Assessment and Risk Prediction of Chronic Kidney Disease and Kidney Fibrosis Using Non-Invasive Biomarkers
Abstract
1. Introduction
- Diagnosis of kidney disease in the early stages: Using conventional diagnostic criteria of eGFR < 60 mL/min/1.73 m2 and/or UACR > 30 or 300 mg/g creatinine, a significant proportion of patients with early and progressing CKD remain undetected and preventive measures are not applied. This is particularly important in the growing population of elderly patients, in whom GFR thresholds are even less consistent than those in the younger population;
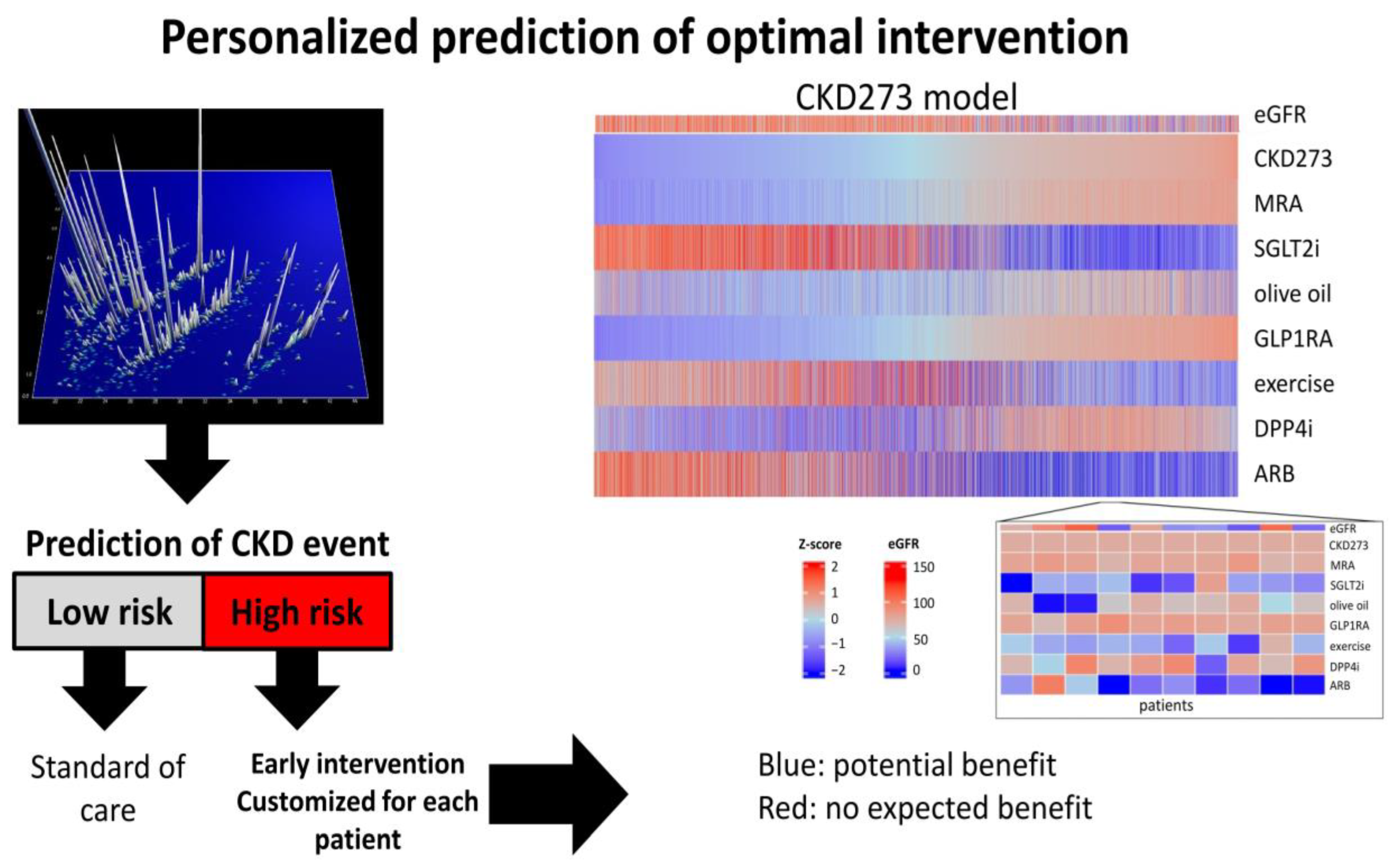
- Prediction of the risk of CKD progression: Identifying patients at a high risk of progression is of utmost importance in order to offer adequate therapy. Likewise, identifying patients who are not at risk of progression would avoid unnecessary therapeutic interventions;
- Estimation of the degree of kidney fibrosis (KF): Interstitial fibrosis and tubular atrophy (IFTA) is the histological marker with the strongest predictive power for progressive loss of kidney function. It would be desirable to predict the degree of IFTA using a non-invasive biomarker;
- Prediction of therapy response: Differentiating responders from non-responders at baseline would be an essential step towards personalized nephrology and individualized therapy;
- Monitoring the effects of therapeutic interventions: Non-invasive sample collection enables multiple samplings throughout the course of the disease, allowing for the monitoring of therapeutic success.
