Dopamine and Norepinephrine Tissue Levels in the Developing Limbic Brain Are Impacted by the Human CHRNA6 3′-UTR Single-Nucleotide Polymorphism (rs2304297) in Rats †
Abstract
1. Introduction
2. Results
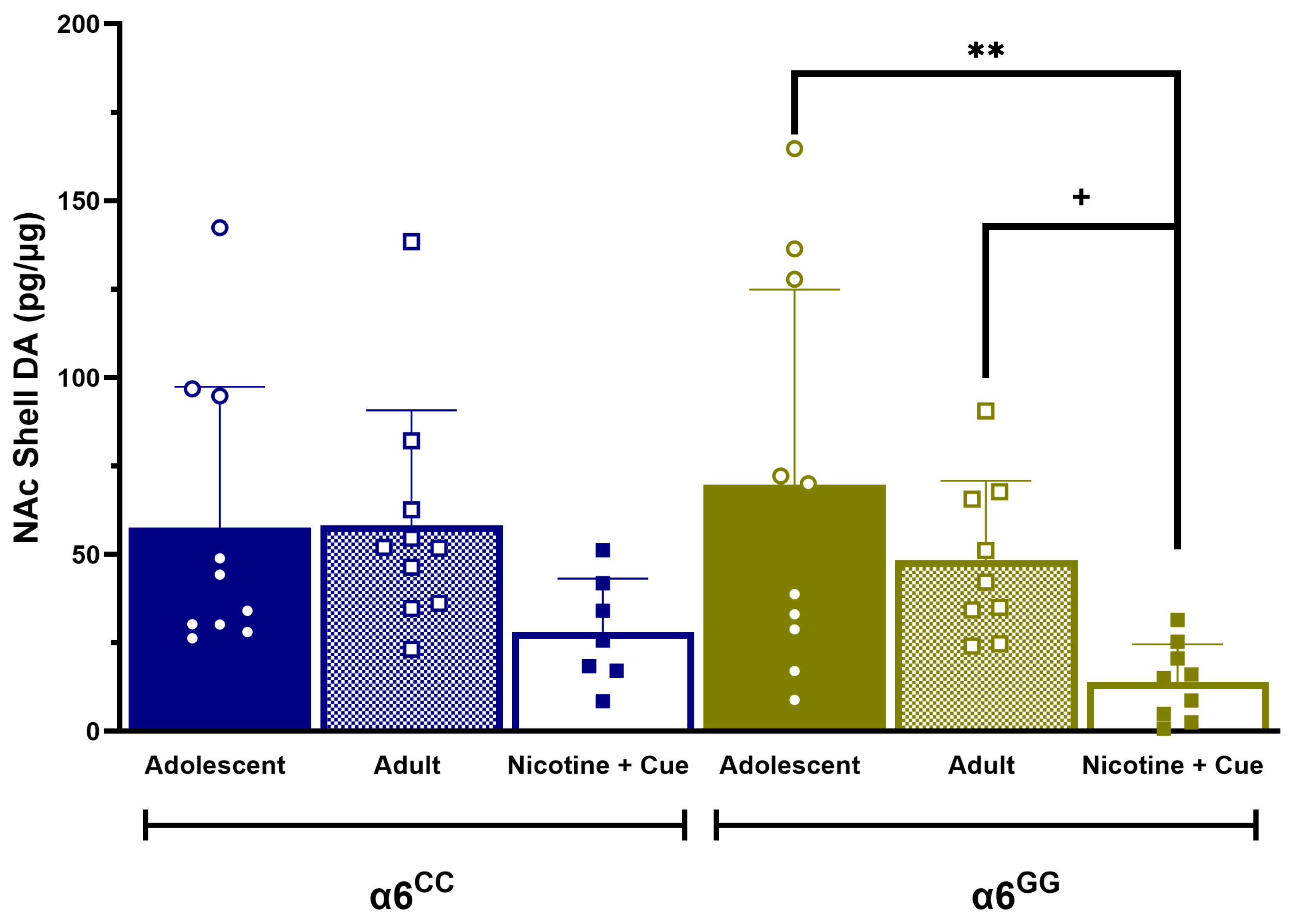
2.1. Comparative DA, NE, and Metabolite Profile Analysis of Adolescent and Adult, α6GG and α6CC in Distinct Limbic Brain Regions
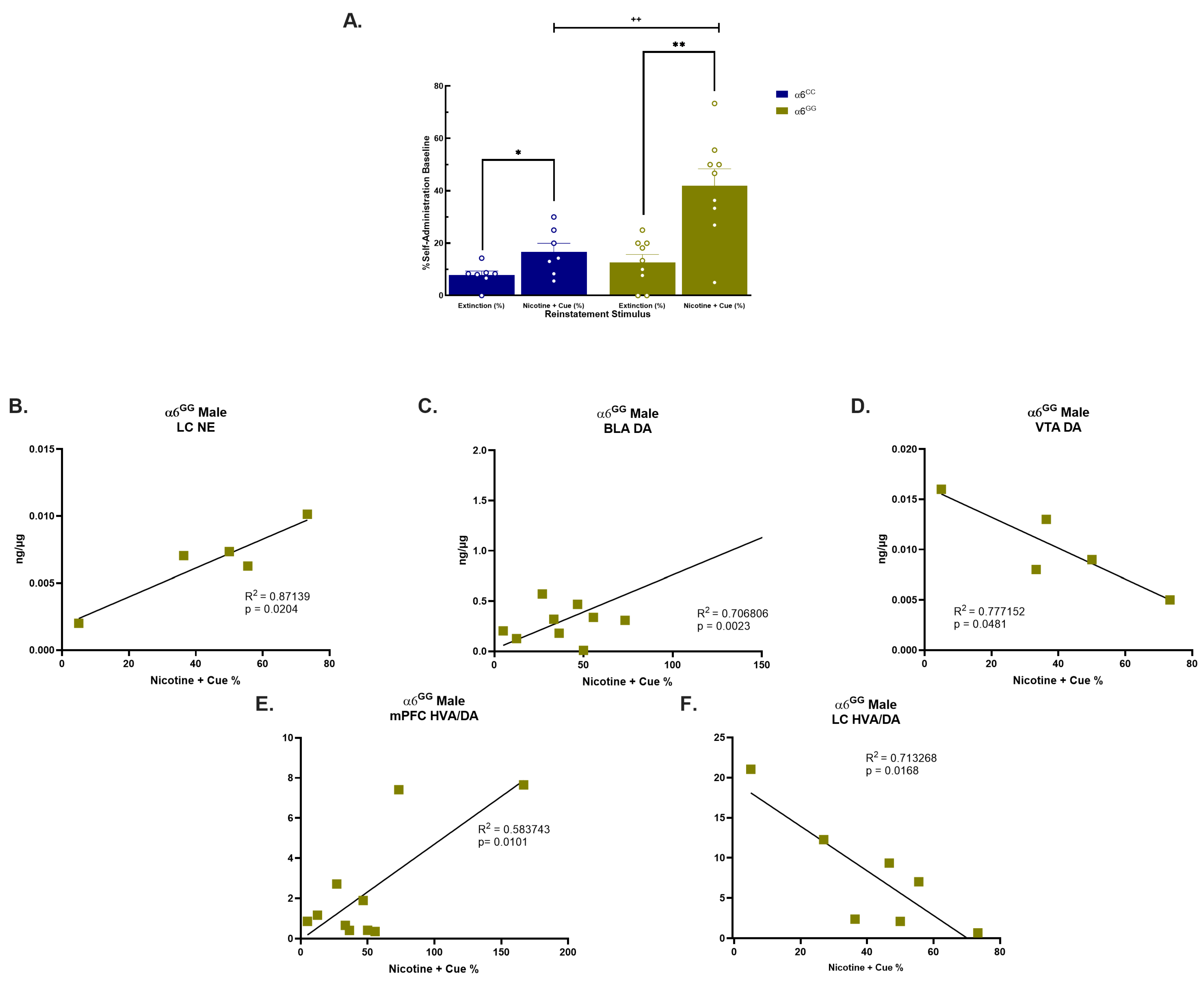
2.2. Sex- and Genotype-Dependent Effects on Tissue Neurotransmitter Levels in Rats Tested for Nicotine plus Cue-Induced Reinstatement
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Catecholamine Levels and HPLC-ED Detection in Naïve CHRNA6 3′-UTR SNP Knock-In Rats
4.3. Apparatus for Behavioral Testing
4.4. Food Self-Administration
4.5. Surgery
4.6. Nicotine Intravenous Self-Administration and Extinction
4.7. Cue and Nicotine-Induced Reinstatement and Tissue Catecholamines Levels
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress A Report of the Surgeon General; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2014. [Google Scholar]
- Birdsey, J.; Cornelius, M.; Jamal, A.; Park-Lee, E.; Cooper, M.R.; Wang, J.; Sawdey, M.D.; Cullen, K.A.; Neff, L. Tobacco Product Use among U.S. Middle and High School Students—National Youth Tobacco Survey, 2023. MMWR 2023, 72, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.B.; McQuown, S.C.; Leslie, F.M. The Dynamic Effects of Nicotine on the Developing Brain. Pharmacol. Ther. 2009, 122, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Cross, S.J.; Loughlin, S.E.; Leslie, F.M. Nicotine and the adolescent brain. J. Physiol. 2015, 593, 3397–3412. [Google Scholar] [CrossRef]
- Changeux, J.-P.; Bertrand, D.; Corringer, P.-J.; Dehaene, S.; Edelstein, S.; Léna, C.; Le Novère, N.; Marubio, L.; Picciotto, M.; Zoli, M. Brain nicotinic receptors: Structure and regulation, role in learning and reinforcement. Brain Res. Rev. 1998, 26, 198–216. [Google Scholar] [CrossRef] [PubMed]
- Leslie, F.M.; Azam, L.; Gallardo, K.; O’Leary, K.; Franke, R.; Lotfipour, S. Nicotinic Receptor Regulation of Developing Catecholamine Systems; Brain Development: New York, NY, USA; Oxford University Press: New York, NY, USA, 2006; pp. 381–398. [Google Scholar] [CrossRef]
