Y.; Bai, S.; Liu, R.; Xu, B. Effect of Leukocytes on Semen Quality in Men from Primary and Secondary Infertile Couples: A Cross-Sectional Study. Health Sci. Rep. 2023, 6, e1683. [Google Scholar] [CrossRef]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The Effect of Bacteriospermia and Leukocytospermia on Conventional and Nonconventional Semen Parameters in Healthy Young Normozoospermic Males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef]
- Plante, M.; de Lamirande, E.; Gagnon, C. Reactive Oxygen Species Released by Activated Neutrophils, but Not by Deficient Spermatozoa, Are Sufficient to Affect Normal Sperm Motility. Fertil. Steril. 1994, 62, 387–393. [Google Scholar] [CrossRef]
- Aitken, R.J.; West, K.; Buckingham, D. Leukocytic Infiltration into the Human Ejaculate and Its Association with Semen Quality, Oxidative Stress, and Sperm Function. J. Androl. 1994, 15, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Fedder, J.; Ellerman-Eriksen, S. Effect of Cytokines on Sperm Motility and Ionophore-Stimulated Acrosome Reaction. Arch. Androl. 1995, 35, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Mathers, M.J.; Degener, S.; Sperling, H.; Roth, S. Hematospermia—A Symptom with Many Possible Causes. Dtsch. Arztebl. Int. 2017, 114, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Soygur, B.; Celik, S.; Celik-Ozenci, C.; Sati, L. Effect of Erythrocyte-Sperm Separation Medium on Nuclear, Acrosomal, and Membrane Maturity Parameters in Human Sperm. J. Assist. Reprod. Genet. 2018, 35, 491–501. [Google Scholar] [CrossRef]
- Yazdinejad, F.; Heydari, L.; Motamed Zadeh, L.; Seifati, S.M.; Agha-Rahimi, A. Application of Erythrocyte Lysing Buffer (ELB) Has Detrimental Effects on Human Sperm Quality Parameters, DNA Fragmentation and Chromatin Structure. Andrologia 2020, 52, e13702. [Google Scholar] [CrossRef] [PubMed]
- Popal, W.; Nagy, Z.P. Laboratory Processing and Intracytoplasmic Sperm Injection Using Epididymal and Testicular Spermatozoa: What Can Be Done to Improve Outcomes? Clinics 2013, 68 (Suppl. S1), 125–130. [Google Scholar] [CrossRef]
- Tournaye, H.; Wieme, P.; Janssens, R.; Verheyen, G.; Devroey, P.; Van Steirteghem, A. Incubation of Spermatozoa from Asthenozoospermic Semen Samples with Pentoxifylline and 2-Deoxyadenosine: Variability in Hyperactivation and Acrosome Reaction Rates. Hum. Reprod. 1994, 9, 2038–2043. [Google Scholar] [CrossRef]
- Rijsselaere, T.; Van Soom, A.; Maes, D.; Verberckmoes, S.; de Kruif, A. Effect of Blood Admixture on in Vitro Survival of Chilled and Frozen-Thawed Canine Spermatozoa. Theriogenology 2004, 61, 1589–1602. [Google Scholar] [CrossRef]
- Bernard, A.; Boumsell, L. Human leukocyte differentiation antigens. Presse Med. 1984, 13, 2311–2316. [Google Scholar] [CrossRef]
- Tian, H.-F.; Xing, J.; Tang, X.-Q.; Chi, H.; Sheng, X.-Z.; Zhan, W.-B. Cluster of Differentiation Antigens: Essential Roles in the Identification of Teleost Fish T Lymphocytes. Mar. Life Sci. Technol. 2022, 4, 303–316. [Google Scholar] [CrossRef]
- Rheinländer, A.; Schraven, B.; Bommhardt, U. CD45 in Human Physiology and Clinical Medicine. Immunol. Lett. 2018, 196, 22–32. [Google Scholar] [CrossRef]
- Karsten, U.; Butschak, G.; Stahn, R.; Goletz, S. A Novel Series of Anti-Human Glycophorin A (CD235a) Antibodies Defining Five Extra- and Intracellular Epitopes. Int. Immunopharmacol. 2010, 10, 1354–1360. [Google Scholar] [CrossRef]
