Synthesis of Antiprotozoal 2-(4-Alkyloxyphenyl)-Imidazolines and Imidazoles and Their Evaluation on Leishmania mexicana and Trypanosoma cruzi
Abstract
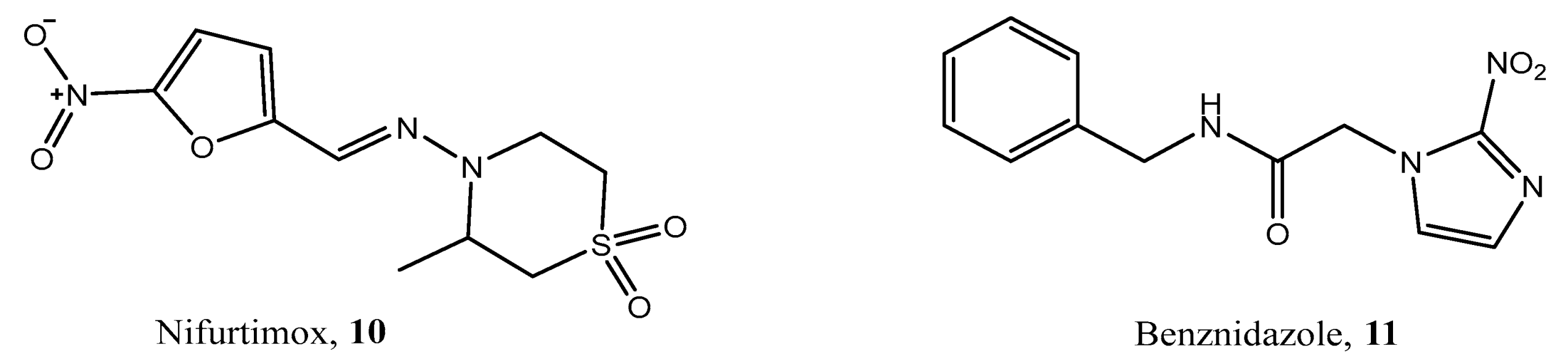
1. Introduction
2. Results and Discussion
2.1. Chemical Basis of Bioisosteres
2.2. Synthesis of Antiprotozoal Compounds
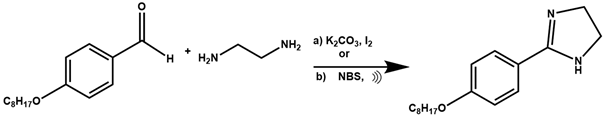
2.2.1. Synthesis of Imidazolines
2.2.2. Synthesis of Imidazoles
2.3. Synthesis of the Diol
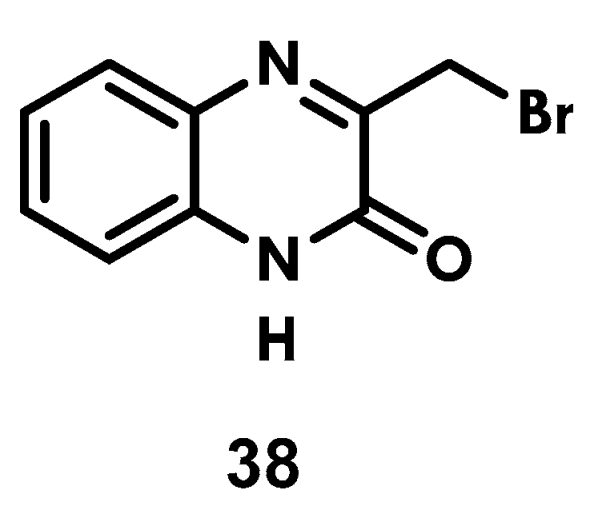
2.4. Synthesis of 3-(Bromomethyl)Quinoxaline-2(1H)-One, 38
2.5. Biological Evaluation
In Vitro Evaluation of Some Compounds on Leishmania mexicana and Trypanosoma cruzi
2.6. Structure–Activity Relationship
3. Materials and Methods
3.1. General
3.2. Synthesis of 4-Alkyloxybenzaldehydes
3.3. Synthesis of Imidazolines
Synthesis of 2-(4-Alkyloxyphenyl)-4,5-Dihydro-1H-Imidazoles
3.4. Synthesis of 2-(4-Alkyloxyphenyl)-1H-Imidazoles
3.5. Synthesis of (1R,2S)-1,2-Bis(4-Alkyloxyphenyl)Ethane-1,2-Diol (37)
3.6. Synthesis of 3-(Bromomethyl)Quinoxalin-2(1H)-One (38)
3.7. Biological Evaluation
3.7.1. Dilution of the Compounds
3.7.2. In Vitro Evaluation of the Metabolic Inhibition of Leishmania mexicana by Fluorometric Analysis with Resazurin
3.7.3. In Vitro Evaluation of the Metabolic Inhibition of Trypanosoma cruzi INC-5 by Colorimetric Analysis with 3-(4,5-Dimethyl-2-Thiazoyl)-2,5-Diphenyltetrazolic Bromide (MTT)
3.7.4. In Vitro Evaluation of the Cytotoxic Effect of the Compounds on Macrophages, Measured by Fluorometric and Colorimetric Analysis with Resazurin
3.7.5. Determination of the Selectivity Index (SI)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Neglected Tropical Diseases. 2024. Available online: https://www.who.int/campaigns/world-ntd-day/2024 (accessed on 15 January 2024).
- Sasidharan, S.; Saudagar, P. Leishmaniasis: Where are we and where are we heading? Parasitol. Res. 2021, 120, 1541–1554. [Google Scholar] [CrossRef]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A.F.; Newman, S.; Ramanan, P.; Suarez, J.A. A review of Leishmaniasis: Current knowledge and future directions. Curr. Trop. Med. Rep. 2021, 8, 121–132. [Google Scholar] [CrossRef]
- Mendes Roatt, B.; de Oliveira Cardoso, J.M.; De Brito, R.C.; Coura-Vital, W.; de Oliveira Aguiar-Soares, R.D.; Reis, A.B. Recent advances and new strategies on leishmaniasis treatment. Appl. Microbiol. Biotechnol. 2020, 104, 8965–8977. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Olmo, F.; Lewis, M.D.; Wilkinson, S.R.; Taylor, M.C.; Kelly, J.M. Challenges in Chagas Disease Drug Development. Molecules 2020, 25, 2799. [Google Scholar] [CrossRef]