2. Non-Invasive Biomarkers for CKD Detection and Assessment of Risk of Progression
2.1. Routine Clinical Markers
2.2. Blood Biomarkers
2.3. Urine Biomarkers
2.4. Multi-Marker Models and Classifiers
3. Non-Invasive Biomarkers to Estimate the Degree of Fibrosis
3.1. Blood Biomarkers
3.2. Urine Biomarkers
3.3. Multi-Marker Models and Classifiers
4. Current Status and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Critselis, E.; Vlahou, A.; Stel, V.S.; Morton, R.L. Cost-effectiveness of screening type 2 diabetes patients for chronic kidney disease progression with the CKD273 urinary peptide classifier as compared to urinary albumin excretion. Nephrol. Dial. Transplant. 2017, 33, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Snider, J.T.; Sullivan, J.; van Eijndhoven, E.; Hansen, M.K.; Bellosillo, N.; Neslusan, C.; O’brien, E.; Riley, R.; Seabury, S.; Kasiske, B.L. Lifetime benefits of early detection and treatment of diabetic kidney disease. PLoS ONE 2019, 14, e0217487. [Google Scholar] [CrossRef]
- Chan, T.-C.; Chuang, Y.-H.; Hu, T.-H.; Lin, H.Y.-H.; Hwang, J.-S. Mortality risk and years of life lost for people with reduced renal function detected from regular health checkup: A matched cohort study. Prev. Med. Rep. 2023, 31, 102107. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Mattace-Raso, F.; Soler, M.J.; Fouque, D. Ageing meets kidney disease. Clin. Kidney J. 2022, 15, 1793–1796. [Google Scholar] [CrossRef]
- Waikar, S.S.; Rebholz, C.M.; Zheng, Z.; Hurwitz, S.; Hsu, C.-Y.; Feldman, H.I.; Xie, D.; Liu, K.D.; Mifflin, T.E.; Eckfeldt, J.H.; et al. Biological Variability of Estimated GFR and Albuminuria in CKD. Am. J. Kidney Dis. 2018, 72, 538–546. [Google Scholar] [CrossRef]
- Leong, A.; Ekinci, E.I.; Nguyen, C.; Milne, M.; Hachem, M.; Dobson, M.; MacIsaac, R.J.; Jerums, G. Long-term intra-individual variability of albuminuria in type 2 diabetes mellitus: Implications for categorization of albumin excretion rate. BMC Nephrol. 2017, 18, 355. [Google Scholar] [CrossRef] [PubMed]
- Thöni, S.; Keller, F.; Denicolò, S.; Buchwinkler, L.; Mayer, G. Biological variation and reference change value of the estimated glomerular filtration rate in humans: A systematic review and meta-analysis. Front. Med. 2022, 9, 1009358. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Sarnak, M.J.; Katz, R.; Fried, L.F.; Seliger, S.L.; Newman, A.B.; Siscovick, D.S.; Stehman-Breen, C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N. Engl. J. Med. 2005, 352, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Shlipak, M.G.; Judd, S.; Cushman, M.; McClellan, W.; Zakai, N.A.; Safford, M.M.; Zhang, X.; Muntner, P.; Warnock, D. Detection of Chronic Kidney Disease With Creatinine, Cystatin C, and Urine Albumin-to-Creatinine Ratio and Association With Progression to End-Stage Renal Disease and Mortality. JAMA 2011, 305, 1545–1552. [Google Scholar] [CrossRef]
- Shlipak, M.G.; Matsushita, K.; Ärnlöv, J.; Inker, L.A.; Katz, R.; Polkinghorne, K.R.; Rothenbacher, D.; Sarnak, M.J.; Astor, B.C.; Coresh, J.; et al. Cystatin C versus Creatinine in Determining Risk Based on Kidney Function. N. Engl. J. Med. 2013, 369, 932–943. [Google Scholar] [CrossRef]
- Grubb, A.; Lindström, V.; Jonsson, M.; Bäck, S.-E.; Åhlund, T.; Rippe, B.; Christensson, A. Reduction in glomerular pore size is not restricted to pregnant women. Evidence for a new syndrome: ‘Shrunken pore syndrome’. Scand. J. Clin. Lab. Investig. 2015, 75, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Grubb, A. Shrunken pore syndrome—A common kidney disorder with high mortality. Diagnosis, prevalence, pathophysiology and treatment options. Clin. Biochem. 2020, 83, 12–20. [Google Scholar] [CrossRef]
- Purde, M.-T.; Nock, S.; Risch, L.; Escobar, P.M.; Grebhardt, C.; Nydegger, U.E.; Stanga, Z.; Risch, M. Ratio of cystatin C and creatinine-based estimates of the glomerular filtration rate predicts mortality in healthy seniors independent of kidney function. Scand. J. Clin. Lab. Investig. 2016, 76, 341–343. [Google Scholar] [CrossRef]