- Le Novere, N.; Zoli, M.; Changeux, J. Neuronal Nicotinic Receptor a6 Subunit mRNA is Selectively Concentrated in Catecholaminergic Nuclei of the Rat Brain. Eur. J. Neurosci. 1996, 8, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.L.; Yoshikami, D.; McIntosh, J.M. The neuronal nicotinic acetylcholine receptors α4* and α6* differentially modulate dopamine release in mouse striatal slices. J. Neurochem. 2008, 105, 1761–1769. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.M.; Azam, L.; Staheli, S.; Dowell, C.; Lindstrom, J.M.; Kuryatov, A.; Garrett, J.E.; Marks, M.J.; Whiteaker, P. Analogs of α-Conotoxin MII Are Selective for α6-Containing Nicotinic Acetylcholine Receptors. Mol. Pharmacol. 2004, 65, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Booker, T.K.; Allen, R.S.; Grady, S.R.; Whiteaker, P.; Marks, M.J.; Salminen, O.; Tritto, T.; Butt, C.M.; Allen, W.R.; et al. The 3 Nicotinic Receptor Subunit: A Component of-Conotoxin MII-Binding Nicotinic Acetylcholine Receptors that Modulate Dopamine Release and Related Behaviors. J. Neurosci. 2003, 23, 11045–11053. [Google Scholar] [CrossRef] [PubMed]
- Champtiaux, N.; Gotti, C.; Cordero-Erausquin, M.; David, D.J.; Przybylski, C.; Léna, C.; Clementi, F.; Moretti, M.; Rossi, F.M.; Le Novère, N.; et al. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J. Neurosci. 2003, 23, 7820–7829. [Google Scholar] [CrossRef]
- Salminen, O.; Drapeau, J.A.; McIntosh, J.M.; Collins, A.C.; Marks, M.J.; Grady, S.R. Pharmacology of α-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol. Pharmacol. 2007, 71, 1563–1571. [Google Scholar] [CrossRef]
- Grady, S.R.; Salminen, O.; McIntosh, J.M.; Marks, M.J.; Collins, A.C. Mouse Striatal Dopamine Nerve Terminals Express α4α5β2 and Two Stoichiometric Forms of α4β2*-Nicotinic Acetylcholine Receptors. J. Mol. Neurosci. 2010, 40, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Göldner, F.M.; Dineley, K.T.; Patrick, J.W. Immunohistochemical localization of the nicotinic acetylcholine receptor subunit α6 to dopaminergic neurons in the substantia nigra and ventral tegmental area. NeuroReport 1997, 8, 2739–2742. [Google Scholar] [CrossRef] [PubMed]
- Vincler, M.A.; Eisenach, J.C. Immunocytochemical localization of the α3, α4, α5, α7, β2, β3 and β4 nicotinic acetylcholine receptor subunits in the locus coeruleus of the rat. Brain Res. 2003, 974, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Léna, C.; D’exaerde, A.d.K.; Cordero-Erausquin, M.; Le Novère, N.; Arroyo-Jimenez, M.d.M.; Changeux, J.-P. Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 12126–12131. [Google Scholar] [CrossRef] [PubMed]