- Andersen, E.M.; Cruz-Saldarriaga, M.; Llanos-Cuentas, A.; Luz-Cjuno, M.; Echevarria, J.; Miranda-Verastegui, C.; Colina, O.; Berman, J.D. Comparison of meglumine antimoniate and pentamidine for peruvian cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2005, 72, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Frézard, F.; Martins, P.C.; Barbosa, M.C.M.; Pimenta, A.M.; Ferreira, W.A.; de Melo, J.E.; Mangrum, J.B.; Demicheli, C. New insights into the chemical structure and composition of the pentavalent antimonial drugs, meglumine antimonate and sodium stibogluconate. J. Inorg. Biochem. 2008, 102, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Jiménez, L.K.; Juárez-Saldivar, A.; Gómez-Escobedo, R.; Delgado-Maldonado, T.; Méndez-Álvare, D.; Palos, I.; Bandyopadhyay, D.; Gaona-Lopez, C.; Ortiz-Pérez, E.; Nogueda-Torres, B.; et al. Ligand-based virtual screening and molecular docking of benzimidazoles as potential inhibitors of triosephosphate isomerase identified new trypanocidal agents. Int. J. Mol. Sci. 2022, 23, 10047. [Google Scholar] [CrossRef] [PubMed]
- García-Huertas, P.; Cardona-Castro, N. Advances in the treatment of Chagas Disease: Promising new drugs, plants and targets. Biomed. Pharmacother. 2021, 142, 112020. [Google Scholar] [CrossRef] [PubMed]
- Meymandi, S.; Hernandez, S.; Park, S.; Sanchez, D.R.; Forsyth, C. Treatment of Chagas Disease in the United States. Curr. Treat. Options Infect. Dis. 2018, 10, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Stass, H.; Just, S.; Weimann, B.; Ince, I.; Willmann, S.; Feleder, E.; Freitas, C.; Yerino, G.; Münster, U. Clinical investigation of the biopharmaceutical characteristics of nifurtimox tablets—Implications for quality control and application. Eur. J. Pharm. Sci. 2021, 166, 105940. [Google Scholar] [CrossRef]
- Piccica, M.; Lagi, F.; Bartoloni, A.; Zammarchi, L. Efficacy and safety of pentamidine isethionate for tegumentary and visceral human leishmaniasis: A systematic review. J. Travel Med. 2021, 28, taab065. [Google Scholar] [CrossRef]
- Kuhlmann, F.M.; Fleckenstein, J.M.; Cohen, J.M.; Powderly, J.; Opal, W.G.; Steven, M. Infectious Diseases, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2017; p. 1345. [Google Scholar] [CrossRef]