- Purde, M.-T.; Nock, S.; Risch, L.; Escobar, P.M.; Grebhardt, C.; Nydegger, U.E.; Stanga, Z.; Risch, M. The cystatin C/creatinine ratio, a marker of glomerular filtration quality: Associated factors, reference intervals, and prediction of morbidity and mortality in healthy seniors. Transl. Res. 2015, 169, 80–90.e2. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, J.; Johansson, B.; Bergdahl, I.A.; Holmgren, A.; Näslund, U.; Hultdin, J.; Söderberg, S. Mild impairment of renal function (shrunken pore syndrome) is associated with increased risk for future surgery for aortic stenosis. Scand. J. Clin. Lab. Investig. 2019, 79, 524–530. [Google Scholar] [CrossRef]
- Herou, E.; Dardashti, A.; Nozohoor, S.; Zindovic, I.; Ederoth, P.; Grubb, A.; Bjursten, H. The mortality increase in cardiac surgery patients associated with shrunken pore syndrome correlates with the eGFR(cystatin C)/eGFR(creatinine)-ratio. Scand. J. Clin. Lab. Investig. 2019, 79, 167–173. [Google Scholar] [CrossRef]
- Åkesson, A.; Lindström, V.; Nyman, U.; Jonsson, M.; Abrahamson, M.; Christensson, A.; Björk, J.; Grubb, A. Shrunken pore syndrome and mortality: A cohort study of patients with measured GFR and known comorbidities. Scand. J. Clin. Lab. Investig. 2020, 80, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Oberg, C.M.; Lindstrom, M.; Grubb, A.; Christensson, A. Potential relationship between eGFR(cystatin C)/eGFR(creatinine) -ratio and glomerular basement membrane thickness in diabetic kidney disease. Physiol. Rep. 2021, 9, e14939. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.C.; Shlipak, M.G.; Scherzer, R.; Bauer, S.R.; Potok, O.A.; Rifkin, D.E.; Ix, J.H.; Muiru, A.N.; Hsu, C.Y.; Estrella, M.M. Association of Intraindividual Difference in Estimated Glomerular Filtration Rate by Creatinine vs Cystatin C and End-stage Kidney Disease and Mortality. JAMA Netw. Open 2022, 5, e2148940. [Google Scholar] [CrossRef]
- Quiroga, B.; Ortiz, A.; Díez, J. Selective glomerular hypofiltration syndrome. Nephrol. Dial. Transplant. 2023, 39, 10–17. [Google Scholar] [CrossRef]
- Tangri, N.; Stevens, L.A.; Griffith, J.; Tighiouart, H.; Djurdjev, O.; Naimark, D.; Levin, A.; Levey, A.S. A Predictive Model for Progression of Chronic Kidney Disease to Kidney Failure. JAMA 2011, 305, 1553–1559. [Google Scholar] [CrossRef]
- Nowak, N.; Skupien, J.; Niewczas, M.A.; Yamanouchi, M.; Major, M.; Croall, S.; Smiles, A.; Warram, J.H.; Bonventre, J.V.; Krolewski, A.S. Increased plasma kidney injury molecule-1 suggests early progressive renal decline in non-proteinuric patients with type 1 diabetes. Kidney Int. 2016, 89, 459–467. [Google Scholar] [CrossRef]
- Schrauben, S.J.; Shou, H.; Zhang, X.; Anderson, A.H.; Bonventre, J.V.; Chen, J.; Coca, S.; Furth, S.L.; Greenberg, J.H.; Gutierrez, O.M.; et al. Association of Multiple Plasma Biomarker Concentrations with Progression of Prevalent Diabetic Kidney Disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study. J. Am. Soc. Nephrol. 2020, 32, 115–126. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Shlipak, M.G.; Katz, R.; Waikar, S.S.; Greenberg, J.H.; Schrauben, S.J.; Coca, S.; Parikh, C.R.; Vasan, R.S.; Feldman, H.I.; et al. Associations of Plasma Biomarkers of Inflammation, Fibrosis, and Kidney Tubular Injury With Progression of Diabetic Kidney Disease: A Cohort Study. Am. J. Kidney Dis. 2021, 79, 849–857.e1. [Google Scholar] [CrossRef]
- Moriya, H.; Mochida, Y.; Ishioka, K.; Oka, M.; Maesato, K.; Hidaka, S.; Ohtake, T.; Kobayashi, S. Plasma neutrophil gelatinase-associated lipocalin (NGAL) is an indicator of interstitial damage and a predictor of kidney function worsening of chronic kidney disease in the early stage: A pilot study. Clin. Exp. Nephrol. 2017, 21, 1053–1059. [Google Scholar] [CrossRef]
- Martin, W.P.; Conroy, C.; Naicker, S.D.; Cormican, S.; Griffin, T.P.; Islam, N.; McCole, E.M.; McConnell, I.; Lamont, J.; FitzGerald, P.; et al. Multiplex Serum Biomarker Assays Improve Prediction of Renal and Mortality Outcomes in Chronic Kidney Disease. Kidney360 2021, 2, 1225–1239. [Google Scholar] [CrossRef]
- Carlsson, A.C.; Nordquist, L.; Larsson, T.E.; Carrero, J.-J.; Larsson, A.; Lind, L.; Ärnlöv, J. Soluble Tumor Necrosis Factor Receptor 1 Is Associated with Glomerular Filtration Rate Progression and Incidence of Chronic Kidney Disease in Two Community-Based Cohorts of Elderly Individuals. Cardiorenal Med. 2015, 5, 278–288. [Google Scholar] [CrossRef]