- Clarke, P.; Reuben, M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: Mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br. J. Pharmacol. 1996, 117, 595–606. [Google Scholar] [CrossRef]
- Lotfipour, S.; Leonard, G.; Perron, M.; Pike, B.; Richer, L.; Séguin, J.R.; Toro, R.; Veillette, S.; Pausova, Z.; Paus, T. Prenatal exposure to maternal cigarette smoking interacts with a polymorphism in the α6 nicotinic acetylcholine receptor gene to influence drug use and striatum volume in adolescence. Mol. Psychiatry 2010, 15, 6–8. [Google Scholar] [CrossRef]
- Pugach, O.; Cannon, D.S.; Weiss, R.B.; Hedeker, D.; Mermelstein, R.J. Classification Tree Analysis as a Method for Uncovering Relations BetweenCHRNA5A3B4andCHRNB3A6in Predicting Smoking Progression in Adolescent Smokers. Nicotine Tob. Res. 2017, 19, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Hoft, N.R.; Corley, R.P.; McQueen, M.B.; Huizinga, D.; Menard, S.; Ehringer, M.A. SNPs in CHRNA6 and CHRNB3 are associated with alcohol consumption in a nationally representative sample. Genes Brain Behav. 2009, 8, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, J.S.; Haberstick, B.C.; Schlaepfer, I.; Collins, A.C.; Corley, R.P.; Crowley, T.J.; Hewitt, J.K.; Hopfer, C.J.; Lessem, J.; McQueen, M.B.; et al. The neuronal nicotinic receptor subunit genes (CHRNA6 and CHRNB3) are associated with subjective responses to tobacco. Hum. Mol. Genet. 2008, 17, 724–734. [Google Scholar] [CrossRef]
- DiFranza, J.R.; Rigotti, N.A.; McNeill, A.D.; Ockene, J.K.; Savageau, J.A.; Cyr, D.S.; Coleman, M. Initial symptoms of nicotine dependence in adolescents. Tob. Control 2000, 9, 313–319. [Google Scholar] [CrossRef]
- Lee, W.; Bergen, A.W.; Swan, G.E.; Li, D.; Liu, J.; Thomas, P.; Tyndale, R.F.; Benowitz, N.L.; Lerman, C.; Conti, D.V. Gender-stratified gene and gene–treatment interactions in smoking cessation. Pharm. J. 2012, 12, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Hoft, N.R.; Corley, R.P.; McQueen, M.B.; Schlaepfer, I.R.; Huizinga, D.; Ehringer, M.A. Genetic association of the CHRNA6 and CHRNB3 genes with tobacco dependence in a nationally representative sample. Neuropsychopharmacology 2009, 34, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Pedneault, M.; Labbe, A.; Roy-Gagnon, M.-H.; Low, N.C.; Dugas, E.; Engert, J.C.; O’Loughlin, J. The association between CHRN genetic variants and dizziness at first inhalation of cigarette smoke. Addict. Behav. 2014, 39, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Cannon, D.S.; Mermelstein, R.J.; Hedeker, D.; Coon, H.; Cook, E.H.; McMahon, W.M.; Hamil, C.; Dunn, D.; Weiss, R.B. Effect of neuronal nicotinic acetylcholine receptor genes (CHRN) on longitudinal cigarettes per day in adolescents and young adults. Nicotine Tob. Res. 2014, 16, 137–144. [Google Scholar] [CrossRef]