- Gopi, E.; Kumar, T.; Menna-Barreto, R.F.S.; Valença, W.O.; da Silva Júnior, E.N.; Namboothiri, I.N.N. Imidazoles from nitroallylic acetates and α-bromonitroalkenes with amidines: Synthesis and trypanocidal activity studies. Org. Biomol. Chem. 2015, 13, 9862–9871. [Google Scholar] [CrossRef]
- Bucio-Cano, A.; Reyes-Arellano, A.; Correa-Basurto, J.; Bello, M.; Torres-Jaramillo, J.; Salgado-Zamora, H.; Curiel-Quesada, E.; Peralta-Cruz, J.; Avila-Sorrosa, A. Targeting quorum sensing by designing azoline derivatives to inhibit the N-hexanoyl homoserine lactone-receptor CviR: Synthesis as well as biological and theoretical evaluations. Bioorg. Med. Chem. 2015, 23, 7565. [Google Scholar] [CrossRef] [PubMed]
- Reyes Arellano, A.R.; Bucio Cano, J.A.; Montenegro Sustaita, M.M. Imidazolinas 8-Hexiloxifenil-2-Imidazolina, 8-Noniloxifenil-2-Imidazolina, 7-Hexiloxifenil-2-Imidazolina y 7-Noniloxifenil-2-Imidazolina y su Proceso de Obtención. Mexican Patent No. 369438, 17 May 2018. [Google Scholar]
- Herrera-Arizmendi, J.L.; Curiel-Quesada, E.; Correa-Basurto, J.; Bello, J.; Reyes-Arellano, A. Effect of New Analogs of Hexyloxy Phenyl Imidazoline on Quorum Sensing in Chromobacterium violaceum and In Silico Analysis of Ligand-Receptor Interactions. J. Chem. 2020, 2020, 8735190. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Camp, J.E. Recent advances in the synthesis of imidazoles. Org. Biomol. Chem. 2020, 18, 3950–3964. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; You, I.; Shin, S. Material Approaches to Stretchable Strain Sensors. Chemphyschem 2015, 16, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, H.; Murai, K.; Ohba, Y.; Hiramatsu, A.; Kita, Y. A mild and efficient one-pot synthesis of 2-dihydroimidazoles from aldehydes. Tetrahedron Lett. 2005, 46, 2197–2199. [Google Scholar] [CrossRef]
- Sant’ Anna, G.; Machado, P.; Sauzem, P.; Rosa, F.; Rubin, M. Ultrasound promoted synthesis of 2-imidazolines in water: A greener approach toward monoamine oxidase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 546–549. [Google Scholar] [CrossRef]
- De la Hoz, A.; Díaz-Ortiz, A.; Mateo, M.; Moral, M.; Moreno, A.; Elguero, J.; Foces-Foces, C.; Rodríguez, M.; Sánchez-Migallón, A. Microwave assisted synthesis and crystal structures of 2-imidazolines and imidazoles. Tetrahedron 2006, 62, 5868–5874. [Google Scholar] [CrossRef]
- Parik, O.; Senauerova, S.; Liskova, V.; Hadlir, K.; Ludwig, M. Study of synthesis of 2-(2-alkoxyphenyl)-1H-imidazoles. Comparison of oxidative aromatization of reactions of imidazolines. J. Heterocycl. Chem. 2006, 43, 835–841. [Google Scholar] [CrossRef]