- Menon, V.; Greene, T.; Wang, X.; Pereira, A.A.; Marcovina, S.M.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Levey, A.S.; Sarnak, M.J. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005, 68, 766–772. [Google Scholar] [CrossRef]
- Shelbaya, S.; Amer, H.; Seddik, S.; A Allah, A.; Sabry, I.M.; Mohamed, T.; El Mosely, M. Study of the role of interleukin-6 and highly sensitive C-reactive protein in diabetic nephropathy in type 1 diabetic patients. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 176–182. [Google Scholar] [PubMed]
- Amdur, R.L.; Feldman, H.I.; Gupta, J.; Yang, W.; Kanetsky, P.; Shlipak, M.; Rahman, M.; Lash, J.P.; Townsend, R.R.; Ojo, A.; et al. Inflammation and Progression of CKD: The CRIC Study. Clin. J. Am. Soc. Nephrol. 2016, 11, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Boutin, L.; Dépret, F.; Gayat, E.; Legrand, M.; Chadjichristos, C.E. Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3124. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Selvin, E.; Liang, M.; Ballantyne, C.M.; Hoogeveen, R.C.; Aguilar, D.; McEvoy, J.W.; Grams, M.E.; Coresh, J. Plasma galectin-3 levels are associated with the risk of incident chronic kidney disease. Kidney Int. 2017, 93, 252–259. [Google Scholar] [CrossRef]
- Alam, M.L.; Katz, R.; Bellovich, K.A.; Bhat, Z.Y.; Brosius, F.C.; de Boer, I.H.; Gadegbeku, C.A.; Gipson, D.S.; Hawkins, J.J.; Himmelfarb, J.; et al. Soluble ST2 and Galectin-3 and Progression of CKD. Kidney Int. Rep. 2018, 4, 103–111. [Google Scholar] [CrossRef]
- Bienaimé, F.; Muorah, M.; Metzger, M.; Broeuilh, M.; Flamant, M.; Haymann, J.-P.; Vonderscher, J.; Mizrahi, J.; Friedlander, G.; Stengel, B.; et al. Combining robust urine biomarkers to assess chronic kidney disease progression. EBioMedicine 2023, 93, 104635. [Google Scholar] [CrossRef]
- Greenberg, J.H.; Abraham, A.G.; Xu, Y.; Schelling, J.R.; Feldman, H.I.; Sabbisetti, V.S.; Ix, J.H.; Jogalekar, M.P.; Coca, S.; Waikar, S.S.; et al. Urine Biomarkers of Kidney Tubule Health, Injury, and Inflammation are Associated with Progression of CKD in Children. J. Am. Soc. Nephrol. 2021, 32, 2664–2677. [Google Scholar] [CrossRef]
- Federico, G.; Meister, M.; Mathow, D.; Heine, G.H.; Moldenhauer, G.; Popovic, Z.V.; Nordström, V.; Kopp-Schneider, A.; Hielscher, T.; Nelson, P.J.; et al. Tubular Dickkopf-3 promotes the development of renal atrophy and fibrosis. J. Clin. Investig. 2016, 1, e84916. [Google Scholar] [CrossRef]
- Meister, M.; Papatriantafyllou, M.; Nordstrã¶M, V.; Kumar, V.; Ludwig, J.; Lui, K.O.; Boyd, A.S.; Popovic, Z.V.; Fleming, T.H.; Moldenhauer, G.; et al. Dickkopf-3, a Tissue-Derived Modulator of Local T-Cell Responses. Front. Immunol. 2015, 6, 78. [Google Scholar] [CrossRef]
- Schunk, S.J.; Speer, T.; Petrakis, I.; Fliser, D. Dickkopf 3-a novel biomarker of the ‘kidney injury continuum’. Nephrol. Dial. Transplant. 2021, 36, 761–767. [Google Scholar] [CrossRef]
- Zewinger, S.; Rauen, T.; Rudnicki, M.; Federico, G.; Wagner, M.; Triem, S.; Schunk, S.J.; Petrakis, I.; Schmit, D.; Wagenpfeil, S.; et al. Dickkopf-3 (DKK3) in Urine Identifies Patients with Short-Term Risk of eGFR Loss. J. Am. Soc. Nephrol. 2018, 29, 2722–2733. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Álamo, B.; García-Iñigo, F.J.; Shabaka, A.; Acedo, J.M.; Cases-Corona, C.; Domínguez-Torres, P.; Diaz-Enamorado, Y.; Landaluce, E.; Navarro-González, J.F.; Gorriz, J.L.; et al. Urinary Dickkopf-3: A new biomarker for CKD progression and mortality. Nephrol. Dial. Transplant. 2021, 36, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Tangri, N.; Grams, M.E.; Levey, A.S.; Coresh, J.; Appel, L.J.; Astor, B.C.; Chodick, G.; Collins, A.J.; Djurdjev, O.; Elley, C.R.; et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure: A Meta-analysis. JAMA 2016, 315, 164–174. [Google Scholar] [CrossRef]
- Owens, E.; Tan, K.-S.; Ellis, R.; Del Vecchio, S.; Humphries, T.; Lennan, E.; Vesey, D.; Healy, H.; Hoy, W.; Gobe, G. Development of a Biomarker Panel to Distinguish Risk of Progressive Chronic Kidney Disease. Biomedicines 2020, 8, 606. [Google Scholar] [CrossRef]