- Cardenas, A.; Bai, Y.; Heydary, Y.H.; Li, J.; Leslie, F.M.; Lotfipour, S. Sex- and Genotype-Dependent Nicotine-Induced Behaviors in Adolescent Rats with a Human Polymorphism (rs2304297) in the 3′-UTR of the CHRNA6 Gene. Int. J. Mol. Sci. 2022, 23, 3145. [Google Scholar] [CrossRef]
- Carreño, D.; Lotfipour, S. Sex- and genotype-dependent nicotine plus cue-primed reinstatement is enhanced in adolescent Sprague Dawley rats containing the human CHRNA6 3′-UTR polymorphism (rs2304297). Front. Psychiatry 2023, 13, 1064211. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Morales, M. The Brain on Drugs: From Reward to Addiction. Cell 2015, 162, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Dao, J.M.; McQuown, S.C.; Loughlin, S.E.; Belluzzi, J.D.; Leslie, F.M. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology 2011, 36, 1319–1331. [Google Scholar] [CrossRef]
- Azam, L.; Chen, Y.; Leslie, F. Developmental Regulation of Nicotinic Acetylcholine receptors within midbrain dopamine neurons. Neuroscience 2007, 144, 1347–1360. [Google Scholar] [CrossRef]
- Venniro, M.; Caprioli, D.; Shaham, Y. Animal models of drug relapse and craving: From drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog. Brain Res. 2016, 224, 25–52. [Google Scholar] [CrossRef]
- Shaham, Y.; Shalev, U.; Lu, L.; de Wit, H.; Stewart, J. The reinstatement model of drug relapse: History, methodology and major findings. Psychopharmacology 2003, 168, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Palombo, P.; Leao, R.M.; Bianchi, P.C.; de Oliveira, P.E.C.; Planeta, C.d.S.; Cruz, F.C. Inactivation of the Prelimbic Cortex Impairs the Context-Induced Reinstatement of Ethanol Seeking. Front. Pharmacol. 2017, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Epstein, D.H.; Preston, K.L.; Stewart, J.; Shaham, Y. Toward a model of drug relapse: An assessment of the validity of the reinstatement procedure. Psychopharmacology 2006, 189, 1–16. [Google Scholar] [CrossRef] [PubMed]