- Avila-Sorrosa, A.; Vega-Ramírez, L.; Rodríguez-Domínguez, R.; Salgado-Zamora, H.; Peralta-Cruz, J.; Reyes-Arellano, A. A Novel Application of [Cr(en)2]2+ in the Synthesis of 1,2-Diols from Aromatic Aldehydes. Chem. Lett. 2010, 39, 500501. [Google Scholar] [CrossRef]
- Jacomini, A.P.; Silva, M.J.; Silva, R.G.; Gonçalves, D.S.; Volpato, H.; Basso, H.A.; Paula, F.R.; Nakamura, C.V.; Sarragiotto, M.H.; Rosa, F. A Synthesis and evaluation against Leishmania amazonensis of novel pyrazolo[3,4-d]pyridazinone-N-acylhydrazone-(bi)thiophene hybrids. Eur. J. Med. Chem. 2016, 124, 340–349. [Google Scholar] [CrossRef]
- Betancourt-Conde, I.; Avitia-Domínguez, C.; Hernández-Campos, A.; Castillo, R.; Yépez-Mulia, L.; Oria-Hernández, J.; Méndez, S.T.; Sierra-Campos, E.; Valdez-Solana, M.; Martínez-Caballero, S.; et al. Benzimidazole Derivatives as New and Selective Inhibitors of Arginase from Leishmania mexicana with Biological Activity against Promastigotes and Amastigotes. Int. J. Mol. Sci. 2022, 22, 13613. [Google Scholar] [CrossRef]
- Olmo, F.; Gómez-Contreras, F.; Navarro, P.; Marín, C.; Yunta, M.J.; Cano, C.; Campayo, L.; Martín-Oliva, D.; Rosales, M.J.; Sánchez-Moreno, M. Synthesis and evaluation of in vitro and in vivo trypanocidal properties of a new imidazole-containing nitrophthalazine derivative. Eur. J. Med. Chem. 2015, 106, 106–119. [Google Scholar] [CrossRef]
- Fernandes, M.C.; Da Silva, E.N., Jr.; Pinto, A.V.; De Castro, S.L.; Menna-Barreto, R.F.S. A novel triazolic naphthofuranquinone induces autophagy in reservosomes and impairment of mitosis in Trypanosoma cruzi. Parasitology 2012, 139, 26–36. [Google Scholar] [CrossRef]
- Galvao, J.; Davis, B.; Tilley, M.; Normando, E.; Duchen, M.R.; Cordeiro, M.F. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014, 28, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Mikus, J.; Steverding, D. A simple colorimetric method to screen drug cytotoxicity against Leishmania using the dye Alamar Blue®. Parasitol. Int. 2000, 48, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Vargas, K.F.; Andrade-Ochoa, S.; Nogueda-Torres, B.; Juárez-Ramírez, D.C.; Lara-Ramírez, E.E.; Mondragón-Flores, R.; Monge, A.; Rivera, G.; Sánchez-Torres, L.E. Isopropyl quinoxaline-7-carboxylate 1,4-di-N-oxide derivatives induce regulated necrosis-like cell death on Leishmania (Leishmania) mexicana. Parasitol. Res. 2018, 117, 45–58. [Google Scholar] [CrossRef] [PubMed]
- AnalystSoft BioStat, version 2007; Programa de Análisis Estadístico; AnalystSoft Inc.: Walnut, CA, USA, 2016.