- Colombo, M.; McGurnaghan, S.J.; Blackbourn, L.A.K.; Dalton, R.N.; Dunger, D.; Bell, S.; Petrie, J.R.; Green, F.; MacRury, S.; McKnight, J.A.; et al. Comparison of serum and urinary biomarker panels with albumin/creatinine ratio in the prediction of renal function decline in type 1 diabetes. Diabetologia 2020, 63, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Ntrinias, T.; Papasotiriou, M.; Balta, L.; Kalavrizioti, D.; Vamvakas, S.; Papachristou, E.; Goumenos, D.S. Biomarkers in Progressive Chronic Kidney Disease. Still a Long Way to Go. Prilozi 2019, 40, 27–39. [Google Scholar] [CrossRef][Green Version]
- Good, D.M.; Zürbig, P.; Argilés, A.; Bauer, H.W.; Behrens, G.; Coon, J.J.; Dakna, M.; Decramer, S.; Delles, C.; Dominiczak, A.F.; et al. Naturally Occurring Human Urinary Peptides for Use in Diagnosis of Chronic Kidney Disease. Mol. Cell. Proteom. 2010, 9, 2424–2437. [Google Scholar] [CrossRef] [PubMed]
- Zürbig, P.; Mischak, H.; Menne, J.; Haller, H. CKD273 Enables Efficient Prediction of Diabetic Nephropathy in Nonalbuminuric Patients. Diabetes Care 2018, 42, e4–e5. [Google Scholar] [CrossRef]
- Schanstra, J.P.; Zürbig, P.; Alkhalaf, A.; Argiles, A.; Bakker, S.J.; Beige, J.; Bilo, H.J.; Chatzikyrkou, C.; Dakna, M.; Dawson, J.; et al. Diagnosis and Prediction of CKD Progression by Assessment of Urinary Peptides. J. Am. Soc. Nephrol. 2015, 26, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.-M.; Thijs, L.; Liu, Y.-P.; Zhang, Z.; Jacobs, L.; Koeck, T.; Zürbig, P.; Lichtinghagen, R.; Brand, K.; Kuznetsova, T.; et al. The urinary proteome as correlate and predictor of renal function in a population study. Nephrol. Dial. Transplant. 2014, 29, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, C.; Jacobs, L.; Staessen, J.A.; Schanstra, J.P.; Rossing, P.; Heerspink, H.J.; Siwy, J.; Mullen, W.; Vlahou, A.; Mischak, H.; et al. A urinary proteome-based classifier for the early detection of decline in glomerular filtration. Nephrol. Dial. Transplant. 2016, 32, 1510–1516. [Google Scholar] [CrossRef]
- Rodriguez-Ortiz, M.E.; Pontillo, C.; Rodriguez, M.; Zurbig, P.; Mischak, H.; Ortiz, A. Novel Urinary Biomarkers for Improved Prediction of Progressive Egfr Loss in Early Chronic Kidney Disease Stages and in High Risk Individuals without Chronic Kidney Disease. Sci. Rep. 2018, 8, 15940. [Google Scholar] [CrossRef]
- Tofte, N.; Lindhardt, M.; Adamova, K.; Beige, J.; Beulens, J.W.J.; Birkenfeld, A.L.; Currie, G.; Delles, C.; Dimos, I.; Francová, L.; et al. Characteristics of high- and low-risk individuals in the PRIORITY study: Urinary proteomics and mineralocorticoid receptor antagonism for prevention of diabetic nephropathy in Type 2 diabetes. Diabet. Med. 2018, 35, 1375–1382. [Google Scholar] [CrossRef]
- Tofte, N.; Lindhardt, M.; Adamova, K.; Bakker, S.J.L.; Beige, J.; Beulens, J.W.J.; Birkenfeld, A.L.; Currie, G.; Delles, C.; Dimos, I.; et al. Early detection of diabetic kidney disease by urinary proteomics and subsequent intervention with spironolactone to delay progression (PRIORITY): A prospective observational study and embedded randomised placebo-controlled trial. Lancet Diabetes Endocrinol. 2020, 8, 301–312. [Google Scholar] [CrossRef]
- Smink, P.; Hoekman, J.; Grobbee, D.; Eijkemans, M.; Parving, H.-H.; Persson, F.; Ibsen, H.; Lindholm, L.; Wachtell, K.; de Zeeuw, D.; et al. A prediction of the renal and cardiovascular efficacy of aliskiren in ALTITUDE using short-term changes in multiple risk markers. Eur. J. Prev. Cardiol. 2013, 21, 434–441. [Google Scholar] [CrossRef]
- Lindhardt, M.; Persson, F.; Oxlund, C.; Jacobsen, I.A.; Zürbig, P.; Mischak, H.; Rossing, P.; Heerspink, H.J. Predicting albuminuria response to spironolactone treatment with urinary proteomics in patients with type 2 diabetes and hypertension. Nephrol. Dial. Transplant. 2017, 33, 296–303. [Google Scholar] [CrossRef]
- Jaimes Campos, M.A.; Mavrogeorgis, E.; Latosinska, A.; Eder, S.; Buchwinkler, L.; Ischak, H.; Iwy, J.; Ossing, P.; Mayer, G.; Jankowski, J. Prediction of response to anti-hypertensive treatment based on urinary peptides: Towards personalised intervention. Nephrol. Dial. Transplant. 2023, in press.