- McFarland, K.; Kalivas, P.W. The Circuitry Mediating Cocaine-Induced Reinstatement of Drug-Seeking Behavior. J. Neurosci. 2001, 21, 8655–8663. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Yu, D.; Bi, Y.; Wang, R.; Li, M.; Zhang, Y.; Dong, M.; Zhai, J.; Li, Y.; Lu, X.; et al. The left dorsolateral prefrontal cortex and caudate pathway: New evidence for cue-induced craving of smokers. Hum. Brain Mapp. 2017, 38, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- Robison, C.L.; Kazan, T.; Miller, R.L.; Cova, N.; Charntikov, S. Inactivation of posterior but not anterior dorsomedial caudate-putamen impedes learning with self-administered nicotine stimulus in male rats. Behav. Brain Res. 2021, 413, 113438. [Google Scholar] [CrossRef] [PubMed]
- Schiltz, C.A.; Kelley, A.E.; Landry, C.F. Contextual cues associated with nicotine administration increase arc mRNA ex-pression in corticolimbic areas of the rat brain. Eur. J. Neurosci. 2005, 21, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Kirch, D.G.; Gerhardt, G.A.; Shelton, R.C.; Freedman, R.; Wyatt, R.J. Effect of Chronic Nicotine Administration on Monoamine and Monoamine Metabolite Concentrations in Rat Brain. Clin. Neuropharmacol. 1987, 10, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, D.; Schroeder, J.P. There and Back Again: A Tale of Norepinephrine and Drug Addiction. Neuropsychopharmacology 2007, 32, 1433–1451. [Google Scholar] [CrossRef]
- Gresch, P.J.; Sved, A.F.; Zigmond, M.J.; Finlay, J.M. Local Influence of Endogenous Norepinephrine on Extracellular Dopamine in Rat Medial Prefrontal Cortex. J. Neurochem. 1995, 65, 111–116. [Google Scholar] [CrossRef]
- Willuhn, I.; Wanat, M.J.; Clark, J.J.; Phillips, P.E.M. Dopamine Signaling in the Nucleus Accumbens of Animals Self-Administering Drugs of Abuse. Curr. Top Behav. Neurosci. 2010, 3, 29–71. [Google Scholar] [CrossRef]
- Bossert, J.M.; Poles, G.C.; Wihbey, K.A.; Koya, E.; Shaham, Y. Differential Effects of Blockade of Dopamine D1-Family Receptors in Nucleus Accumbens Core or Shell on Reinstatement of Heroin Seeking Induced by Contextual and Discrete Cues. J. Neurosci. 2007, 27, 12655–12663. [Google Scholar] [CrossRef]
- Bossert, J.M.; Stern, A.L.; Theberge, F.R.M.; Cifani, C.; Koya, E.; Hope, B.T.; Shaham, Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat. Neurosci. 2011, 14, 420–422. [Google Scholar] [CrossRef]
- Bossert, J.M.; Gray, S.M.; Lu, L.; Shaham, Y. Activation of Group II Metabotropic Glutamate Receptors in the Nucleus Accumbens Shell Attenuates Context-Induced Relapse to Heroin Seeking. Neuropsychopharmacology 2006, 31, 2197–2209. [Google Scholar] [CrossRef]