- Krausz, C.; West, K.; Buckingham, D.; Aitken, R.J. Development of a Technique for Monitoring the Contamination of Human Semen Samples with Leukocytes. Fertil. Steril. 1992, 57, 1317–1325. [Google Scholar] [CrossRef]
- Ochsendorf, F.R.; Beschmann, H.A.; Neuhauser, S.; Milbradt, R. CD 45/67 Immunobead Preparation of Human Semen Activates Granulocytes. Andrologia 1997, 29, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hipler, U.C.; Schreiber, G.; Wollina, U. Reactive Oxygen Species in Human Semen: Investigations and Measurements. Arch. Androl. 1998, 40, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Maietta, G.; Fiore, J.R.; Pellegrino, V.; Milillo, G.; Tagliaferro, L.; Corbelli, M.; Pastore, G. Leucocyte Identification and Analysis in Human Semen. Arch. Androl. 1997, 38, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.D.; Kim, U.; Soh, H.T. Multitarget Magnetic Activated Cell Sorter. Proc. Natl. Acad. Sci. USA 2008, 105, 18165–18170. [Google Scholar] [CrossRef] [PubMed]
- Plouffe, B.D.; Murthy, S.K.; Lewis, L.H. Fundamentals and Application of Magnetic Particles in Cell Isolation and Enrichment: A Review. Rep. Prog. Phys. 2015, 78, 16601. [Google Scholar] [CrossRef] [PubMed]
- Frenea-Robin, M.; Marchalot, J. Basic Principles and Recent Advances in Magnetic Cell Separation. Magnetochemistry 2022, 8, 11. [Google Scholar] [CrossRef]
- Gil Juliá, M.; Hervas, I.; Navarro-Gomezlechon, A.; Mossetti, L.; Quintana, F.; Amoros, D.; Pacheco, A.; Gonzalez-Ravina, C.; Rivera-Egea, R.; Garrido, N. Semen Processing Using Magnetic-Activated Cell Sorting before ICSI Is Deemed Safe for Obstetric and Perinatal Outcomes: A Retrospective Multicentre Study. Reprod. Biomed. Online 2023, 47, 103172. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ahmad, G.; Esteves, S.C.; Agarwal, A. Terminal Deoxynucleotidyl Transferase DUTP Nick End Labeling (TUNEL) Assay Using Bench Top Flow Cytometer for Evaluation of Sperm DNA Fragmentation in Fertility Laboratories: Protocol, Reference Values, and Quality Control. J. Assist. Reprod. Genet. 2016, 33, 291–300. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czétány, P.; Balló, A.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. An Alternative Application of Magnetic-Activated Cell Sorting: CD45 and CD235a Based Purification of Semen and Testicular Tissue Samples. Int. J. Mol. Sci. 2024, 25, 3627. https://doi.org/10.3390/ijms25073627
Czétány P, Balló A, Márk L, Török A, Szántó Á, Máté G. An Alternative Application of Magnetic-Activated Cell Sorting: CD45 and CD235a Based Purification of Semen and Testicular Tissue Samples. International Journal of Molecular Sciences. 2024; 25(7):3627. https://doi.org/10.3390/ijms25073627
Chicago/Turabian StyleCzétány, Péter, András Balló, László Márk, Attila Török, Árpád Szántó, and Gábor Máté. 2024. "An Alternative Application of Magnetic-Activated Cell Sorting: CD45 and CD235a Based Purification of Semen and Testicular Tissue Samples" International Journal of Molecular Sciences 25, no. 7: 3627. https://doi.org/10.3390/ijms25073627
APA StyleCzétány, P., Balló, A., Márk, L., Török, A., Szántó, Á., & Máté, G. (2024). An Alternative Application of Magnetic-Activated Cell Sorting: CD45 and CD235a Based Purification of Semen and Testicular Tissue Samples. International Journal of Molecular Sciences, 25(7), 3627. https://doi.org/10.3390/ijms25073627