- Eder, S.; Leierer, J.; Kerschbaum, J.; Rosivall, L.; Wiecek, A.; de Zeeuw, D.; Mark, P.B.; Heinze, G.; Rossing, P.; Heerspink, H.L.; et al. A Prospective Cohort Study in Patients with Type 2 Diabetes Mellitus for Validation of Biomarkers (PROVALID)—Study Design and Baseline Characteristics. Kidney Blood Press. Res. 2018, 43, 181–190. [Google Scholar] [CrossRef]
- Lindhardt, M.; Persson, F.; Zürbig, P.; Stalmach, A.; Mischak, H.; de Zeeuw, D.; Heerspink, H.L.; Klein, R.; Orchard, T.; Porta, M.; et al. Urinary proteomics predict onset of microalbuminuria in normoalbuminuric type 2 diabetic patients, a sub-study of the DIRECT-Protect 2 study. Nephrol. Dial. Transplant. 2016, 32, 1866–1873. [Google Scholar] [CrossRef]
- Campos, M.A.J.; Andújar, I.; Keller, F.; Mayer, G.; Rossing, P.; Staessen, J.A.; Delles, C.; Beige, J.; Glorieux, G.; Clark, A.L.; et al. Prognosis and Personalized In Silico Prediction of Treatment Efficacy in Cardiovascular and Chronic Kidney Disease: A Proof-of-Concept Study. Pharmaceuticals 2023, 16, 1298. [Google Scholar] [CrossRef]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef]
- Lovisa, S.; Zeisberg, M.; Kalluri, R. Partial Epithelial-to-Mesenchymal Transition and Other New Mechanisms of Kidney Fibrosis. Trends Endocrinol. Metab. 2016, 27, 681–695. [Google Scholar] [CrossRef]
- Li, L.; Fu, H.; Liu, Y. The fibrogenic niche in kidney fibrosis: Components and mechanisms. Nat. Rev. Nephrol. 2022, 18, 545–557. [Google Scholar] [CrossRef]
- Breyer, M.D.; Susztak, K. The next generation of therapeutics for chronic kidney disease. Nat. Rev. Drug Discov. 2016, 15, 568–588. [Google Scholar] [CrossRef]
- Latosinska, A.; Siwy, J.; Faguer, S.; Beige, J.; Mischak, H.; Schanstra, J.P. Value of Urine Peptides in Assessing Kidney and Cardiovascular Disease. Proteom.—Clin. Appl. 2020, 15, e2000027. [Google Scholar] [CrossRef]
- LeBleu, V.S.; Teng, Y.; O’Connell, J.T.; Charytan, D.; A Müller, G.; A Müller, C.; Sugimoto, H.; Kalluri, R. Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat. Med. 2013, 19, 227–231. [Google Scholar] [CrossRef]
- Nagy, B.; Krasznai, Z.T.; Balla, H.; Csobán, M.; Antal-Szalmás, P.; Hernádi, Z.; Kappelmayer, J.; Nagy, J.B. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2012, 49, 377–380. [Google Scholar] [CrossRef]
- Luo, J.; Wang, F.; Wan, J.; Ye, Z.; Huang, C.; Cai, Y.; Liu, M.; Wu, B.-Q.; Li, L. Serum human epididymis secretory protein 4 as a potential biomarker of renal fibrosis in kidney transplantation recipients. Clin. Chim. Acta 2018, 483, 216–221. [Google Scholar] [CrossRef]
- Glowacki, F.; Savary, G.; Gnemmi, V.; Buob, D.; Van der Hauwaert, C.; Lo-Guidice, J.-M.; Bouyé, S.; Hazzan, M.; Pottier, N.; Perrais, M.; et al. Increased Circulating miR-21 Levels Are Associated with Kidney Fibrosis. PLoS ONE 2013, 8, e58014. [Google Scholar] [CrossRef]
- Zhong, X.; Tu, Y.J.; Li, Y.; Zhang, P.; Wang, W.; Chen, S.S.; Li, L.; Chung, A.C.; Lan, H.Y.; Chen, H.Y.; et al. Serum levels of WNT1-inducible signaling pathway protein-1 (WISP-1): A noninvasive biomarker of renal fibrosis in subjects with chronic kidney disease. Am. J. Transl. Res. 2017, 9, 2920–2932. [Google Scholar]
- Zhao, Y.; Tang, K.; Tianbao, X.; Wang, J.; Yang, J.; Li, D. Increased serum lysyl oxidase-like 2 levels correlate with the degree of left atrial fibrosis in patients with atrial fibrillation. Biosci. Rep. 2017, 37, BSR20171332. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Li, X.; Zhou, W.-Q.; Liu, X.; Huang, J.-L.; Zhang, Y.-Y.; Lindholm, B.; Yu, C. Serum Lysyl Oxidase Is a Potential Diagnostic Biomarker for Kidney Fibrosis. Am. J. Nephrol. 2020, 51, 907–918. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, I.M.; Colona, M.R.; Kestenbaum, B.R.; Alexopoulos, L.G.; Palsson, R.; Srivastava, A.; Liu, J.; Stillman, I.E.; Rennke, H.G.; Vaidya, V.S.; et al. Cadherin-11, Sparc-related modular calcium binding protein-2, and Pigment epithelium-derived factor are promising non-invasive biomarkers of kidney fibrosis. Kidney Int. 2021, 100, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Y.; Liu, X.S.; Huang, X.R.; Yu, X.Q.; Lan, H.Y. Diverse Role of TGF-beta in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, E.; Teppo, A.M.; Tornroth, T.; Groop, P.H.; Gronhagen-Riska, C. Urinary transforming growth factor-beta 1 in membranous glomerulonephritis. Nephrol. Dial. Transplant. 1997, 12, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
- De, M.P.; Faedda, R.; Fresu, P.; Masala, A.; Cigni, A.; Concas, G.; Mela, M.G.; Satta, A.; Carcassi, A.; Sanna, G.M.; et al. Urinary transforming growth factor-beta 1 in various types of nephropathy. Pharmacol. Res. 2004, 49, 293–298. [Google Scholar] [CrossRef]