- Markou, A.; Li, J.; Tse, K.; Li, X. Cue-induced nicotine-seeking behavior after withdrawal with or without extinction in rats. Addict. Biol. 2018, 23, 111–119. [Google Scholar] [CrossRef]
- Pons, S.; Fattore, L.; Cossu, G.; Tolu, S.; Porcu, E.; McIntosh, J.M.; Changeux, J.P.; Maskos, U.; Fratta, W. Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J. Neurosci. 2008, 28, 12318–12327. [Google Scholar] [CrossRef]
- Ehringer, M.A.; McQueen, M.B.; Hoft, N.R.; Saccone, N.L.; Stitzel, J.A.; Wang, J.C.; Bierut, L.J. Association of CHRN genes with “dizziness” to tobacco. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2010, 153, 600–609. [Google Scholar] [CrossRef]
- Powers, M.S.; Broderick, H.J.; Drenan, R.M.; Chester, J.A. Nicotinic acetylcholine receptors containing α6 subunits contribute to alcohol reward-related behaviours. Genes Brain Behav. 2013, 12, 543–553. [Google Scholar] [CrossRef]
- Mackey, E.D.; Engle, S.E.; Kim, M.R.; O’Neill, H.C.; Wageman, C.R.; Patzlaff, N.E.; Wang, Y.; Grady, S.R.; McIntosh, J.M.; Marks, M.J.; et al. 6* Nicotinic Acetylcholine Receptor Expression and Function in a Visual Salience Circuit. J. Neurosci. 2012, 32, 10226–10237. [Google Scholar] [CrossRef]
- Jackson, K.J.; McIntosh, J.M.; Brunzell, D.H.; Sanjakdar, S.S.; Damaj, M.I. The Role of 6-Containing Nicotinic Acetylcholine Receptors in Nicotine Reward and Withdrawal. J. Pharmacol. Exp. Ther. 2009, 331, 547–554. [Google Scholar] [CrossRef]
- Drenan, R.M.; Grady, S.R.; Whiteaker, P.; McClure-Begley, T.; McKinney, S.; Miwa, J.M.; Bupp, S.; Heintz, N.; McIntosh, J.M.; Bencherif, M.; et al. In Vivo Activation of Midbrain Dopamine Neurons via Sensitized, High-Affinity α6∗ Nicotinic Acetylcholine Receptors. Neuron 2008, 60, 123–136. [Google Scholar] [CrossRef]
- Drenan, R.M.; Grady, S.R.; Steele, A.D.; McKinney, S.; Patzlaff, N.E.; McIntosh, J.M.; Marks, M.J.; Miwa, J.M.; Lester, H.A. Cholinergic Modulation of Locomotion and Striatal Dopamine Release Is Mediated by 6 4* Nicotinic Acetylcholine Receptors. J. Neurosci. 2010, 30, 9877–9889. [Google Scholar] [CrossRef]
- Cohen, B.; Mackey, E.; Grady, S.; Mckinney, S.; Patzlaff, N.; Wageman, C.; Mcintosh, J.; Marks, M.; Lester, H.; Drenan, R. Nicotinic cholinergic mechanisms causing elevated dopamine release and abnormal locomotor behavior. Neuroscience 2012, 200, 31–41. [Google Scholar] [CrossRef]