- González-Morales, L.D.; Moreno-Rodríguez, A.; Vázquez-Jiménez, L.K.; Delgado-Maldonado, T.; Juárez-Saldivar, A.; Ortiz-Pérez, E.; Paz-Gonzalez, A.D.; Lara-Ramírez, E.E.; Yépez-Mulia, L.; Meza, P.; et al. Triose Phosphate Isomerase Structure-based virtual screening and in vitro biological activity of natural products as Leishmania Mexicana inhibitors. Pharmaceutics 2023, 15, 2046. [Google Scholar] [CrossRef]



 | |
| Compound | Yield (%) |
| 12: R1 = CH3 | 95 |
| 13: R1 = C2H5 | 95 |
| 14: R1 = C3H7 | 92 |
| 15: R1 = iC3H7 | 90 |
| 16: R1 = C4H9 | 90 |
| 17: R1 = C5H11 | 89 |
| 18: R1 = C6H13 | 88 |
| 19: R1 = C7H15 | 86 |
| 20: R1 = C8H17 | 86 |
| 21: R1 = C9H19 | 85 |
 | ||||
| Experiment | Reagents and Reaction Conditions | Solvent | Time | Yield (%) |
| A | a* | t-BuOH | 4 h | 61 |
| B | b | H2O | 2 h | NR |
| C | b | H2O:EtOH (1:1) | 2 h | NR |
| D | b | CH2Cl2 | 24 min | 34 |
| E | b | AcOEt | 24 min | 48 |
| F | b | CH3CN | 20 min | 60 |
 : Ultrasound (P = 60 W, f = 20–25 KHz); NR, no reaction and the recovery of the raw materials.
: Ultrasound (P = 60 W, f = 20–25 KHz); NR, no reaction and the recovery of the raw materials.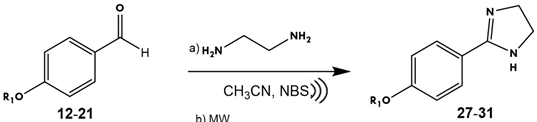 | |||||
| Experiment | R | Energy | T (°C) | t (min) | Yield (%) |
| A | 27: C5H11 | MW | 50 | 40 | 80 |
| B | 28: C6H13 | 70 | |||
| C | 29: C7H15 | 62 | |||
| D | 30: C8H17 | 61 | |||
| E | 31: C9H19 | 61 | |||
| F | 27: C5H11 |  | 50 | 20 | 80 |
| G | 28: C6H13 | 71 | |||
| H | 29: C7H15 | 64 | |||
| I | 30: C8H17 | 60 | |||
| J | 31: C9H19 | 62 | |||
 : Ultrasound (P = 60 W, f = 20–25 KHz).
: Ultrasound (P = 60 W, f = 20–25 KHz).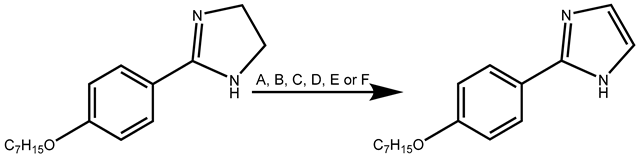 | |||||
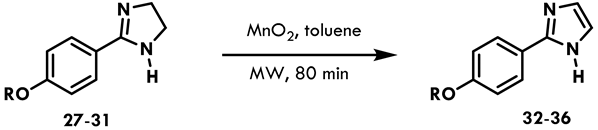
| Experiment | Reagents | Energy | T (°C) | t (min) | Yield % |
| A | NBS, K2CO3, CH2Cl2 | Δ | Reflux | 60 | NR |
| B | MnO2 (5 eq), toluene | Δ | 65 | 960 | NR |
| C | MnO2 (5 eq), CH2Cl2 | Δ | RT | 960 | NR |
| D | MnO2 (5 eq), toluene | MW | 65 | 110 | 10 |
| E | MnO2 (12 eq), toluene | MW | 65 | 110 | 22 |
| F | MnO2 (18 eq), toluene | MW | 65 | 80 | 57 |
 | ||
| Experiment | R | Yield (%) |
| A | 32: C5H11 | 67 |
| B | 33: C6H13 | 65 |
| C | 34: C7H15 | 57 |
| D | 35: C8H17 | 51 |
| E | 36: C9H19 | 47 |
| L. mexicana Promastigotes | T. cruzi INC-5 Epimastigotes | Macrophages J774 | SI (CC50/IC50) | ||
|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | CC50 (µg/mL) | L. mexicana | T. cruzi | |
| (22) | 16.04 (14.65–17.42) | >50 | >100 | >6.23 | ND |
| (23) | 7.62 (6.01–9.22) | >50 | >100 | >13.12 | ND |
| (26) | 2.63 (2.39–2.87) | 48.74 (45.88–51.61) | >100 | >37.98 | >0.48 |