- Ghoul, B.E.; Squalli, T.; Servais, A.; Elie, C.; Meas-Yedid, V.; Trivint, C.; Vanmassenhove, J.; Grunfeld, J.P.; Olivo-Marin, J.C.; Thervet, E.; et al. Urinary procollagen III aminoterminal propeptide (PIIINP): A fibrotest for the nephrologist. Clin. J. Am. Soc. Nephrol. 2010, 5, 205–210. [Google Scholar] [CrossRef]
- Teppo, A.-M.; Törnroth, T.; Honkanen, E.; Grönhagen-Riska, C. Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation 2003, 75, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Sparding, N.; Neprasova, M.; Maixnerova, D.; Genovese, F.; Karsdal, M.A.; Kollar, M.; Koprivova, H.; Hruskova, Z.; Tesar, V. Unique Biomarkers of Collagen Type III Remodeling Reflect Different Information Regarding Pathological Kidney Tissue Alterations in Patients with IgA Nephropathy. Biomolecules 2023, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Tian, Y.; Sun, L.; Zhou, L.; Xiao, L.; Tan, R.J.; Tian, J.; Fu, H.; Hou, F.F.; Liu, Y. Matrix Metalloproteinase-7 Is a Urinary Biomarker and Pathogenic Mediator of Kidney Fibrosis. J. Am. Soc. Nephrol. 2016, 28, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.-M.; Tsai, M.-T.; Chen, H.-Y.; Li, F.-A.; Lee, K.-H.; Tseng, W.-C.; Chang, F.-P.; Lin, Y.-P.; Yang, R.-B.; Tarng, D.-C. Urinary Galectin-3 as a Novel Biomarker for the Prediction of Renal Fibrosis and Kidney Disease Progression. Biomedicines 2022, 10, 585. [Google Scholar] [CrossRef]
- Wang, X.; Lieske, J.C.; Alexander, M.P.; Jayachandran, M.; Denic, A.; Mathew, J.; Lerman, L.O.; Kremers, W.K.; Larson, J.J.; Rule, A.D. Tubulointerstitial Fibrosis of Living Donor Kidneys Associates with Urinary Monocyte Chemoattractant Protein 1. Am. J. Nephrol. 2016, 43, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Furuichi, K.; Sakai, N.; Iwata, Y.; Kitagawa, K.; Ishida, Y.; Kondo, T.; Hashimoto, H.; Ishiwata, Y.; Mukaida, N.; et al. Gene Therapy via Blockade of Monocyte Chemoattractant Protein-1 for Renal Fibrosis. J. Am. Soc. Nephrol. 2004, 15, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Melchinger, H.; Calderon-Gutierrez, F.; Obeid, W.; Xu, L.; Shaw, M.M.; Luciano, R.L.; Kuperman, M.; Moeckel, G.W.; Kashgarian, M.; Wilson, F.P.; et al. Urine Uromodulin as a Biomarker of Kidney Tubulointerstitial Fibrosis. Clin. J. Am. Soc. Nephrol. 2022, 17, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Papasotiriou, M.; Genovese, F.; Klinkhammer, B.M.; Kunter, U.; Nielsen, S.H.; Karsdal, M.A.; Floege, J.; Boor, P. Serum and urine markers of collagen degradation reflect renal fibrosis in experimental kidney diseases. Nephrol. Dial. Transplant. 2015, 30, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Siwy, J.; Zürbig, P.; Argiles, A.; Beige, J.; Haubitz, M.; Jankowski, J.; Julian, B.A.; Linde, P.G.; Marx, D.; Mischak, H.; et al. Noninvasive diagnosis of chronic kidney diseases using urinary proteome analysis. Nephrol. Dial. Transplant. 2016, 32, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, P.; Pejchinovski, M.; Markoska, K.; Banasik, M.; Klinger, M.; Svec-Billa, D.; Rychlik, I.; Rroji, M.; Restivo, A.; Ca-passo, G.; et al. Association of kidney fibrosis with urinary peptides: A path towards non-invasive liquid biopsies? Sci. Rep. 2017, 7, 16915. [Google Scholar] [CrossRef] [PubMed]
- Catanese, L.; Siwy, J.; Mavrogeorgis, E.; Amann, K.; Mischak, H.; Beige, J.; Rupprecht, H. A Novel Urinary Proteomics Classifier for Non-Invasive Evaluation of Interstitial Fibrosis and Tubular Atrophy in Chronic Kidney Disease. Proteomes 2021, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Pontillo, C.; Zhang, Z.-Y.; Schanstra, J.P.; Jacobs, L.; Zürbig, P.; Thijs, L.; Ramírez-Torres, A.; Heerspink, H.J.; Lindhardt, M.; Klein, R.; et al. Prediction of Chronic Kidney Disease Stage 3 by CKD273, a Urinary Proteomic Biomarker. Kidney Int. Rep. 2017, 2, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Waijer, S.W.; Sen, T.; Arnott, C.; Neal, B.; Kosterink, J.G.; Mahaffey, K.W.; Parikh, C.R.; de Zeeuw, D.; Perkovic, V.; Neuen, B.L.; et al. Association between TNF Receptors and KIM-1 with Kidney Outcomes in Early-Stage Diabetic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2022, 17, 251–259. [Google Scholar] [CrossRef]
- Chen, T.K.; Estrella, M.M.; Appel, L.J.; Coresh, J.; Luo, S.; Reiser, J.; Obeid, W.; Parikh, C.R.; Grams, M.E. Biomarkers of Immune Activation and Incident Kidney Failure With Replacement Therapy: Findings From the African American Study of Kidney Disease and Hypertension. Am. J. Kidney Dis. 2021, 78, 75–84.e1. [Google Scholar] [CrossRef]