- Azam, L.; McIntosh, J.M. Alpha-conotoxins as pharmacological probes of nicotinic acetylcholine receptors. Acta Pharmacol. Sin. 2009, 30, 771–783. [Google Scholar] [CrossRef]
- Azam, L.; Maskos, U.; Changeux, J.-P.; Dowell, C.D.; Christensen, S.; De Biasi, M.; McIntosh, J.M.; Barloscio, D.; Cerri, E.; Domenici, L.; et al. α-Conotoxin BuIA[T5A;P6O]: A novel ligand that discriminates between α6β4 and α6β2 nicotinic acetylcholine receptors and blocks nicotine-stimulated norepinephrine release. FASEB J. 2010, 24, 5113–5123. [Google Scholar] [CrossRef]
- Lenoir, M.; Starosciak, A.K.; Ledon, J.; Booth, C.; Zakharova, E.; Wade, D.; Vignoli, B.; Izenwasser, S. Sex differences in conditioned nicotine reward are age-specific. Pharmacol. Biochem. Behav. 2015, 132, 56–62. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 4th ed.; Elsevier Academic Press: London, UK, 1989. [Google Scholar]
- Tian, G.; Hui, M.; Macchia, D.; Derdeyn, P.; Rogers, A.; Hubbard, E.; Liu, C.; Vasquez, J.J.; Taniguchi, L.; Bartas, K.; et al. An extended amygdala-midbrain circuit controlling cocaine withdrawal-induced anxiety and reinstatement. Cell Rep. 2022, 39, 110775. [Google Scholar] [CrossRef]
- Capriles, N.; Rodaros, D.; Sorge, R.E.; Stewart, J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology 2003, 168, 66–74. [Google Scholar] [CrossRef]
- Carreño, D.; Lotfipour, S. Male and Female Sprague Dawley Rats Exhibit Equivalent Natural Reward, Nicotine Self-Administration, Extinction, and Reinstatement During Adolescent-Initiated Behaviors. Nicotine Tob. Res. 2023, 25, 1039–1046. [Google Scholar] [CrossRef]
- Costello, M.R.; Reynaga, D.D.; Mojica, C.Y.; Zaveri, N.T.; Belluzzi, J.D.; Leslie, F.M. Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats. Neuropsychopharmacology 2014, 39, 1843–1851. [Google Scholar] [CrossRef]
- Cross, S.J.; Reynaga, D.D.; Cano, M.; Belluzzi, J.D.; Zaveri, N.T.; Leslie, F.M. Differences in mechanisms underlying reinstatement of cigarette smoke extract- and nicotine-seeking behavior in rats. Neuropharmacology 2020, 162, 107846. [Google Scholar] [CrossRef]
- Belluzzi, J.D.; Wang, R.; Leslie, F.M. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology 2005, 30, 705–712. [Google Scholar] [CrossRef]
- Gellner, C.A.; Belluzzi, J.D.; Leslie, F.M. Self-administration of nicotine and cigarette smoke extract in adolescent and adult rats. Neuropharmacology 2016, 109, 247–253. [Google Scholar] [CrossRef]