| (27) | 0.808 (0.747–0.868) | 37.52 (33.90–41.15) | 71.33 (68.33–74.33) | 88.28 | 1.90 |
| (28) | 0.175 (0.098–0.253) | 25.09 (21.098–29.083) | 29.21 (25.94–32.48) | 166.31 | 1.16 |
| (29) | 0.2022 (0.125–0.279) | 4.08 (2.15–6.02) | 21.48 (18.37–24.59) | 106.21 | 5.25 |
| (30) | 0.2020 (0.183–0.2207) | 0.6284 (0.580–0.660) | 9.72 (8.99–10.45) | 48.11 | 15.46 |
| (31) | 0.5468 (0.4840–0.6096) | 21.03 (19.64–22.42) | 50.61 (47.63–53.60) | 92.56 | 2.40 |
| (33) | 3.40 (3.115–3.64) | 6.86 (5.36–8.36) | 85.74 (80.95–90.53) | 25.21 | 12.58 |
| (35) | 2.694 (2.47–2.91) | 2.109 (1.959–2.259) | 40.18 (37.58–42.79) | 14.92 | 19.05 |
| (36) | 1.095 (1.038–1.153) | 0.6337 (0.2137–1.053) | 31.28 (28.28–34.27) | 28.53 | 49.35 |
| (37) | >50 | >50 | ND | ND | ND |
| (38) | >50 | >50 | ND | ND | ND |
| AmB | 0.19 (0.16–0.21) | - | 48.12 (46.89–49.35) | 253.26 | - |
| Bnz | - | 11.02 (9.52–12.52) | 91.61 (87.18–96.04) | - | 8.31 |
| Nfx | - | 2.50 (1.4–3.6) | 57.76 (54.18–61.34) | - | 23.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Jaramillo, J.; Blöcher, R.; Chacón-Vargas, K.F.; Hernández-Calderón, J.; Sánchez-Torres, L.E.; Nogueda-Torres, B.; Reyes-Arellano, A. Synthesis of Antiprotozoal 2-(4-Alkyloxyphenyl)-Imidazolines and Imidazoles and Their Evaluation on Leishmania mexicana and Trypanosoma cruzi. Int. J. Mol. Sci. 2024, 25, 3673. https://doi.org/10.3390/ijms25073673
Torres-Jaramillo J, Blöcher R, Chacón-Vargas KF, Hernández-Calderón J, Sánchez-Torres LE, Nogueda-Torres B, Reyes-Arellano A. Synthesis of Antiprotozoal 2-(4-Alkyloxyphenyl)-Imidazolines and Imidazoles and Their Evaluation on Leishmania mexicana and Trypanosoma cruzi. International Journal of Molecular Sciences. 2024; 25(7):3673. https://doi.org/10.3390/ijms25073673
Chicago/Turabian StyleTorres-Jaramillo, Jenifer, René Blöcher, Karla Fabiola Chacón-Vargas, Jorge Hernández-Calderón, Luvia E. Sánchez-Torres, Benjamín Nogueda-Torres, and Alicia Reyes-Arellano. 2024. "Synthesis of Antiprotozoal 2-(4-Alkyloxyphenyl)-Imidazolines and Imidazoles and Their Evaluation on Leishmania mexicana and Trypanosoma cruzi" International Journal of Molecular Sciences 25, no. 7: 3673. https://doi.org/10.3390/ijms25073673
APA StyleTorres-Jaramillo, J., Blöcher, R., Chacón-Vargas, K. F., Hernández-Calderón, J., Sánchez-Torres, L. E., Nogueda-Torres, B., & Reyes-Arellano, A. (2024). Synthesis of Antiprotozoal 2-(4-Alkyloxyphenyl)-Imidazolines and Imidazoles and Their Evaluation on Leishmania mexicana and Trypanosoma cruzi. International Journal of Molecular Sciences, 25(7), 3673. https://doi.org/10.3390/ijms25073673