- Azukaitis, K.; Ju, W.; Kirchner, M.; Nair, V.; Smith, M.; Fang, Z.; Thurn-Valsassina, D.; Bayazit, A.; Canpolat, N.; Bulut, I.K.; et al. Low levels of urinary epidermal growth factor predict chronic kidney disease progression in children. Kidney Int. 2019, 96, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Govender, M.A.; Brandenburg, J.-T.; Fabian, J.; Ramsay, M. The Use of ‘Omics for Diagnosing and Predicting Progression of Chronic Kidney Disease: A Scoping Review. Front. Genet. 2021, 12, 682929. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.G.; Xu, Y.; Roem, J.L.; Greenberg, J.H.; Weidemann, D.K.; Sabbisetti, V.S.; Bonventre, J.V.; Denburg, M.; Warady, B.A.; Furth, S.L. Variability in CKD Biomarker Studies: Soluble Urokinase Plasminogen Activator Receptor (suPAR) and Kidney Disease Progression in the Chronic Kidney Disease in Children (CKiD) Study. Radiology 2021, 3, 712–721.e1. [Google Scholar] [CrossRef]
- Cañadas-Garre, M.; Anderson, K.; McGoldrick, J.; Maxwell, A.P.; McKnight, A.J. Genomic approaches in the search for molecular biomarkers in chronic kidney disease. J. Transl. Med. 2018, 16, 292. [Google Scholar] [CrossRef]
- Dakna, M.; Harris, K.; Kalousis, A.; Carpentier, S.; Kolch, W.; Schanstra, J.P.; Haubitz, M.; Vlahou, A.; Mischak, H.; Girolami, M. Addressing the Challenge of Defining Valid Proteomic Biomarkers and Classifiers. BMC Bioinform. 2010, 11, 594. [Google Scholar] [CrossRef] [PubMed]
- Mavrogeorgis, E.; He, T.; Mischak, H.; Latosinska, A.; Vlahou, A.; Schanstra, J.P.; Catanese, L.; Amann, K.; Huber, T.B.; Beige, J.; et al. Urinary peptidomic liquid biopsy for non-invasive differential diagnosis of chronic kidney disease. Nephrol. Dial. Transplant. 2023, 39, 453–462. [Google Scholar] [CrossRef]
- Sirolli, V.; Pieroni, L.; Di Liberato, L.; Urbani, A.; Bonomini, M. Urinary Peptidomic Biomarkers in Kidney Diseases. Int. J. Mol. Sci. 2019, 21, 96. [Google Scholar] [CrossRef] [PubMed]
- Decramer, S.; Gonzalez de, P.A.; Breuil, B.; Mischak, H.; Monsarrat, B.; Bascands, J.L.; Schanstra, J.P. Urine in clinical proteomics. Mol. Cell Proteom. 2008, 7, 1850–1862. [Google Scholar] [CrossRef]


| Intended Use | Biomarker | Findings | References |
|---|---|---|---|
| (1) Early diagnosis | CKD273 | Enables detection of CKD at early stage, superior to albuminuria or eGFR | [46,49,50,52,88] |
| (2) Assessment of fibrosis | FPP_29BH | Detection of fibrosis | [87] |
| C3M | Association with fibrosis | [78] | |
| HE4 | Increased in fibrosis | [66,67] | |
| LOX | Increased in fibrosis | [71] | |
| DKK3 | Increased in fibrosis | [36] | |
| (3) Prognosis of progression | KIM-1 | Increased in progressive CKD | [23,24,34,35] |
| NGAL | Increased in progressive CKD | [26,34] | |
| TNFR1 | Increased in progressive CKD | [24,26,35,89,90] | |
| TNFR2 | Increased in progressive CKD | [23,24,35,89,90] | |
| EGF | Reduced levels indicate progression | [34,35,91] | |
| MCP-1 | Increased in progressive CKD | [35] | |
| DKK3 | Increased in progressive CKD | [39,40] | |
| CKD273 | Increased in progressive CKD | [46,47,52,88,92] | |
| (4) Prediction of drug response | DKDp189 | Prediction of response to anti-hypertensive treatment | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupprecht, H.; Catanese, L.; Amann, K.; Hengel, F.E.; Huber, T.B.; Latosinska, A.; Lindenmeyer, M.T.; Mischak, H.; Siwy, J.; Wendt, R.; et al. Assessment and Risk Prediction of Chronic Kidney Disease and Kidney Fibrosis Using Non-Invasive Biomarkers. Int. J. Mol. Sci. 2024, 25, 3678. https://doi.org/10.3390/ijms25073678
Rupprecht H, Catanese L, Amann K, Hengel FE, Huber TB, Latosinska A, Lindenmeyer MT, Mischak H, Siwy J, Wendt R, et al. Assessment and Risk Prediction of Chronic Kidney Disease and Kidney Fibrosis Using Non-Invasive Biomarkers. International Journal of Molecular Sciences. 2024; 25(7):3678. https://doi.org/10.3390/ijms25073678
Chicago/Turabian StyleRupprecht, Harald, Lorenzo Catanese, Kerstin Amann, Felicitas E. Hengel, Tobias B. Huber, Agnieszka Latosinska, Maja T. Lindenmeyer, Harald Mischak, Justyna Siwy, Ralph Wendt, and et al. 2024. "Assessment and Risk Prediction of Chronic Kidney Disease and Kidney Fibrosis Using Non-Invasive Biomarkers" International Journal of Molecular Sciences 25, no. 7: 3678. https://doi.org/10.3390/ijms25073678
APA StyleRupprecht, H., Catanese, L., Amann, K., Hengel, F. E., Huber, T. B., Latosinska, A., Lindenmeyer, M. T., Mischak, H., Siwy, J., Wendt, R., & Beige, J. (2024). Assessment and Risk Prediction of Chronic Kidney Disease and Kidney Fibrosis Using Non-Invasive Biomarkers. International Journal of Molecular Sciences, 25(7), 3678. https://doi.org/10.3390/ijms25073678