- Richardson, N.R.; Roberts, D.C.S. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods 1996, 66, 1–11. [Google Scholar] [CrossRef]

| HVA/DA | ||||||
|---|---|---|---|---|---|---|
| α6CC | α6GG | |||||
| Adolescents | Adults | Reinstatement | Adolescents | Adults | Reinstatement | |
| mPFC | 0.63 ± 0.13 | 0.45 ± 0.12 | 3.81 ± 1.57 **++ | 0.65 ± 0.13 | 0.6 ± 0.12 | 2.86 ± 1.06 *+ |
| dCPu | 0.05 ± 0.01 | 0.07 ± 0.01 | 0.03 ± 0.01 ++ | 0.04 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.00 ++ |
| NAc Core | 0.04 ± 0.01 | 0.09 ± 0.01 | 0.07 ± 0.01 | 0.03 ± 0.01 | 0.11 ± 0.01 | 0.06 ± 0.01 ++ |
| NAc Shell | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.15 ± 0.11 | 0.05 ± 0.01 | 0.04 ± 0.01 | 0.29 ± 0.07 ***+++ |
| BLA | 0.14 ± 0.03 | 0.06 ± 0.03 | 0.31 ± 0.15 | 0.14 ± 0.03 | 0.1 ± 0.03 | 0.35 ± 0.10 ++ |
| VTA | 0.08 ± 0.02 | 0.1 ± 0.02 | 0.3 ± 0.33 | 0.13 ± 0.02 | 0.11 ± 0.02 | 0.62 ± 0.20 ***+++ |
| IPN | 1.22 ± 0.32 | 0.25 ± 0.33 | 5.27 ± 1.82 **+++ | 1.06 ± 0.32 | 0.38 ± 0.32 | 2.24 ± 0.86 + |
| LC | 1.08 ± 0.40 | 0.56 ± 0.40 | 5.27 ± 3.17 + | 1.16 ± 0.40 | 0.91 ± 0.40 | 6.86 ± 1.94 ***+++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreño, D.; Facundo, A.; Nguyen, M.T.T.; Lotfipour, S. Dopamine and Norepinephrine Tissue Levels in the Developing Limbic Brain Are Impacted by the Human CHRNA6 3′-UTR Single-Nucleotide Polymorphism (rs2304297) in Rats. Int. J. Mol. Sci. 2024, 25, 3676. https://doi.org/10.3390/ijms25073676
Carreño D, Facundo A, Nguyen MTT, Lotfipour S. Dopamine and Norepinephrine Tissue Levels in the Developing Limbic Brain Are Impacted by the Human CHRNA6 3′-UTR Single-Nucleotide Polymorphism (rs2304297) in Rats. International Journal of Molecular Sciences. 2024; 25(7):3676. https://doi.org/10.3390/ijms25073676
Chicago/Turabian StyleCarreño, Diana, Antonella Facundo, My Trang Thi Nguyen, and Shahrdad Lotfipour. 2024. "Dopamine and Norepinephrine Tissue Levels in the Developing Limbic Brain Are Impacted by the Human CHRNA6 3′-UTR Single-Nucleotide Polymorphism (rs2304297) in Rats" International Journal of Molecular Sciences 25, no. 7: 3676. https://doi.org/10.3390/ijms25073676
APA StyleCarreño, D., Facundo, A., Nguyen, M. T. T., & Lotfipour, S. (2024). Dopamine and Norepinephrine Tissue Levels in the Developing Limbic Brain Are Impacted by the Human CHRNA6 3′-UTR Single-Nucleotide Polymorphism (rs2304297) in Rats. International Journal of Molecular Sciences, 25(7), 3676. https://doi.org/10.3390/ijms25073676





